Abstract
Background
In many settings, recent or prior injection drug use remains a barrier to accessing direct-acting antiviral treatment (DAA) for hepatitis C virus (HCV) infection. We examined patterns of drug and alcohol use and injection equipment sharing among people with recent injecting drug use or receiving opioid agonist treatment (OAT) during and following DAA-based treatment.
Methods
SIMPLIFY and D3FEAT are phase 4 trials evaluating the efficacy of DAA among people with past 6-month injecting drug use or receiving OAT through a network of 25 international sites. Enrolled in 2016–2017, participants received sofosbuvir/velpatasvir (SIMPLIFY) or paritaprevir/ritonavir/dasabuvir/ombitasvir ± ribavirin (D3FEAT) for 12 weeks and completed behavioral questionnaires before, during, and up to 2 years posttreatment. The impact of time in HCV treatment and follow-up on longitudinally measured longitudinally measured behaviors was estimated using generalized estimating equations.
Results
At screening, of 190 participants (mean age, 47 years; 74% male), 62% reported any past-month injecting 16% past-month injection equipment sharing, and 61% current OAT. Median alcohol use was 2 (Alcohol Use Disorders Identification Test–Consumption; range, 1–12). During follow-up, opioid injecting (odds ratio [OR], 0.95; 95% confidence interval [CI], 0.92–0.99) and sharing (OR, 0.87; 95% CI, 0.80–0.94) decreased, whereas no significant changes were observed for stimulant injecting (OR, 0.98; 95% CI, 0.94–1.02) or alcohol use (OR, 0.99; 95% CI, 0.95–1.04).
Conclusions
Injecting drug use and risk behaviors remained stable or decreased following DAA-based HCV treatment. Findings further support expanding HCV treatment to all, irrespective of injection drug use.
Clinical Trials Registration
SIMPLIFY, NCT02336139; D3FEAT, NCT02498015.
Keywords: DAA, drug use, hepatitis C, injecting drug use, PWID
We examined patterns of drug, alcohol use and injection equipment sharing following treatment with direct-acting antivirals for hepatitis C virus infection among people with recent injecting or receiving opioid agonist treatment. Behaviors remained stable or decreased throughout the 2-year follow-up.
Hepatitis C virus (HCV) infection is a leading cause of chronic liver disease, cirrhosis, and liver cancer, affecting more than 71 million people globally [1, 2]. The burden of HCV infection is disproportionately high among people who inject drugs (PWID) currently or formerly, such as those receiving opioid agonist treatment (OAT) for the management of opioid dependence [3–5]. The development of direct-acting antiviral (DAA) therapies, which are considerably more efficacious and tolerable than previous interferon-based combinations, makes HCV infection a curable disease in nearly all patients with access to treatment. Several studies, including some conducted by our group, have demonstrated high efficacy of DAA therapy among PWID, irrespective of whether or not they receive OAT or report recent injection [6]. However, uptake of treatment is low [7–9].
The high cost of DAA therapies led to restricted reimbursement in many settings [10–12]. Despite clinical guidelines that recommend DAA treatment for nearly all patients with HCV [13, 14], recent drug and/or alcohol use persists as a restriction to accessing therapy [10–12]. Even in settings where such restrictions do not exist, many physicians are hesitant to treat people who are actively injecting drugs or receiving OAT given concerns regarding continuing or increasing injecting risk behaviors with a consequent risk of HCV reinfection [15].
To date, no study has examined whether and how patterns of drug use and injection risk behaviors change following DAA treatment. Three studies, all conducted in the pre-DAA era, reported stable or decreasing drug-related behaviors during and in the immediate period posttreatment with pegylated interferon alpha (± ribavirin) [16–18]. Among 124 people with a history of injecting in Australia, injection drug use remained stable and ancillary injection equipment sharing decreased during and 6 months posttreatment [17]. Among 87 PWID in Montreal, Canada, those who engaged in HCV treatment were less likely to report injecting drug use at 1-year follow-up compared to those who chose not to engage in care [16]. Finally, among 93 PWID followed in an international multicenter clinical trial, drug injecting and alcohol use decreased during and/or 6 months posttreatment, yet no changes were noted for sharing behaviors [18]. In addition to being limited by a short follow-up posttreatment, these investigations only reported average changes in drug use behaviors within the population and over time. Exploring whether and how trends evolve differently for some patients can help clinicians tailor therapeutic actions to optimize health outcomes. Therefore, our aim in this study was to examine longitudinal patterns of drug and alcohol use and injection equipment sharing among people with recent injecting drug use or receiving OAT during and following DAA-based treatment for chronic HCV infection.
METHODS
Study Design and Sample
This study is a pooled analysis of 2 international, multicenter, open-label, single-arm, phase 4 trials to evaluate the efficacy and safety of HCV DAA treatment and its impact on clinical and nonclinical outcomes in HCV-infected people with recent injecting drug use or currently receiving OAT: SIMPLIFY and D3FEAT. Study procedures are similar across the 2 studies and have been previously published along with efficacy and safety findings [19, 20]. Briefly, participants received sofosbuvir/velpatasvir once daily (SIMPLIFY) or paritaprevir/ritonavir/dasabuvir/ombitasvir ± ribavirin twice daily (D3FEAT) for 12 weeks. Recruitment was conducted through a network of drug and alcohol, hospital, and community clinics and private practices at 25 sites in Australia (n = 7), Canada (n = 6), France (n = 2), New Zealand (n = 2), Norway (n = 1), Switzerland (n = 4), the United Kingdom (n = 1), and the United States (n = 1). Recruitment occurred between March 2016 and October 2016 in SIMPLIFY and June 2016 and February 2017 in D3FEAT. Participants had to be aged >18 years, have chronic HCV infection, and be HCV treatment-naive. In SIMPLIFY, participants must have injected drugs in the last 6 months. In D3FEAT, participants must have injected drugs in the last 6 months or be receiving OAT. A total of 190 participants were recruited (SIMPLIFY, N = 103; D3FEAT, N = 87).
