Abstract
A joint moment also causes motion at other joints of the body. This joint coupling-perspective allows more insight into two age-related phenomena during gait. First, whether increased hip kinetic output compensates for decreased ankle kinetic output during positive joint work. Second, whether preserved joint kinetic patterns during negative joint work in older age have any functional implication. Therefore, we examined how age and surface inclination affect joint moment strategies to accelerate and/or decelerate individual leg joints during walking. Healthy young (age: 22.5±4.1 years, n=18) and older (age: 76.0±5.7 years, n=22) adults walked at 1.4 m/s on a split-belt instrumented treadmill at three grades (0%, 10%, −10%). Lower-extremity moment-induced angular accelerations were calculated for the hip (0% and 10%) and knee (0% and −10%) joints. During level and uphill walking, both age groups showed comparable ankle moment-induced ipsilateral (p=0.774) and contralateral (p=0.047) hip accelerations, although older adults generated lower ankle moments in late stance. However, ankle moment-induced contralateral hip accelerations were smaller (p=0.001) in an older adult subgroup (n=13) who showed larger hip extension moments in early stance than youngsss adults. During level and downhill walking, leg joint moment-induced knee accelerations were unaffected by age (all p>0.05). These findings suggest that during level and uphill walking increased hip flexor mechanical output in older adults does not arise from reduced ankle moments, contrary to increased hip extensor mechanical output. Additionally, results during level and downhill walking imply that preserved eccentric knee extensor function is important in maintaining knee stabilization in older age.
Keywords: induced acceleration analysis, eccentric, concentric, gait, aging
Introduction
Joint motion results from the net moment generated by muscles spanning a joint as well as from net moments at more distal and proximal joints. This mechanism is the result of the dynamic coupling between segments. Zajac and Gordon (1989) determined intersegmental coupling by induced acceleration analysis (IAA) and showed that moments arising from the uni-articular soleus muscle accelerate both ankle and knee into extension (Zajac & Gordon, 1989). IAA provides insights into inter-joint moment effects on motion at any joint, which cannot be revealed or quantified using conventional inverse dynamics analysis, thus enriching our current understanding of inter-joint moment coordination during movement (Moniz-Pereira et al. 2018; Siegel et al. 2006; Riley et al. 2001; Kepple et al. 1997).
When walking at similar speeds, older compared with young adults show decreased ankle joint kinetics (i.e., moment, power, work) and increased hip joint kinetics during concentric muscle function (Anderson & Madigan, 2014; DeVita & Hortobagyi, 2000; McGibbon, 2003; Silder et al., 2008). These age-related differences, which are magnified by walking speed and surface incline (Waanders et al. 2018; Franz and Kram 2014; Anderson & Madigan 2014; Silder et al. 2008), are most likely mediated by leg strength and other factors caused by various age-associated biological changes (see Hortobagyi et al., 2016). The agerelated compensation by the hip musculature during gait, referred to as an ankle-hip tradeoff, is further supported by simulation studies (Goldberg & Neptune, 2007; van der Krogt et al., 2012), clinical studies (Nadeau et al., 1999; Waterval et al., 2018), and studies where participants deliberately walk with altered ankle power outputs (Browne & Franz, 2019; Lewis & Ferris, 2008). This age-related hip compensation is expected given that, during gait, plantarflexion occurs at the same time as ipsilateral hip flexion and contralateral hip extension. These actions also share common functions, i.e., ipsilateral plantarflexion and hip flexion both occur during late stance, and both contribute to body propulsion and leg swing (Neptune et al., 2001; Sadeghi et al., 2001). In addition, plantarflexion and contralateral hip extension both occur during double-support and both contribute to propulsion (Kepple et al., 1997; Pickle et al., 2016). However, little empirical data conclusively demonstrate that the hip moments compensate for the lower ankle joint moments. IAA, unlike inverse dynamics, can be used to understand the direct, instantaneous effects of the ankle and hip moments on hip motion and whether these effects are age-related. We hypothesize that during level walking the ankle moments in older adults would induce lower hip joint angular accelerations than in young adults. We also hypothesize that this age effect would become larger during uphill walking.
In contrast to concentric function, joint kinetics during eccentric muscle function are largely unaffected by advancing age, even during downhill walking (Waanders et al., 2019). However, its functional significance is unclear. Knee flexion deceleration is attributed to eccentric knee extensor function, based on its amount of energy absorbed (Montgomery & Grabowski, 2018; Alexander et al. 2017; Kuster et al. 1995), and likely helps decelerate the downward moving center of mass (Pickle et al., 2016). Another inference from inverse dynamics results is that hip and ankle moments assist in knee flexion control (Rose & Gamble, 2006), while these moments also remain unchanged in older adults during downhill walking (Waanders et al., 2019). Currently it is unclear to what extent the knee moment, and also proximal and distal moments, contribute to knee flexion deceleration. IAA allows us to quantify how age and surface inclination affect the inter-joint moment strategy for knee flexion control. Increasing our understanding of how young and older adults control knee flexion under different biomechanical demands during walking is functionally relevant because gait tasks become more hazardous with increasing descent (Startzell et al. 2000; Redfern & DiPasquale 1997). We hypothesize that the effects of individual joint moments on knee flexion deceleration during weight acceptance in both level and downhill walking is unaffected by age. The purpose of this study was to examine how age and surface inclination affect joint moment strategies to accelerate and/or decelerate individual leg joints during walking.
