Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a syndrome of excessive immune system activation driven mainly by high levels of interferon gamma. The clinical presentation of HLH can have considerable overlap with other inflammatory conditions. We present a cohort of patients with therapy refractory HLH referred to our center who were found to have a simultaneous presentation of complement-mediated thrombotic microangiopathy (TMA). Twenty-three patients had therapy refractory HLH (13 primary, 4 EVB-HLH, 6 HLH without known trigger). Sixteen (69.6%) met high-risk TMA criteria. Renal failure requiring renal replacement therapy, severe hypertension, serositis, and gastrointestinal bleeding were documented only in patients with HLH who had concomitant complement-mediated TMA. Patients with HLH and without TMA required ventilator support mainly due to CNS symptoms, while those with HLH and TMA had respiratory failure predominantly associated with pulmonary hypertension, a known presentation of pulmonary TMA. Ten patients received eculizumab for complement-mediated TMA management while being treated for HLH. All patients who received the complement blocker eculizumab in addition to the interferon gamma blocker emapalumab had complete resolution of their TMA and survived. Our observations suggest co-activation of both interferon and complement pathways as a potential culprit in the evolution of thrombotic microangiopathy in patients with inflammatory disorders like refractory HLH and may offer novel therapeutic approaches for these critically ill patients. TMA should be considered in children with HLH and multi-organ failure, as an early institution of a brief course of complement blocking therapy in addition to HLH-targeted therapy may improve clinical outcomes in these patients.
Electronic supplementary material
The online version of this article (10.1007/s10875-020-00789-4) contains supplementary material, which is available to authorized users.
Keywords: Hemophagocytic lymphohistiocytosis, Thrombotic microangiopathy, Complement, Interferon gamma, Eculizumab, Emapalumab
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a rare clinical syndrome of excessive immune activation, characterized by signs and symptoms of extreme inflammation, driven mainly by interferon gamma (IFNγ) and other pro-inflammatory cytokines. Our understanding of HLH pathogenesis has significantly evolved in the past two decades. Though there are varied molecular etiologies, genetic HLH disorders most often develop due to either defective lymphocyte cytotoxicity or abnormal inflammasome activity. Defects in lymphocyte cytotoxicity due to perforin deficiency or degranulation defects lead to prolonged synapse times, increased production of inflammatory cytokines, and abnormal reciprocal activation of mononuclear phagocytes and cytotoxic NK cells and T cells. Activated mononuclear phagocytes promote hemophagocytosis and release of HLH biomarkers like ferritin, CXCL9, and IL-18BP. SAP deficiency uniquely impairs restimulation-induced cell death and, like CD27 and CD70 deficiency, prevents normal killing of EBV-infected B cells. The absence of XIAP permits pathogenic NLRP3 inflammasome activity and abnormal lymphocyte apoptosis, while activating mutations in NLRC4 drive pathologic NLRC4 inflammasome activity [1–6].
The current diagnostic criteria for HLH include either a genetic HLH diagnosis or five of the following eight clinical and/or laboratory criteria: fever, splenomegaly, cytopenias, hypertriglyceridemia and/or hypofibrinogenemia, hemophagocytosis, low or absent NK cell activity, elevated ferritin, and elevated sIL-2 receptor (sIL2R) (Table 1) [7]. The most commonly affected organs are the liver, spleen, central nervous system (CNS), and bone marrow. HLH is a syndrome with a variable clinical presentation, requiring comprehensive clinical evaluation to rule out other disorders and clinical syndromes, as clinical diagnostic criteria are not specific and there is considerable overlap with other conditions [8–11]. We present a group of patients referred to our center for the management of HLH, who also had a simultaneous presentation of clinically significant thrombotic microangiopathy (TMA). TMA is an increasingly recognized clinical syndrome that results from vascular endothelial injury and may occur in the setting of excessive inflammation such as that seen following a toxic injury (spider or snake bite, radiation, or chemotherapy), in highly inflammatory diseases like systemic lupus erythematosus (SLE), and after stem cell transplantation (TA-TMA) or solid organ transplant [12]. TMA presents with a constellation of laboratory features and a specific pattern of organ injury [13]. TMA diagnostic criteria include either a histologic diagnosis on tissue biopsy of microangiopathic changes or at least five of the seven laboratory and clinical markers listed in Table 1. If not recognized, TMA can progress to multi-organ failure. Complement system dysregulation is a known pathogenic pathway leading to TMA, and complement blocking agents are used therapeutically with some success [14]. However, other inflammatory pathways may contribute to the endothelial injury that initiate and sustain TMA, and can likely be targeted to improve clinical outcomes further [15, 16].
Table 1.
