Abstract
The outbreak of COVID-19 caused by 2019–nCov/SARS-CoV-2 has become a pandemic with an urgent need for understanding the mechanisms and identifying a treatment. Viral infections including SARS-CoV are associated with increased levels of reactive oxygen species, disturbances of Ca++ caused by unfolded protein response (UPR) mediated by endoplasmic reticulum (ER) stress and is due to the exploitation of virus's own protein i.e., viroporins into the host cells. Several clinical trials are on-going including testing Remdesivir (anti-viral), Chloroquine and Hydroxychloroquine derivatives (anti-malarial drugs) etc. Unfortunately, each drug has specific limitations. Herein, we review the viral protein involvement to activate ER stress transducers (IRE-1, PERK, ATF-6) and their downstream signals; and evaluate combination therapies for COVID-19 mediated ER stress alterations. Melatonin is an immunoregulator, anti-pyretic, antioxidant, anti-inflammatory and ER stress modulator during viral infections. It enhances protective mechanisms for respiratory tract disorders. Andrographolide, isolated from Andrographis paniculata, has versatile biological activities including immunomodulation and determining SARS-CoV-2 binding site. Considering the properties of both compounds in terms of anti-inflammatory, antioxidant, anti-pyrogenic, anti-viral and ER stress modulation and computational approaches revealing andrographolide docks with the SARS-CoV2 binding site, we predict that this combination therapy may have potential utility against COVID-19.
Keywords: COVID-19, 2019-nCov/SARS-CoV-2, Endoplasmic reticulum stress, Unfolded protein response, Andrographolide, Melatonin
Graphical abstract
1. Introduction
Viral diseases continue to emerge and represent a serious issue to public health [1], Coronavirus is part of a family of enveloped viruses with positive sense non-segmented single-stranded RNA genomes. There are two human α-corona viruses, HCoV-229E and HCoV-NL63 and two β-corona viruses, HCoV-OC43 and HCoV-HKU1. Among these, HCoV-NL63 and HCoV-HKU1 have been identified as SARS-CoV and account for the recent outbreaks. These viruses are endemic in the human populations, causing 15–30% of respiratory tract infections each year [2]. In December 2019, a novel strain of the 2019-nCov/SARS-CoV-2, a β-coronavirus, emerged in Wuhan, Hubei province, China. This etiologic agent of this new lung disease, COVID-19 caused by SARS-CoV-2, poses a global health emergency affecting millions of lives worldwide [3,4]. Recent studies demonstrated the crystal structure of CR3022, a neutralizing antibody isolated from a convalescent SARS patient. This antibody targets a highly conserved epitope, distal from the receptor-binding site, that enables cross-reactive binding between SARS-CoV-2 and SARS-CoV [5] . On March 11, the COVID-19 outbreak was characterized as a pandemic by the WHO [6]. As of April 2020, this pandemic has taken the lives of ~250 k people and infected 3.5 M individuals worldwide. In the US, ~1.2 M laboratory confirmed infectious were reported including >65 k deaths. There is an urgent need for the development of an effective mechanism to treat and prevent 2019–nCov/SARS-CoV-2 outbreaks. In this review article, we provide an indication of future research in order to understand the molecular mechanism related to COVID-19 and possible drug targets to regulate the impact of this viral infection.
2. COVID-19 and its pathogenesis
According to the Centers for Disease Control and Prevention (CDC), people with COVID- 19 have had a wide range of symptoms ranging from mild to severe illness and which may appear within 2–14 days after exposure to the virus. The high risk of fatality due to COVID-19 is a consequence of age-associated conditions, such as cardiovascular, pulmonary, and diabetic disorders as well as immune-compromised conditions [7,8]. From the perspective of cell biology, COVID-19 can be divided into three phases that correspond to different clinical stages of the disease. The stages are as follows: i) Asymptomatic state (initial 1–2 days of infection). In this stage the inhaled virus binds to the receptor of angiotensin converting enzyme 2; (ACE2) on epithelial cells in the nasal cavity and replicates. ii) Upper airway and conducting airway response (next few days). The virus propagates and migrates down the respiratory tract along the conducting airways, and a more robust innate immune response is triggered. For about 80% of the infected patients, the disease will be mild and mostly restricted to the upper and conducting airways. iii) Hypoxia, ground glass infiltrates and progression to ARDS (Acute Respiratory Distress Syndrome). Unfortunately, about 20% of the infected patients will progress to stage 3 disease and will develop pulmonary infiltrates and some of these will develop severe disease. The pathological results of SARS and COVID-19 are diffuse alveolar damage with fibrin rich hyaline membranes and a few multinucleated giant cells [9,10].
