Abstract
Background
Influenza causes a substantial burden worldwide, and current seasonal influenza vaccine has suboptimal effectiveness. To develop better, more broadly protective vaccines, a more thorough understanding is needed of how antibodies that target the influenza virus surface antigens, hemagglutinin (HA) (including head and stalk regions) and neuraminidase (NA), impact influenza illness and virus transmission.
Methods
We used a case-ascertained, community-based study of household influenza virus transmission set in Managua, Nicaragua. Using data from 170 reverse transcriptase–polymerase chain reaction (RT-PCR)–confirmed influenza virus A(H1N1)pdm infections and 45 household members with serologically confirmed infection, we examined the association of pre-existing NA, hemagglutination inhibiting, and HA stalk antibody levels and influenza viral shedding and disease duration using accelerated failure time models.
Results
Among RT-PCR–confirmed infections in adults, pre-existing anti-NA antibody levels ≥40 were associated with a 69% (95% confidence interval [CI], 34–85%) shortened shedding duration (mean, 1.0 vs 3.2 days). Neuraminidase antibody levels ≥80 were associated with further shortened shedding and significantly shortened symptom duration (influenza-like illness, 82%; 95% CI, 39–95%). Among RT-PCR–confirmed infections in children, hemagglutination inhibition titers ≥1:20 were associated with a 32% (95% CI, 13–47%) shortened shedding duration (mean, 3.9 vs 6.0 days).
Conclusions
Our results suggest that anti-NA antibodies play a large role in reducing influenza illness duration in adults and may impact transmission, most clearly among adults. Neuraminidase should be considered as an additional target in next-generation influenza virus vaccine development.
We found that antibodies against neuraminidase were associated with significantly shortened viral shedding, and among adults they were also associated with shortened symptom duration. These results support neuraminidase as a potential target of next-generation influenza virus vaccines.
Keywords: influenza, neuraminidase, hemagglutination inhibition (HAI), household, shedding
The burden of influenza is large, with an estimated annual global burden of 3–5 million severe cases and 290 000–650 000 deaths [1]. Current vaccine effectiveness is only 10–60% [2] and new formulations are needed each year, prompting a new push to develop a more effective and longer lasting influenza virus vaccine suitable for all age groups [3, 4]. In addition to preventing disease, next-generation vaccines might also aim to reduce influenza symptoms and virus transmission. Thus, a better understanding of how antibodies affect both illness duration and viral shedding is needed.
Influenza virus has 2 surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA). Current seasonal vaccines are designed to elicit antibody responses to the HA head but not specifically to NA or the HA stalk [5]. Neuraminidase evolves more slowly than the HA head, which could allow NA-based immunity to protect against otherwise drifted strains, making it an attractive vaccine target [6]. Anti-NA antibodies are also suggested to limit influenza disease once a person has been infected [6], potentially lessening severity and decreasing transmission, but evidence of this association is limited [5, 7].
Human transmission studies in natural settings offer a unique opportunity to study the effects of pre-existing antibody titers on influenza infection and symptoms. The Household Influenza Transmission Study (HITS) is a case-ascertained study of natural influenza transmission in households in Managua, Nicaragua [8]. Here we assess the effects of pre-existing influenza A(H1N1)pdm hemagglutination inhibiting (HI), anti-HA stalk, and anti-NA antibodies on viral shedding and symptom duration in children and adults.
METHODS
Ethics Statement
This study was approved by the institutional review boards at the Nicaraguan Ministry of Health and the University of Michigan. Informed consent or parental permission for minors was obtained from all participants. Assent was obtained for children aged 6 years and older.
Study Design and Population
The HITS is a case-ascertained study of households in the catchment area of the Health Center Sócrates Flores Vivas (HCSFV) in District II of Managua, the capital of Nicaragua. Briefly, influenza index cases were recruited at the HCSFV and through the ongoing Nicaraguan Pediatric Influenza Cohort Study [9], and their households were invited to enroll. Inclusion criteria for index cases in HITS were as follows: (1) a positive influenza QuickVue A+B rapid test (Quidel), (2) experienced onset of acute respiratory illness within the previous 48 hours, and (3) living with at least 1 other household member. Two seasons with influenza A(H1N1)pdm activity were included for this analysis: May–October 2013 and November–December 2015.
Data Collection
Demographic information and clinical history were collected at enrollment. Household members and index cases were monitored through home visits. Nasal and oropharyngeal swabs were collected at enrollment and every 2–3 days thereafter for a total of 5 visits over a period of 10–14 days. Daily symptom data were collected by study staff. Blood samples were collected from participants aged 6 months or older at enrollment and 30–45 days later. If participants sought care at the HCSFV while enrolled, data from the visit were recorded [9].
Laboratory Testing and Influenza Infection Definitions
Pooled nasal and oropharyngeal swabs maintained in viral transport media at 4°C and blood samples were sent within 48 hours to the Nicaraguan National Virology Laboratory at the Nicaraguan Ministry of Health. Real-time reverse transcriptase–polymerase chain reaction (RT-PCR) was performed on RNA extracted from the swab samples per validated Centers for Disease Control and Prevention protocols for influenza A(H1N1)pdm detection [10, 11].