Diagnostic criteria for HLH and TMA
| Hemophagocytic lymphohistiocytosis (HLH) | Thrombotic microangiopathy (TMA) |
|---|---|
| A. Genetic HLH diagnosis | A. Tissue diagnosis |
| or | or |
| B. Five of the eight criteria met | B. Five of the seven criteria met |
| 1. Fever ≥ 38.5 °C | 1. LDH above normal value for age |
| 2. Splenomegaly | 2. Schistocytes on peripheral blood smear |
| 3. Cytopenias (affecting at least 2 cells lines) -Hemoglobin < 9 g/dL-Platelets < 100 × 109/L-Neutrophils < 1 × 109/L | 3. De novo thrombocytopenia or require platelet transfusions |
| 4. Hypertriglyceridemia and/or hypofibrinogenemia (< 150 mg/dL) | 4. De novo anemia or require RBC transfusions |
| 5. Hemophagocytosis | 5. Hypertension > 99% for age (< 18 years of age) or 140/90 (≥ 18 years of age) or receiving antihypertensive therapy |
| 6. Low or absent NK cell activity | 6. Proteinuria ≥ 30 mg/dL on random urinalysis ×2 or random urine protein creatinine ratio ≥ 2 mg/mg |
| 7. Elevated ferritin > 500 ng/mL | 7. Terminal complement activation: elevated plasma sC5b-9 above normal limit of ≥ 244 ng/mL, or elevated above defined normal laboratory value |
| 8. Elevated sIL-2 receptor (sIL2R) > 2400 U/mL or elevated above defined normal laboratory value | Note: 6 and 7 are high-risk TMA features |
Our observations suggest that co-activation of both interferon and complement pathways is important in the evolution of TMA and offers novel therapeutic approaches for these critically ill patients.
Methods
Study Subjects
Consecutive patients of any age with hemophagocytic lymphohistiocytosis (HLH) refractory to first-line therapy who were treated at our institution from January 2012 through December 2018 were identified by retrospective chart review after Institutional Review Board (IRB) approval. All patients were prospectively screened for TMA as part of clinical care as previously described [17]. Patient demographics, disease characteristics, HLH and TMA-targeted therapy, complications, intervention, and laboratory assessment were captured from the electronic medical record.
Diagnostic Testing
HLH and TMA diagnoses were made prospectively using published diagnostic criteria (Table 1) [7, 17]. Laboratory tests were performed as part of routine clinical care in a CLIA-certified laboratory. Routine institutional TMA monitoring included daily complete blood count (CBC) with attention to schistocytes; twice a week lactate dehydrogenase (LDH); and weekly haptoglobin, free plasma hemoglobin, and urinalysis with a random urine protein/creatinine ratio. For subjects < 18 years of age, hypertension was defined as a blood pressure > 99% for age, sex, and height [18]. Hypertension for subjects ≥ 18 years of age was defined as a blood pressure ≥ 140/90 mmHg. The number of antihypertensive medications, not including diuretics, required to maintain the blood pressure below the targeted hypertension threshold (99th centile for age) were counted for each patient, and hypertension was classified as severe if management included > 2 antihypertensive medications or a continuous antihypertensive medication infusion for > 12 h.
Terminal complement activation was tested by measuring the level of sC5b-9 in blood and was checked in patients with elevated LDH and nephrotic range proteinuria (random urine protein/creatinine ratio ≥ 2 mg/mg). sC5b-9 was reported as elevated if the measurement in the blood was > 244 ng/mL by enzyme immunoassay (EIA).
Patients with hematologic signs of TMA who had both nephrotic range proteinuria and complement activation as measured by elevated sC5b-9, or evidence of multi-organ injury syndrome (MODS), were classified as high-risk TMA and were offered therapy with the terminal complement blocker, eculizumab. Eculizumab dosing was based on drug pharmacokinetics and pharmacodynamics (PK/PD) as previously published [17, 19]. All patients on eculizumab therapy received antimicrobial prophylaxis against meningococcus until the drug was cleared after therapy completion and complement function was restored.
MODS was diagnosed when a patient had symptoms of HLH/TMA and dysfunction of two or more organ systems: renal failure requiring renal replacement therapy (RRT) or cystatin C glomerular filtration rate (GFR) < 50 mL/min, invasive or non-invasive positive pressure ventilator support for > 24 h, diagnosis of pulmonary hypertension (as determined by echocardiogram and cardiology consultation), serositis (pleural or pericardial effusions), severe hypertension requiring either ≥ 2 medications or continuous infusion of an antihypertensive for > 12 h to maintain blood pressure < 99% for age, CNS symptoms (seizures, bleeding, posterior reversible encephalopathy syndrome (PRES), or altered mental status), or gastrointestinal symptoms (ileus and/or bleeding) [20–24].
Results
Twenty-three patients with frontline therapy refractory HLH were treated at our institution during the study period. All patients met clinical HLH criteria, as listed in Table 1. Twelve (52%) had confirmed genetic HLH, 4 (17.4%) had EBV-associated secondary HLH, and 7 (30.4%) met HLH clinical diagnostic criteria but did not have any identified genetic abnormality, nor EBV infection. Sixteen of 23 patients (70%) had CNS symptomatology such as altered mental status, encephalopathy, or seizures, and seven of these patients also had a documented CNS bleed, as well as radiographic evidence of posterior reversible encephalopathy syndrome (PRES). All patients were initially treated with etoposide and dexamethasone except for one patient who received cytarabine and methylprednisolone. All patients had either disease progression on therapy or had an HLH disease relapse after achieving brief remission with additional agents and high dose dexamethasone (≥ 10 mg/m2) (Supplemental Table 1A). Two patients had HLH relapse after allogenic HCT. Seventeen patients received emapalumab a monoclonal antibody directed against interferon gamma (IFNγ), as a targeted HLH therapy for refractory disease [25]. Fourteen of them received emapalumab as part of the clinical study and three for compassionate use. All patients had active HLH signs including elevated sIL2R at the start of emapalumab.