3. Endoplasmic reticulum stress and corona virus
In eukaryotic cells, one of the largest organelles, the endoplasmic reticulum (ER) is the site of synthesis and folding of membrane, secretory proteins, lipids, sterols, and storage of free calcium [11]. Alterations of protein folding in the ER due to physiological stress such as disturbances in redox, Ca++levels, glycosylation or other environmental elements cause accumulation of misfolded proteins leading to ER stress. The increased levels of reactive oxygen species (ROS) triggered by ER stress activate not only proinflammatory signals but also inflammasome formation, suggesting that ER stress exerts immunogenic effects [12] and can be activated by excessive lipids or pro-inflammatory cytokines [13]. As a result, a series of signal transduction cascades or an unfolded protein response (UPR) occurs. The hallmark of the UPR is the expression of ER-resident chaperones, such as immunoglobulin heavy chain binding protein (BiP/GRP78) and glucose-regulated protein 94 (GRP94). In addition, PERK, IRE-1, and ATF-6 serve as proximal sensors which regulate components that upregulate the capacity of the ER to fold newly synthesized proteins and degrade misfolded/unfolded proteins [14]. In addition, UPR is associated with several major cellular activities including apoptosis, angiogenesis, autophagy, the mitogen-activated protein (MAP) kinase pathways, innate immunity, and pro-inflammatory response.
Accumulating evidence suggests that ER stress and sustained UPR signaling are major contributors to the pathogenesis of several diseases, including inflammatory disorders and viral infections [15] and can increase the severity of these events [16]. Viruses may interact with the host UPR to maintain an environment favorable for establishment of persistent infection [17]. The mechanism is the imbalance of calcium concentration by the expression of viroporins, small virally encoded hydrophobic proteins that oligomerize in the membrane of host cells. This leads to the formation of hydrophilic pores, and consequent depletion of ER membrane due to the release of virions [18] which cause ER stress in the host cells by generating large amounts of unfolded or misfolded proteins [19].
It is well documented that the replication of corona virus occurs in the cytoplasm and is strongly associated with ER and its transducers. In brief, cells infected with SARS-CoV or cells overexpressing the SARS-CoVS2 subunit showed increased levels of GRP94 (ER stress associated gene) and GRP78 gene expression. Like GRP, a significant phosphorylation of PKR and PERK has been observed in SARS-CoV infected cells [20]. However, SARS-CoV is resistant to the antiviral activity of PKR in vitro and PERK and responsible for eIF2 phosphorylation induced by SARS-CoV. Therefore, studies on the UPR stress mediated ER are decisive in elucidating the complicated issue of coronavirus host interaction. However, activation of ATF4 and CHOP promoter activities by the accessory protein 3a of SARS-CoV leads to the activation of PERK [21]. In terms of IRE-1 and its downstream effects, coronavirus infected cells induce significant splicing of XBP1 mRNA but not at the protein level. This may be due to the sustained translation attenuation by this virus-induced eIF2 phosphorylation which blocks the translation of XBP1 protein [22,23] In case of severe acute respiratory syndrome, coronavirus (SARS-CoV) accessory viral protein binds to ATF6 domain thus inducing proteolysis of ATF6. The cleaved DNA binding and transcription activation domains of ATF6 then move from ER to the nucleus [24]. These findings suggest that viruses may exploit their own protein (s) to directly modulate UPR responses. Therefore, UPR induction may modulate host anti-viral response and constitute a major aspect of corona-virus-host interaction.
4. Impact of melatonin on viral infections
Melatonin exhibits a circadian rhythm in the blood and orchestrates many physiologic changes [25,26]. It is involved in regulation of immune function, the tumor microenvironment, and acts as an antioxidant agent [27,28] and anti-pyretic agent [29]. A decreased level of melatonin often occurs in the elderly and in conditions associated with high susceptibility to severe viral infection which has a significant impact on the mitochondrial metabolism and immune cells phenotype. Therefore, the interactions of pineal melatonin with mitochondrial metabolism provide an important point of impact for viral infections [30,31]. Though melatonin is not a viricidal, it likely has an indirect impact on viral actions due to its anti-inflammation, antioxidation, and immune enhancing features [32].
Several well-documented studies have shown that melatonin has a protective role in infections induced by encephalitis virus due to its activity in the central nervous system, associated with its capability to regulate immune function [33]. Another study also confirmed its protective mechanism in bronchiolitis, a severe inflammatory lower respiratory tract disorder mediated by RSV (Respiratory syncytial virus) infection [34]. It is suggested that respiratory disorders induced by many other human pathogens may result from an exuberant generation of reactive oxygen species by inflammatory cells in response to infection [35]. A recent review article documented melatogenergic pathway's role in viral infections, emphasizing influenza and COVID-19 infections. Therefore, melatonin has the potential to be a therapeutic target of COVID-19 infection due to its anti-inflammation, anti-oxidation, and immune enhancing properties [36].
5. Viral infection, melatonin and ER stress
Melatonin modulates ER stress and activates UPR response during viral infections. Due to its antioxidant properties, it regulates ER stress and controls autophagic and apoptotic processes. An earlier study showed that melatonin reduces macrophage inflammation by controlling the ER stress associated signaling pathways [37]. During RHDV (rabbit hemorrhagic disease virus) infection, melatonin induced a decrease in the autophagy associated with this infection and inhibited RHDV RNA replication. The molecular mechanism involved an interplay of RHDV-induced autophagy with oxidative stress, ER stress and apoptosis [38]. A recent study illustrated that melatonin treatment attenuated viral myocarditis via sustaining cardiomyocyte viability, repressing mitochondrial dysfunction and inhibiting ER stress [39]. The rationale for using melatonin in viral diseases is supported by its capability to modulate UPR during viral infection due to its immune enhancing actions, anti-inflammatory and antioxidant properties.