Antibody levels were measured as hemagglutination inhibition (HAI) titers (reciprocals) and enzyme-linked immunosorbent assay (ELISA) areas under the curve (AUCs). HAI assays were performed following standard World Health Organization (WHO) protocols, using A/Nicaragua/1815/01/TR2/2013 (as a 6:2 reassortant with A/PR/8/34) as the antigen for 2013 because the HA head had drifted that year and A/Michigan/45/15 as an antigen for 2015 [12]. An initial sample dilution of 1:10 was used and serial 2-fold dilutions were made in 96-well plates. HAI titers were determined by visual detection of red blood cell agglutination in wells.
ELISAs were performed to determine the quantity of anti-HA stalk and anti-NA antibodies, as described previously, on the subset of participants who had sufficient sample volume for both baseline and convalescent draws [13]. For HA stalk, a chimeric HA was used, consisting of the stalk domain of A(H1N1)pdm A/California/04/09 and the head domain from an H6N1 virus (which has not infected humans and to which no antibodies should be present). For NA, the NA of A(H1N1)pdm A/California/04/09 was used. Both HA and NA were expressed as soluble proteins, maintaining correct protein folding, conformational epitopes, and enzymatic activity, as previously described [14, 15]. AUCs were calculated with GraphPad Prism and are hereafter referred to as antibody levels. All assays were performed by personnel who were blinded to the influenza status of samples. Geometric mean antibody levels were calculated from log2-transformed variables.
Influenza virus infections were defined in 2 ways, as those that were (1) RT-PCR positive for influenza A(H1N1)pdm and (2) RT-PCR positive or serologically confirmed for A(H1N1)pdm infection. Serologically confirmed infections were defined as a 4-fold or greater increase in HAI titer [16]. Since household contacts were tested for influenza every 2–3 days, it is possible that individuals may have shed influenza virus that was not detected. The second definition allowed us to examine the potential effects of the limit of viral shedding detection due to sampling frequency. Outcome values for nondetected shedding and symptoms were set to a nonzero value of 0.5 days for analysis.
Influenza Shedding and Symptom Duration
Influenza viral shedding duration was defined using the RT-PCR results of up to 6 samples, as the time from first RT-PCR–positive sample to shedding cessation. Because shedding cessation occurred in the interval between the last RT-PCR–positive and subsequent negative sample (interval censoring), it was defined by using the bounds of this interval, as previously described [17, 18]. Symptom duration was defined as time from symptom onset to resolution of influenza-like illness (ILI), cough, and runny nose. Influenza-like illness was defined as having fever with a cough or sore throat.
Statistical Methods
Parametric accelerated failure time (AFT) models were used to calculate event time ratios (ETRs), as done previously [15, 19], comparing disease duration in influenza infections with high and low pre-existing antibody levels. Weibull distributions were selected to account for censoring [20, 21]. Analyses were stratified by age, as children aged 0–14 years and adults aged 15–85 years, and were adjusted for age and sex. A series of cutoffs was used to categorize high and low HAI titers and anti-HA stalk and anti-NA ELISA AUCs: ≥1:20/20, 1:40/40, 1:80/80, 1:160/160, and 1:320/320 versus lower. Statistical analyses were conducted in R version 3.5.2 (https://www.R-project.org/), using the package “Survival” version 2.43.3 (https://CRAN.R-project.org/package=survival) to run the AFT models and to predict disease duration accounting for age and sex, “SurvRegCensCov” version 1.4 (https://CRAN.R-project.org/package=SurvRegCensCov) to convert the model output to ETRs, and “ggplot2” version 3.1.0 (http://ggplot2.org) for plotting.
RESULTS
Study Population
A total of 777 individuals in 161 households were enrolled in HITS over the 2013 and 2015 influenza seasons. In 2013, both influenza A(H3N2) and A(H1N1)pdm circulated, and in 2015, A(H1N1)pdm predominated (Supplementary Figure S1). An average of 4.3 swabs and a median of 10 days of symptom diaries were collected per participant. Index cases presented to the study health clinic, on average, 0.9 days from symptom onset. There were 91 A(H1N1)pdm index cases, all except 6 of whom were children aged 0–14 years, and a total of 196 RT-PCR–confirmed influenza A(H1N1)pdm infections. Two of the participants had coinfections with A(H3N2) and were excluded. Of the remaining participants, 170 had HAI results. An additional 45 had serologically confirmed infections, for a total of 215 influenza virus infections included in these analyses (Table 1, Supplementary Figure S2).
Table 1.