Patients with HLH and TMA (n = 16)
All patients initiated prospective monitoring for thrombotic microangiopathy (TMA) as part of clinical care on arrival to our institution as listed in “Methods” section. Sixteen of 23 patients (70%) met clinical and laboratory criteria for high-risk TMA in addition to their diagnosis of HLH, including elevated lactate dehydrogenase (LDH), presence of schistocytes in the blood, transfusion-dependent Coombs negative hemolytic anemia, transfusion-dependent thrombocytopenia, nephrotic range proteinuria, and severe hypertension. High-risk TMA features included nephrotic range proteinuria and complement activation (Table 1) and/or multi-organ injury. Nine patients were tested for complement gene abnormalities using an institutional aHUS genetic susceptibility panel (C3, CFB, CFH, CFHR1, CFHR3, CFHR5, CFI, and MLPA analysis for CHFR1/CHFR3 deletion). Six patients had at least one complement gene variant identified. Three subjects were heterozygous for CFHR3-CFHR1 by multiple ligation-dependent probe amplification (MLPA) analysis that has been reported in heterozygous state in stem cell transplant recipients with TMA. One subject had CFHR3 and CFHR1 partial duplication, which is of uncertain clinical significance (VUCS). Three subjects had more than 1 gene variant identified, including a likely pathogenic variant in CFI (c.1246A>C(p.I416L, het), CFI (c.1217G>A(p.R406H),het) variant reported in macular degeneration and CFHR5 (c.486_487insAA(p.E163fs) heterozygous for a frameshift mutation that has been associated with aHUS (Supplemental Table 1A, B).
Fourteen patients were tested for the evidence of terminal complement pathway activation by measuring blood sC5b-9 (or soluble membrane attack complex), and thirteen of them had elevated sC5b-9 level (sC5b-9 range was 262 to > 1890 ng/mL, normal is < 244 ng/mL). Thirteen patients had nephrotic range proteinuria (random urine protein/creatinine ratio > 2 mg/mg). Fifteen patients had multi-organ dysfunction syndrome (MODS) with two or more organ systems involved, and 12 of them required intensive care support. Twelve patients required positive pressure ventilation for respiratory failure, and four of them had pulmonary hypertension. One patient required ECMO support who did not survive and was found on autopsy to have diffuse alveolar hemorrhage. This patient had CMV viremia that could be a possible trigger for inflammatory process. Eight patients had renal failure; seven received renal replacement therapy (RRT), and one patient’s family elected not to proceed with RRT. Two patients had histologic evidence of TMA in kidney on autopsy (Supplemental Table 1A). Eight additional patients had a > 50% decline in cystatin C GFR from their pre-transplant baseline. All patients with TMA had severe hypertension requiring more than two antihypertensive medications or a continuous antihypertensive medication infusion to maintain blood pressure below 99th percentile for age. Six had clinically significant serositis requiring pericardial or pleural drain placement. Eleven patients in this group had CNS symptoms such as seizure or encephalopathy attributed to HLH diagnosis, but six of these patients also had a documented CNS bleed clinically attributed to PRES (Table 2).
Table 2.
Demographic and disease characteristics
| HLH with TMA 16/23 (70%) | HLH without TMA 7/23 (30%) | |
|---|---|---|
| Median age in years (range) | 1.5 (0.4–23) | 1 (0.1–4) |
| Female gender | 10 | 4 |
|
Genetic HLH diagnosis No genetic diagnosis/no other triggers EBV-HLH (no genetic diagnosis) |
7 6 3 |
5 1 1 |
| TMA diagnosis (requires 5 of 7 criteria to be met) | ||
| 11/16 met 7 criteria | 1/7 met 4 criteria | |
| 4/16 met 6 criteria | 2/7 met 3 criteria | |
| 1/16 met 5 criteria | 4/7 met 2 criteria | |
| Organ injury | ||
| Multi-organ dysfunction syndrome (MODS) | 15/16 (94%) | 4/7 (57%) |
| Hypertension, severe | 16/16 (100%) | 0/7 (0%) |
| Nephrotic range proteinuria | 13/16 (81%) | 1/7 (14%) |
| Elevated sC5b-9 (> 244 ng/mL) | 13/14 (93%) | 0/4 (0%) |
| Renal failure | 8/16 (50%) | 0 (0%) |
| Renal replacement therapy | 7/16 (44%) | 0/7 (0%) |
| Cystatin C GFR < 50 mL/min | 13/16 (81%) | 2/6 (33%) |
| Positive pressure ventilation | 12/16 (75%) | 4/7 (57%) |
| Pulmonary hypertension | 4/15 (27%) | 1/7 (14%) |
| Serositis requiring surgical interventions | 6/16 (38%) | 0 (0%) |
| GI symptoms (bleeding, ileus) | 7/16 (44%) | 0/7 (0%) |
| CNS symptoms (any)a | 11/16 (69%) | 5/7 (71%) |
| CNS bleeding | 6/11 (55%) | 1/7 (14%)b |
| PICU admission | 12/16 (75%) | 4/7 (57%) |
| Death | 6/16 (37.5%) | 3/7 (43%) |
Additional information is listed in Supplemental Table 1
aClinically attributed to HLH diagnosis
bPerinatal intraventricular hemorrhage (IVH)
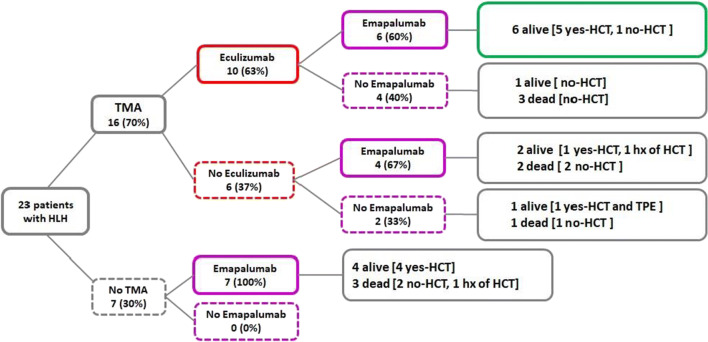
Ten of 16 patients in the HLH plus TMA group received complement blockade with eculizumab as a targeted therapy for thrombotic microangiopathy using personalized PK/PD dosing (Fig. 1). All of these patients had documented activation of terminal complement (elevated sC5b9). Four of these 10 treated patients received eculizumab together with primary HLH therapy with corticosteroid with or without etoposide. None of these four patients proceeded to HSCT, and two died with active TMA. The other six patients received eculizumab after starting treatment with the IFNγ blocker emapalumab for refractory HLH. One patient started eculizumab 8 days after initiation of emapalumab and had resolution of TMA. The patient did not proceed to HCST due to a lack of suitable donor and is alive and well for more than 3 years after therapy. The other five patients in this group who proceeded to HSCT after HLH-targeted therapy had active TMA at the start of hematopoietic stem cell transplantation (HSCT). They continued complement blockade with eculizumab during HSCT with successful resolution of their TMA. All patients were alive, with only one developing liver GVHD after HSCT. The median number of eculizumab doses given to patients with TMA was 9 (range 2–11). The median time to complete TMA resolution and organ function recovery was 16 weeks (9–21 weeks). Eculizumab therapy was well tolerated without any adverse events in this cohort.
