Abstract
Background
Monovalent rotavirus vaccine, Rotarix (GlaxoSmithKline), was introduced in Kenya in July 2014 and is recommended to infants as oral doses at ages 6 and 10 weeks. A multisite study was established in 2 population-based surveillance sites to evaluate vaccine impact on the incidence of rotavirus-associated hospitalizations (RVHs).
Methods
Hospital-based surveillance was conducted from January 2010 to June 2017 for acute diarrhea hospitalizations among children aged <5 years in 2 health facilities in Kenya. A controlled interrupted time-series analysis was undertaken to compare RVH pre– and post–vaccine introduction using rotavirus-negative cases as a control series. The change in incidence post–vaccine introduction was estimated from a negative binomial model that adjusted for secular trend, seasonality, and multiple health worker industrial actions (strikes).
Results
Between January 2010 and June 2017 there were 1513 and 1652 diarrhea hospitalizations in Kilifi and Siaya; among those tested for rotavirus, 28% (315/1142) and 23% (197/877) were positive, respectively. There was a 57% (95% confidence interval [CI], 8–80%) reduction in RVHs observed in the first year post–vaccine introduction in Kilifi and a 59% (95% CI, 20–79%) reduction in Siaya. In the second year, RVHs decreased further at both sites, 80% (95% CI, 46–93%) reduction in Kilifi and 82% reduction in Siaya (95% CI. 61–92%); this reduction was sustained at both sites into the third year.
Conclusions
A substantial reduction in RVHs and all-cause diarrhea was observed in 2 demographic surveillance sites in Kenya within 3 years of vaccine introduction.
Keywords: rotavirus vaccine, interrupted time series, control, vaccine impact
Following the national introduction of the rotavirus vaccine in Kenya, our impact evaluation across 2 surveillance sites indicates a substantial reduction in childhood hospitalization due to rotavirus-associated and all-cause severe diarrhea.
(See the Major Article by Khagayi et al on pages 2298–305 and Editorial Commentary by Steele and Groome, on pages 2314–6.)
Rotavirus is a major contributor to severe diarrhea illness and related mortality, especially in low- and middle-income countries [1–3]. Global estimates suggest that, in 2013, rotavirus accounted for 215 000 or 3.4% of all deaths in children aged less than 5 years [4]. In 2009, the World Health Organization recommended that all countries, especially those with high diarrhea-associated child mortality rates, implement rotavirus immunization programs [5]. Kenya adopted this recommendation in July 2014 [6, 7]. Since then, 2 doses of live-attenuated rotavirus vaccine (Rotarix), in addition to oral polio, pneumococcal conjugate, and pentavalent vaccines, have been recommended to children in Kenya, targeted at 6 and 10 weeks of life, as part of the routine child immunization program [8, 9]. It was estimated that introduction of this vaccine in Kenya would avert over 60 000 deaths and over 200 000 hospitalizations among children aged younger than 5 years during the first 20 years of introduction [10]. Following vaccine introduction, we used demographic and hospital data from 2 population-based surveillance sites in Kenya to estimate the impact of the program on rotavirus hospitalizations in children younger than 5 years.
METHODS
Geographical Location
The study was conducted in 2 regions with established demographic surveillance systems (DSSs): the Kilifi Health and Demographic Surveillance System (Kilifi HDSS) [11], which is located on the Kenyan coast, and the Kenya Medical Research Institute (KEMRI) and the Centers for Disease Control and Prevention (CDC) Health and Demographic Surveillance System (Siaya HDSS) [12] in rural western Kenya near Lake Victoria. Hospitalization, diarrhea, and rotavirus counts for children under 5 years of age were obtained from 2 government hospitals: Kilifi County Hospital located in the Kilifi HDSS and Siaya County Referral Hospital located in the Siaya HDSS.
Vaccine Introduction and Coverage
Rotavirus vaccine was introduced into the childhood vaccination program in both Kilifi and Siaya counties in July 2014. Children who were aged 6 weeks or younger or born after the start of the rotavirus immunization program were eligible to receive 1 dose at 6 weeks and 1 dose at 10 weeks, although vaccine stock-outs in November and December 2014 kept some eligible children from receiving vaccine. There was no catch-up campaign in either Kilifi or Siaya.