6. Impact of andrographolide and viral infections
Andrographolide is a lactone (bicyclic diterpenoid) derived from Andrographis paniculata [40]. Like melatonin, it has several biological activities including anti-carcinogenic [[41], [42], [43], [44]], anti-inflammatory [45,46], immunomodulator [47], antioxidant [[48], [49], [50]], anti-pyrogenic [51] and anti-viral properties [[52], [53], [54], [55], [56], [57]]. Andrographolide induces ER stress leading to cancer cell death due to apoptosis through the induction of ROS [48], which can inhibit virus-induced carcinogenesis. Additional inhibitory effects of andrographolide include that of cell migration, invasion, matrix metalloproteinase expression, anti-angiogenesis, autophagy, and dysregulation of signaling pathway has been reported for inflammatory disorders including cancer [41,50,58,59]. Upregulation of CTLs and NK cell activity has been found after andrographolide treatment [47] which demonstrates its antiviral properties. Oral administration of the leaves of A. paniculata is effective in the treatment of upper respiratory tract infections, liver toxicity and a variety of other ailments [60]. Moreover, several clinical trials demonstrate its positive effects on infectious disease, autoimmune disorders and it has a potential effect against viral defenses [44,50,52]. Moreover, it exerts anti-viral activity towards a number of different viruses including HIV, hepatitis B, herpes simplex, influenza, hepatitis C, chikungunya virus (CHIKV), Epstein-Barr virus (EBV), human papillomavirus (HPV) dengue virus (DENV) and others [51,56,57,[61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72]]. Recent studies showed that andrographolide is a potential inhibitor of the main protease of SARS-CoV-2 through in silico studies, such as molecular docking, target analysis, toxicity prediction and ADME prediction (absorption, distribution, metabolism, and excretion) [57]. The molecular mechanisms of the antiviral properties of andrographolide are as follows: 1). Enhanced H1N1 virus-I, induced cell death through the inhibition of viral-induced activation of the retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) signaling pathway [53] and diminished lung virus titer through its immune-modulatory activity [51] 2). Alteration of ER stress mediated UPR pathway on virus replication pathway [55,73]. 3) Induction of heme oxygenase 1 (HO-1) expression [74,75]. 4) Involvement of multiple pathway including NFkβ and JAK-STAT. 5). Inhibition of protease activity. 6). Reduction of antigen expression. 7). Inhibition of glycoprotein expression. 8). Suppresses lytic protein expression [56].
Cytokine storm stimulated by influenza virus infection is thought to be an important event in the infection of highly pathogenic influenza virus including corona virus. It has been reported that SC75741, an inhibitor of NF-kβ signaling pathway, which plays a pivotal role in cytokine expression, has a promising anti-influenza capacity in vivo and in vitro. One study demonstrated that delayed treatment of andrographolide (initiated at 4 days post infection) protects mice infected with a lethal dose of influenza combined with CL-385319, a potent influenza entry inhibitor which has been proved to suppress H5N1 replication in vitro [54]. With potent antiviral activity and potentially defined mechanism of action, andrographolide may warrant further evaluation as a possible therapy for COVID-19.
7. Present and future treatment aspects of corona virus
Current antiviral drugs only have a single target. Moreover, these drugs focus on antagonism of the invasion and replication of the virus, not virus recognition and activation of the immune system. Moreover, high mutation rates of influenza virus limit the application of the classic anti-influenza agents targeting viral particles.
Several attempts and collaborative studies are underway to discover and develop full-human neutralizing antibodies targeting SARS-CoV-2 to potentially prevent or treat CoVID-19 [[76], [77], [78], [79], [80]]. Antimalarial drugs such as Chloroquine and Hydroxychloroquine derivatives are being used in emergency cases; however, they are not suitable for patients with conditions such as diabetes, hypertension and cardiac issues [81]. Social isolation is currently the best way to manage the spread of COVID-19 in the absence of an effective treatment. It is revealed that Remdesivir, a drug thought to be one of the best prospects for treating COVID-19, has severe side effects, leading to its discontinuation in trial. Therefore, a novel combination therapy drug with immunomodulators might be a promising therapeutic approach for COVID-19.
A recent study screened a medicinal plant database containing 32,297,216 potential anti-viral phytochemicals and selected the top nine with the potential to inhibit SARS-CoV-2 11 3CLpro 217 activity and hence virus replication [82]. The current review reveals that andrographolide has a broad spectrum of anti-viral properties. The integrity of the vascular endothelial barrier is crucial in the immunoregulation within alveoli.
Our previous study demonstrated that andrographolide suppressed angiogenic signaling and Akt activation and a recent publication reported that melatonin inhibits VEGF (aggravates edema and the extravasation of the immune cells from blood vessels) expression in vascular endothelial cells [32,83]. In the ICU, deep sedation is associated with increased long-term mortality, and the application of melatonin reduces sedation use and the frequency of pain, agitation, and anxiety.