Fig. 1.
Outcomes of patients with HLH based on treatment received
Six patients with HLH and TMA did not receive eculizumab (Fig. 1). One patient had resolution of TMA with plasma exchange therapy before eculizumab was available and successfully underwent allogeneic HSCT. In two other patients, TMA resolved with emapalumab given for HLH. Of these two, one patient’s family opted not to proceed to HSCT and one patient subsequently underwent HSCT. Two patients died shortly after transfer to our institution with both HLH and TMA after initiation of emapalumab, but prior to HSCT. One patient died prior to the use of either eculizumab or emapalumab.
Patients With HLH and Without TMA (n = 7)
Seven of 23 patients (30%) with HLH did not meet criteria for TMA and did not have evidence of complement activation in the blood (Fig. 1). Four of these seven patients required intensive care admission for respiratory support, mostly due to CNS symptoms such as status epilepticus. Five patients developed CNS symptoms, all attributed to HLH. One patient who was diagnosed with HLH 3 days after birth had evidence of a perinatal intraventricular bleed (IVH). None of the patients without TMA required renal replacement therapy, and none had severe hypertension. MODS was present in four of these patients.
Discussion
We observed a high incidence of clinically significant complement-mediated thrombotic microangiopathy (TMA) associated with multi-organ injury in children and young adults with a diagnosis of HLH. TMA has not been previously associated with interferon gamma-driven diseases like HLH [26], but TMA has been reported as an iatrogenic therapy-limiting side effect in patients receiving interferon alpha and beta therapy [27–31]. We hypothesized that high levels of interferon gamma in HLH might contribute directly to endothelial damage or injure endothelium through complement system activation. Our previous gene expression analysis in autologous stem cell recipients showed upregulation of interferon-responsive complement genes at the onset of TA-TMA that resolved after therapy with complement blocking agent eculizumab [32]. High levels of IFNγ promote the formation of neutrophil extracellular traps (NETs), leading to complement activation via complement factor P (CFP) [33]. Terminal complement activation resulting in vascular endothelial injury is one of the important pathogenic pathways leading to high-risk TMA with multi-organ dysfunction syndrome. Uncontrolled terminal complement activation can maintain IFNγ secretion via intracellular C5a receptor production resulting in a cycle of continuing tissue injury [34]. Vascular endothelium injured by terminal complement can secrete interferons in addition to the robust production of IFNγ by activated T cells, NK cells, and macrophages in patients with immune over-activation syndromes like HLH. While both interferons and complement are essential components of our immune response, over-activation of these pathways results in organ injury as “collateral damage” during high inflammatory states as seen in HLH and TMA.
In our prior prospective study examining complement genes in HSCT, we observed a significant association between TMA severity and outcomes in HLH patients undergoing HSCT who had complement gene variants identified. In this study, complement gene variants were more common in HLH subjects with TMA then those without TMA (7/9, 78% vs 0/8, 0%, p = 0.0023). The median number of complement variants detected in these 7 HSCT recipients with HLH was 2 (range 1–5), and all patients with complement variants identified had severe TMA and multi-organ injury with an elevated blood sC5b-9 level indicating terminal complement over-activation. Transplant-related mortality was also significantly higher in HLH patients with TMA with six patients of the 9 patients dying from TMA-associated complications, while all HLH patients without TMA survived after transplant (6/9 67% vs 0/8, 0%, p = 0.09) [35]. Based on this prior observation, it is possible that patients with HLH who have complement gene variants in addition to the genetic defects of their primary disease may be more prone to rapid complement activation during active HLH, and may have more severe organ damage resulting from both interferon and complement co-injury of endothelial cells. Prompt control of over-activation of the interferon and complement systems is likely needed for successful resolution of both clinical conditions, HLH and TMA. Our study was limited by small sample size, so we were unable to perform any statistical analysis confirming benefit of eculizumab therapy in subjects with TMA. However, all patients with HLH and TMA who received the complement blocker eculizumab with the IFNγ blocker emapalumab recovered and are doing well, supporting further investigation of this approach. Such targeted therapies can only be successfully applied if these co-existent hyperinflammatory conditions are appropriately recognized.