In the Kilifi HDSS, vaccinations have been recorded comprehensively since 2008 through a network of approximately 30 health facilities (both private and public) (Supplementary Figure 1), as part of the Kilifi Vaccine-Monitoring Study (KiVMS) [13]. In the Siaya HDSS, over 20 health facilities administer vaccines to children (Supplementary Figure 2); since 2007, immunization data have been collected for children aged less than 5 years during household data collection rounds, which occur 2–3 times per year [12]. In addition, the vaccination status of children enrolled in the rotavirus surveillance at Siaya County Referral Hospital is recorded at the time of hospital admission. In both sites, card-confirmed vaccine information was captured by the field staff.
Rotavirus Surveillance
In 2009, to prepare for vaccine introduction [12, 14], rotavirus surveillance was intensified at Siaya County Referral Hospital among children aged less than 5 years who presented with 3 or more loose stools within a 24-hour period and 1 or more vomiting episodes. In Kilifi, rotavirus surveillance was initiated in late 2009 in inpatient children aged less than 13 years with 3 or more loose stools within a 24-hour period. Parents of eligible children were briefed on the potential risks and benefits of the study before being asked to give consent by the study team. Stool samples were collected from eligible children whose parents provided voluntary informed consent and tested for rotavirus antigen by enzyme-linked immunosorbent assay, as described previously [14, 15].
Surveillance was interrupted by 12 strikes, including 7 before and 5 after rotavirus vaccine introduction, by different cadres of healthcare workers as indicated in Supplementary Table 1.
Statistical Analysis
We used a controlled interrupted time-series analysis in which rotavirus-negative diarrhea hospitalizations acted as the control series to compare monthly rotavirus-associated hospitalizations (RVHs) before and after rotavirus vaccine introduction in children younger than 5 years of age. Specifically, RVHs from the period 54 months prior to (January 2010 to June 2014) were compared with hospitalizations from the period 36 months after (July 2014 to June 2017) vaccine introduction at each facility. For a month in which less than 100% of diarrhea cases were tested for rotavirus, the number of rotavirus-positive cases was scaled by multiplying the total number of diarrhea cases by the proportion who were rotavirus positive among those tested in that month.
A negative binomial regression was used to estimate the change in rotavirus hospitalizations in the first year (July 2014–June 2015), second year (July 2015–June 2016), and third year (July 2016–June 2017) post–vaccine introduction. The model included rotavirus-negative diarrhea (the control series) as an offset and calendar month and health worker industrial actions as covariates. Lag-1 Newey-West standard errors were used to adjust for autocorrelation [16]. A similar analysis was conducted for all-cause diarrhea using non–diarrhea hospitalizations as the control series.
For the analysis of rotavirus-positive diarrhea, a sensitivity analysis was conducted using a synthetic control [17–19] in the regression model instead of rotavirus-negative cases. The synthetic control was generated by weighting 3 conditions (rotavirus-negative diarrhea, pneumonia, malaria)—using the SYNTH [20] command in Stata—so that their sum closely resembled the prevaccine rotavirus-positive time series.
All statistical analyses were conducted using Stata13.1 software (Stata Corporation). All datasets, Stata files, and related documentation are available on an online data repository (https://doi.org/10.7910/DVN/PH4COG) [21].
Ethics Review
Ethical approval to conduct this study was granted by the KEMRI Scientific and Ethics Review Unit (SERU; SSC 3049) and the CDC's institutional review board (protocol 6968).
RESULTS
Incidence and Seasonality
During the study period, there were 1513 and 1652 diarrhea hospitalizations in Kilifi County Hospital and Siaya County Referral Hospital, respectively. In Kilifi, 1142 (75.5%) of the diarrhea cases were tested, and among those tested, 315 (27.6%) were positive for rotavirus. In Siaya, 877 (53.1%) were tested and 197 (22.5%) were positive for rotavirus.