Procedures
Participants completed a self-administered behavioral questionnaire on a tablet computer at screening (pretreatment assessment), baseline (treatment commencement), every fourth week during treatment (weeks 4, 8, 12 [end of treatment]), weeks 24 (sustained virological response [SVR]12) and 36 (SVR24), and 6-month intervals thereafter (weeks 60, 84, and 108) for a total of 10 visits. Questionnaires were developed through focus-testing with PWID and have been used by our group previously in the ACTIVATE study [21]. They collected information on demographics, drug and alcohol use, injecting equipment sharing, and drug treatment. In addition to behavioral surveys, study visits included standard laboratory testing (eg, liver function tests, full blood count), an assessment of adverse events, and, at prespecified select intervals, physical examinations (screening, baseline, weeks 4 and 12), HCV RNA testing (screening, baseline, weeks 12 and 24), HCV genotyping, and fibrosis stage (screening). During treatment, participants attended the clinic on a weekly basis to receive their medication supply. Study nurses and physicians provided counseling and access to ancillary services (eg, injection equipment, OAT) as per the standard of care in their country. All participants provided written informed consent to participate and received the equivalent of AUS$20 reimbursement for their time at each visit. The study protocol was approved by the St Vincent’s Hospital, Sydney Human Research Ethics Committee and local ethics committees at all study sites and was conducted according to the Declaration of Helsinki and International Conference on Harmonisation Good Clinical Practice guidelines.
Measures
The following 5 behavioral outcomes were evaluated in relation to HCV treatment: injection drug use (any), opioid and stimulant injection, injection equipment sharing, and alcohol use. Opioids included heroin or prescription opioids, and stimulants included cocaine or amphetamine. Injection equipment sharing was defined as receptive sharing of needles, syringes, spoons or mixing containers, drug solution, water, or filters. Alcohol use was assessed using the Alcohol Use Disorders Identification Test–Consumption (AUDIT-C; score range, 1–12) [22]. Scores of 3 or more (women) and 4 or more (men) indicate hazardous consumption or active alcohol use disorders [22]. Receipt of OAT was also evaluated in relation to HCV treatment and defined as treatment with methadone, buprenorphine, or buprenorphine-naloxone. Noninjected opioids and stimulants were examined as secondary outcomes, given their limited connection to HCV infection and liver-related outcomes. Except for alcohol, all variables were assessed on a binary scale (yes/no) with respect to the previous month (drugs) or currently (OAT). Alcohol use was evaluated in count form.
Statistical Analyses
Descriptive statistics were used to summarize participants’ characteristics at screening. Main analyses involved estimating average changes in behaviors over time using a generalized estimating equation (GEE) extension of logistic regression. GEE models were specified using a binomial family function and a logit link for binary variables and an identity family function and a Poisson link for count variables. Models estimated the effect of time since screening on each outcome using the odds ratio (OR) and 95% confidence interval (CI). The time effect was assessed in incremental study visits, irrespective of varying time lapses between visits. To control for behavioral changes attributed to changing OAT patterns over time, models were adjusted for this factor as a time-varying covariate. Fixed covariates (eg, age, sex) had no influence on parameter estimates and were therefore not included in the models.
Since assessment of average behavioral patterns could mask heterogeneity among individuals over time, in secondary analyses, group-based trajectory modeling was used to visually inspect the presence of distinct longitudinal patterns. This method is used to identify relatively homogeneous clusters of trajectories of stability or change over time in the presence of repeated observations [23, 24]. For each behavioral outcome, the number of groups and their shape were informed by previous studies examining drug use trajectories [25, 26] and several statistical criteria [23, 24]. We considered models with up to 4 and 5 groups for binary and count outcomes, respectively. For each outcome, the final number of groups was determined by selecting the model that maximized the Bayesian information criteria as long as the Bayes factor was <0.1 and membership in each trajectory group was more than 5%. To describe the shape of trajectories, quadratic and cubic polynomials were considered sufficiently flexible for binary and count variables, respectively. We then obtained more parsimonious models by excluding polynomial terms that did not attain statistical significance at the 5% level.
At the time of this analysis (November 2018), follow-up was still ongoing, and analyses were conducted on available data. To minimize the potential for selection bias due to losses to follow-up while accounting for participants who had yet to come back for a study visit, we developed a conservative definition of study retention a priori and refitted the GEE models among individuals who met this criterion. Study retainment was defined as having completed all 5 visits of screening and during treatment and an additional any 2 afterward. Overall, 151 (79.5%) met our predefined criteria for study retention.
Missing values due to participant nonresponse were infrequent (<4% for any one variable) and were left as is. Analyses were performed in SAS 9.4 (SAS Institute Inc, Cary, NC) and the traj macro [25].
RESULTS
Characteristics of Study Participants
Table 1 presents the characteristics of the 190 participants at screening. Overall, nearly three-quarters of participants were male (74%) with mean age of 47 years (standard deviation, 9). Most had injected drugs in the past month (62%) and were receiving OAT (61%). Major drug classes injected were opioids (47%) and stimulants (32%). Sixteen percent reported sharing injection equipment in the past month. The median alcohol use score, evaluated using the AUDIT-C test, was 2 (interquartile range [IQR], 0–4). Although similar in age and gender distribution, compared to participants recruited in D3FEAT, those enrolled in SIMPLIFY were more likely to report recent unstable housing (22% vs 12%), drug use (eg, injection drug use: 75% vs 47%), and injection equipment sharing (22% vs 8%) and were less likely to be receiving OAT (52% vs 72%), consistent with study inclusion criteria.
Table 1.