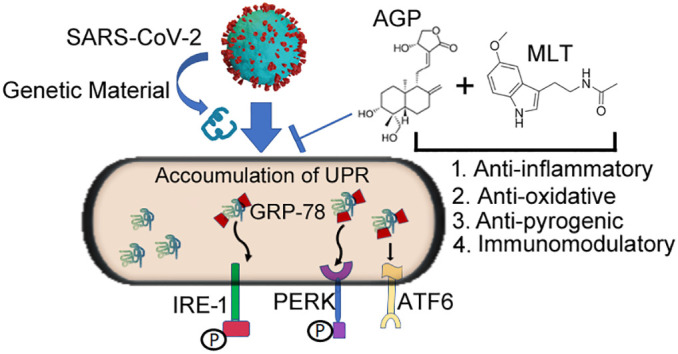
The rationale of combination therapeutic approach for the treatment of COVID-19 is as follows: 1) Andrographolide is a plant derived biocomponent and melatonin is a natural peptide hormone. 2) Both andrographolide and melatonin have significant antiviral properties. 3) Both compounds have anti-inflammatory, antioxidative and immunomodulatory actions (Fig. 1 ). 4) Melatonin has a protective role in infectious disease including many virus-related conditions [30]. 5) Both compounds impact the UPR signaling pathways by inhibiting the ER stress transducers (Fig. 1). 6) Melatonin has favorable safety profile even at remarkably high doses. 7) Both drugs are readily available at a low cost. However, previous reports of adverse reactions to andrographolide in a phase I clinical trial [71] suggests that a combination therapy of andrographolide and melatonin could unveil a potentially useful treatment for COVID-19. Melatonin has been shown to protect against the toxicity of a variety of drugs and toxins. This may increase the efficacy of the combined therapy.
Fig. 1.
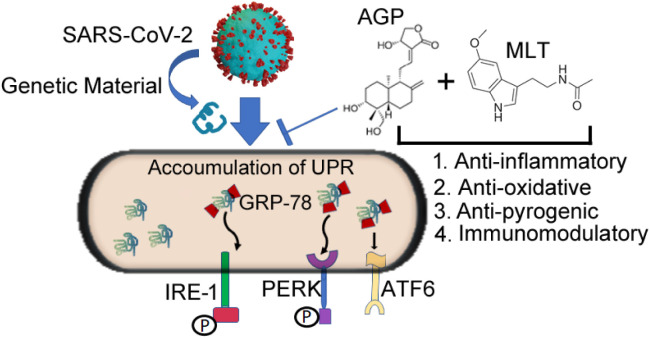
Schematic representation of the modulation of the UPR arms on SARS-CoV-2 infection illustrating the potential benefits of melatonin and andrographolide as an adjuvant use of melatonin and andrographolide. We postulated that genetic material transmitted from SARS-CoV-2 causes elevation of ER stress master regulator (GRP-78) and ER stress transducers IRE-1, PERK and ATF-6. The combined anti-inflammatory, anti-oxidative, anti-pyrogenic and immunomodulatory properties of andrographolide and melatonin could provide a useful adjuvant therapy for COVID-19 by altering ER stress signals.
Acknowledgments
Acknowledgements
We wish to thank the Department of Pediatrics at the University of Maryland School of Medicine for support. We acknowledge Vivekjyoti Banerjee for creating the corona virus graphic using Blender software.
Author contributions
A.B wrote the original draft, T.G.B., S. J. C. and R. J. R reviewed, and edited the manuscript.
Declaration of competing interest
The authors declare that they have no competing interests.