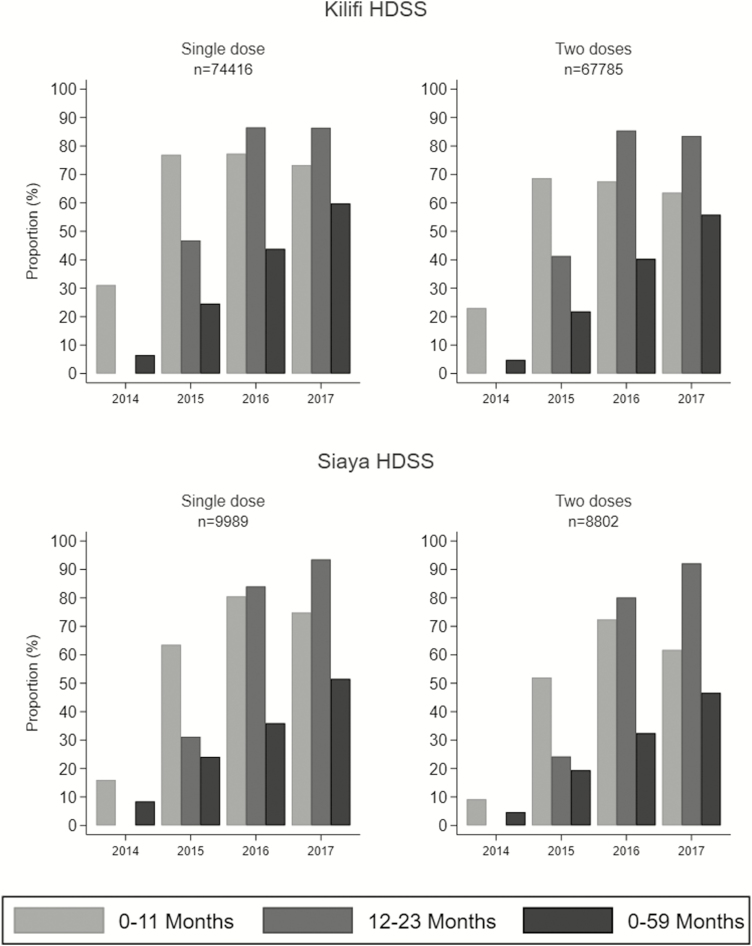
The proportion of children living in the study areas who received rotavirus vaccine increased with time. Between 2014 (year of introduction) and 2017, the proportion of those aged less than 1 year with at least 1 dose increased from 31% to 73% in Kilifi and from 16% to 75% in Siaya; the proportion who were fully vaccinated (2 doses) in 2017 was 65% in Kilifi and 62% in Siaya. The highest coverage among infants was observed in 2016. Among children aged 12–23 months, the proportion with at least 1 dose increased from 0% to 86% in Kilifi and from 0% to 94% in Siaya; coverage with 2 doses in 2017 was 84% and 92%, respectively (Figure 1). The lowest proportions of vaccinated children were observed in 2014 across both sites due to vaccine roll-out in midyear and the unexpected stock-out. Among vaccinated children, the median age at receipt of the first dose was 6 weeks (interquartile range [IQR], 6–7 weeks) in Kilifi and 6 weeks (IQR, 6–7 weeks) in Siaya; median age at receipt of the second dose was 10 weeks (IQR, 10–13 weeks) in Kilifi and 10 weeks (IQR, 10–12 weeks) in Siaya.
Figure 1.
Proportion of children aged less than 5 years who had received 1 or 2 doses of rotavirus vaccine between 2014 and 2017 in Kilifi and Siaya demographic surveillance sites. Abbreviation: HDSS, Health and Demographic Surveillance System; n, number of children vaccinated.
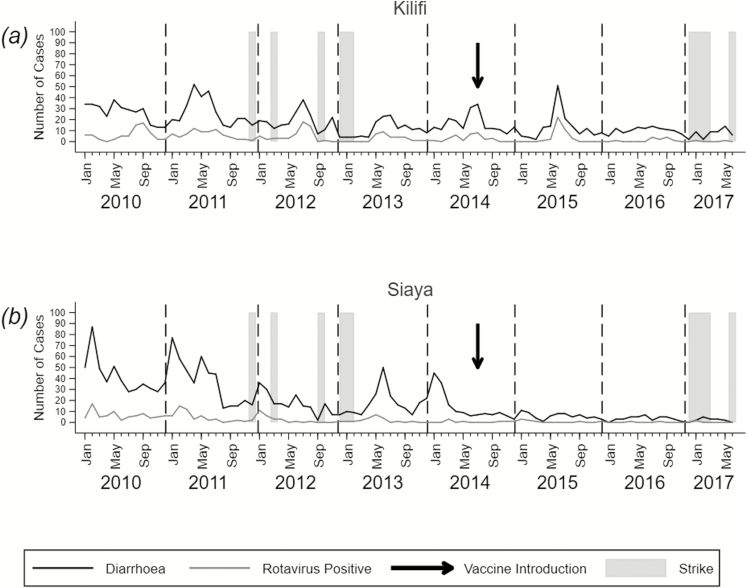
Across the 2 DSSs, overall diarrheal illness and rotavirus-specific illness displayed seasonal patterns, which were dampened after the introduction of the vaccine (Figure 2). Siaya generally recorded higher diarrhea and rotavirus-associated diarrhea incidences compared with Kilifi in the prevaccination era (Tables 1 and 2). In Kilifi, the highest numbers of rotavirus cases were recorded from April/May to September/November of the same year (Figure 2A). In Siaya, the RVH season began earlier in the year, usually in January /February (Figure 2B). At both sites, there was a declining trend in annual incidence of diarrhea and RVHs that began prior to the rotavirus immunization program and continued following the introduction of vaccine (2015–2017). During the years affected by industrial actions of health workers (Supplementary Table 1) lower rotavirus incidence rates were recorded at both sites (Table 2).