Descriptive Characteristics at Study Entry for People With Recent Injection Drug Use or Receiving Opioid Agonist Treatment Recruited and Followed in SIMPLIFY and D3FEAT
| Variable | Total (N = 190) |
SIMPLIFY (n = 103) |
D3FEAT (n = 87) |
|---|---|---|---|
| Age, mean (standard deviation), y | 47 (9) | 47 (9) | 47 (10) |
| Male sex | 141 (74%) | 74 (72%) | 67 (78%) |
| Completed high school education | 92 (49%) | 50 (49%) | 42 (49%) |
| Unstable housing, past 6 months | 33 (18%) | 23 (22%) | 10 (12%) |
| Any injection drug use, past month | 117 (62%) | 77 (75%) | 40 (47%) |
| Opioid injection, past month | 88 (47%) | 58 (56%) | 30 (35%) |
| Stimulant injection, past month | 58 (39%) | 39 (39%) | 19 (23%) |
| Noninjecting opioid use, past month | 42 (23%) | 26 (26%) | 16 (19%) |
| Noninjecting stimulant use, past month | 48 (26%) | 28 (28%) | 20 (24%) |
| Sharing of injection equipment, past month | 28 (16%) | 22 (22%) | 6 (8%) |
| Currently receives opioid agonist treatment | 114 (61%) | 53 (52%) | 61 (72%) |
| Alcohol use score,a median (interquartile range) | 2 (0–4) | 2 (0–4) | 1 (0–4) |
| ≥Advanced fibrosisb | 32 (18%) | 18 (19%) | 14 (16%) |
Except for age and gender, data were unavailable for 2 participants recruited in D3FEAT. Missing values are reflected in the frequency distributions for each variable.
a Measured using the Alcohol Use Disorders Identification Test–Consumption.
bAdvanced fibrosis was defined as having a METAVIR score of F3 or higher.
Average Behavioral Changes During and Following HCV Treatment
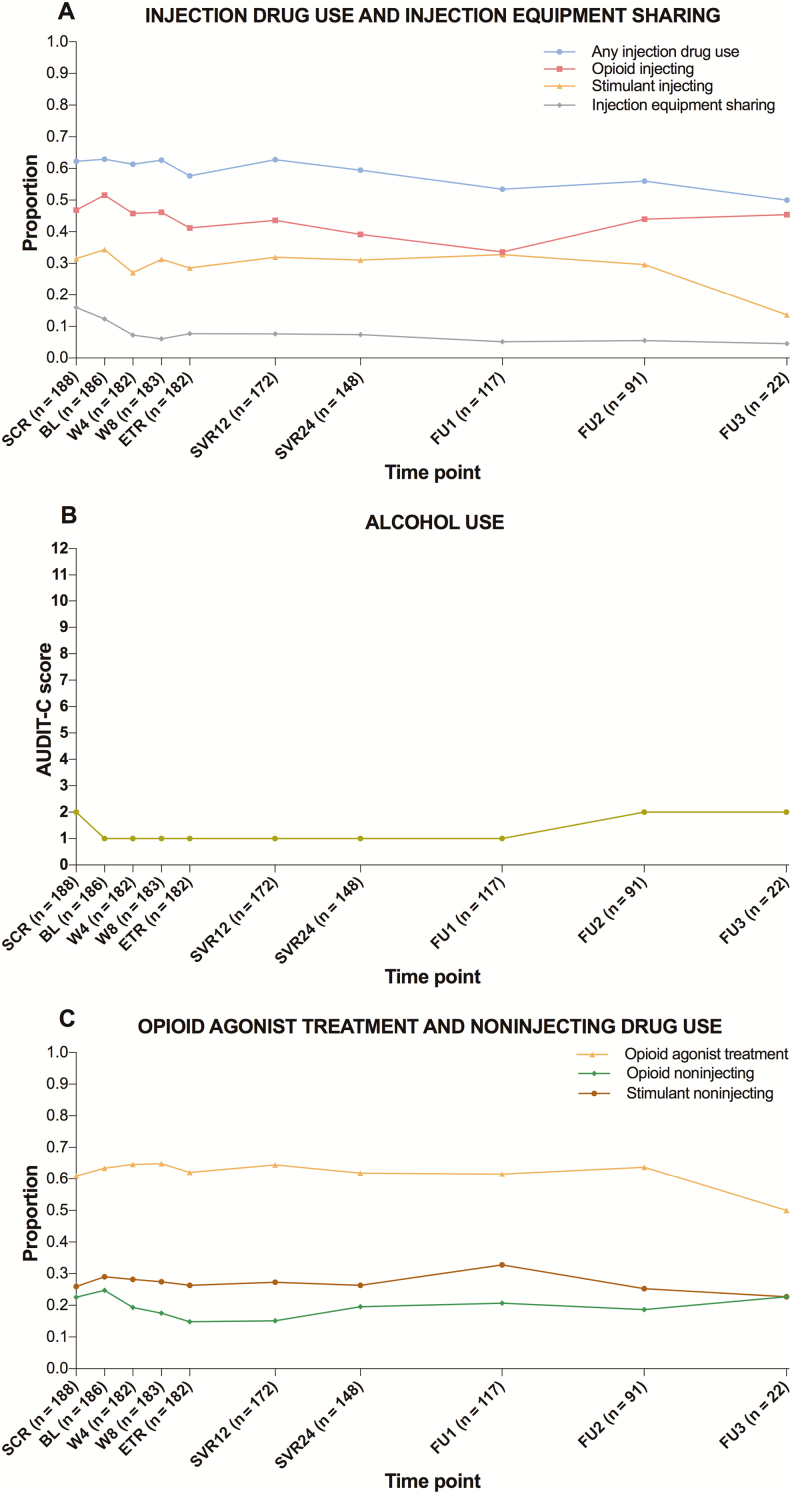
During follow-up, participants had a median of 8 visits (IQR, 7–9) and contributed to a total of 1471 observations. Figure 1 presents the overall proportion of participants reporting each behavioral outcome and their median alcohol use at each visit. Table 2 presents the results of GEE analyses. As ORs remained unchanged after adjusting for OAT, only adjusted estimates are presented. A modest decrease was noted for any injection drug use; each additional study visit was associated, on average, with a 4% decrease in odds of past-month injecting. When examining classes of injected drugs separately, only opioid injecting decreased over time, whereas stimulant injecting did not. For sharing of injection equipment, a more pronounced decrease was noted; each additional study visit was associated, on average, with a 13% decrease in odds of past-month sharing. Alcohol use, receipt of OAT, and noninjecting stimulant use did not appear to change during follow-up. A modest and nonstatistically significant decrease was observed for noninjecting opioid use.