References
- 1.Graham B.S., Sullivan N.J. Emerging viral diseases from a vaccinology perspective: preparing for the next pandemic. Nat. Immunol. 2018;19:20–28. doi: 10.1038/s41590-017-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carbajo-Lozoya J., Müller M.A., Kallies S., Thiel V., Drosten C., von Brunn A. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012;165:112–117. doi: 10.1016/j.virusres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan M., Wu N.C., Zhu X., Lee C.-C.D., So R.T.Y., Lv H., Mok C.K.P., Wilson I.A. A highly conserved cryptic epitope in the receptor-binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020 doi: 10.1126/science.abb7269. (eabb 7269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S., Zhi K., Mukherji A., Gerth K. Repurposing antiviral protease inhibitors using extracellular vesicles for potential therapy of COVID-19. Viruses. 2020;12:486. doi: 10.3390/v12050486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., Madhur M.S., Tomaszewski M., Maffia P., D’Acquisto F., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020 doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceriello A., Stoian A.P., Rizzo M. COVID-19 and diabetes management: what should be considered? Diabetes Res. Clin. Pract. 2020;163 doi: 10.1016/j.diabres.2020.108151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu J., Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am. J. Pathol. 2007;170:1136–1147. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin J.H., Walter P., Yen T.S.B. Endoplasmic reticulum stress in disease pathogenesis. Ann. Rev. Pathol. Mech. Dis. 2008;3:399–425. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menu P., Mayor A., Zhou R., Tardivel A., Ichijo H., Mori K., Tschopp J. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis. 2012;3 doi: 10.1038/cddis.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fung T.S., Liu D.X. Coronavirus infection, ER stress, apoptosis and innate immunity. Front. Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee A., Ahmed H., Yang P., Czinn S.J., Blanchard T.G. Vol. 7. Oncotarget; 2016. Endoplasmic Reticulum Stress and IRE-1 Signaling Cause Apoptosis in Colon Cancer Cells in Response to Andrographolide Treatment; pp. 41432–41444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oakes S.A., Papa F.R. The role of endoplasmic reticulum stress in human pathology. Ann. Rev. Pathol. Mech. of Dis. 2015;10:173–194. doi: 10.1146/annurev-pathol-012513-104649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.So J.S. Roles of endoplasmic reticulum stress in immune responses. Mol. Cell. 2018;41:705–716. doi: 10.14348/molcells.2018.0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi J.A., Song C.H. Insights into the role of endoplasmic reticulum stress in infectious diseases. Front. Immunol. 2020;10 doi: 10.3389/fimmu.2019.03147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fung T.S., Torres J., Liu D.X. The emerging roles of viroporins in ER stress response and autophagy induction during virus infection. Viruses. 2015;7:2834–2857. doi: 10.3390/v7062749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim I., Xu W., Reed J.C. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 20.Pfefferle S., Krähling V., Ditt V., Grywna K., Mühlberger E., Drosten C. Reverse genetic characterization of the natural genomic deletion in SARS-Coronavirus strain Frankfurt-1 open reading frame 7b reveals an attenuating function of the 7b protein in-vitro and in-vivo. Virol. J. 2009;6:131. doi: 10.1186/1743-422X-6-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minakshi R., Padhan K., Rani M., Khan N., Ahmad F., Jameel S. The SARS coronavirus 3a protein causes endoplasmic reticulum stress and induces ligand-independent downregulation of the type 1 interferon receptor. PLoS One. 2009;4 doi: 10.1371/journal.pone.0008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fung T.S., Huang M., Liu D.X. Coronavirus-induced ER stress response and its involvement in regulation of coronavirus–host interactions. Virus Res. 2014;194:110–123. doi: 10.1016/j.virusres.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bechill J., Chen Z., Brewer J.W., Baker S.C. Coronavirus infection modulates the unfolded protein response and mediates sustained translational repression. J. Virol. 2008;82:4492–4501. doi: 10.1128/JVI.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sung S.-C., Chao C.-Y., Jeng K.-S., Yang J.-Y., Lai M.M.C. The 8ab protein of SARS-CoV is a luminal ER membrane-associated protein and induces the activation of ATF6. Virology. 2009;387:402–413. doi: 10.1016/j.virol.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiter R., Tan D., Rosales-Corral S., Galano A., Zhou X., Xu B. Mitochondria: central organelles for melatonin’s antioxidant and anti-aging actions. Molecules. 2018;23:509. doi: 10.3390/molecules23020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshikawa T., Obayashi K., Miyata K., Saeki K., Ogata N. Decreased melatonin secretion in patients with glaucoma: quantitative association with glaucoma severity in the LIGHT study. J. Pineal Res. 2020 doi: 10.1111/jpi.12662. [DOI] [PubMed] [Google Scholar]