Participant Characteristics
| Characteristics | RT-PCR–Positive Only | RT-PCR–Positive and 4-fold HAI Increase | ||||||
|---|---|---|---|---|---|---|---|---|
| HAI Titer ≥40a | HAI Titer ≥ 40a | |||||||
| No | Yes | P | Total | No | Yes | P | Total | |
| Total N (row %) | 145 (85.3) | 18 (10.6) | 170 | 178 (82.8) | 29 (13.5) | 215 | ||
| Age 0–14 years | ||||||||
| n (row %) | 96 (81.3) | 16 (13.6) | 118 | 107 (78.7) | 22 (16.2) | 136 | ||
| Mean (SD), years | 5.6 (3.8) | 7.9 (4.5) | .03 | 5.9 (4.0) | 5.9 (4.0) | 7.6 (4.5) | .08 | 6.2 (4.2) |
| Sex, n (%) | ||||||||
| F | 46 (47.9) | 6 (37.5) | .62 | 55 (46.6) | 51 (47.7) | 8 (36.4) | .46 | 63 (46.3) |
| M | 50 (52.1) | 10 (62.5) | 63 (53.4) | 56 (52.3) | 14 (63.6) | 73 (53.7) | ||
| Type of case, n (%) | ||||||||
| Index | 59 (61.5) | 8 (50.0) | .56 | 73 (61.9) | 62 (57.9) | 10 (45.5) | .4 | 78 (57.4) |
| Secondary | 37 (38.5) | 8 (50.0) | 45 (38.1) | 45 (42.1) | 12 (54.5) | 58 (42.6) | ||
| Age ≥15 years | ||||||||
| n (row %) | 49 (94.2) | 2 (3.1) | 52 | 71 (89.9) | 7 (8.9) | 79 | ||
| Mean (SD), years | 37.7 (17.5) | 17.0 (2.8) | .11 | 36.8 (17.5) | 37.7 (17.7) | 36.0 (17.0) | .81 | 37.5 (17.4) |
| Sex, n (%) | ||||||||
| F | 37 (75.5) | 1 (50.0) | 1 | 38 (73.1) | 53 (74.6) | 6 (85.7) | .85 | 59 (74.7) |
| M | 12 (24.5) | 1 (50.0) | 14 (26.9) | 18 (25.4) | 1 (14.3) | 20 (25.3) | ||
| Type of case, n (%) | ||||||||
| Index | 3 (6.1) | 0 (0.0) | 1 | 4 (7.7) | 3 (4.2) | 0 (0.0) | 1 | 4 (5.1) |
| Secondary | 46 (93.9) | 2 (100.0) | 48 (92.3) | 68 (95.8) | 7 (100.0) | 75 (94.9) | ||
Abbreviations: ELISA, enzyme-linked immunosorbent assay; F, female; HAI, hemagglutination inhibition; M, male; RT-PCR, reverse transcriptase–polymerase chain reaction; SD, standard deviation
aColumns stratified by HAI titer may not add to total because some participants are missing HAI but had ELISA results.
Pre-existing Antibody Levels
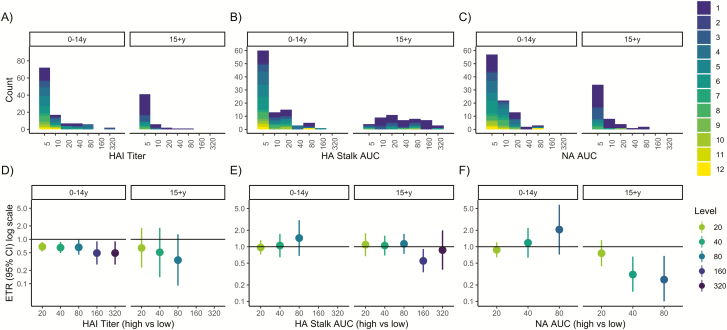
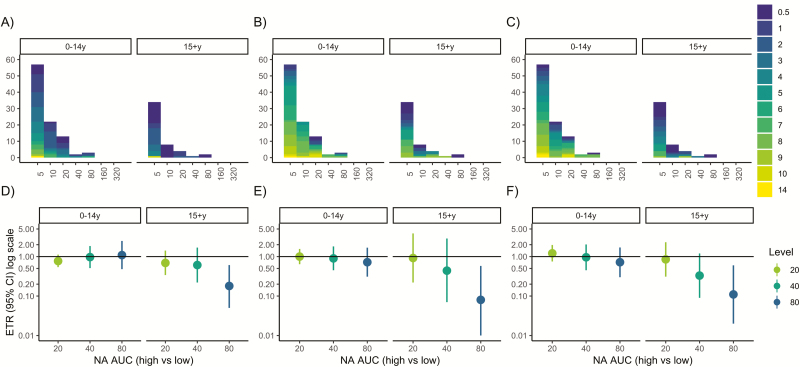
Pre-existing antibody levels were low in participants with influenza A(H1N1)pdm infections. Only 14% of children and 3% of adults had HAI titers of 1:40 or greater, 9% of children and 51% of adults had anti-HA stalk levels of 40 or greater, and 5% of children and 6% of adults had anti-NA levels of 40 or greater (Figure 1A–C). Serologically confirmed RT-PCR–negative infections (shown in dark purple, Figures 1 and 2) had higher antibody levels than RT-PCR–positive infections—geometric mean HAI titers: 1:10 vs 1:8.27; and HA stalk and NA ELISA AUCs: 23.5 vs 13.2 and 12.63 vs 10.87, respectively (Table 1). Children with HAI antibody titers of 1:40 or greater were older than those with lower titers (mean age, 8 vs 6 years), while adults with HAI antibody titers of 1:40 or greater were younger than those with titers less than 1:40 (mean age, 17 vs 37 years) (Table 1).