Figure 1.
Proportion of participants reporting injecting drug use and sharing of injection equipment (A), median alcohol use (B), and proportion receiving opioid agonist treatment (OAT) and noninjecting drugs (C) at each visit, during and following direct-acting antiviral treatment for hepatitis C virus infection among people with recent injection drug use or receiving OAT recruited and followed in SIMPLIFY and D3FEAT (N = 190). Drug use outcomes and OAT refer to the past month and current period, respectively. Baseline visit refers to the date of treatment initiation. Follow-up periods 1, 2, and 3 correspond to weeks 60, 84, and 108 since treatment initiation, respectively. At screening, the sample size was 188 rather than 190 because behavioral data were unavailable for 2 participants recruited in D3FEAT. Abbreviations: BL, baseline; ETR, end of treatment; FU, follow-up; SCR, screening; SVR, sustained virological response; W, week.
Table 2.
Changes in Behavioral Outcomes During and Following Direct-acting Antiviral Treatment for Hepatitis C Virus Infection Among People With Recent Injection Drug Use or Receiving Opioid Agonist Treatment Recruited and Followed in SIMPLIFY and D3FEAT, by Incremental Study Visits
| Variable | Adjusted Odds Ratio (95% Confidence Interval)a,b | P Value |
|---|---|---|
| Injection drug use, past month | .96 (.92–.99) | .02 |
| Opioid injection, past month | .95 (.92–.99) | .01 |
| Stimulant injection, past month | .98 (.94–1.02) | .33 |
| Sharing injection equipment, past month | .87 (.80–.94) | <.01 |
| Alcohol use, past month | .99 (.95–1.04) | .75 |
| Current opioid agonist treatment | .99 (.97–1.02) | .45 |
| Noninjecting opioid use, past month | .96 (.91–1.01) | .16 |
| Noninjecting stimulant use, past month | 1.00 (.97–1.03) | .94 |
aAdjusted for opioid agonist treatment at each visit.
bThe estimated odds ratio indicates the average behavior change across 2 consecutive visits, irrespective of time lapses between visits.
Trajectories of Behavioral Outcomes During and Following HCV Treatment
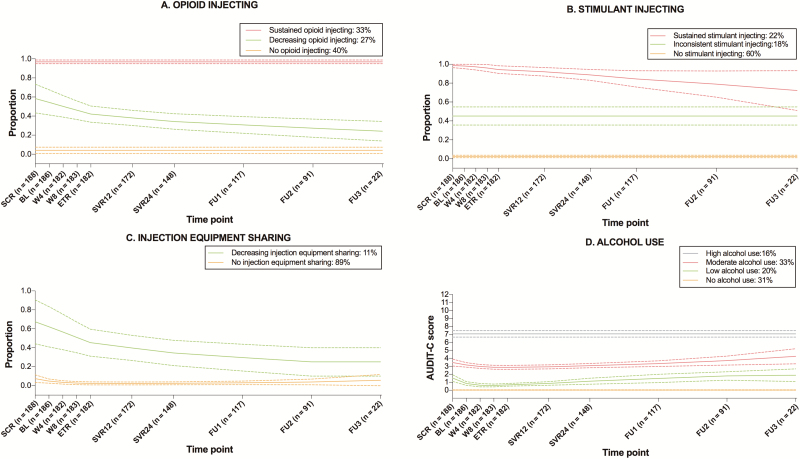
Figure 2 presents results of group-based trajectory analyses for 4 behavioral outcomes: opioid and stimulant injecting, injection equipment sharing, and alcohol use. Supplementary Table 1 presents the model selection process. For opioids, 3 distinct injection probability trajectories were identified: no (40%), sustained (33%), and decreasing (27%) injection. Among participants presenting with a decrease in opioid injecting, the decline was gradual and persistent throughout treatment and during the 2-year follow-up period. For stimulant injecting, 3 trajectories were identified: no (60%), sustained (22%), and inconsistent (18%) injection. For injection equipment sharing, a 2-trajectory group was identified, 1 of no sharing (89%) and 1 of decreasing sharing (11%). As with opioids, the decline in sharing probability was gradual and persistent across the follow-up period. For alcohol, 4 distinct trajectories were identified, all of which remained stable during and following HCV treatment: no (31%), low (20%), moderate (33%), and high (16%) use. Trajectories for OAT receipt and noninjecting opioid and stimulant use remained stable throughout follow-up (Supplementary Figure 1).
Figure 2.
Trajectories of behavioral outcomes during and following direct-acting antiviral treatment for hepatitis C virus infection among people with recent injection drug use or receiving opioid agonist treatment recruited and followed in SIMPLIFY and D3FEAT (N = 190). Solid lines represent the estimated probability of each behavioral outcome for each group, and dashed lines represent 95% confidence intervals. Behaviors refer to the past month period. Baseline visit refers to the date of treatment initiation. Follow-up periods 1, 2, and 3 correspond to weeks 60, 84, and 108 since treatment initiation, respectively. At screening, the sample size was 188 rather than 190 because behavioral data were unavailable for 2 participants recruited in D3FEAT. Example of interpretation: for stimulant injecting, 22% of participants injected stimulants at nearly all visits, 18% oscillated between injecting and noninjecting patterns, and 60% never injected. Abbreviations: BL, baseline; AUDIT-C, Alcohol Use Disorders Identification Test–Consumption; BL, baseline; ETR, end of treatment; FU, follow-up; SCR, screening; SVR, sustained virological response; W, week.