Methods
Participants
Prospective participants were screened using a telephone interview. Inclusion criteria were: aged 18–35 (young) or 65+ (older) years; able to walk without an assistive aid; no current lower-extremity injury; not taking medication that causes dizziness; no score below 24 on the Mini-Mental State Examination, which would indicate mild cognitive impairment (Folstein et al., 1975). Participants provided written informed consent. The study was approved by the University of North Carolina Institutional Review Board (#16–3217). N=18 young and n=22 older adults participated; all were mobility independent (Short Physical Performance Battery score ≥ 9) (Guralnik et al., 1994) (Table 1).
Table 1.
Participant characteristics
| Young (8 M, 9 F) |
Older (9 M, 13 F) |
|
|---|---|---|
| Age, years | 22.5 ± 4.1 | 76.0 ± 5.7 |
| Body height, m | 1.78 ± 0.08 | 1.69 ± 0.09 |
| Body weight, kg | 72.3 ± 12.5 | 69.5 ± 10.5 |
| BMI, kg/m2 | 22.8 ± 2.7 | 24.1 ± 2.8 |
| MMSE score | 29.8 ± 0.5 | 29.5 ± 0.8 |
| SPPB score | 11.9 ± 0.2 | 11.1 ± 0.9 |
Values are mean ± SD. MMSE: Mini-Mental State Examination, SPPB: Short Physical Performance Battery
Instrumentation and treadmill walking protocol
Participants first walked for five minutes at 1.2 m/s on a split-belt instrumented treadmill (Bertec Corp., Columbus, OH, USA) to warm up their muscles and become familiar with equipment and safety harness. Participants then walked for one minute at 1.4 m/s at each grade in a fixed order (i.e. 0%, 10%, −10%) without reporting difficulty, with rest provided between conditions as needed to avoid fatigue. This speed was selected as it was similar to the subjects’ preferred over-ground walking speed (young: 1.44±0.18 m/s, older: 1.34±0.22 m/s) as part of a larger protocol (Waanders et al., 2019). Continuously during each condition, bilateral ground reaction forces (GRFs) were collected at 960 Hz and an 8-camera passive motion capture system recorded marker positions at 120 Hz (Vicon, Centennial, CO, USA). Participants wore 36 reflective markers attached to lower-extremity landmarks (for details see Waanders et al., 2019).
Inverse kinematics and dynamics analyses
Raw GRF and marker position data from each trial were imported into a movement analysis software (Visual3D, C-Motion, Inc., Germantown, MD, USA) and low-pass filtered (4th-order Butterworth) with cutoff frequencies of 45 Hz and 6 Hz, respectively. Using marker position data and participant’s body mass and height, the lower-extremities were modelled as seven rigid segments (pelvis: cylinder shaped; thighs, shanks, and feet: cone shaped). Outcome variables were step length, duty cycle (for details see Waanders et al., 2019), and sagittal plane hip, knee, and ankle joint angles and net moments using inverse kinematics and dynamics.
Induced acceleration analysis
Segment positions, joint angles, and net joint moments were used as inputs for IAA performed using Visual3D. Equation 1 (Zajac & Gordon, 1989) was solved to estimate the instantaneous effect of individual net joint moments or gravity on joint angular accelerations and GRFs:
| (1) |
Where matrix contains generalized joint accelerations, inverse inertia matrix M−1 includes segmental positions and its inertial properties, and matrices T and G include all net joint moments and gravitational terms, respectively. Coriolis and centripetal terms were set to zero and not included in Eq. 1.
A seven-segment model (see segments above) was used with three rotational degreesof-freedom (DOFs) at the hip, one rotational DOF (flexion/extension) at the knee, and two rotational DOFs (flexion/extension, inversion/eversion) at the ankle. Segment masses and its inertial properties were based on subject’s body weight and height, regression equations (Dempster, 1955), and segment shape (Hanavan, 1964). During stance, the foot was constrained to the floor during foot-flat (‘fixed-foot’), but able to rotate around the foot’s medio-lateral axis passing through the center of pressure before and after foot-flat (‘free-foot’). This prevented foot translation into and over the floor. During leg swing, foot movement was unconstrained (Moniz-Pereira et al. 2018). Each net joint moment or gravity was separately entered into the model frame-by-frame across each gait cycle. All the remaining moments and gravity were set to zero, to estimate specific independent effects on the outcome variables: induced GRFs, hip, and knee angular accelerations (Zajac & Gordon, 1989). Within each walking condition for each participant, induced accelerations were extracted and averaged from five gait cycles, all identified after the 10th second of the trial to ensure stable movement patterns.