Figure 2.
Monthly counts of diarrhea and rotavirus-positive cases in Kilifi (A) and Siaya (B) from January 2010 to June 2017. Strikes by health workers are indicated by gray shading, and the start of rotavirus vaccination in Kenya (July 2014) is indicated by an arrow.
Table 1.
Monthly Diarrhea and Rotavirus Counts and Incidence Rates per 100 000 Children per Year by Site Prior to Rotavirus Vaccine Introduction (January 2010–June 2014)
| Kilifi | Siaya | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Person-years | Diarrhea | Rotavirus (Observed) | Rotavirus (Scaled) | Diarrhea Incidence | Rotavirus Incidence | Person-years | Diarrhea | Rotavirus (Observed) | Rotavirus (Scaled) | Diarrhea Incidence | Rotavirus Incidence | |
| January | 20 041 | 90 | 19 | 22 | 449 | 110 | 8169 | 218 | 22 | 49 | 2669 | 600 |
| February | 20 041 | 86 | 12 | 16 | 429 | 80 | 8169 | 220 | 39 | 70 | 2693 | 857 |
| March | 20 041 | 103 | 15 | 19 | 514 | 95 | 8169 | 136 | 25 | 51 | 1665 | 624 |
| April | 20 041 | 113 | 21 | 29 | 564 | 145 | 8169 | 116 | 16 | 30 | 1420 | 367 |
| May | 20 041 | 125 | 22 | 29 | 624 | 145 | 8169 | 160 | 24 | 37 | 1959 | 453 |
| June | 20 041 | 158 | 37 | 47 | 788 | 235 | 8169 | 164 | 9 | 18 | 2008 | 220 |
| July | 16 055 | 118 | 38 | 52 | 735 | 324 | 6769 | 111 | 8 | 13 | 1640 | 192 |
| August | 16 055 | 78 | 39 | 46 | 486 | 287 | 6769 | 73 | 8 | 13 | 1078 | 192 |
| September | 16 055 | 65 | 25 | 33 | 405 | 206 | 6769 | 65 | 9 | 12 | 960 | 177 |
| October | 16 055 | 58 | 12 | 14 | 361 | 87 | 6769 | 70 | 7 | 11 | 1034 | 163 |
| November | 16 055 | 68 | 5 | 6 | 424 | 37 | 6769 | 73 | 6 | 12 | 1078 | 177 |
| December | 16 055 | 40 | 4 | 6 | 249 | 37 | 6769 | 81 | 9 | 19 | 1197 | 281 |
Rotavirus incidence rates are scaled as described in the Methods section.
Table 2.
Annual Diarrhea and Rotavirus Incidence Rates per 100 000 Children Under 5 Years in Kilifi and Siaya, Kenya
| Kilifi | Siaya | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Population Estimate | Diarrhea | Rotavirus (Observed) | Rotavirus (Scaled)a | Diarrhea Incidence | Rotavirus Incidence | Population Estimate | Diarrhea | Rotavirus (Observed) | Rotavirus (Scaled)a | Diarrhea Incidence | Rotavirus Incidence |
| 2010 | 47 746 | 319 | 70 | 93 | 668 | 195 | 21 885 | 500 | 78 | 124 | 2285 | 567 |
| 2011 | 48 083 | 323 | 74 | 90 | 672 | 187 | 20 953 | 446 | 53 | 107 | 2129 | 511 |
| 2012 | 48 412 | 213 | 56 | 70 | 440 | 145 | 19 947 | 201 | 26 | 50 | 1008 | 251 |
| 2013 | 48 414 | 140 | 31 | 42 | 289 | 87 | 18 439 | 218 | 21 | 45 | 1182 | 244 |
| 2014 | 47 835 | 196 | 31 | 40 | 410 | 84 | 16 803 | 162 | 7 | 13 | 964 | 77 |
| 2015 | 47 943 | 157 | 39 | 51 | 327 | 106 | 14 613 | 71 | 8 | 8 | 486 | 55 |
| 2016 | 46 109 | 116 | 12 | 13 | 252 | 28 | 12 806 | 39 | 2 | 2 | 305 | 16 |
| 2017 | 23 310 | 49 | 2 | 3 | 210 | 13 | 10 891 | 15 | 2 | 2 | 138 | 18 |
aRotavirus incidence rates are scaled as described in the Methods section.