Figure 1.
Pre-existing antibodies and influenza virus shedding duration among reverse transcriptase–polymerase chain reaction–confirmed infections. A–C, Histograms of hemagglutination inhibition, anti-hemagglutinin stalk, and anti-neuraminidase antibody levels by age. Histograms are colored according to influenza virus shedding duration. D–F, Event time ratios from accelerated failure time models, adjusted for age and sex, compare the shedding duration in those with high (threshold or higher) vs low (lower than threshold) antibody levels. An ETR <1 corresponds to a shorter duration in the high antibody group. Abbreviations: AFT, accelerated failure time; AUC, area under the curve; CI, confidence interval; ETR, event time ratio; HA, hemagglutinin; HAI, hemagglutination inhibition; NA, neuraminidase; RT-PCR, reverse transcriptase–polymerase chain reaction.
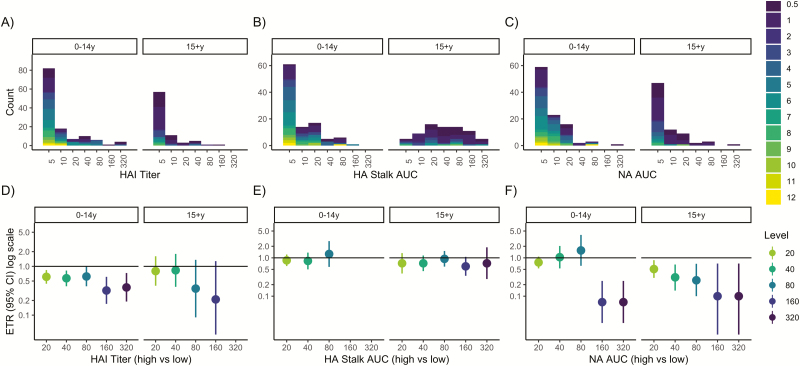
Figure 2.
Pre-existing antibody levels and influenza shedding duration among reverse transcriptase–polymerase chain reaction (RT-PCR)–and serologically confirmed infections. A–C, Histograms of hemagglutination inhibition, anti-hemagglutinin stalk, and anti-neuraminidase antibody levels by age. Histograms are colored according to influenza virus shedding duration. Shedding duration was set to 0.5 days for serologically confirmed, RT-PCR–negative individuals. D–F, event time ratios (ETRs) from accelerated failure time models, adjusted for age and sex, compare the shedding duration in those with high (threshold or higher) vs low (lower than threshold) antibody levels. An ETR <1 corresponds to a shorter duration in the high antibody group. Abbreviations: AFT, accelerated failure time; AUC, area under the curve; CI, confidence interval; ETR, event time ratio; HA, hemagglutinin; HAI, hemagglutination inhibition; NA, neuraminidase; RT-PCR, reverse transcriptase–polymerase chain reaction.
Pre-existing Antibodies and Shedding Duration in Adults With RT-PCR–Confirmed Infection
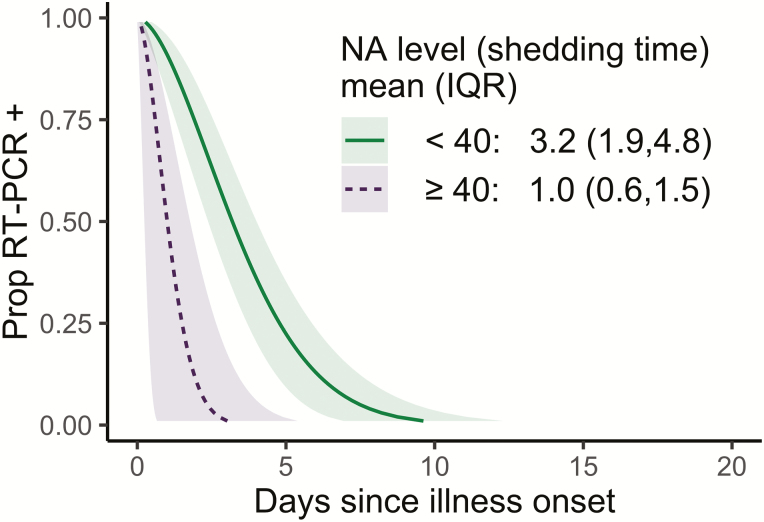
Anti-NA antibodies in adults were associated with decreased influenza shedding duration (Figure 1C, F). An anti-NA antibody level of 40 or higher (vs <40) was associated with a 69% decrease in shedding duration (adjusted ETR, 0.31; 95% confidence interval [CI], 0.15–0.66) (Supplementary Table S1), with predicted mean shedding times of 1.0 versus 3.2 days (Figure 3). Further, the relationship between anti-NA antibody level and shedding had a dose–response pattern, with antibody levels of 80 or higher associated with further shortening. We observed no associations with HAI or anti-HA stalk antibodies and shedding duration in adults (Figure 1).