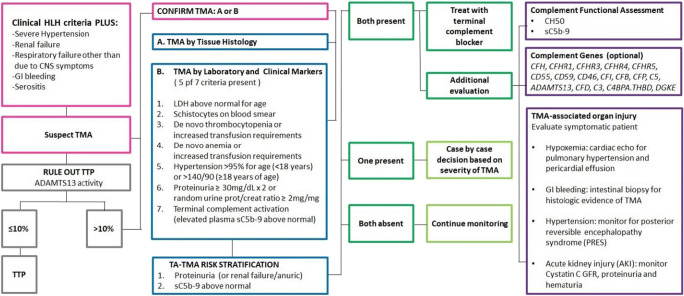
HLH often presents with liver failure and a hyperinflammatory syndrome mimicking sepsis that can ultimately lead to MODS due to coagulopathy, hypotension, and capillary leak. However, HLH rarely causes direct renal and pulmonary failure, or gastrointestinal bleeding in the absence of sepsis [36]. HLH commonly causes neutropenia, but the white blood cell counts are rarely affected in TMA. Patients presenting with a diagnosis of HLH and MODS (in particular renal failure, pulmonary failure, and uncontrolled hypertension that is more severe than expected with dexamethasone use) should be evaluated for co-existing TMA. These patients can benefit from evaluation for terminal complement activation by measuring sC5b-9 blood levels in addition to routine hematologic and renal TMA markers and organ function assessment (Fig. 2).
Fig. 2.
TMA evaluation schema for patients with clinical diagnosis of HLH
In our patient cohort, ventilator support in patients who had HLH but not TMA was generally required because of CNS symptoms, while patients with TMA were more likely to have respiratory failure due to cardiorespiratory pathology, often pulmonary hypertension. HLH patients with hypoxemic acute respiratory failure should undergo diagnostic evaluation for pulmonary hypertension, a known presentation of TMA [21].
In our cohort of patients, renal failure associated with severe hypertension was exclusively seen in patients with HLH and TMA. While hypertension in a patient with HLH can be due to the use of dexamethasone, severe hypertension requiring multiple medications or a continuous infusion of antihypertensive medication should trigger evaluation for TMA. In contrast, the hyperinflammatory state seen in patients with HLH without TMA usually mimics sepsis and is more likely to present with hypotension. Nephrotic range proteinuria measured by random urine protein/creatinine ratio of ≥ 2 mg/mg in addition to severe hypertension should also prompt consideration of TMA in the differential diagnosis of patients with HLH and renal insufficiency. Patients with HLH are more likely to have fluid overload from liver failure leading to hepato-renal syndrome, whereas TMA primarily affects the renal vasculature resulting in direct kidney injury requiring renal replacement therapy, especially associated with hypertension.
CNS involvement in HLH is quite common at clinical presentation, and CNS bleeding can also occur, especially in thrombocytopenic patients with coagulopathy. CNS injury in HLH and TMA can be similar in clinical presentation, but certain features help distinguish CNS HLH, such as CSF pleocytosis, elevated CSF protein, and neopterin, along with radiologic abnormalities such as meningeal enhancement, polymorphic white matter changes, often with non-specific periventricular distribution. CNS injury in TMA most commonly occurs due to uncontrolled hypertension resulting in altered mental status, PRES, seizures, and/or CNS bleeding [37].
Intestinal bleeding has been reported in patients with HLH, but is not common, while TMA is known to cause bowel injury quite often resulting in severe abdominal pain and bleeding, as was seen in our study population [24, 38]. If available, histologic tissue evaluation for TMA should be considered in patients with HLH and GI bleeding. Patients with clinically significant intestinal bleeding may require higher doses of therapeutic monoclonal antibodies due to increased drug clearance [22].
Our clinical observations suggest that HLH patients co-presenting with complement-mediated TMA often suffer multi-organ injury and likely can benefit from a brief course of therapy with the complement blocking agent eculizumab while receiving therapy for HLH. Co-administration of both an interferon blocker and a complement blocker targeting both pathways in TMA might provide faster control. While complement activation in our patients with HLH and TMA was quite prominent, so PK/PD-based eculizumab dosing should be considered as drug clearance can be rapid, especially during a high inflammatory state. Based on our data, we anticipate that a brief course of eculizumab should be sufficient to control complement over-activation while HLH treatment is ongoing. Our patients with HLH and TMA received a median of six doses of eculizumab. Some of our patients started eculizumab late in disease presentation with severe organ injury and were not able to recover. Those who survived were able to discontinue eculizumab without recurrence of TMA successfully. Six patients who received both drugs, an interferon gamma blocker and a complement blocker, resolved both HLH and TMA, and are alive and well.