Retention in Follow-up
With the exception of being slightly older (mean age, 47 vs 44 years; P = .09), participants classified as retained in follow-up (n = 151) were similar to those who were not (n = 39) with respect to all other characteristics (Supplementary Table 2). In GEE analyses restricted to participants retained in follow-up, ORs remained largely unchanged (Supplementary Table 3).
DISCUSSION
This pooled analysis of 2 international multicenter studies evaluated longitudinal patterns of drug use behaviors during and following DAA-based HCV treatment among people with recent injecting drug use or receiving OAT, who often face difficulties accessing treatment [10–12, 15]. Our study has 2 main findings. First, drug and alcohol use remained stable during follow-up or decreased slightly. Second, sharing of injection equipment underwent a gradual decrease over time. These findings are encouraging, given that sharing behaviors are the main driver of HCV reinfection among PWID [27]. Importantly, behavioral patterns were not transient but appeared to be persistent during the 2-year follow-up. Taken together, our findings do not support concerns of increasing injection drug use or risk behaviors following DAA-based HCV treatment and further endorse the removal of barriers to access for all infected PWID, irrespective of ongoing injection drug use.
The introduction of DAA treatment marks a shift away from the demanding therapeutic engagements of injectable interferon-based treatments to the relatively simple management of well-tolerated, all-oral regiments [28]. While previous studies, conducted in the interferon-era, showed some decreases in drug use and/or injection equipment sharing following treatment [16–18], concerns have arisen that the simplified treatment provision with DAAs may diminish opportunities to have a positive impact on nonclinical outcomes such as risk behaviors [28] or possibly even lead to increases [15]. Our study does not support this. Rather, it seems that for the majority of DAA-treated patients, engagement in treatment is unlikely to modify their drug use patterns. For some, however, it may be a cue to prompt motivation to decrease HCV risk behaviors and injection drug use. This finding underscores the importance of providing counseling and access to ancillary services alongside HCV treatment to ensure that patients have access to all the tools necessary to support them in making broader drug use changes.
Aside from a potential impact of treatment, it is also possible that the stable or decreasing drug use patterns observed reflect a moment in time when individuals were ready to make broader health changes, which in turn motivated HCV treatment-seeking. Supporting this premise is the stable OAT pattern observed throughout follow-up in those reporting OAT at screening, which contrasts with more common patterns of cycling-in and cycling-out of addiction treatment [29]. In a study that examined determinants of DAA treatment initiation among PWID, participants identified similar circumstances as “the right time for treatment” [30]. For more vulnerable and marginalized populations, HCV treatment is situated within a context of competing every day concerns [31]. It is therefore important that personal attitudes be considered in decisions around HCV treatment readiness and that any opportunity for engagement in care is fully seized upon.
While most participants reported low or moderate levels of alcohol use, approximately 16% reported heavy use, according to AUDIT-C criteria [22], and this pattern remained consistent throughout follow-up. Among people with chronic hepatitis C, heavy alcohol use has been linked to excess mortality [32]. While additional research is needed to document its impact on liver-related outcomes among people who achieved viral eradication, there is some evidence that suggests that liver complications post-SVR are lowest in people who do not drink alcohol [33]. Clinical practice guidelines on the management of HCV recommend that all patients who undertake treatment be offered counseling and support to avoid harmful alcohol consumption [13, 14, 34].
Our study has several limitations. First, given the absence of a comparison group of PWID not receiving DAA treatment, we cannot attribute behavioral changes to HCV treatment or any one intervention. Second, behavioral outcomes were based on self-reported data, which are prone to socially desirable responding and recall error. Although behaviors may be underestimated, self-reported information on drug use has been shown to be reliable and valid, particularly if assessed through computer-assisted surveys [35, 36]. Third, even though follow-up was fairly high for a drug-using population and no differences in drug use patterns were found among participants who were and were not retained, our data may have been influenced by losses to follow-up. Long-term changes should be interpreted with caution given the smaller number of participants followed up in later years.
Our findings may not be generalizable to the broader population of current and former PWID. Our study sample was fairly well engaged in health services, and a relatively modest proportion (16%) reported sharing behaviors compared to the prevalence typically reported among community-recruited PWID [37, 38]. Despite a broad geographic distribution of study participants, all were recruited in high-income settings, where there is typically greater capacity for HCV and addiction care delivery relative to low- and middle-income countries. Finally, participants had weekly contacts with healthcare professionals while on treatment, and it is unclear whether findings would be similar in the context of a simplified monitoring strategy, for which there is growing interest [39]. However, even if simplified HCV treatment options become available, many PWID may continue to benefit from regular monitoring and support while on treatment [40].