Figure 3.
Predicted influenza virus shedding duration by high and low pre-existing neuraminidase antibody levels. Curves show the proportion of people still positive by reverse transcriptase-polymerase chain reaction (y axis) each day since the start of shedding (x axis) for an adult female of average age (36 y). Mean (interquartile range) shedding times are presented in the inset. Abbreviations: IQR, interquartile range; NA, neuraminidase; Prop, proportion; RT-PCR, reverse transcriptase–polymerase chain reaction.
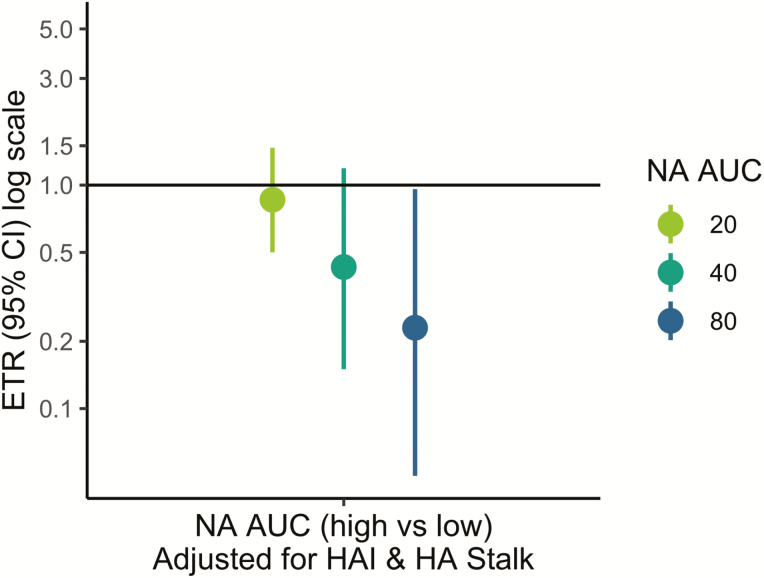
To assess whether anti-NA antibodies in adults were independently associated with shortened shedding, the same models were adjusted for HAI and anti-HA stalk antibodies (Figure 4). After adjusting, the same dose–response relationship was seen in adults and an anti-NA antibody level of 80 or greater was still associated with significantly shortened shedding duration (ETR, 0.23; 95% CI, 0.05–0.96) (Figure 4).
Figure 4.
Pre-existing neuraminidase (NA) antibodies and influenza virus shedding duration among reverse transcriptase–polymerase chain reaction–confirmed infections, adjusted for hemagglutination inhibition (HAI) and antihemagglutinin (HA) stalk antibodies. Event time ratios from accelerated failure time models compare the shedding duration in those with high (threshold or higher) vs low (lower than threshold) NA antibody levels. All models are adjusted for age and sex, and antibody-adjusted models are adjusted for HAI (titer ≥1:80) and anti-HA stalk antibodies (area under the curve ≥160). Abbreviations: AFT, accelerated failure time; AUC, area under the curve; CI, confidence interval; ETR, event time ratio; HA, hemagglutinin; HAI, hemagglutination inhibition; NA, neuraminidase; RT-PCR, reverse transcriptase–polymerase chain reaction.
Pre-existing Antibodies and Shedding Duration in Adults With RT-PCR– or Serologically Confirmed Infection
When serologically confirmed infections were included, all anti-NA antibody levels (AUC ≥20) were associated with significantly shortened shedding duration and showed a strong dose–response relationship (Figure 2); an anti-NA level of 20 or higher was associated with a 49% decrease in shedding (adjusted ETR, 0.51; 95% CI, 0.30–0.87) (Supplementary Table S2), while an anti-NA antibody level of 80 or higher was associated with a 74% decrease in shedding duration (adjusted ETR, 0.26; 95% CI, 0.10–0.70). Hemagglutination inhibition titers were not associated with shortened shedding (Figure 2).
Pre-existing Antibodies and Shedding Duration in Children With RT-PCR–Confirmed Infection
In RT-PCR–positive children, we did not observe an association between anti-NA antibody level and viral shedding duration. However, HAI antibody titers were significantly associated with decreased influenza shedding duration (Figure 1D). Indeed, shedding was reduced by 32% for children with HAI titers of 1:20 or higher (adjusted ETR, 0.68; 95% CI, 0.53–0.87) (Supplementary Table S1), with predicted mean shedding of 3.9 versus 6.0 days (Supplementary Table S1). As with adults, we observed no association with HA stalk.
Pre-existing Antibodies and Shedding Duration in Children With RT-PCR– or Serologically Confirmed Infection
When we included serologically confirmed infections, anti-NA antibody levels of 160 or higher in children were associated with shortened shedding (Figure 2). HAI titers remained associated with shortened shedding duration; however, the association was no longer significant at all titer levels (Figure 2).