Our study is limited by retrospective review of the data but provides preliminary insights into additional pathogenetic pathways of TMA that could be monitored and targeted, and brings awareness of the co-existence of HLH and TMA, especially in patients with multi-organ injury. While both HLH and TMA can have overlapping clinical presentation, they also have some distinctive clinical features and organ injury patterns that can be recognized using prospective monitoring (Table 3). Emerging new therapies like chimeric antigen receptor T cells (CART) cause cytokine release syndrome (CRS) due to high IFNγ production, and disorders like systemic lupus erythematosus and Dego’s syndrome present with high IFNα along with complement activation. The resolution of TMA without TMA-directed therapy has been observed in mild to moderate cases of TMA triggered by infection or engraftment after HSCT. However, severe TMA, especially presenting with significant complement activation, leads to high mortality due to multi-organ failure. Early institution of complement blocking therapy along with treatment of the primary disease may improve clinical outcomes in these patients.
Table 3.
Organ injury at disease presentation
| Organ | Hemophagocytic lymphohistiocytosis (HLH) | Thrombotic microangiopthy (TMA) |
|---|---|---|
| Liver | Transaminitis, coagulopathy, liver failure | Transaminitis, no coagulopathy |
| CNS | Seizures, encephalophagy due to CNS-HLH. Meningeal enhancement, polymorphic white matter changes on MRI. Elevated CSF protein and neopterin. | Mental status changes. Seizures often associated with hypertension. Evidence of PRES on brain MRI. CNS bleed in non-coagulopathic patient. |
| Kidney | Primary injury is not common: secondary AKI due to medications, capillary leak, hypotension | AKI, nephrotic range proteinuria, renal failure, severe hypertension. |
| Heart | Primary injury is not common, serositis due to third spacing, liver failure | Right heart failure as a result of pulmonary hypertension due to pulmonary TMA. Pericardial effusion |
| Vascular system | Hypotension mimicking sepsis due to capillary leak | Hypertension more severe that can be attributed to dexamethasone use |
| Lungs | Primary injury is not common, respiratory failure often related to CNS symptomatology (airway protection), or capillary leak (“wet lungs”) |
Hypoxemia often with clear lungs. Pulmonary hypertension, interstitial pulmonary bleeding leading to ARDS |
| GI | Primary injury is not common | Intestinal bleeding due to ischemic colitis |
| Hemato-poietic | Neutropenia, anemia, thrombocytopenia | Anemia, thrombocytopenia, schistocytosis |
AKI acute kidney injury, PRES posterior reversable encephalopathy syndrome, ARDS acute respiratory distress syndrome
Conclusions
Our observations suggest co-activation of both interferon and complement pathways as a potential culprit in the evolution of thrombotic microangiopathy in patients with inflammatory disorders like HLH and may offer novel therapeutic approaches for these critically ill patients. TMA should be considered in children with HLH and multi-organ failure, as an early institution of a brief course of complement blocking therapy in addition to HLH-targeted therapy may improve clinical outcomes in these patients.
Electronic Supplementary Material
(PDF 144 kb)
Acknowledgments
Part of the research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institute of Health (NIH) under award number R01HD093773 (S.Jodele). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Samples used in this study were collected and provided by the staff of the Cincinnati Children’s Hospital Bone Marrow Tissue Repository who we thank for outstanding ongoing technical assistance. The authors would like to thank the clinical staff, patients, and families at Cincinnati Children’s Hospital Medical Center.
Author Contributions
NJG and SJ conceived the project, collected, analyzed, and interpreted the data, and wrote the manuscript. CED, SMD, KCM, MBJ, RAM, AK, JB, and ATC analyzed and interpreted the data and edited the manuscript.
Compliance with Ethical Standards
Conflict of Interest
NJG, CED, RAM, and JB have no interests to disclose. SJ and SMD have US patents pending. MBJ and AK are consultants for Sobi. ATC is on the speaker’s bureau for Sobi. None of these funding sources had any input in the study design, analysis, manuscript preparation, or decision to submit for publication.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Buatois V, Chatel L, Cons L, Lory S, Richard F, Guilhot F, Johnson Z, Bracaglia C, De Benedetti F, de Min C, Kosco-Vilbois MH, Ferlin WG. Use of a mouse model to identify a blood biomarker for IFNgamma activity in pediatric secondary hemophagocytic lymphohistiocytosis. Transl Res. 2017;180:37–52. doi: 10.1016/j.trsl.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prencipe G, Caiello I, Pascarella A, Grom AA, Bracaglia C, Chatel L, Ferlin WG, Marasco E, Strippoli R, de Min C, De Benedetti F. Neutralization of IFN-gamma reverts clinical and laboratory features in a mouse model of macrophage activation syndrome. J Allergy Clin Immunol. 2018;141:1439–1449. doi: 10.1016/j.jaci.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 3.Henter JI, Elinder G, Soder O, Hansson M, Andersson B, Andersson U. Hypercytokinemia in familial hemophagocytic lymphohistiocytosis. Blood. 1991;78:2918–2922. doi: 10.1182/blood.V78.11.2918.2918. [DOI] [PubMed] [Google Scholar]