- 27.Ganguly K., Kundu P., Banerjee A., Reiter R.J., Swarnakar S. Hydrogen peroxide-mediated downregulation of matrix metalloprotease-2 in indomethacin-induced acute gastric ulceration is blocked by melatonin and other antioxidants. Free Radic. Biol. Med. 2006;41:911–925. doi: 10.1016/j.freeradbiomed.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 28.Moradkhani F., Moloudizargari M., Fallah M., Asghari N., Heidari Khoei H., Asghari M.H. Immunoregulatory role of melatonin in cancer. J. Cell. Physiol. 2020;235:745–757. doi: 10.1002/jcp.29036. [DOI] [PubMed] [Google Scholar]
- 29.Nava F., Calapai G., Facciolà G., Cuzzocrea S., Giuliani G., de Sarro A., Caputi A.P. Melatonin effects on inhibition of thirst and fever induced by lipopolysaccharide in rat. Eur. J. Pharmacol. 1997;331:267–274. doi: 10.1016/S0014-2999(97)01049-2. [DOI] [PubMed] [Google Scholar]
- 30.Reiter R.J., Ma Q., Sharma R. Melatonin in mitochondria: mitigating clear and present dangers. Physiology. 2020;35:86–95. doi: 10.1152/physiol.00034.2019. [DOI] [PubMed] [Google Scholar]
- 31.Anderson G., Reiter R.J. Melatonin: roles in influenza, Covid-19, and other viral infections. Rev. Med. Virol. 2020 doi: 10.1002/rmv.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang R., Wang X., Ni L., Di X., Ma B., Niu S., Liu C., Reiter R.J. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020;250 doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silvestri M., Rossi G.A. Melatonin: its possible role in the management of viral infections-a brief review. Ital. J. Pediatr. 2013;39:61. doi: 10.1186/1824-7288-39-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang S.-H., Cao X.-J., Liu W., Shi X.-Y., Wei W. Inhibitory effect of melatonin on lung oxidative stress induced by respiratory syncytial virus infection in mice. J. Pineal Res. 2010;48:109–116. doi: 10.1111/j.1600-079X.2009.00733.x. [DOI] [PubMed] [Google Scholar]
- 35.Davis I., Matalon S. Reactive species in viral pneumonitis: lessons from animal models. Physiology. 2001;16:185–190. doi: 10.1152/physiologyonline.2001.16.4.185. [DOI] [PubMed] [Google Scholar]
- 36.Reiter R.J., Ma Q., Sharma R. Treatment of Ebola and other infectious diseases: melatonin “goes viral”. Melatonin Res. 2020;3:43–57. doi: 10.32794/mr11250047. [DOI] [Google Scholar]
- 37.Chen Y., Zhao Q., Sun Y., Jin Y., Zhang J., Wu J. Melatonin induces anti-inflammatory effects via endoplasmic reticulum stress in RAW264.7 macrophages. Mol. Med. Rep. 2018 doi: 10.3892/mmr.2018.8613. [DOI] [PubMed] [Google Scholar]
- 38.San-Miguel B., Crespo I., Vallejo D., Álvarez M., Prieto J., González-Gallego J., Tuñón M.J. Melatonin modulates the autophagic response in acute liver failure induced by the rabbit hemorrhagic disease virus. J. Pineal Res. 2014;56:313–321. doi: 10.1111/jpi.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouyang H., Zhong J., Lu J., Zhong Y., Hu Y., Tan Y. Inhibitory effect of melatonin on Mst1 ameliorates myocarditis through attenuating ER stress and mitochondrial dysfunction. J. Mol. Histol. 2019;50:405–415. doi: 10.1007/s10735-019-09836-w. [DOI] [PubMed] [Google Scholar]
- 40.Islam M.T., Ali E.S., Uddin S.J., Islam Md.A., Shaw S., Khan I.N., Saravi S.S.S., Ahmad S., Rehman S., Gupta V.K., et al. Andrographolide, a diterpene lactone from Andrographis paniculata and its therapeutic promises in cancer. Cancer Lett. 2018;420:129–145. doi: 10.1016/j.canlet.2018.01.074. [DOI] [PubMed] [Google Scholar]
- 41.Zhang R., Zhao J., Xu J., Jiao D.-X., Wang J., Gong Z.-Q., Jia J.-H. Andrographolide suppresses proliferation of human colon cancer SW620 cells through the TLR4/NF-κB/MMP-9 signaling pathway. Oncol. Lett. 2017;14:4305–4310. doi: 10.3892/ol.2017.6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuwen D., Mi S., Ma Y., Guo W., Xu Q., Shen Y., Shu Y. Andrographolide enhances cisplatin-mediated anticancer effects in lung cancer cells through blockade of autophagy. Anti-Cancer Drugs. 2017;28:967–976. doi: 10.1097/CAD.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 43.Soo H.L., Quah S.Y., Sulaiman I., Sagineedu S.R., Lim J.C.W., Stanslas J. Advances and challenges in developing andrographolide and its analogues as cancer therapeutic agents. Drug Discov. Today. 2019;24:1890–1898. doi: 10.1016/j.drudis.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 44.Rajagopal S., kumar R.A., Deevi D.S., Satyanarayana C., Rajagopalan R. Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata. J. Exp. Ther. Oncol. 2003;3:147–158. doi: 10.1046/j.1359-4117.2003.01090.x. [DOI] [PubMed] [Google Scholar]
