Abstract
Background
New therapeutic options to address the ongoing coronavirus disease 2019 (COVID-19) pandemic are urgently needed. One possible strategy is the repurposing of existing drugs approved for other indications as antiviral agents for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Due to the commercial unavailability of SARS-CoV-2 drugs for treating COVID-19, we screened approximately 250 existing drugs or pharmacologically active compounds for their inhibitory activities against feline infectious peritonitis coronavirus (FIPV) and human coronavirus OC43 (HCoV-OC43), a human coronavirus in the same genus (Betacoronavirus) as SARS-CoV-2.
Methods
FIPV was proliferated in feline Fcwf-4 cells and HCoV-OC43 in human HCT-8 cells. Viral proliferation was assayed by visualization of cytopathic effects on the infected Fcwf-4 cells and immunofluorescent assay for detection of the nucleocapsid proteins of HCoV-OC43 in the HCT-8 cells. The concentrations (EC50) of each drug necessary to diminish viral activity to 50% of that for the untreated controls were determined. The viabilities of Fcwf-4 and HCT-8 cells were measured by crystal violet staining and MTS/PMS assay, respectively.
Results
Fifteen out of the 252 drugs or pharmacologically active compounds screened were found to be active against both FIPV and HCoV-OC43, with EC50 values ranging from 11 nM to 75 μM. They are all old drugs as follows, anisomycin, antimycin A, atovaquone, chloroquine, conivaptan, emetine, gemcitabine, homoharringtonine, niclosamide, nitazoxanide, oligomycin, salinomycin, tilorone, valinomycin, and vismodegib.
Conclusion
All of the old drugs identified as having activity against FIPV and HCoV-OC43 have seen clinical use in their respective indications and are associated with known dosing schedules and adverse effect or toxicity profiles in humans. Those, when later confirmed to have an anti-viral effect on SARS-CoV-2, should be considered for immediate uses in COVID-19 patients.
Keywords: Coronavirus, COVID-19, Cytopathic effect, HCoV-OC43, SARS-CoV-2, Drug repurpose
At a glance commentary
Scientific background on the subject
Repurposing of existing drugs as antiviral agents for SARS-CoV-2 is a practical strategy for the urgent need in treating COVID-19. Thus, we screened 252 existing drugs or pharmacologically active compounds for their inhibitory activities against FIPV and HCoV-OC43, a human coronavirus in the same genus (Betacoronavirus) as SARS-CoV-2.
What this study adds to the field
All of the old drugs identified against FIPV and HCoV-OC43 have seen clinical use in their respective indications and are associated with known dosing schedules and adverse or toxicity profiles in humans. Those, when later confirmed to have an anti-SARS-CoV-2 effect, should be considered for immediate uses in COVID-19 patients.
Coronavirus disease 2019 (COVID-19), the disease caused by severe acute respiratory syndrome associated coronavirus-2 (SARS-CoV-2), has infected over 2,970,000 people and killed more than 206,000 in 213 countries since the first outbreak in WuHan, China [1] was reported in December 2019. To control this disease, effective treatments, including SARS-CoV-2 inhibitors, are being actively pursued [1]. One potentially efficient approach is the repurposing of drugs previously approved to treat other diseases, since they are immediately available for use in clinical trials with COVID-19 patients and have known safety profiles.
Coronavirus (CoV) has a large genome and its mutation rate is higher than that of other RNA viruses [2,3]. Animal CoVs cause persistent enzootic infections that inevitably infect new host species and become zoonotic, as happened for SARS-CoV, Middle East respiratory syndrome coronavirus (MERS-CoV), and SARS-CoV-2. Due to the diversity and relentlessness of these viruses, their individual mitigation is difficult [4]; and therefore broad-spectrum inhibitors of emerging and endemic CoVs are needed, especially for those prone to be periodically cycling in and out of humans and livestock. Therefore, the small molecule inhibitors, either old drugs or new chemical entities, were actively pursued and developed aiming to prevent or cure the SARS-CoV and MERS-CoV infections [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14]]. Previous work established the clinical efficacy of pegylated interferon-α-2a, ribavirin, and lopinavir/ritonavir for the treatment of the MERS-CoV [15,16]. Therefore, the strategy of repurposing existing drugs to treat patients infected with novel or zoonotic CoV has been validated and merits consideration in the contest of tackling the SARS-CoV-2 and COVID-19 pandemic.