Excluding 1 Child With High Pre-existing Antibodies and 12 Days of Viral Shedding
A single child with high pre-existing anti-HA stalk and anti-NA antibody levels and a shedding duration of 12 days was identified in the study (see Figure 1B, C). To examine the effect of this child, we reconducted the analysis excluding the child. On exclusion, in RT-PCR–confirmed infections, an NA antibody level of 40 or greater in children was significantly associated with shortened shedding (adjusted ETR, 0.50; 95% CI, 0.26–0.96) (Supplementary Figure S3).
When serologically confirmed infections were included (Figure S4), anti-NA levels of 20 or higher and 40 or higher were significantly associated with shortened shedding duration in children (adjusted ETRs: anti-NA AUC ≥20, 0.63; 95% CI: 0.45–0.89) (Supplementary Figure S4).
Pre-existing Antibody Levels and Symptom Duration Among RT-PCR–Confirmed Infections
Among adult RT-PCR–positive influenza cases, anti-NA levels of 80 or higher were associated with a shorter duration of ILI, cough, and runny nose (Figure 5). An anti-NA level of 80 or higher was associated with shortening of symptom duration by 82% for ILI (ETR, 0.18; 95% CI, 0.05–0.61), 92% for cough (ETR, 0.08; 95% CI, 0.01–0.58), and 89% for runny nose (ETR, 0.11; 95% CI, 0.02–0.60). Anti-NA antibody levels were not significantly associated with symptom duration in children.
Figure 5.
Pre-existing neuraminidase (NA) antibodies and symptom duration among reverse transcriptase–polymerase chain reaction–confirmed infections. A–C, Histograms of NA antibody levels by age. Histograms are colored according to duration of symptoms for influenza-like illness (ILI) (A), cough (B), and runny nose (C). Symptom duration was set to 0.5 days for individuals with no recorded symptoms. D–F, event time ratios (ETRs) from accelerated failure time models, adjusted for age and sex, compare the duration of ILI (D), cough (E), and runny nose (F) in those with high (threshold or higher) vs low (lower than threshold) antibody levels. An ETR <1 corresponds to a shorter duration in the high antibody group. Abbreviations: AFT, accelerated failure time; AUC, area under the curve; CI, confidence interval; ETR, event time ratio; ILI, influenza-like illness; NA, neuraminidase; RT-PCR, reverse transcriptase–polymerase chain reaction.
DISCUSSION
We found that pre-existing anti-NA antibodies were associated with shorter viral shedding duration in natural influenza A(H1N1)pdm infections in a community-based household transmission study. We also found that the association differed by age: in adults, the association was stronger and had a dose-dependent relationship with increasing anti-NA antibody levels associated with even shorter shedding, while in children there was a threshold effect with only very high anti-NA levels (≥160) associated with shortened shedding. After adjusting for HI and anti-HA stalk antibodies, the relationship of anti-NA antibodies and shedding duration in adults persisted. Further, anti-NA antibodies were also associated with shorter symptom duration in adults.
We observed that HI antibodies were not significantly associated with shortened shedding duration in adults. However, it is important to note that there were very few influenza A(H1N1)pdm–infected adults with high HI titers. In children, HI antibodies were associated with moderately shortened shedding duration. We saw no association with anti-HA stalk antibodies and shedding duration.
Recently, in this study population, we identified HI and anti-HA stalk, but not anti-NA antibodies, as independent correlates of protection from infection from influenza A H1N1pdm [22]. Taken together with our findings here, these results fit nicely with the mechanism of action of these antibodies laid out by molecular studies. Anti-HA antibodies prevent viral attachment and entry into host cells, while anti-NA antibodies prevent viral particles from budding and spreading from host cells, and to some degree also prevent virus from leaving the mucosal entryways (reviewed in [7]). This last mechanism of action suggests that the importance of anti-NA antibodies might vary by the route of infection.
Our findings advance those of previous human studies to establish the relationship of anti-HA and anti-NA antibodies and protection against influenza. A previous human challenge study among prisoners of an NA-containing vaccine found similar numbers of infections but less illness among those who were vaccinated [23], while several studies of natural infections additionally found that anti-NA antibodies correlated with protection from serologically confirmed infection [24] and PCR-confirmed symptomatic infection [25, 26]. While our results that anti-NA antibodies affect shedding duration but not infection risk appear discordant, it is important to note that studies of PCR-confirmed symptomatic infections likely may have missed milder or asymptomatic infections and would not have actually been able to differentiate between shortened shedding duration and protection from influenza infection. It is also important to note that because anti-NA and anti-HA stalk antibodies correlate, anti-NA antibodies would appear to independently correlate with protection from infection in serologically confirmed infections not adjusted for anti-HA stalk antibody [22]. A recent human challenge study also found that anti-NA antibodies were associated with shorter shedding and symptom duration, but limitations of this model include restricting to healthy adults, infecting intranasally with a high dose, and only seeing mild disease, less than in a community setting [27]. Another important difference between this study and previous studies [24, 27] is that antibodies in this study were measured in a binding assay using recombinant NA while previous studies used viruses expressing an N1 in combination with an H6 HA. While the H6 HA is not recognized by anti-H1 HA head antibodies, antistalk antibodies can bind to it and can also inhibit the NA by steric hindrance [28, 29]. It is therefore possible that anti-HA stalk antibodies were also detected in neuraminidase-inhibiting (NI) assays leading a composite correlate of protection driven by antibodies to the HA stalk and the NA. Since anti-HA stalk antibodies were recently identified to correlate with protection from infection, it is reasonable that the NI titer could also correlate with protection from infection.