CONCLUSIONS
Our study results indicate that drug and alcohol use remains stable or decreases slightly and injection equipment sharing decreases during and following DAA-based HCV treatment among people with recent injecting or receiving OAT. These findings further support expanding HCV treatment to all infected PWID, irrespective of ongoing injection drug use. More broadly, our results suggest that even in the era of simplified DAA therapies, there are ways to enhance the delivery of treatment to afford opportunities for harm reduction. Additional research is needed to elucidate which interventions during HCV treatment can promote reductions in injection equipment sharing. While the majority of people who undergo HCV DAA treatment will achieve cure, reductions in sharing behaviors and risk of HCV reinfection posttreatment will likely not be achieved unless treatment services include HCV counseling and are integrated with addiction treatment and harm-reduction services.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the study participants for their contribution to research, as well as current and past researchers and staff. They acknowledge the following members of the study group: Protocol Steering Committee—Jason Grebely (chair, University of New South Wales [UNSW] Sydney, Sydney, Australia), Gregory Dore (UNSW Sydney, Sydney, Australia), Philippa Marks (UNSW Sydney, Sydney, Australia), Olav Dalgard (Akershus University Hospital, Oslo, Norway), Philip Bruggmann (Arud Centres for Addiction Medicine, Zurich, Switzerland), Catherine Stedman (Christchurch Hospital, Christchurch, New Zealand), Karine Lacombe (Hôpital Saint-Antoine, Paris, France), Jeff Powis (South Riverdale Community Health Centre, Toronto, Canada), Margaret Hellard (Alfred Hospital, Melbourne, Australia), Sione Crawford (Hepatitis Victoria, Melbourne, Australia), Tracy Swan (International Network on Hepatitis in Substance Users, New York, New York), Jude Byrne (Australian Injecting & Illicit Drug Users League, Canberra, Australia), and Melanie Lacalamita (Poliklinik fur Infektiologie, Inselspital, Bern, Switzerland). Coordinating Centre—Amanda Erratt (study coordinator), Evan Cunningham (associate lecturer), Behzad Hajarizadeh (lecturer), Jason Grebely (coprincipal investigator), Gregory Dore (coprincipal investigator), Pip Marks (clinical trials manager), Ineke Shaw (systems manager), Sharmila Siriragavan (data manager), Janaki Amin (statistician), Sophie Quiene (clinical project coordinator), and Kathy Petoumenos (biostatistics and databases program). Site Principal Investigators—Philip Bruggmann (Arud Centres for Addiction Medicine, Zurich, Switzerland), Patrick Schmid (Kantonsspital St Gallen, St Gallen, Switzerland), Erika Castro (Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland), Alberto Moriggia (Fondazione Epatocentro Ticino, Lugano, Switzerland), Karine Lacombe (Hôpital Saint-Antoine, Paris, France), Jean-Pierre Daulouede (Csapa Bizia, Bayonne, France), Olav Dalgard (Akershus University Hospital, Oslo, Norway), Brian Conway (Vancouver Infectious Diseases Center, Vancouver, Canada), Christopher Fraser (Cool Aid Community Health Centre, Victoria, Canada), Jeff Powis (South Riverdale Community Health Centre, Toronto, Canada), Jordan Feld (Toronto General Hospital, Toronto, Canada), Julie Bruneau (Centre Hôspitalier de l’Université de Montréal, Montréal, Canada), Curtis Cooper (Ottawa Hospital, Ottawa, Canada), Ed Gane (Auckland Hospital, Auckland, New Zealand), Catherine Stedman (Christchurch Hospital, Christchurch, New Zealand), Gail Matthews (St Vincent’s Hospital, Sydney Australia), Adrian Dunlop (Newcastle Pharmacotherapy Service, Newcastle, Australia), Margaret Hellard (Alfred Hospital, Melbourne, Australia), Ian Kronborg (Footscray Hospital, Footscray, Australia), David Shaw (Royal Adelaide Hospital, Adelaide, Australia), Alain Litwin and Brianna Norton (Montefiore Medical Centre, New York, New York; Division of General Internal Medicine, Department of Medicine, Albert Einstein College of Medicine, New York, New York), Maria Christine Thurnheer (Poliklinik fur Infektiologie, Inselspital, Bern, Switzerland), Martin Weltman (Nepean Hospital, Kingswood, Australia), Philip Read (Kirby Institute, UNSW Sydney, Sydney, Australia; Kirketon Road Centre, Sydney, Australia), and John Dillon (Ninewells Hospital, Dundee, United Kingdom). Site Coordinators—Tina Horschik (Arud Centres for Addiction Medicine, Zurich, Switzerland), Simone Kessler and Cornelia Knapp (Kantonsspital St Gallen, St Gallen, Switzerland), Lorenza Oprandi (Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland), Paola Messina and Marzia Pantic (Fondazione Epatocentro Ticino, Lugano, Switzerland), Manuela Le Cam (Hôpital Saint-Antoine, Paris, France), Cecilia Maitre (Csapa Bizia, Bayonne, France), Jessica Andreassen, Ingunn Melkeraaen, and Merete Moen Tollefsen (Akershus University Hospital, Oslo, Norway), Hannah Pagarigan (Vancouver Infectious Diseases Center, Vancouver, Canada), Rozalyn Milne (Cool Aid Community Health Centre, Victoria, Canada), Kate Mason (South Riverdale Community Health Centre, Toronto, Canada), Diana Kaznowski and Lily Zou (Toronto General Hospital, Toronto, Canada), Rachel Bouchard and Barbara Kotsoros (Centre Hôspitalier de l’Université de Montréal, Montréal, Canada), Miriam Muir and Jessica Milloy (Ottawa Hospital, Ottawa, Canada), Victoria Oliver (Auckland Hospital, Auckland, New Zealand), Tracy Noonan (Christchurch Hospital, Christchurch, New Zealand), Alison Sevehon (St Vincent’s Hospital, Sydney, Australia), Susan Hazelwood and Michelle Hall (Newcastle Pharmacotherapy Service, Newcastle, Australia), Michelle Hagenauer (Alfred Hospital, Melbourne, Australia), Rachel Liddle (Footscray Hospital, Footscray, Australia), Catherine Ferguson (Royal Adelaide Hospital, Adelaide, Australia), Linda Agyemang, Hiral Patel, and Irene Soloway (Montefiore Medical Centre, New York, New York), Orlando Cerocchi (Toronto General Hospital, Toronto, Canada), Melanie Lacalamita (Poliklinik fur Infektiologie, Inselspital, Bern, Switzerland), Vincenzo Fragomeli (Nepean Hospital, Kingswood, Australia), Rosie Gilliver, Rebecca Lothian (Kirby Institute, UNSW Sydney, Sydney, Australia; Kirketon Road Centre, Sydney, Australia), Shirley Cleary and Linda Johnston (Ninewells Hospital, Dundee, United Kingdom), and Sarah Middleton (Auckland City Hospital, Auckland, New Zealand). The authors also thank Ronald D’Amico, Barbara McGovern, Jonathan Anderson, Ze Zhong, Fiona Keane, Fernando Tatsch, and others at AbbVie and Diana Brainard, John McHutchison, and others at Gilead Sciences for their support.
Disclaimer. The sponsors had no role in the analysis and interpretation of the study results. The views expressed in this publication do not necessarily represent the position of the Australian Government.