Two representative CoVs were selected for existing drugs to be tested against. Feline infectious peritonitis coronavirus (FIPV) belongs to the genus Alphacoronavirus and causes enteritis in domestic and wild cats. Approximately 5–15% of infected cats develop feline infectious peritonitis, which is usually fatal [17,18]. The pathogenesis, epidemiology, and pulmonary lesions associated with FIPV are similar to those associated with human SARS [19]. Human coronavirus OC43 (HCoV-OC43) belongs to the same viral genus (Betacoronavirus) as SARS-CoV and SARS-CoV-2, infects humans and cattle, and is one of the viruses responsible for the common cold [20,21].
252 old drugs or pharmacologically active compounds were assessed for their inhibitory activity of FIPV [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16]], of which 23 were found to exhibit some anti-FIPV activity and advanced to testing against HCoV-OC43. In total, 15 exerted an inhibitory effect on both FIPV and HCoV-OC43 and they turned out are old drugs. These included chloroquine, which was recently demonstrated to be capable of inhibiting SARS-CoV-2 in vitro, and exhibited some efficacy in the clinical treatment of COVID-19 patients [22]. Moreover, GS-441524, the active metabolite of remdesivir [23,24] which is currently being investigated in clinical trials for severe cases of COVID-19 [24,25], also exerted potent inhibitory activities against both FIPV and HCoV-OC43 herein.
Materials and methods
Cells, viruses, and antibodies
Felis catus whole fetus-4 (Fcwf-4) cells (ATCC®CRL-2787) were maintained in Dulbecco's modified Eagle's medium (DMEM, Hyclone Laboratories, Logan, UT, USA) containing 10% fetal bovine serum (FBS) with 1% penicillin/streptomycin at 37 °C with 5% CO2. The serotype Ⅱ FIPV Taiwan isolate NTU156 strain, a kind gift from National Taiwan University, was propagated and titrated in Fcwf-4 cells [26]. Confluent Fcwf-4 cells were seeded in 96-well plates and treated with various concentrations of testing compounds of up to 100 μM at 37 °C under an atmosphere of 5% CO2 for 48 h. Sixteen h post inoculation, cells were infected with FIPV NTU156 strain at 300 TCID50 per well and incubated at 37 °C. After 1 h, the supernatant was discarded and a series of 7 concentrations at different dilution of testing compounds in DMEM containing 2% FBS added. Plates were incubated at 37 °C under an atmosphere of 5% CO2 for additional 48 h; then, the cells were fixed with 10% formalin and stained with 0.1% crystal violet. The cytopathic effect (CPE) of the virus was assumed to correlate with the intensity of the crystal violet staining and measured visually for determination of the 50% effective concentrations (EC50). Cell cytotoxicity was also measured by crystal violet staining. The 50% cytotoxicity concentration (CC50) was calculated according to the Reed and Muench method [27].
HCT-8 colon epithelial cells (ATCC®CCL-244™) were grown as monolayers in a growth medium consisting of DMEM and 10% FBS, (Biological Industries, Cromwell, CT, USA). HCoV-OC43 (ATCC®VR1558™) was grown and propagated in HCT-8 cells cultured with DMEM and 2% FBS. EC50 was measured using an indirect immunofluorescent assay (IFA). HCT-8 cells (5 x104 cells/well) were deposited in 96-well plates, pre-treated with solutions of the compounds to be tested for 30 min, and then infected with HCoV-OC43 at a multiplicity of infection (MOI) of 0.05, and incubated at 37 °C (see above) for 72 h. For the IFA assay, HCT-8 cells were fixed with 80% acetone and subjected to IFA with (i) an antibody against nucleocapsid proteins of HCoV-OC43 (Mab9013; Merck Millipore, Burlington, MA, USA) and (ii) antibody fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin (#55499; MP Biomedicals, Irvine, CA, USA). After three washes with phosphate-buffered saline, cells were incubated with the FITC-conjugated anti-mouse immunoglobulin for 60 min at room temperature. The cells were washed three times with PBS and the fluorescence intensities measured using either a SpectraMax® Paradigm® system (Molecular Devices, San Jose, CA, USA) (excitation and emission wavelengths, 485 and 535 nm, respectively) to determine the EC50 for inhibiting nucleocapsid protein expression; or viewed by a fluorescence microscopy. Hoechst 33258 dye (H3569, Invitrogen™, Waltham, MA, USA) was used to stain the nuclear DNA of live cells. Images of the cells after IFA or Hoechst 33258 staining were captured using a charge-coupled device linked to a Nikon Image-Pro Express. The cells were treated with a series of 5 concentrations of the test compounds at 5-fold dilution; and the results of these assays used to obtain concentration–response curves from which EC50 values were determined. The % area of immunofluorescent staining of the cells was used to correct for EC50 values since the fluorescence intensity was disproportionately higher when only small portion of the cells were infected. For the cytotoxicity assay, HCT-8 cells cultured in DMEM and 10% FBS in 96-well plates were treated with a designed series of 5 concentrations at 5-fold dilution of the test compounds for 72 h. The results of these assays were used to obtain the concentration–response curves from which the CC50 concentrations were obtained.