Impact of Vaccine Using a Rotavirus-Negative Control Series
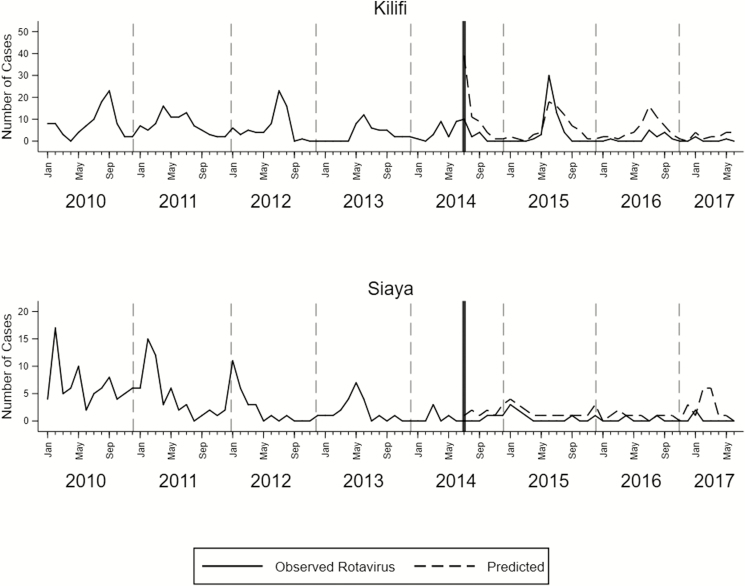
In Kilifi, we observed reduced rates of RVHs among Kilifi HDSS resident children younger than 5 years throughout the postvaccination period (Table 3 and Figure 3). When compared with rates from the prevaccine era, reductions were observed during the first year (57%; 95% confidence interval [CI], 8.0–80.0%), the second year (80%; 95% CI, 46–93%), and the third year (76%; 95% CI, 56–87%) (Supplementary Figure 3a). Similarly, in Siaya, there were reductions in the first year (59%; 95% CI, 20–79%), second year (82%; 95% CI, 61–92%), and the third year (81%; 95% CI, 7–96%) post–vaccine introduction (Supplementary Figure 3b).
Table 3.
Incidence Rate Ratios Comparing Rotavirus and Diarrhea Incidence Pre– (January 2010–June 2014) and Post–Vaccine (July 2014–June 2017) Introduction in a Vaccine Impact Study in Kenya, 2010–2017
| Rotavirus | All-cause Diarrhea | |||||
|---|---|---|---|---|---|---|
| Post–Vaccine Period | IRR | 95% CI | P value | IRR | 95% CI | P value |
| Kilifi | ||||||
| July 2014–June 2015 | 0.43 | .20–.92 | .031 | 0.59 | .41–.84 | .003 |
| July 2015–June 2016 | 0.20 | .07–.54 | .002 | 0.52 | .45–.60 | <.001 |
| July 2016–June 2017 | 0.24 | .13–.44 | <.001 | 0.54 | .45–.66 | <.001 |
| Siaya | ||||||
| July 2014–June 2015 | 0.41 | .21–.80 | .009 | 0.59 | .48–.73 | <.001 |
| July 2015–June 2016 | 0.18 | 0.08–0.39 | <.001 | 0.40 | .28–.57 | <.001 |
| July 2016–June 2017 | 0.19 | .04–.93 | .040 | 0.38 | .23–.62 | <.001 |
See the Methods section for statistical details.Abbreviations: CI, confidence interval; IRR, incidence rate ratio.
Figure 3.
Monthly counts of rotavirus-positive cases (solid lines) and the number of cases (dashed lines) predicted by the model assuming no vaccine introduction. The vertical line indicates the time of vaccine introduction in Kenya (July 2014).