Outcome variable
A custom MATLAB-script was used for further analysis (Mathworks, Natick, MA, USA). Following the hypotheses, induced hip angular accelerations (level, uphill) were extracted during specific intervals (Figure 4) corresponding to joint work phases identified previously (Waanders et al., 2019). In addition, induced knee angular accelerations (level, downhill) were extracted during one interval, defined between the onset of heel strike and the end of energy absorption at the knee during weight acceptance. The induced joint angular accelerations and net joint moments were then integrated within these intervals to obtain joint angular velocity changes and joint angular impulses, then normalized to stride time since this was 4.0% shorter in older compared to young adults across walking slopes (Waanders et al., 2019), and used in the statistical analysis. Joint moment-induced joint angular velocities were obtained as these relate to power production (i.e., moment*angular velocity).
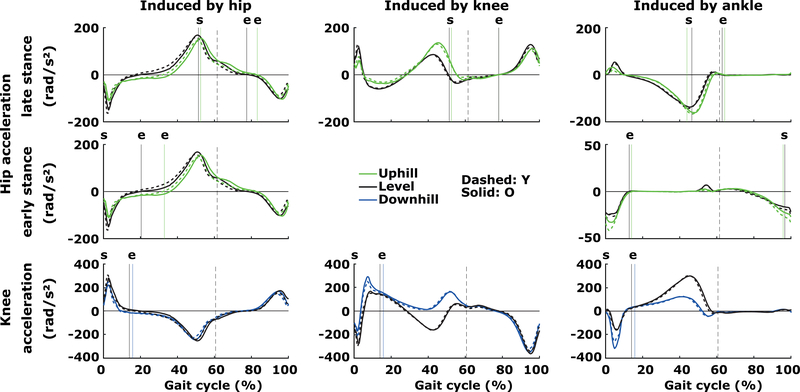
Figure 4.
Induced hip and knee angular accelerations across the gait cycle in young and older adults. Vertical dashed lines reflect toe-off during level walking and the colored, vertical lines reflect the start (‘s’) and end (‘e’) of the analyzed interval consistent with the direction (e.g. uphill) of walking for young adults only, for clarity.
To estimate the model’s accuracy, the sum of all the moment and gravity-induced vertical and horizontal GRFs was compared to the experimental GRFs for each participant and walking condition by calculating the coefficient of multiple correlation (CMC) (Ferrari et al., 2010) and average root mean square-difference (RMS) (see Supplementary Table S1). CMC assesses the similarity of two GRF time-series obtained using IAA and force plates respectively, within each gait cycle, taking into account the difference in offset, correlation, and gain. CMC-scores≥0.90 were considered representative and included in our analysis. The model’s sensitivity to the type of foot-floor constraint (fixed-foot vs. free-foot) and knee angle change was estimated using as outputs the ankle moment-induced peak hip acceleration during level and uphill walking, and knee-moment-induced peak knee acceleration during level and downhill walking, for n=5 participants.
Statistical analysis
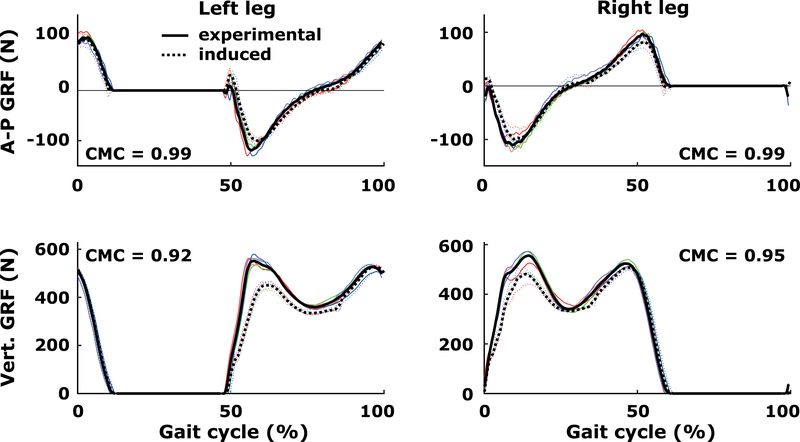
One young participant was excluded from the analyses, because of a CMC-score of 0.86 for one of the GRF-components during level walking (see also Figure 1). Seventeen out of 22 variables had normal distributions and equal variances, and so did five after logtransformation, according to the Shapiro-Wilks test and Levene’s test, respectively. Two-way mixed factorial ANOVAs were performed (between-participant factor age: young, older; within-participant factor slope (0%, ±10%) on gait kinematics and kinetics, and on joint moment-induced hip (separately for early and late stance) and knee angular velocity changes. Effect sizes of r=0.1, r=0.3, and r=0.5 represent small, moderate, and large effects, respectively (Cohen, 1992). A Holm-Bonferroni correction, which has greater power (1-β) than a simple Bonferroni correction, was performed to account for the number of mixed ANOVAs (i.e., m=8) to test the hypotheses (Holm, 1979). The correction works as follows: all p-values are sorted first, from the lowest to highest p-value. Second, if the first (lowest) p-value is greater than p*=alpha/m, the procedure is stopped and the first and all consecutive p-values are non-significant. Otherwise, the p-value is significant and the second p-value is compared to p*=alpha/(m-1), et cetera. Alpha was 0.05. IBM SPSS (SPSS Inc., Chicago, IL, USA) was used for the analyses. Detailed statistical results are reported only for the most relevant outcomes.