Financial support. This work was supported by funding from AbbVie and Gilead Sciences. The Kirby Institute, which sponsored this study, is funded by the Australian Government Department of Health and Ageing. A. A. A. is supported through a Canadian Institute for Health Research Doctoral Award: Frederick Banting and Charles Best Canada Graduate Scholarships and a Canadian Network on Hepatitis C (CanHepC) PhD fellowship. E. B. C. holds a UNSW Sydney Tuition Fee Scholarship. B. H. is supported by a National Health and Medical Research Council Early Career Fellowship. G. J. D. is supported by a National Health and Medical Research Council Practitioner Research Fellowship. J. G. is supported by a National Health and Medical Research Council Career Development Fellowship. J. G., G. V. M., and G. J.D. have received funding from the US National Institute on Drug Abuse, US National Institutes of Health for an unrelated project (1R01DA040506-01).
Potential conflicts of interest. G. J. D. reports grants, personal fees, and nonfinancial support from Gilead Sciences, AbbVie, and Merck and grants from Bristol-Myers Squibb. B. C. reports grants, personal fees, and nonfinancial support from Gilead Sciences, Merck, and AbbVie. O. D. reports grants and personal fees Merck Sharp & Dohme, Gilead Sciences, and AbbVie. J. P. reports grants from Gilead Sciences. P. B. reports grants and personal fees from AbbVie, Gilead Sciences, Merck Sharp & Dohme, and Mundipharma. M. H. reports grants from Gilead Sciences, AbbVie, and Bristol-Myers Squibb. C. C. reports grants and personal fees from Merck, Gilead Sciences, AbbVie, and Bristol-Myers Squibb. P. R. reports grants from Gilead Sciences and fees for educational talks from Gilead Sciences and Merck Sharp & Dohme. J. J .F. reports grants from AbbVie, Gilead Sciences, Janssen, and Wako/Fujifilm and personal fees from AbbVie, Abbott, Gilead Sciences, Enanta, and Roche. B. H. reports grants from AbbVie and Gilead Sciences. K. L. reports personal fees from Gilead Sciences, Janssen, and Merck Sharp & Dohme. C. S. reports personal fees from AbbVie and Merck Sharp & Dohme. A. H. L. reports grants and personal fees from Gilead Sciences and Merck and personal fees from AbbVie. G. V. M. reports grants from Gilead Sciences and AbbVie. J. B. reports a grant from Gilead Sciences and personal fees from Gilead Sciences, Merck Sharp & Dohme, and AbbVie. J. G. reports grants and personal fees from AbbVie, Cepheid, Gilead Sciences, and Merck/MSD. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Polaris Observatory HCVC. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017; 2(3): 161–76. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Hepatitis C fact sheet no. 164 Geneva, Switzerland: WHO, 2017. [Google Scholar]
- 3. Grebely J, Larney S, Peacock A, et al. Global, regional, and country-level estimates of hepatitis C infection among people who have recently injected drugs. Addiction 2019; 114:150–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Larney S, Grebely J, Hickman M, De Angelis D, Dore GJ, Degenhardt L. Defining populations and injecting parameters among people who inject drugs: implications for the assessment of hepatitis C treatment programs. Int J Drug Policy 2015; 26:950–7. [DOI] [PubMed] [Google Scholar]
- 5. Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health 2018; 5:e1192–e1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hajarizadeh B, Cunningham EB, Reid H, Law M, Dore GJ, Grebely J. Direct-acting antiviral treatment for hepatitis C among people who use or inject drugs: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2018; 3:754–67. [DOI] [PubMed] [Google Scholar]
- 7. Nitulescu R, Young J, Saeed S, et al. ; Canadian Co-Infection Cohort Study Variation in hepatitis C virus treatment uptake between Canadian centres in the era of direct-acting antivirals. Int J Drug Policy 2019; 65:41–9. [DOI] [PubMed] [Google Scholar]
- 8. Socias ME, Ti L, Wood E, et al. Disparities in uptake of direct-acting antiviral therapy for hepatitis C among people who inject drugs in a Canadian setting. Liver Int 2019. doi:10.1111/liv.14043. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Falade-Nwulia O, Irvin R, Merkow A, et al. Barriers and facilitators of hepatitis C treatment uptake among people who inject drugs enrolled in opioid treatment programs in Baltimore. J Subst Abuse Treat 2019; 100:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marshall AD, Cunningham EB, Nielsen S, et al. ; International Network on Hepatitis in Substance Users Restrictions for reimbursement of interferon-free direct-acting antiviral drugs for HCV infection in Europe. Lancet Gastroenterol Hepatol 2018; 3:125–33. [DOI] [PubMed] [Google Scholar]
- 11. Marshall AD, Saeed S, Barrett L, et al. ; Canadian Network on Hepatitis C Restrictions for reimbursement of direct-acting antiviral treatment for hepatitis C virus infection in Canada: a descriptive study. CMAJ Open 2016; 4:E605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ooka K, Connolly JJ, Lim JK. Medicaid reimbursement for oral direct antiviral agents for the treatment of chronic hepatitis C. Am J Gastroenterol 2017; 112:828–32. [DOI] [PubMed] [Google Scholar]
- 13. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C, 2018. Available at: https://www.hcvguidelines.org/sites/default/files/full-guidance-pdf/HCVGuidance_May_24_2018b.pdf. Accessed 9 February 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. European Association for the Study of the Liver recommendations on treatment of hepatitis C 2018. J Hepatol 2018; 69:461–511. [DOI] [PubMed] [Google Scholar]