This study is the first to look at the effect of antibodies specifically targeting NA on shedding duration in children, and in our main analyses we found a threshold effect with high anti-NA antibody levels associated with shorter influenza virus shedding duration. Because there were so few influenza A(H1N1) pdm–infected children with high anti-NA levels, the 1 outlying child with high anti-NA levels and very long viral shedding strongly influenced the results. On exclusion of this child, there was a dose–response relationship similar to that in adults. Thus, it is possible that children and adults might have the same association with anti-NA and shedding duration, but the outlier combined with the small number of children with high anti-NA levels prevented us from observing it. In addition, it is possible that the difference between children and adults could be due to (1) glycoproteins evolving over time and epitopes of the first exposure shaping future immune responses (adults may have developed antibodies to different/more [and potentially more important] NA epitopes) [30] and (2) adults might additionally have non–antibody-mediated immune responses that are important along with the anti-NA antibodies [31].
We were limited by the number of infected participants we could observe with high levels of pre-existing antibodies because such levels can prevent infection. By including serologically confirmed infections, we increased the number of observed infections and were able to capture individuals who may have shed virus too briefly for us to detect, although we could not distinguish between those who shed very briefly and those who did not shed virus. Another limitation is that we measured circulating rather than mucosal immunity. Multimeric mucosal immunoglobulin (Ig) A (IgA) immunity may be more broadly immunogenic than systemic monomeric IgG immunity [32], suggesting that mucosal immunity may be even more protective than the systemic immunity we measured. Future studies should also measure mucosal immunity, which is likely more important for protection.
The anti-NA antibodies measured in this study resulted primarily from natural infections, because the vaccination rate is low in this setting, but we also know that current influenza vaccines containing both HA and NA favor an anti-HA response and that natural infections have been shown to produce more anti-NA antibodies [14]. Following the early human studies of anti-NA as a correlate of protection [24], an NA-only vaccine was developed [33], which was then not pursued in part because the clinical endpoints used to evaluate the vaccine did not allow the development of symptoms [34]; however, interest in NA as a vaccine antigen is growing again with the rising need for improved seasonal influenza vaccine effectiveness [3–7].
Our results suggest that anti-NA antibodies play a large and independent role in shortening influenza disease duration in both children and adults. Increasing anti-NA immunity could reduce influenza illness, severity, and transmission and should be a priority for future influenza vaccines.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the many dedicated study personnel in Nicaragua at the Centro Nacional de Diagnóstico y Referencia and the Sócrates Flores Vivas Health Center.
Financial support. This work was supported by the National Institute for Allergy and Infectious Disease (grant numbers U01 AI088654 and R01 AI120997 and contract numbers HHSN272201400006C and HHSN272201400008C).
Potential conflicts of interest. The Icahn School of Medicine at Mount Sinai has filed patent applications regarding influenza virus vaccines. F. K.’s laboratory receives funding for universal influenza virus vaccine projects from the Department of Defense, PATH, the Bill and Melinda Gates Foundation, and GlaxoSmithKline. A. G. reports consultancy fees from the Centers for Disease Control and Prevention, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Influenza (seasonal). Available at: http://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal). Accessed 15 October 2018. [Google Scholar]
- 2. Centers for Disease Control and Prevention. Seasonal influenza vaccine effectiveness, 2004–2018 | seasonal influenza (Flu) | CDC 2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed 15 October 2018.
- 3. Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the national institute of allergy and infectious diseases. J Infect Dis 2018; 218:347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paules CI, Marston HD, Eisinger RW, Baltimore D, Fauci AS. The pathway to a universal influenza vaccine. Immunity 2017; 47:599–603. [DOI] [PubMed] [Google Scholar]
- 5. Petrie JG, Gordon A. Epidemiological studies to support the development of next generation influenza vaccines. Vaccines 2018; 6:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eichelberger MC, Morens DM, Taubenberger JK. Neuraminidase as an influenza vaccine antigen: a low hanging fruit, ready for picking to improve vaccine effectiveness. Curr Opin Immunol 2018; 53:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krammer F, Fouchier RAM, Eichelberger MC, et al. NAction! How can neuraminidase-based immunity contribute to better influenza virus vaccines? mBio 2018; 9 Available at: http://mbio.asm.org/lookup/doi/10.1128/mBio.02332-17. Accessed 15 October 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gordon A, Tsang TK, Cowling BJ, et al. Influenza transmission dynamics in urban households, Managua, Nicaragua, 2012–2014. Emerg Infect Dis 2018; 24:1882–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gordon A, Kuan G, Aviles W, et al. The Nicaraguan pediatric influenza cohort study: design, methods, use of technology, and compliance. BMC Infect Dis 2015; 15:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. WHO | CDC protocol of realtime RTPCR for influenza A (H1N1) 2009. Available at: http://www.who.int/csr/resources/publications/swineflu/realtimeptpcr/en/. Accessed 15 October 2018.