- 4.Xu XJ, Tang YM, Song H, Yang SL, Xu WQ, Zhao N, Shi SW, Shen HP, Mao JQ, Zhang LY, Pan BH. Diagnostic accuracy of a specific cytokine pattern in hemophagocytic lymphohistiocytosis in children. J Pediatr. 2012;160:984–990. doi: 10.1016/j.jpeds.2011.11.046. [DOI] [PubMed] [Google Scholar]
- 5.Bracaglia C, de Graaf K, Pires Marafon D, Guilhot F, Ferlin W, Prencipe G, Caiello I, Davi S, Schulert G, Ravelli A, Grom AA, de Min C, De Benedetti F. Elevated circulating levels of interferon-gamma and interferon-gamma-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann Rheum Dis. 2017;76:166–172. doi: 10.1136/annrheumdis-2015-209020. [DOI] [PubMed] [Google Scholar]
- 6.Lounder DT, Bin Q, de Min C, Jordan MB. Treatment of refractory hemophagocytic lymphohistiocytosis with emapalumab despite severe concurrent infections. Blood Adv. 2019;3:47–50. doi: 10.1182/bloodadvances.2018025858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Risma KA, Marsh RA. Hemophagocytic lymphohistiocytosis: clinical presentations and diagnosis. J Allergy Clin Immunol Pract. 2019;7:824–832. doi: 10.1016/j.jaip.2018.11.050. [DOI] [PubMed] [Google Scholar]
- 8.Gurunathan A, Boucher AA, Mark M, Prus KM, O’Brien MM, Breese EH, Mizukawa BE, Absalon MJ, Nelson AS, Jordan MB, Grimley MS, Lorsbach RB, Rotz SJ, Mathanda R, Kumar AR. Limitations of HLH-2004 criteria in distinguishing malignancy-associated hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2018;65:e27400. doi: 10.1002/pbc.27400. [DOI] [PubMed] [Google Scholar]
- 9.La Rosee P, Horne A, Hines M, von Bahr Greenwood T, Machowicz R, Berliner N, Birndt S, Gil-Herrera J, Girschikofsky M, Jordan MB, Kumar A, van Laar JAM, Lachmann G, Nichols KE, Ramanan AV, Wang Y, Wang Z, Janka G, Henter JI. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133:2465–2477. doi: 10.1182/blood.2018894618. [DOI] [PubMed] [Google Scholar]
- 10.Jordan MB, Allen CE, Greenberg J, Henry M, Hermiston ML, Kumar A, Hines M, Eckstein O, Ladisch S, Nichols KE, Rodriguez-Galindo C, Wistinghausen B, McClain KL. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: recommendations from the North American Consortium for Histiocytosis (NACHO) Pediatr Blood Cancer. 2019;66:e27929. doi: 10.1002/pbc.27929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118:4041–4052. doi: 10.1182/blood-2011-03-278127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jodele S, Dandoy CE, Myers KC, El-Bietar J, Nelson A, Wallace G, Laskin BL. New approaches in the diagnosis, pathophysiology, and treatment of pediatric hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Transfus Apher Sci. 2016;54:181–190. doi: 10.1016/j.transci.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jodele S, Laskin BL, Dandoy CE, Myers KC, El-Bietar J, Davies SM, Goebel J, Dixon BP. A new paradigm: diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. 2015;29(3):191–204. doi: 10.1016/j.blre.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jodele S. Complement in pathophysiology and treatment of transplant-associated thrombotic microangiopathies. Semin Hematol. 2018;55:159–166. doi: 10.1053/j.seminhematol.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Dvorak CC, Higham C, Shimano KA. Transplant-associated thrombotic microangiopathy in pediatric hematopoietic cell transplant recipients: a practical approach to diagnosis and management. Front Pediatr. 2019;7:133. doi: 10.3389/fped.2019.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia H, Thelwell C, Dilger P, Bird C, Daniels S, Wadhwa M. Endothelial cell functions impaired by interferon in vitro: insights into the molecular mechanism of thrombotic microangiopathy associated with interferon therapy. Thromb Res. 2018;163:105–116. doi: 10.1016/j.thromres.2018.01.039. [DOI] [PubMed] [Google Scholar]
- 17.Jodele S, Davies SM, Lane A, Khoury J, Dandoy C, Goebel J, Myers K, Grimley M, Bleesing J, El-Bietar J, Wallace G, Chima RS, Paff Z, Laskin BL. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124:645–653. doi: 10.1182/blood-2014-03-564997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.C. National High Blood Pressure Education Program Working Group on High Blood Pressure in, Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. doi: 10.1542/peds.114.2.S2.555. [DOI] [PubMed] [Google Scholar]
- 19.Jodele S, Fukuda T, Mizuno K, Vinks AA, Laskin BL, Goebel J, Dixon BP, Chima RS, Hirsch R, Teusink A, Lazear D, Lane A, Myers KC, Dandoy CE, Davies SM. Variable eculizumab clearance requires pharmacodynamic monitoring to optimize therapy for thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2016;22(2):307–315. doi: 10.1016/j.bbmt.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dandoy C, Davies SM, Hirsch R, Chima RS, Paff Z, Cash M, Ryan TD, Lane A, El-Bietar J, Myers KC, Jodele S. Abnormal echocardiography seven days after stem cell transplant may be an early indicator of thrombotic microangiopathy. Biol Blood Marrow Transplant. 2015;21(1):113–118. doi: 10.1016/j.bbmt.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dandoy CE, Hirsch R, Chima R, Davies SM, Jodele S. Pulmonary hypertension after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:1546–1556. doi: 10.1016/j.bbmt.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Warren M, Jodele S, Dandoy C, Myers KC, Wallace G, Nelson A, El-Bietar J. A complete histologic approach to gastrointestinal biopsy from hematopoietic stem cell transplant patients with evidence of transplant-associated gastrointestinal thrombotic microangiopathy. Arch Pathol Lab Med. 2017;141:1558–1566. doi: 10.5858/arpa.2016-0599-RA. [DOI] [PubMed] [Google Scholar]