Impact of Vaccine on All-Cause Diarrhea
When examining trends in all-cause diarrhea using non–diarrhea hospitalizations as the control series, significant reductions were observed at both sites in post–vaccine introduction years (Table 3), although by a smaller magnitude compared with reductions in RVHs. In Kilifi, the impact of the vaccine on all-cause diarrhea increased from 41% (95% CI, 16–59%) in the first year to 48% (95% CI, 40–55%) and 46% (95% CI, 34–55%) in the second and third year, respectively (Supplementary Figure 3e). In Siaya, there was a 41% (95% CI, 27–52%) reduction in all-cause diarrhea in the first year followed by 60% (95% CI, 43–72%) in the second year and 62% (95% CI, 38–77%) in the third year (Supplementary Figure 3f).
Impact of the Vaccine on Rotavirus-Associated Hospitalizations Using Synthetic Controls
The associated weights for each component of the synthetic control are shown in Supplementary Table 2 and the results of vaccine impact analysis in Supplementary Table 3 and Supplementary Figure 3. Vaccine impact estimates from this analysis are similar to those presented in Table 3, except that the estimate for the last year in Kilifi was slightly lower than the corresponding estimate in Table 3. In Kilifi, the impact of the vaccine was 67% (95% CI, 27–85%) in the first year, 86% (95% CI, 64–94%) in the second year, and 69% (95% CI, 45–83%) in the third year. Similarly, in Siaya, vaccine impact increased from 68% (95% CI, 33–84%) in the first year to 89% (95% CI, 68–96%) in the second year and 82% (95% CI, 8–96%) in the third year.
DISCUSSION
We present results of an evaluation of the impact of rotavirus vaccine in Kenya using data from 2 population-based surveillance systems. Kenya introduced Rotarix vaccine into the national program as a 2-dose schedule (6 and 10 weeks of age). We observed that most vaccinated children at both sites received either 1 or both doses of the vaccine within 3 weeks of the recommended time of vaccination. We estimated the impact of the vaccine on RVHs using non–rotavirus hospitalizations and synthetic controls to control for any secular trends unrelated to the vaccine. Despite differences in the incidence of rotavirus disease in Kilifi and Siaya, as observed in this study and previous studies [15, 22], the impact of vaccination was similar at both sites. In the first year post–vaccine introduction, RVHs declined by close to 60% in both Kilifi and Siaya, and all-cause diarrhea declined by over 40% at both sites. In the second year of vaccination, RVHs further declined by over 80%, and this decline was sustained into the third post–vaccine introduction year with increasing coverage. Similarly, the positive impact of the vaccine on all-cause diarrhea increased in the second and third years to as high as 60% reduction, thus providing clear evidence of the substantial public health value of rotavirus immunization. These reductions suggest that vaccination of children aged less than 2 years, who are most likely to transmit rotavirus, optimizes direct and indirect protection against severe diarrheal infections.
Our estimates of rotavirus vaccine impact in the first year of vaccination are consistent with estimates from other African studies, which have ranged between 54% and 61% reduction for RVHs and between 43% and 48% for all-cause diarrhea hospitalizations [23, 24]. The same studies reported increasing impact in the second year of vaccination as was observed in our study.
Using the average of the 2013 Kilifi and Siaya rotavirus disease incidence estimates (166 per 100 000 per year) to represent disease incidence in Kenya in the absence of rotavirus vaccination, and assuming an under-5 population size of 6 million [25], an 80% vaccine impact equates to approximately 8000 rotavirus-related hospitalizations prevented per year in Kenyan children younger than 5 years. Over 20 years this corresponds to 160 000 hospitalizations prevented, which is similar to a previous estimate of 200 000 [10].
Our study was impacted by health worker industrial actions and a prevaccine secular trend in RVHs, possibly reflecting improved sanitation and hygiene [26]. To mitigate against these potential sources of bias we included the monthly rotavirus negative cases (or synthetic controls) as an offset term in our regression model. Additionally, we excluded the longest strike period (July–December 2017) from our analysis. An important assumption of this analysis is that the rotavirus-negative count reflects the counterfactual trend in rotavirus-positive cases that would have been observed in the absence of vaccination. Another limitation we considered was that only a fraction (75% in Kilifi and 53% in Siaya) of eligible diarrhea cases were tested for rotavirus, which represents another potential source of bias. To adjust for this, we imputed the number of rotavirus cases by multiplying the number of diarrhea cases by the fraction that tested positive. This imputation assumes that the fraction of rotavirus-positive cases is the same in those tested as it is in those who were not tested and may itself introduce bias, particularly if the fraction tested changes over time.