- 11. Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team; Dawood FS, Jain S, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009; 360:2605–15. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization. WHO | Manual for the laboratory diagnosis and virological surveillance of influenza. 2011. Available at: http://www.who.int/influenza/gisrs_laboratory/manual_diagnosis_surveillance_influenza/en/. Accessed 15 October 2018. [Google Scholar]
- 13. Jacobsen H, Rajendran M, Choi A, et al. Influenza virus hemagglutinin stalk-specific antibodies in human serum are a surrogate marker for in vivo protection in a serum transfer mouse challenge model. mBio 2017; 8 Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5605943/. Accessed 13 February 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen Y-Q, Wohlbold TJ, Zheng N-Y, et al. Influenza infection in humans induces broadly cross-reactive and protective neuraminidase-reactive antibodies. Cell 2018; 173:417–29.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Margine I, Palese P, Krammer F. Expression of functional recombinant hemagglutinin and neuraminidase proteins from the novel H7N9 influenza virus using the baculovirus expression system. J Vis Exp 2013. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3970794/. Accessed 13 February 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cauchemez S, Horby P, Fox A, et al. Influenza infection rates, measurement errors and the interpretation of paired serology. PLoS Pathog 2012; 8:e1003061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ng S, Lopez R, Kuan G, et al. The timeline of influenza virus shedding in children and adults in a household transmission study of influenza in Managua, Nicaragua. Pediatr Infect Dis J 2016; 35:583–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza a virus shedding in adults. J Infect Dis 2018; 218:1378–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wohlbold TJ, Nachbagauer R, Xu H, et al. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. MBio 2015; 6:e02556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Therneau T. A package for survival analysis in S 2015. Available at: https://CRAN.R-project.org/package=survival. Accessed 15 October 2018.
- 21. Kalbfleisch JD, Prentice RL. Failure time models. In: The statistical analysis of failure time data. New York: Wiley-Blackwell, 2002: 31–51. Available at: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781118032985.ch2. Accessed 15 October 2018. [Google Scholar]
- 22. Ng S, Nachbagauer R, Balmaseda A, et al. Novel correlates of protection against pandemic H1N1 influenza A virus infection. Nat Med 2019; 25:962–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Couch RB, Kasel JA, Gerin JI, Schulman JL, Kilbourne ED. Induction of partial immunity to influenza by a neuraminidase-specific vaccine. J Infect Dis 1974; 129:10. [DOI] [PubMed] [Google Scholar]
- 24. Monto AS, Kendal AP. Effect of neuraminidase antibody on Hong Kong influenza. Lancet 1973; 1:623–5. [DOI] [PubMed] [Google Scholar]
- 25. Couch RB, Atmar RL, Franco LM, et al. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J Infect Dis 2013; 207:974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Monto AS, Petrie JG, Cross RT, et al. Antibody to influenza virus neuraminidase: an independent correlate of protection. J Infect Dis 2015; 212:1191–9. [DOI] [PubMed] [Google Scholar]
- 27. Memoli MJ, Shaw PA, Han A, et al. Evaluation of antihemagglutinin and antineuraminidase antibodies as correlates of protection in an influenza A/H1N1 virus healthy human challenge model. MBio 2016; 7:e00417–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wohlbold TJ, Chromikova V, Tan GS, et al. Hemagglutinin stalk- and neuraminidase-specific monoclonal antibodies protect against lethal H10N8 influenza virus infection in mice. J Virol 2016; 90:851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rajendran M, Nachbagauer R, Ermler ME, et al. Analysis of anti-influenza virus neuraminidase antibodies in children, adults, and the elderly by ELISA and enzyme inhibition: evidence for original antigenic sin. mBio 2017; 8 Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5362038/. Accessed 16 February 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cobey S, Hensley SE. Immune history and influenza virus susceptibility. Curr Opin Virol 2017; 22:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol 2010; 17:1055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krammer F. The human antibody response to influenza A virus infection and vaccination. Nat Rev Immunol 2019. Available at: http://www.nature.com/articles/s41577-019-0143-6. Accessed 11 April 2019. [DOI] [PubMed]
- 33. Kilbourne ED, Couch RB, Kasel JA, et al. Purified influenza A virus N2 neuraminidase vaccine is immunogenic and non-toxic in humans. Vaccine 1995; 13:1799–803. [DOI] [PubMed] [Google Scholar]
- 34. Eichelberger MC, Monto AS. Neuraminidase, the forgotten surface antigen, emerges as an influenza vaccine target for broadened protection. J Infect Dis 2019. Available at: http://academic.oup.com/jid/advance-article/doi/10.1093/infdis/jiz017/5304892. Accessed 8 February 2019. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.