Figure 1.
Comparison of five waveforms (one per gait cycle) between experimental and induced GRF-components during level walking for one representative participant. RMS differences between the experimental and induced GRF-components during stance across participants were about 10% (for full reporting, see Supplementary Table 1) and deemed acceptable.
Results
The model was generally more sensitive to the modeled foot-floor contact than altered knee angle (see Figure 2 caption), as visualized for one representative subject.
Figure 2.
Model sensitivity analysis-outcomes shown for one representative subject. Per knee flexion angle-change (n=5 subjects), ankle moment-induced peak hip extension acceleration decreased by 7.8±0.2% (uphill, A) and 6.6±0.8% (level, B), and knee moment-induced peak knee extension acceleration decreased by 2.6±1.4% (level, C) and 2.6±0.7% (downhill, D). Between modeled foot-floor contacts (10 vs. 11 DOFs, i.e. fixed- vs. free-foot, n=5 subjects), ankle moment-induced peak hip extension acceleration were 18.2±21.7% (uphill, E) and 11.7±11.6% (level, F) different, and knee moment-induced peak knee extension acceleration were 6.3±10.6% (level, G) and 2.6±10.5% (downhill, H) different.
Age-related changes in gait kinematics and kinetics
On average across conditions, older compared with young adults took 3.7% shorter steps, and adopted 5.0° greater stance phase hip flexion and 4.4° lower peak plantarflexion during late stance (Figure 3) (all p<0.05). During level walking, older adults produced an 8.5% lower plantarflexion impulse and performed 13.9% less positive plantarflexion work in late stance than young adults; these differences were magnified during uphill walking (+16.6% and +19.1%, respectively) (all p<0.05). Across level and uphill walking, older adults performed more (p<0.05) positive hip extension (+19.0%) and flexion (+15.8%) work than young adults but both groups produced comparable (p>0.05) hip extension and flexion (0% and −2.5% difference, respectively) impulses. Therefore, a subgroup of older adults (n=13) was identified including those with 18.6±13.9% greater (F1,28=4.57, p=0.041, r=0.37) hip extension impulses than the young adults’ average across level and uphill walking. This subgroup produced a 13.8% lower plantarflexion impulse, performed 19.1% less positive plantarflexion work, more positive hip extension (+42.8%) and flexion (+15.0%) work (all p<0.05), but comparable hip flexion impulse (−3.7%) than young adults. Across level and downhill walking, both age groups showed comparable hip, knee, and ankle angular impulses (all p>0.05).
Figure 3.
Joint angles and net joint moments during walking in young (dashed lines) and older (solid lines) adults
Age-effects on induced hip angular accelerations in level and uphill walking
No significant age or age*slope-interaction effects were observed between the young (n=17) and older adults (n=22) across conditions (Figure 4, Table 2). Specifically, as the hip was extended during early stance, both groups showed comparable induced hip extensions by the ipsilateral hip moment (age: F1,37=3.28, p=0.078, r=0.29) and contralateral ankle moment (age: F1,37=4.22, p=0.047>p*, r=0.32). Both moment-induced effects increased from level to uphill walking, i.e., 16.5% and 52% respectively, in a comparable manner between groups (age×slope, hip: p>0.05; ankle: p=0.035>p*). As the hip flexed during late stance, both groups showed comparable induced hip flexion induced by the ipsilateral hip flexion moment (age: F1,37=5.38, p=0.026>p*, r=0.36), and hip extension induced by the ipsilateral ankle (age: F1,37=0.08, p=0.774, r=0.05) and knee extension moments (age: F1,37=7.33, p=0.010>0.008* (α/6), r=0.41). These moment-induced effects increased by 96% (ankle) and 7.2% (hip) or decreased by 128% (knee) from level to uphill walking, all in a comparable manner between groups (age×slope, ankle & hip: both p>0.05; age×slope, knee: p=0.043>p*).
Table 2.