- 23.Pfeiffer TM, Rotz SJ, Ryan TD, Hirsch R, Taylor M, Chima R, Pate A, Hlavaty J, Grimley M, Myers K, El-Bietar J, Davies SM, Jodele S, Dandoy C. Pericardial effusion requiring surgical intervention after stem cell transplantation: a case series. Bone Marrow Transplant. 2017;52:630–633. doi: 10.1038/bmt.2016.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Bietar J, Warren M, Dandoy C, Myers KC, Lane A, Wallace G, Davies SM, Jodele S. Histologic features of intestinal thrombotic microangiopathy in pediatric and young adult patients after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:1994–2001. doi: 10.1016/j.bbmt.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vallurupalli M, Berliner N. Emapalumab for the treatment of relapsed/refractory hemophagocytic lymphohistiocytosis. Blood. 2019;134(21):1783–1786. doi: 10.1182/blood.2019002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bae MN, Kwak DH, Park SJ, Choi BS, Park CW, Choi YJ, Lee JW, Yang CW, Kim YS, Chung BH. Acute kidney injury induced by thrombotic microangiopathy in a patient with hemophagocytic lymphohistiocytosis. BMC Nephrol. 2016;17:4. doi: 10.1186/s12882-015-0217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allinovi M, Cirami CL, Caroti L, Antognoli G, Farsetti S, Amato MP, Minetti EE. Thrombotic microangiopathy induced by interferon beta in patients with multiple sclerosis: three cases treated with eculizumab. Clin Kidney J. 2017;10:625–631. doi: 10.1093/ckj/sfw143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kundra A, Wang JC. Interferon induced thrombotic microangiopathy (TMA): analysis and concise review. Crit Rev Oncol Hematol. 2017;112:103–112. doi: 10.1016/j.critrevonc.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Magro CM, Poe JC, Kim C, Shapiro L, Nuovo G, Crow MK, Crow YJ. Degos disease: a C5b-9/interferon-alpha-mediated endotheliopathy syndrome. Am J Clin Pathol. 2011;135:599–610. doi: 10.1309/AJCP66QIMFARLZKI. [DOI] [PubMed] [Google Scholar]
- 30.Kavanagh D, McGlasson S, Jury A, Williams J, Scolding N, Bellamy C, Gunther C, Ritchie D, Gale DP, Kanwar YS, Challis R, Buist H, Overell J, Weller B, Flossmann O, Blunden M, Meyer EP, Krucker T, Evans SJ, Campbell IL, Jackson AP, Chandran S, Hunt DP. Type I interferon causes thrombotic microangiopathy by a dose-dependent toxic effect on the microvasculature. Blood. 2016;128:2824–2833. doi: 10.1182/blood-2016-05-715987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alperin JM, Ortiz-Fernandez L, Sawalha AH. Monogenic lupus: a developing paradigm of disease. Front Immunol. 2018;9:2496. doi: 10.3389/fimmu.2018.02496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jodele S, Medvedovic M, Luebbering N, Chen J, Dandoy CE, Laskin BL, Davies SM. Interferon-complement loop in transplant-associated thrombotic microangiopathy. Blood Adv. 2020;4:1166–1177. doi: 10.1182/bloodadvances.2020001515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuen J, Pluthero FG, Douda DN, Riedl M, Cherry A, Ulanova M, Kahr WH, Palaniyar N, Licht C. NETosing neutrophils activate complement both on their own NETs and bacteria via alternative and non-alternative pathways. Front Immunol. 2016;7:137. doi: 10.3389/fimmu.2016.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cross AR, Glotz D, Mooney N. The role of the endothelium during antibody-mediated rejection: from victim to accomplice. Front Immunol. 2018;9:106. doi: 10.3389/fimmu.2018.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jodele S, Zhang K, Zou F, Laskin B, Dandoy CE, Myers KC, Lane A, Meller J, Medvedovic M, Chen J, Davies SM. The genetic fingerprint of susceptibility for transplant associated thrombotic microangiopathy. Blood. 2016;127(8):989–996. doi: 10.1182/blood-2015-08-663435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karapinar B, Yilmaz D, Balkan C, Akin M, Ay Y, Kvakli K. An unusual cause of multiple organ dysfunction syndrome in the pediatric intensive care unit: hemophagocytic lymphohistiocytosis. Pediatr Crit Care Med. 2009;10:285–290. doi: 10.1097/PCC.0b013e318198868b. [DOI] [PubMed] [Google Scholar]
- 37.Dandoy CE, Linscott LL, Davies SM, Leach JL, Myers KC, El-Bietar J, Chima RS, Pate A, Nelson A, Wallace G, Wong HR, Jodele S. Clinical utility of computed tomography and magnetic resonance imaging for diagnosis of posterior reversible encephalopathy syndrome after stem cell transplantation in children and adolescents. Biol Blood Marrow Transplant. 2015;21:2028–2032. doi: 10.1016/j.bbmt.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Popeskou S, Gavillet M, Demartines N, Christoforidis D. Hemophagocytic lymphohistiocytosis and gastrointestinal bleeding: what a surgeon should know. Case Rep Surg. 2015;2015:745848. doi: 10.1155/2015/745848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 144 kb)