- 45.Tan W.S.D., Liao W., Zhou S., Wong W.S.F. Is there a future for andrographolide to be an anti-inflammatory drug? Deciphering its major mechanisms of action. Biochem. Pharmacol. 2017;139:71–81. doi: 10.1016/j.bcp.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 46.Lai C.-Y., Su Y.-W., Lin K.-I., Hsu L.-C., Chuang T.-H. Natural modulators of endosomal toll-like receptor-mediated psoriatic skin inflammation. J Immunol Res. 2017;2017:1–15. doi: 10.1155/2017/7807313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carretta M.D., Alarcón P., Jara E., Solis L., Hancke J.L., Concha I.I., Hidalgo M.A., Burgos R.A. Andrographolide reduces IL-2 production in T-cells by interfering with NFAT and MAPK activation. Eur. J. Pharmacol. 2009;602:413–421. doi: 10.1016/j.ejphar.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 48.Banerjee A., Banerjee V., Czinn S., Blanchard T. Vol. 8. Oncotarget; 2017. Increased Reactive Oxygen Species Levels Cause ER Stress and Cytotoxicity in Andrographolide Treated Colon Cancer Cells; pp. 26142–26153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khole S., Mittal S., Jagadish N., Ghosh D., Gadgil V., Sinkar V., Ghaskadbi S. Andrographolide enhances redox status of liver cells by regulating microRNA expression. Free Radic. Biol. Med. 2019;130:397–407. doi: 10.1016/j.freeradbiomed.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Mussard E., Cesaro A., Lespessailles E., Legrain B., Berteina-Raboin S., Toumi H. Andrographolide, a natural antioxidant: an update. Antioxidants. 2019;8:571. doi: 10.3390/antiox8120571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen JX., Xue HJ., Ye WC., Fang BH., Liu YH., Yuan SH., Yu P., Wang YQ. Activity of Andrographolide and Its Derivatives Against Influenza Virus in Vivo and in Vitro. Biol. Pharm. Bull. 2009;32:1385–1391. doi: 10.1248/bpb.32.1385. [DOI] [PubMed] [Google Scholar]
- 52.Seubsasana S., Pientong C., Ekalaksananan T., Thongchai S., Aromdee C. A potential andrographolide analogue against the replication of herpes simplex virus type 1 in vero cells. Med. Chem. 2011;7:237–244. doi: 10.2174/157340611795564268. [DOI] [PubMed] [Google Scholar]
- 53.Yu B., Dai C., Jiang Z., Li E., Chen C., Wu X., Chen J., Liu Q., Zhao C., He J., et al. Andrographolide as an Anti-H1N1 drug and the mechanism related to retinoic acid-inducible gene-I-like receptors signaling pathway. Chin. J. Integr. Med. 2014;20:540–545. doi: 10.1007/s11655-014-1860-0. [DOI] [PubMed] [Google Scholar]
- 54.Ding Y., Chen L., Wu W., Yang J., Yang Z., Liu S. Andrographolide inhibits influenza A virus-induced inflammation in a murine model through NF-κB and JAK-STAT signaling pathway. Microbes Infect. 2017;19:605–615. doi: 10.1016/j.micinf.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 55.Diwaker D., Mishra K.P., Ganju L. Effect of modulation of unfolded protein response pathway on dengue virus infection. Acta Biochim. Biophys. Sin. 2015:gmv108. doi: 10.1093/abbs/gmv108. [DOI] [PubMed] [Google Scholar]
- 56.Gupta S., Mishra K.P., Ganju L. Broad-spectrum antiviral properties of andrographolide. Arch. Virol. 2017;162:611–623. doi: 10.1007/s00705-016-3166-3. [DOI] [PubMed] [Google Scholar]
- 57.Enmozhi S.K., Raja K., Sebastine I., Joseph J. Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: an in silico approach. J. Biomol. Struct. Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1760136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peng Y., Wang Y., Tang N., Sun D., Lan Y., Yu Z., Zhao X., Feng L., Zhang B., Jin L., et al. Andrographolide inhibits breast cancer through suppressing COX-2 expression and angiogenesis via inactivation of p300 signaling and VEGF pathway. J. Exp. Clin. Cancer Res. 2018;37:248. doi: 10.1186/s13046-018-0926-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou J., Ong C.-N., Hur G.-M., Shen H.-M. Inhibition of the JAK-STAT3 pathway by andrographolide enhances chemosensitivity of cancer cells to doxorubicin. Biochem. Pharmacol. 2010;79:1242–1250. doi: 10.1016/j.bcp.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 60.Sareer O., Ahmad S., Umar S. Andrographis paniculata: a critical appraisal of extraction, isolation and quantification of andrographolide and other active constituents. Nat. Prod. Res. 2014;28:2081–2101. doi: 10.1080/14786419.2014.924004. [DOI] [PubMed] [Google Scholar]
- 61.Ekalaksananan T., Sookmai W., Fangkham S., Pientong C., Aromdee C., Seubsasana S., Kongyingyoes B. Activity of Andrographolide and its derivatives on HPV16 Pseudovirus infection and viral oncogene expression in cervical carcinoma cells. Nutr. Cancer. 2015;67:687–696. doi: 10.1080/01635581.2015.1019630. [DOI] [PubMed] [Google Scholar]
- 62.Lin T.-P., Chen S.-Y., Duh P.-D., Chang L.-K., Liu Y.-N. Inhibition of the Epstein–Barr virus lytic cycle by andrographolide. Biol. Pharm. Bull. 2008;31:2018–2023. doi: 10.1248/bpb.31.2018. [DOI] [PubMed] [Google Scholar]