Induced joint angular velocity changes due to net joint moments
| Induced velocity changes due to |
Group | Hip, early stance |
Hip, late stance |
Knee, weigh acceptance |
|||
|---|---|---|---|---|---|---|---|
| Level | Uphill | Level | Uphill | Level | Downhill | ||
| Hip moment | Y | −8.4±1.7 | −9.2±3.0# | 12.1±2.4 | 12.6±2.0 | 14.3±3.3 | 8.4±3.4# |
| O | −9.6±3.0 | −11.0±3.6 | 13.5±3.3 | 15.0±3.1 | 15.4±4.8 | 9.2±3.2 | |
| Subgr | −10.5±3.4 | −13.0±2.6ψ | |||||
| Knee moment | Y | −3.7±1.4 | −0.9±1.5# | 2.8±5.3 | 14.5±4.1# | ||
| O | −4.7±2.1 | −2.7±1.8 | 3.1±7.1 | 19.3±6.6 | |||
| Ankle moment | Y | −2.7±0.7 | −4.2±1.0# | −6.0±5.0 | −12.7±7.0# | −8.0±5.9 | −11.4±4.6# |
| O | −2.5±0.8 | −3.4±1.0 | −6.3±3.8 | −11.5±4.7 | −6.8±6.5 | −13.6±6.6 | |
| Subgr | −2.0±0.5 | −3.0±0.6ψ | |||||
Values are presented in mean±SD, radians/s. Negative (positive) values: flexion (extension) velocity change (i.e., acceleration). Y = young adults, O = older adults, Subgr = Subgroup of older adults showing the age-related redistribution of joint moments.
age effect (Y vs. Subgr): p < p* (explained in statistical analysis-section),
slope effect (level vs. non-level walking): p < 0.001
Age-related differences were observed between the young (n=17) and older adult subgroup (n=13) (Table 2). That is, across conditions, a 34% greater and 27% lower hip extension induced by the ipsilateral hip moment (age: F1,28=11.53, p=0.002<0.007* (α/7), r=0.54) and contralateral ankle moment (age: F1,28=16.91, p=0.001<0.006* (α/8), r=0.61) for the older subgroup, respectively. Both hip- and ankle moment-induced effects increased from level to uphill walking, 24% and 59% respectively, comparably across age groups (age×slope: both p>0.05).
Age-effects on induced knee angular accelerations in level and downhill walking
No significant age or age*slope-interaction effects were observed between the young (n=17) and older adults (n=22) across conditions (Figure 4, Table 2). As the knee flexed during the weight acceptance phase, knee extension induced by the ipsilateral hip moment (age: F1,37=0.68, p=0.414, r=0.13) and knee moment (age: F1,37=0.29, p=0.594, r=0.08), and knee flexion induced by the ankle moment (age: F1,37=0.09, p=0.768, r=0.05) were comparable between groups. These moment-induced effects increased by 756% (knee) and 69% (ankle) or decreased by 69% (hip) similarly between groups (age×slope, all p>0.05).
Discussion
We used IAA to reveal how age and surface inclination affect joint coupling mechanisms to accelerate and/or decelerate lower-extremity joints during walking. Unexpectedly, the accelerating effect of ankle moment in late stance on ipsilateral and contralateral hip motion was comparable between age groups across level and uphill walking. These effects also increased comparably between age groups from level to uphill walking, despite the lower ankle moment in older vs. young adults during level walking being even more pronounced during uphill walking. As hypothesized, the inter-joint moment effects on knee flexion during weight acceptance across level and downhill walking were unaffected by age.
The observed age-related decrease in ankle angular impulse and redistribution of positive leg joint work during level and uphill walking agrees with previous literature (Anderson & Madigan, 2014; DeVita & Hortobagyi, 2000; Franz & Kram, 2014; Silder et al., 2008). However, we and others (Franz & Kram, 2014) did not observe the age-related increased hip extensor impulse using a treadmill, as opposed to the over-ground walking studies cited above. Others suggested that the lower hip extension impulse during treadmill vs. over-ground walking in young adults (Riley et al., 2007) is more pronounced in older adults (Watt et al., 2010). However, further research should explore whether treadmill walking truly attenuates the age-difference in hip extensor impulse.
The IAA results suggest that the hip musculature itself contributes to increases in hip mechanical output in older adults, during walking. Across our entire cohort, the hip moments appeared not to compensate for lower ankle moments in older adults, as young and older adults showed comparable ankle-to-hip effects across level and uphill walking. This can be partially explained by the observed age-related differences in hip and ankle angles, given that joint kinematics are the only other independent input to IAA, alongside the joint moments. However, because older adults did not walk with a larger hip extension moment, which characterizes elderly gait, it is not possible to draw definitive cause-and-effect conclusions from these findings alone. Indeed, the older adult subgroup that showed an age-related increase in hip extensor impulse had lower ankle moment- and greater hip moment-induced hip accelerations compared to young adults, at least during the early stance phase. Nevertheless, the lower ankle-to-hip effect in the older adult subgroup accounted only partially for their increased hip-to-hip effect, suggesting that the contribution of the ankle moment to hip extension is small. We hypothesize that an ankle-hip tradeoff is more applicable to power generation. This is supported by a recent study observation that older adults can redistribute positive power between the hip and ankle joint during walking by altering their ankle angular velocity more than their ankle moment (Browne & Franz, 2019). In the present study, the ankle moment surprisingly induced almost no ipsilateral hip flexion acceleration during late stance. This may suggest that the plantarflexors as a whole contribute less to leg swing initiation than suggested by others (Neptune et al., 2001).