In conclusion, results from this study suggest that the burden of rotavirus and all-cause diarrhea declined substantially in Kenyan children in 2 regions of Kenya after rotavirus vaccine introduction. The estimates from this study represent total impact, indicating potential herd effects of the vaccine. Our estimates of vaccine impact on rotavirus and all-cause diarrhea were consistent across the 2 study sites and are consistent with findings from other African countries. These results contribute to the global estimates of rotavirus vaccine impact, especially in low- and middle-income countries, and will likely inform future decisions by policy makers on rotavirus vaccination.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. G. P. O.: formal analysis, validation, writing—original draft and review and editing; data curation. C. B.: formal analysis, validation, writing—review and editing. I. A.: validation, investigation, methodology, writing—review and editing. S. K., M. N., R. O., B. O., B. E. O., G. B., J. B. O., C. O., J. J., J. M., C. T.: validation, investigation, writing—review and editing. J. E. T., Y. A., T. B.: investigation, methodology, writing—review and editing. U. D. P.: conceptualization, investigation, methodology, writing—review and editing. R. F. B.: conceptualization, funding acquisition, investigation, methodology, writing—review and editing. J. R. V.: conceptualization, funding acquisition, investigation, methodology, writing—review and editing. D. J. N.: funding acquisition, methodology, supervision (mentorship), writing—review and editing.
Acknowledgments. The authors thank the study participants and clinical and research team members from both Kilifi and Siaya Health and Demographic Surveillance Systems who were involved at various stages of this study including sample collection and processing. This paper has been approved for publication by the director of the Kenya Medical Research Institute.
Disclaimer. The funding sources had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, approval of this paper or choice of journal. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Financial support. This study was funded by the GAVI (Global Alliance for Vaccines and Immunization) alliance under the Rotavirus Immunization Programme Evaluation in Kenya (RIPEK) Study, a collaboration between Emory University (USA); the Centers for Disease Control and Prevention in Atlanta, Georgia, USA; the Kenya Medical Research Institute (KEMRI)–Wellcome Trust Research Programme, Centre for Geographic Medicine Research-Coast, Kilifi; and the KEMRI Centre for Global Health Research, Kisumu, Kenya. The study was also supported by the Wellcome Trust (grant numbers 203077 and 102975).
Potential conflicts of interest. The authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bajolet O, Chippaux-Hyppolite C. Rotavirus and other viruses of diarrhea. Bull Soc Pathol Exot 1990 1998; 91:432–437. [PubMed] [Google Scholar]
- 2. Neuzil KM, Kotloff KL. Community-acquired diarrhoea in a world with rotavirus vaccine: a glimpse into the future. Lancet Glob Health 2015; 3:e510–1. [DOI] [PubMed] [Google Scholar]
- 3. Nguyen TV, Le Van P, Le Huy C, Weintraub A. Diarrhea caused by rotavirus in children less than 5 years of age in Hanoi, Vietnam. J Clin Microbiol 2004; 42:5745–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. Estimated rotavirus deaths for children under 5 years of age: 2013, 215 000. Available at: http://www.who.int/immunization/monitoring_surveillance/burden/estimates/rotavirus/en/. Accessed 25 January 2018.
- 5. Tate JE, Burton AH, Boschi-Pinto C, Parashar UD; World Health Organization–Coordinated Global Rotavirus Surveillance Network Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis 2016; 62(Suppl 2):S96–105. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization. Rotavirus vaccines WHO position paper 2013. Available at: https://www.who.int/immunization/policy/position_papers/rotavirus/en/. Accessed 1 July 2019.
- 7. Wandera EA, Mohammad S, Ouko JO, Yatitch J, Taniguchi K, Ichinose Y. Variation in rotavirus vaccine coverage by sub-counties in Kenya. Trop Med Health 2017; 45:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ministry of Health, Unit of Vaccines and Immunisation Services. Introduction of Rotavirus vaccine into routine immunization in Kenya—a guide for health workers. Available at: http://guidelines.health.go.ke:8000/media/RotaTraining_Guide.pdf#targetText=The%20Ministry%20of%20Health%2C%20through,with%20effect%20from%20July%202014.&targetText=Hence%2C%20rotavirus%20vaccine%20is%20strongly,prevent%20rotavirus%20disease%20in%20infants. Accessed 5 February 2018.