- 63.Wintachai P., Kaur P., Lee R.C.H., Ramphan S., Kuadkitkan A., Wikan N., Ubol S., Roytrakul S., Chu J.J.H., Smith D.R. Activity of andrographolide against chikungunya virus infection. Sci. Rep. 2015;5 doi: 10.1038/srep14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li F., Lee E.M., Sun X., Wang D., Tang H., Zhou G.-C. Design, synthesis and discovery of andrographolide derivatives against Zika virus infection. Eur. J. Med. Chem. 2020;187 doi: 10.1016/j.ejmech.2019.111925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chandramohan V., Kaphle A., Chekuri M., Gangarudraiah S., Bychapur Siddaiah G. Evaluating andrographolide as a potent inhibitor of NS3-4A protease and its drug-resistant mutants using in silico approaches. Adv. Virol. 2015;2015:1–9. doi: 10.1155/2015/972067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee J.-C., Tseng C.-K., Young K.-C., Sun H.-Y., Wang S.-W., Chen W.-C., Lin C.-K., Wu Y.-H. Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/Nrf2 pathway in human hepatoma cells. Br. J. Pharmacol. 2014;171:237–252. doi: 10.1111/bph.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aromdee C., Suebsasana S., Ekalaksananan T., Pientong C., Thongchai S. Stage of action of naturally occurring andrographolides and their semisynthetic analogues against herpes simplex virus type 1 in vitro. Planta Med. 2011;77:915–921. doi: 10.1055/s-0030-1250659. [DOI] [PubMed] [Google Scholar]
- 68.Chen H., Ma Y.-B., Huang X.-Y., Geng C.-A., Zhao Y., Wang L.-J., Guo R.-H., Liang W.-J., Zhang X.-M., Chen J.-J. Synthesis, structure–activity relationships and biological evaluation of dehydroandrographolide and andrographolide derivatives as novel anti-hepatitis B virus agents. Bioorg. Med. Chem. Lett. 2014;24:2353–2359. doi: 10.1016/j.bmcl.2014.03.060. [DOI] [PubMed] [Google Scholar]
- 69.Vora J., Patel S., Sinha S., Sharma S., Srivastava A., Chhabria M., Shrivastava N. Molecular docking, QSAR and ADMET based mining of natural compounds against prime targets of HIV. J. Biomol. Struct. Dyn. 2019;37:131–146. doi: 10.1080/07391102.2017.1420489. [DOI] [PubMed] [Google Scholar]
- 70.Yi Z., Ouyang S., Zhou C., Xie L., Fang Z., Yuan H., Yang J., Zou L., Jia T., Zhao S., et al. Andrographolide inhibits mechanical and thermal hyperalgesia in a rat model of HIV-induced neuropathic pain. Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calabrese C., Berman S.H., Babish J.G., Ma X., Shinto L., Dorr M., Wells K., Wenner C.A., Standish L.J. A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother. Res. 2000;14:333–338. doi: 10.1002/1099-1573(200008)14:5<333::AID-PTR584>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 72.Tan W.S.D., Peh H.Y., Liao W., Pang C.H., Chan T.K., Lau S.H., Chow V.T., Wong W.S.F. Cigarette smoke-induced lung disease predisposes to more severe infection with nontypeable Haemophilus influenzae: protective effects of andrographolide. J. Nat. Prod. 2016;79:1308–1315. doi: 10.1021/acs.jnatprod.5b01006. [DOI] [PubMed] [Google Scholar]
- 73.Peña J., Harris E. Dengue virus modulates the unfolded protein response in a time-dependent manner. J. Biol. Chem. 2011;286:14226–14236. doi: 10.1074/jbc.M111.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu A.-L., Lu C.-Y., Wang T.-S., Tsai C.-W., Liu K.-L., Cheng Y.-P., Chang H.C., Lii C.-K., Chen H.-W. Induction of heme oxygenase 1 and inhibition of tumor necrosis factor α-induced intercellular adhesion molecule expression by andrographolide in EA.hy926 cells. J. Agric. Food Chem. 2010;58:7641–7648. doi: 10.1021/jf101353c. [DOI] [PubMed] [Google Scholar]
- 75.Tseng C.-K., Lin C.-K., Wu Y.-H., Chen Y.-H., Chen W.-C., Young K.-C., Lee J.-C. Human heme oxygenase 1 is a potential host cell factor against dengue virus replication. Sci. Rep. 2016;6 doi: 10.1038/srep32176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van der Heide V. Neutralizing antibody response in mild COVID-19. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rubin E.J., Baden L.R., Morrissey S. Audio interview: approaches to Covid-19 vaccines and antivirals. N. Engl. J. Med. 2020;382:e58. doi: 10.1056/NEJMe2012889. [DOI] [PubMed] [Google Scholar]
- 78.Chen W.-H., Hotez P.J., Bottazzi M.E. Potential for developing a SARS-CoV receptor-binding domain (RBD) recombinant protein as a heterologous human vaccine against coronavirus infectious disease (COVID)-19. Hum. Vaccines Immunotherapeutics. 2020:1–4. doi: 10.1080/21645515.2020.1740560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang S., Hillyer C., Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41:355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dhama K., Sharun K., Tiwari R., Dadar M., Malik Y.S., Singh K.P., Chaicumpa W. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum. Vaccines Immunotherapeutics. 2020:1–7. doi: 10.1080/21645515.2020.1735227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brufsky A. Hyperglycemia, hydroxychloroquine, and the COVID-19 pandemic. J. Med. Virol. 2020 doi: 10.1002/jmv.25887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.ul Qamar M.T., Alqahtani S.M., Alamri M.A., Chen L.-L. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J. Pharm. Anal. 2020 doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blanchard T.G., Lapidus R., Banerjee V., Bafford A.C., Czinn S.J., Ahmed H., Banerjee A. Upregulation of RASSF1A in colon cancer by suppression of angiogenesis signaling and Akt activation. Cell. Physiol. Biochem. 2018;48:1259–1273. doi: 10.1159/000492012. [DOI] [PubMed] [Google Scholar]