The hip musculature itself may contribute to age-related increases in hip mechanical output through postural differences. In spite of comparable hip moments, older compared with young adults showed larger contributions from the ipsilateral hip moment to hip accelerations during early (r=0.29) and late stance (r=0.36). This reflects the mediating effect of posture on function. Indeed, older compared with young adults averaged 5° greater peak hip flexion during stance. Although not measured in the present study, this may imply that older adults walked with greater trunk flexion, typical of elderly gait (Kerrigan et al., 1998; Miyazaki et al., 2013). Greater trunk flexion requires increased and prolonged hip extensor mechanical output to stabilize the trunk (Kluger et al., 2014; Leteneur et al., 2009). Trunk flexion may also shift the body’s center of mass forward, thereby increasing the demand for hip flexor power generation to initiate leg swing more vigorously. However, future studies should confirm whether decreasing trunk forward lean during gait also lowers hip joint kinetics in older compared to young adults.
We previously determined that lower-extremity joint kinetics during eccentric muscle function are similar between young and older adults even during downhill walking (Waanders et al., 2019). The present results show that the inter-joint moment mechanism used to stabilize the knee during weight acceptance is preserved in older age, with the knee moment as the largest contributor to knee stabilization during downhill walking. These results imply that preserved eccentric knee extensor function plays an important role in maintaining knee stabilization in older age. The large contribution by the knee moment was partly foreshadowed by large increases (young: 329%, old: 330%) in knee extensor impulse from level to downhill walking, also observed by others (Kuster et al., 1995; Redfern & DiPasquale, 1997). However, the current analysis also took the effects of the hip and ankle moment on knee motion into account. Surprisingly, the hip extensor moment contributed more to knee flexion deceleration than the knee moment during level walking, reflecting a more important role of the hip in stabilizing the knee in gait than previously inferred (Rose & Gamble, 2006). This role is supported by other research observations showing that higher hip extension moments during stiff versus soft landings caused less knee flexion in the former task (Devita & Skelly, 1992). Generally, our IAA findings at the knee agree well with the qualitative inferences from inverse dynamics results.
IAA has received some critique in that it is particularly sensitive to modeling decisions, such as the number of DOFs (Chen, 2006) and complexity of foot-floor interaction (Dorn et al., 2012), and results are difficult to validate (Silverman, 2017). Furthermore, IAA results represent instantaneous, isolated effects and do not account for past behavior effects. The present sensitivity analyses showed larger changes in induced joint angular accelerations when the number of DOFs changed compared to when knee angle is altered. However, the present modeled foot-floor interaction yields comparable results to more complex models, at least in the sagittal plane (Dorn et al., 2012). Additionally, results of the few experimental studies that performed functional electrical stimulation are in line with IAA results (Hunter et al., 2009; Stewart et al., 2007). The present results are limited to fit healthy elderly, because pathological gait can show compensatory inter-joint moment effects to control joint motion (Siegel et al., 2006, 2007). Lastly, a potential for condition ordering effects cannot be fully excluded.
In conclusion, the increased hip flexor mechanical output in older adults during level and uphill walking does not arise from reduced ankle moments, contrary to the hip extensors’ increased mechanical output. Finally, IAA revealed comparable inter-joint moment effects on knee flexion deceleration during walking between young and older adults, including a more important role of the hip moment than previously inferred from inverse dynamics results. The well-preserved knee-to-knee contribution in older age imply that preserved eccentric knee extensor function is important in maintaining knee stabilization.
Supplementary Material
Acknowledgements
We would like to thank Dr. Gregory Sawicki for the use of some laboratory equipment. This work was funded in part by the National Institutes of Health (grant no. R01AG051748) awarded to J. R. F.
Footnotes
statement no conflict of interest
The authors declare no conflicts of interest.
References