- 15. Asher AK, Portillo CJ, Cooper BA, Dawson-Rose C, Vlahov D, Page KA. Clinicians’ views of hepatitis C virus treatment candidacy with direct-acting antiviral regimens for people who inject drugs. Subst Use Misuse 2016; 51:1218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Artenie AA, Zang G, Daniel M, et al. Short-term injection drug use changes following hepatitis C virus (HCV) assessment and treatment among persons who inject drugs with acute HCV infection. Int J Drug Policy 2017; 47:239–43. [DOI] [PubMed] [Google Scholar]
- 17. Alavi M, Spelman T, Matthews GV, et al. ; ATAHC Study Group Injecting risk behaviours following treatment for hepatitis C virus infection among people who inject drugs: the Australian trial in acute hepatitis C. Int J Drug Policy 2015; 26:976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Midgard H, Hajarizadeh B, Cunningham EB, et al. ; ACTIVATE Study Group Changes in risk behaviours during and following treatment for hepatitis C virus infection among people who inject drugs: the ACTIVATE study. Int J Drug Policy 2017; 47:230–8. [DOI] [PubMed] [Google Scholar]
- 19. Grebely J, Conway B, Cunningham EB, et al. ; D3FEAT Study Group Paritaprevir, ritonavir, ombitasvir, and dasabuvir with and without ribavirin in people with HCV genotype 1 and recent injecting drug use or receiving opioid substitution therapy. Int J Drug Policy 2018; 62:94–103. [DOI] [PubMed] [Google Scholar]
- 20. Grebely J, Dalgard O, Conway B, et al. ; SIMPLIFY Study Group Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol 2018; 3:153–61. [DOI] [PubMed] [Google Scholar]
- 21. Grebely J, Dalgard O, Cunningham EB, et al. ; ACTIVATE Study Group Efficacy of response-guided directly observed pegylated interferon and self-administered ribavirin for people who inject drugs with hepatitis C virus genotype 2/3 infection: the ACTIVATE study. Int J Drug Policy 2017; 47:177–86. [DOI] [PubMed] [Google Scholar]
- 22. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory care quality improvement project (ACQUIP). Alcohol use disorders identification test. Arch Intern Med 1998; 158:1789–95. [DOI] [PubMed] [Google Scholar]
- 23. Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 2010; 6:109–38. [DOI] [PubMed] [Google Scholar]
- 24. Frankfurt S, Frazier P, Syed M, Jung KR. Using group-based trajectory and growth mixture modeling to identify classes of change trajectories. Couns Psychol 2016; 44:622–60. [Google Scholar]
- 25. Genberg BL, Gange SJ, Go VF, Celentano DD, Kirk GD, Mehta SH. Trajectories of injection drug use over 20 years (1988–2008) in Baltimore, Maryland. Am J Epidemiol 2011; 173:829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mikolajczyk RT, Horn J, Prins M, Wiessing L, Kretzschmar M. Trajectories of injecting behavior in the Amsterdam Cohort Study among drug users. Drug Alcohol Depend 2014; 144:141–7. [DOI] [PubMed] [Google Scholar]
- 27. Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol 2013; 10:553–62. [DOI] [PubMed] [Google Scholar]
- 28. Harris M, Rhodes T. Caring and curing: considering the effects of hepatitis C pharmaceuticalisation in relation to non-clinical treatment outcomes. Int J Drug Policy 2018; 60:24–32. [DOI] [PubMed] [Google Scholar]
- 29. Bell J, Burrell T, Indig D, Gilmour S. Cycling in and out of treatment; participation in methadone treatment in NSW, 1990–2002. Drug Alcohol Depend 2006; 81:55–61. [DOI] [PubMed] [Google Scholar]
- 30. Madden A, Hopwood M, Neale J, Treloar C. Beyond interferon side effects: what residual barriers exist to DAA hepatitis C treatment for people who inject drugs? PLoS One 2018; 13:e0207226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harris M, Rhodes T. Methadone diversion as a protective strategy: the harm reduction potential of ‘generous constraints’. Int J Drug Policy 2013; 24:e43–50. [DOI] [PubMed] [Google Scholar]
- 32. Innes H, McAuley A, Alavi M, Valerio H, Goldberg D, Hutchinson SJ. The contribution of health risk behaviors to excess mortality in American adults with chronic hepatitis C: a population cohort-study. Hepatology 2018; 67:97–107. [DOI] [PubMed] [Google Scholar]
- 33. Vandenbulcke H, Moreno C, Colle I, et al. Alcohol intake increases the risk of HCC in hepatitis C virus-related compensated cirrhosis: a prospective study. J Hepatol 2016; 65:543–51. [DOI] [PubMed] [Google Scholar]
- 34. Terrault NA, Hassanein TI. Management of the patient with SVR. J Hepatol 2016; 65:120–9. [DOI] [PubMed] [Google Scholar]
- 35. Darke S. Self-report among injecting drug users: a review. Drug Alcohol Depend 1998; 51:253–63. [DOI] [PubMed] [Google Scholar]
- 36. Islam MM, Topp L, Conigrave KM, et al. The reliability of sensitive information provided by injecting drug users in a clinical setting: clinician-administered versus audio computer-assisted self-interviewing (ACASI). AIDS Care 2012; 24:1496–503. [DOI] [PubMed] [Google Scholar]
- 37. Folch C, Casabona J, Espelt A, et al. ; REDAN Study Group High prevalence and incidence of HIV and HCV among new injecting drug users with a large proportion of migrants–is prevention failing? Subst Use Misuse 2016; 51:250–60. [DOI] [PubMed] [Google Scholar]
- 38. Bruneau J, Arruda N, Zang G, Jutras-Aswad D, Roy É. The evolving drug epidemic of prescription opioid injection and its association with HCV transmission among people who inject drugs in Montréal, Canada. Addiction 2019; 114:366–73. [DOI] [PubMed] [Google Scholar]
- 39. Dore G, Feld J, Thompson A, et al. PS-178-Simplified monitoring for hepatitis C virus treatment with glecaprevir plus pibrentasvir: the SMART-C study. J Hepatol 2019; 70:e110. [DOI] [PubMed] [Google Scholar]
- 40. Chronister KJ, Lothian R, Gilliver R, Kearley J, Read P. Feasibility and acceptability of adherence support for direct acting antiviral therapy for hepatitis C in a low-threshold primary health-care opioid agonist treatment program. Drug Alcohol Rev 2019; 38:185–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.