- 9. Adetifa IMO, Karia B, Mutuku A, et al. . Coverage and timeliness of vaccination and the validity of routine estimates: insights from a vaccine registry in Kenya. Vaccine 2018; 36:7965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sigei C, Odaga J, Mvundura M, Madrid Y, Clark AD; Kenya ProVac Technical Working Group; Uganda ProVac Technical Working Group Cost-effectiveness of rotavirus vaccination in Kenya and Uganda. Vaccine 2015; 33(Suppl 1):A109–18. [DOI] [PubMed] [Google Scholar]
- 11. Scott JA, Bauni E, Moisi JC, et al. . Profile: the Kilifi Health and Demographic Surveillance System (KHDSS). Int J Epidemiol 2012; 41:650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Odhiambo FO, Laserson KF, Sewe M, et al. . Profile: the KEMRI/CDC health and demographic surveillance system—Western Kenya. Int J Epidemiol 2012; 41:977–87. [DOI] [PubMed] [Google Scholar]
- 13. Adetifa IMO, Bwanaali T, Wafula J, et al. . Cohort profile: the Kilifi Vaccine Monitoring Study. Int J Epidemiol 2017; 46:792–792h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khagayi S, Burton DC, Onkoba R, et al. . High burden of rotavirus gastroenteritis in young children in rural western Kenya, 2010–2011. Pediatr Infect Dis J 2014; 33(Suppl 1):S34-40. [DOI] [PubMed] [Google Scholar]
- 15. Nokes DJ, Abwao J, Pamba A, et al. . Incidence and clinical characteristics of group A rotavirus infections among children admitted to hospital in Kilifi, Kenya. PLoS Med 2008; 5:e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Newey WK, West KD. A simple, positive semi-definite, heteroskedasticity and autocorrelation consistent covariance matrix. Econometrica 1987; 55:703–708. [Google Scholar]
- 17. Abadie A, Diamond A, Hainmueller J. Synthetic control methods for comparative case studies: estimating the effect of California's tobacco control program National Bureau of Economic Research, 2007. Available at: http://www.nber.org/papers/w12831. Accessed 5 February 2018. [Google Scholar]
- 18. Abadie A, Gardeazabal J. The economic costs of conflict: a case study of the Basque Country. Am Econ Rev 2003; 93:113–132. [Google Scholar]
- 19. Bouttell J, Craig P, Lewsey J, Robinson M, Popham F. Synthetic control methodology as a tool for evaluating population-level health interventions. J Epidemiol Community Health 2018; 72:673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abadie A, Diamond A, Hainmueller J. SYNTH: Stata module to implement synthetic control methods for comparative case studies 2014. Available at: https://econpapers.repec.org/software/bocbocode/s457334.htm. Accessed 5 February 2018.
- 21. Otieno GP, Bottomley C, Khagayi S, Ogwel B, Nokes DJ. Replication data for: Impact of the introduction of rotavirus vaccine on hospital admissions for diarrhoea among children in Kenya: a controlled interrupted time series analysis Available at: https://dataverse.harvard.edu/dataset.xhtml;jsessionid=0db858744eeb505b20a5b0d68f60?persistentId=doi%3A10.7910%2FDVN%2FPH4COG&version=DRAFT. Accessed 23 October 2018. [DOI] [PMC free article] [PubMed]
- 22. Tate JE, Rheingans RD, O'Reilly CE, et al. . Rotavirus disease burden and impact and cost-effectiveness of a rotavirus vaccination program in Kenya. J Infect Dis 2009; 200(Suppl 1):S76–84. [DOI] [PubMed] [Google Scholar]
- 23. Ngabo F, Tate JE, Gatera M, et al. . Effect of pentavalent rotavirus vaccine introduction on hospital admissions for diarrhoea and rotavirus in children in Rwanda: a time-series analysis. Lancet Glob Health 2016; 4:e129–36. [DOI] [PubMed] [Google Scholar]
- 24. Armah G, Pringle K, Enweronu-Laryea CC, et al. . Impact and effectiveness of monovalent rotavirus vaccine against severe rotavirus diarrhea in Ghana. Clin Infect Dis 2016; 6(Suppl 2):S200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kenya National Bureau of Statistics. Kenya Population and Housing Census 2009 2013. Available at: https://www.knbs.or.ke/kenya-population-and-housing- census-2009/. Accessed 10 April 2018.
- 26. Parashar UD, Gibson CJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis 2006; 12:304–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.