- Alexander N, Strutzenberger G, Ameshofer LM, & Schwameder H. (2017). Lower limb joint work and joint work contribution during downhill and uphill walking at different inclinations. Journal of Biomechanics, 61, 75–80. 10.1016/j.jbiomech.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Anderson DE, & Madigan ML (2014). Healthy older adults have insufficient hip range of motion and plantar flexor strength to walk like healthy young adults. Journal of Biomechanics, 47(5), 1104–1109. 10.1016/j.jbiomech.2013.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne MG, & Franz JR (2019). Ankle power biofeedback attenuates the distal-toproximal redistribution in older adults. Gait & Posture, 71, 44–49. 10.1016/j.gaitpost.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. (2006). Induced acceleration contributions to locomotion dynamics are not physically well defined. Gait & Posture, 23(1), 37–44. 10.1016/j.gaitpost.2004.11.016 [DOI] [PubMed] [Google Scholar]
- Cohen J. (1992). QUANTITATIVE METHODS IN PSYCHOLOGY: A Power Primer, 112(1), 155–159. [DOI] [PubMed] [Google Scholar]
- Dempster WT (1955). Space Requirements of the Seated Operator (WADC Technical report). Wright-Patterson Air Force Base, Ohio. [Google Scholar]
- DeVita P, & Hortobagyi T. (2000). Age causes a redistribution of joint torques and powers during gait. Journal of Applied Physiology (Bethesda, Md. : 1985), 88(5), 1804–1811. [DOI] [PubMed] [Google Scholar]
- Devita P, & Skelly WA (1992). Effect of landing stiffness on joint kinetics and energetics in the lower extremity. Medicine and Science in Sports and Exercise, 24(1), 108–115. [PubMed] [Google Scholar]
- Dorn TW, Lin Y-C, & Pandy MG (2012). Estimates of muscle function in human gait depend on how foot-ground contact is modelled. Computer Methods in Biomechanics and Biomedical Engineering, 15(6), 657–668. 10.1080/10255842.2011.554413 [DOI] [PubMed] [Google Scholar]
- Ferrari A, Cutti AG, & Cappello A. (2010). A new formulation of the coefficient of multiple correlation to assess the similarity of waveforms measured synchronously by different motion analysis protocols. Gait & Posture, 31(4), 540–542. 10.1016/j.gaitpost.2010.02.009 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Franz JR, & Kram R. (2014). Advanced age and the mechanics of uphill walking: a jointlevel, inverse dynamic analysis. Gait & Posture, 39(1), 135–140. 10.1016/j.gaitpost.2013.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EJ, & Neptune RR (2007). Compensatory strategies during normal walking in response to muscle weakness and increased hip joint stiffness. Gait & Posture, 25(3), 360–367. 10.1016/j.gaitpost.2006.04.009 [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, … Wallace RB (1994). A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of Gerontology, 49(2), M85–94. [DOI] [PubMed] [Google Scholar]
- Hanavan EP Jr (1964). A mathematical model of the human body. Air Force Aerospace Medical Research. [PubMed] [Google Scholar]
- Holm S. (1979). A Simple Sequentially Rejective Multiple Test Procedure. Scandinavian Journal of Statistics, 6(2), 65–70. 10.2307/4615733 [DOI] [Google Scholar]
- Hortobagyi T, Rider P, Gruber AH, & DeVita P. (2016). Age and muscle strength mediate the age-related biomechanical plasticity of gait. European Journal of Applied Physiology, 116(4), 805–814. 10.1007/s00421-015-3312-8 [DOI] [PubMed] [Google Scholar]
- Hunter BV, Thelen DG, & Dhaher YY (2009). A three-dimensional biomechanical evaluation of quadriceps and hamstrings function using electrical stimulation. IEEE Transactions on Neural Systems and Rehabilitation Engineering : A Publication of the IEEE Engineering in Medicine and Biology Society, 17(2), 167–175. 10.1109/TNSRE.2009.2014235 [DOI] [PubMed] [Google Scholar]
- Kepple TM, Siegel KL, & Stanhope SJ (1997). Relative contributions of the lower extremity joint moments to forward progression and support during gait. Gait and Posture, 6(1), 1–8. 10.1016/S0966-6362(96)01094-6 [DOI] [Google Scholar]
- Kerrigan DC, Todd MK, Della Croce U, Lipsitz LA, & Collins JJ (1998). Biomechanical gait alterations independent of speed in the healthy elderly: evidence for specific limiting impairments. Archives of Physical Medicine and Rehabilitation, 79(3), 317–322. [DOI] [PubMed] [Google Scholar]
- Kluger D, Major MJ, Fatone S, & Gard SA (2014). The effect of trunk flexion on lower-limb kinetics of able-bodied gait. Human Movement Science, 33, 395–403. 10.1016/j.humov.2013.12.006 [DOI] [PubMed] [Google Scholar]
- Kuster M, Sakurai S, & Wood GA (1995). Kinematic and kinetic comparison of downhill and level walking. Clinical Biomechanics (Bristol, Avon), 10(2), 79–84. [DOI] [PubMed] [Google Scholar]
- Leteneur S, Gillet C, Sadeghi H, Allard P, & Barbier F. (2009). Effect of trunk inclination on lower limb joint and lumbar moments in able men during the stance phase of gait. Clinical Biomechanics (Bristol, Avon), 24(2), 190–195. 10.1016/j.clinbiomech.2008.10.005 [DOI] [PubMed] [Google Scholar]
- Lewis CL, & Ferris DP (2008). Walking with increased ankle pushoff decreases hip muscle moments. Journal of Biomechanics, 41(10), 2082–2089. 10.1016/j.jbiomech.2008.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGibbon CA (2003). Toward a better understanding of gait changes with age and disablement: neuromuscular adaptation. Exercise and Sport Sciences Reviews, 31(2), 102–108. [DOI] [PubMed] [Google Scholar]
- Miyazaki J, Murata S, Horie J, Uematsu A, Hortobagyi T, & Suzuki S. (2013). Lumbar lordosis angle (LLA) and leg strength predict walking ability in elderly males. Archives of Gerontology and Geriatrics, 56(1), 141–147. 10.1016/j.archger.2012.09.004 [DOI] [PubMed] [Google Scholar]
- Moniz-Pereira V, Kepple TM, Cabral S, João F, & Veloso AP (2018). Joint moments’ contributions to vertically accelerate the center of mass during stair ambulation in the elderly: An induced acceleration approach. Journal of Biomechanics, 79, 105–111. 10.1016/j.jbiomech.2018.07.040 [DOI] [PubMed] [Google Scholar]
- Montgomery JR, & Grabowski AM (2018). The contributions of ankle, knee and hip joint work to individual leg work change during uphill and downhill walking over a range of speeds. Royal Society Open Science, 5(8), 180550. 10.1098/rsos.180550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau S, Gravel D, Arsenault AB, & Bourbonnais D. (1999). Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors. Clinical Biomechanics (Bristol, Avon), 14(2), 125–135. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, & Zajac FE (2001). Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. Journal of Biomechanics, 34(11), 1387–1398. 10.1016/S0021-9290(01)00105-1 [DOI] [PubMed] [Google Scholar]
- Pickle NT, Grabowski AM, Auyang AG, & Silverman AK (2016). The functional roles of muscles during sloped walking. Journal of Biomechanics, 49(14), 3244–3251. 10.1016/j.jbiomech.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley PO, Dellacroce U, & Kerrigan DC (2001). Effect of age on lower extremity joint moment contributions to gait speed. Journal of Biomechanics, 14, 264–270. [DOI] [PubMed] [Google Scholar]
- Riley PO, Paolini G, Della Croce U, Paylo KW, & Kerrigan DC (2007). A kinematic and kinetic comparison of overground and treadmill walking in healthy subjects. Gait & Posture, 26(1), 17–24. 10.1016/j.gaitpost.2006.07.003 [DOI] [PubMed] [Google Scholar]
- Rose J, & Gamble JG (2006). Human Walking (3rd ed.). Lippincott Williams & Wilkins. [Google Scholar]
- S.Redfern M, & DiPasquale J. (1997). Biomechanics of descending ramps. Gait & Posture, 6(2), 119–125. 10.1016/s0966-6362(97)01117-x [DOI] [Google Scholar]
- Sadeghi H, Sadeghi S, Prince F, Allard P, Labelle H, & Vaughan CL (2001). Functional roles of ankle and hip sagittal muscle moments in able-bodied gait. Clinical Biomechanics (Bristol, Avon), 16(8), 688–695. [DOI] [PubMed] [Google Scholar]
- Siegel KL, Kepple TM, & Stanhope SJ (2006). Using induced accelerations to understand knee stability during gait of individuals with muscle weakness. Gait & Posture, 23(4), 435–440. 10.1016/j.gaitpost.2005.05.007 [DOI] [PubMed] [Google Scholar]
- Siegel KL, Kepple TM, & Stanhope SJ (2007). A case study of gait compensations for hip muscle weakness in idiopathic inflammatory myopathy. Clinical Biomechanics (Bristol, Avon), 22(3), 319–326. 10.1016/j.clinbiomech.2006.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silder A, Heiderscheit B, & Thelen DG (2008). Active and passive contributions to joint kinetics during walking in older adults. Journal of Biomechanics, 41(7), 1520–1527. 10.1016/j.jbiomech.2008.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman AK (2017). Induced Acceleration and Power Analyses of Human Motion. Handbook of Human Motion, 1–18. 10.1007/978-3-319-30808-1_175-1 [DOI] [Google Scholar]
- Startzell JK, Owens DA, Mulfinger LM, & Cavanagh PR (2000). Stair negotiation in older people: a review. Journal of the American Geriatrics Society, 48(5), 567–580. [DOI] [PubMed] [Google Scholar]
- Stewart C, Postans N, Schwartz MH, Rozumalski A, & Roberts A. (2007). An exploration of the function of the triceps surae during normal gait using functional electrical stimulation. Gait & Posture, 26(4), 482–488. 10.1016/j.gaitpost.2006.12.001 [DOI] [PubMed] [Google Scholar]
- van der Krogt MM, Delp SL, & Schwartz MH (2012). How robust is human gait to muscle weakness? Gait & Posture, 36(1), 113–119. 10.1016/j.gaitpost.2012.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waanders JB, Hortobagyi T, Murgia A, DeVita P, & Franz JR (2019). Advanced Age Redistributes Positive but Not Negative Leg Joint Work during Walking. Medicine and Science in Sports and Exercise. 10.1249/MSS.0000000000001828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterval NFJ, Brehm M-A, Ploeger HE, Nollet F, & Harlaar J. (2018). Compensations in lower limb joint work during walking in response to unilateral calf muscle weakness. Gait & Posture, 66, 38–44. 10.1016/j.gaitpost.2018.08.016 [DOI] [PubMed] [Google Scholar]
- Watt JR, Franz JR, Jackson K, Dicharry J, Riley PO, & Kerrigan DC (2010). A three-dimensional kinematic and kinetic comparison of overground and treadmill walking in healthy elderly subjects. Clinical Biomechanics (Bristol, Avon), 25(5), 444–449. 10.1016/j.clinbiomech.2009.09.002 [DOI] [PubMed] [Google Scholar]
- Zajac FE, & Gordon ME (1989). Determining muscle’s force and action in multiarticular movement. Exercise and Sport Sciences Reviews, 17, 187–230. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.