Chemicals
Emetine (HY–B1479A, 99.81%, LCMS), salinomycin (HY-15597, >98%, NMR), tilorone (HY–B1080, 99.9%, LCMS), chloroquine (HY-17589, 99.9%, LCMS), homoharringtonine (HY-14944, 99.2%, LCMS), gemcitabine (HY–B0003, 99.9%, LCMS), vismodegib (HY-10440, 99.9%, LCMS), conivaptan (HY-18347A, 99.9%, LCMS), and atovaquone (HY-13832, 99.8%, LCMS) were purchased from MedChem Express (Monmouth Junction, NJ, USA); niclosamide (S3030, 99.8%, HPLC) and nitazoxanide (S1627, 99.3%, HPLC) were from Selleckchem (Houston, TX, USA); antimycin A (A8674, 97.64%, HPLC), anisomycin (A9789, >98%, HPLC) oligomycin (O4876, >90%, HPLC), valinomycin (V0627, ≧90%, HPLC) and crystal violet (C0775, Dye content ≥90%) were from Sigma–Aldrich (St. Louis, MO, USA); GS-441524 (AG167808, >98%, HPLC) were from Carbosynth (San Diego, CA, USA). All chemicals were used as supplied.
Results and discussion
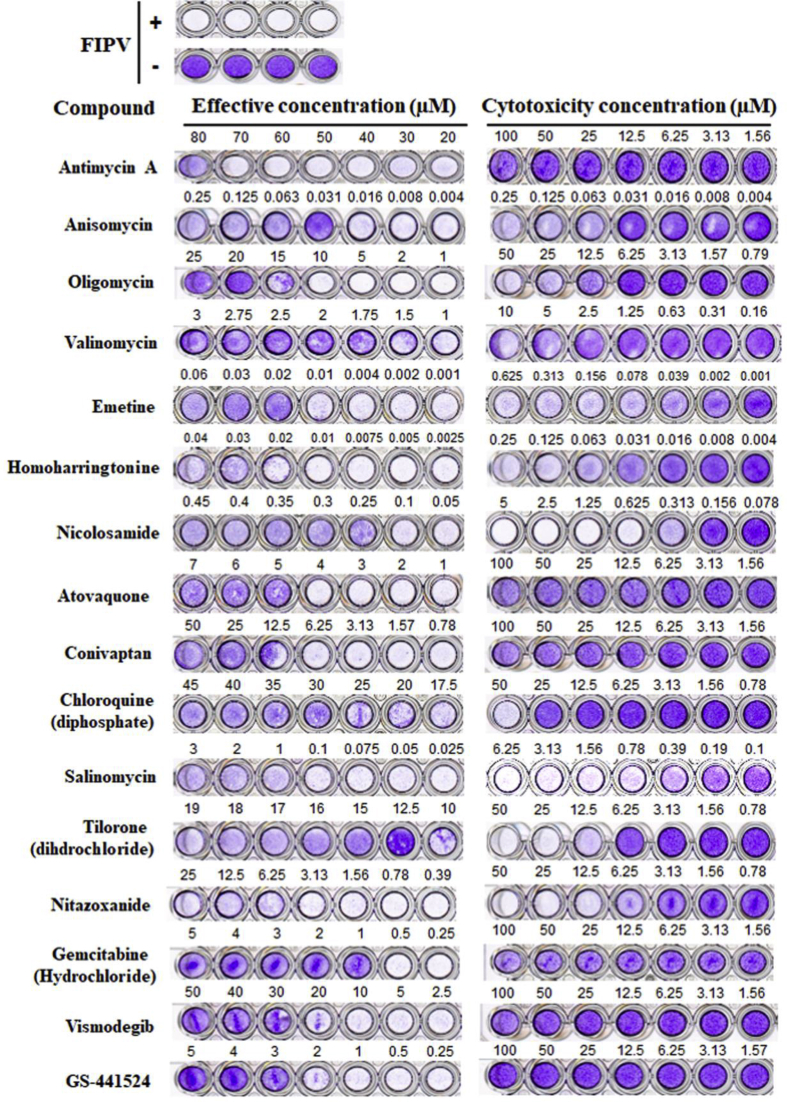
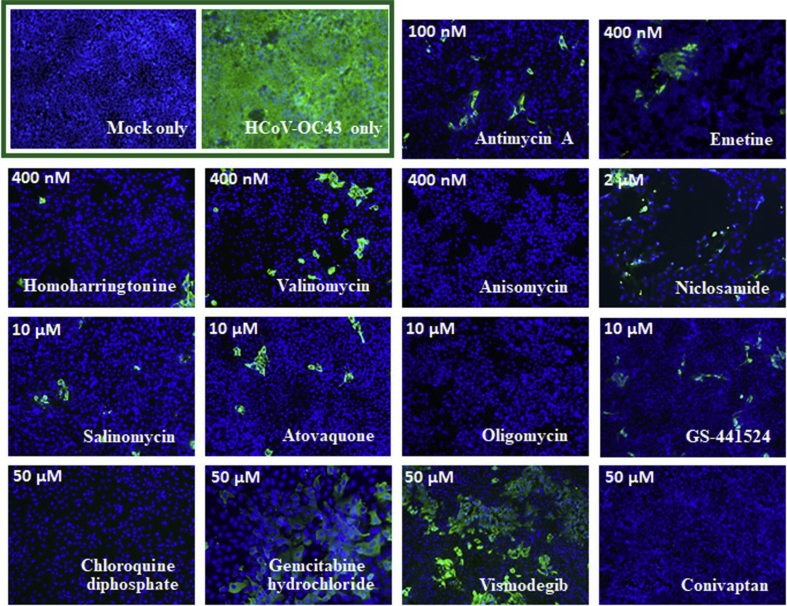
252 drugs were collected and screened for their inhibitory activity against FIPV; this activity was ascertained by visual observation of their cytopathic effects. Of these 252, 23 were also tested for their inhibition of HCoV-OC43 by an IFA against HCoV-OC43 nucleocapsid protein (Table 1). Fifteen of these, all old drugs, exhibited inhibitory activity against HCoV-OC43 and exhibited - EC50 values ranging from 11 nM to 75 μM for FIPV and 62 nM to 48 μM for HCoV-OC43 (Table 1). Representative results from the cytopathy (FIPV) and IFA assays (HCoV-OC43) are depicted in Fig. 1, Fig. 2, respectively. These 15 drugs include 4 antibiotics antimycin A, anisomycin, valinomycin, and oligomycin, with EC50 values of 75 μM, 23 nM, 1.63 μM, and 17.78 μM against FIPV and 62 nM, 0.2 μM, 0.4 μM, and 5.2 μM against HCoV-OC43 respectively.
Table 1.
Inhibitory activities of 15 drugs and GS-441524 against FIPV and HCoVOC43 coronaviruses.
| Compound Name | Feline infectious peritonitis virusa |
Human coronavirus OC43a |
||||
|---|---|---|---|---|---|---|
| EC50 (μM) | CC50 (μM) | Selectivity index | EC50 (μM) | CC50 (μM) | Selectivity index | |
| (Visual assay) | (Visual assay) | (IFA) | (MTS) | |||
| Antimycin A | 75.00 ± 0.00 | 81.94 ± 6.36 | 1.10 | 0.062 ± 0.003 | >50 | >806 |
| Anisomycin | 0.023 ± 0.00 | 0.047 ± 0.012 | 2.05 | 0.20 ± 0.03 | 22.8 ± 0.94 | 114 |
| Oligomycin | 17.78 ± 0.48 | 18.75 ± 0.00 | 1.06 | 5.22 ± 0.96 | >50 | >9.58 |
| Valinomycin | 1.63 ± 0.10 | 3.98 ± 0.39 | 2.45 | 0.41 ± 0.02 | >50 | >122 |
| Emetine | 0.011 ± 0.00 | 0.03 ± 0.00 | 3.00 | 0.21 ± 0.07 | >50 | >238 |
| Homoharringtonine | 0.031 ± 0.003 | 0.049 ± 0.002 | 1.58 | 0.29 ± 0.03 | 7.61 ± 0.60 | 26.7 |
| Niclosamide | 0.29 ± 0.02 | 0.23 ± 0.00 | 0.80 | 1.36 ± 0.56 | >50 | >36.8 |
| Atovaquone | 4.78 ± 0.51 | >100 | 20.92 | 6.78 ± 0.73 | >50 | >7.37 |
| Conivaptan | 16.89 ± 1.51 | >100 | 5.92 | 12.2 ± 4.20 | >50 | >4.10 |
| Chloroquine (diphosphate) | 27.92 ± 0.72 | 37.50 ± 0.00 | 1.35 | 27.4 ± 2.51 | >50 | >1.82 |
| Salinomycin | 0.70 ± 0.13 | 0.34 ± 0.10 | 0.49 | 5.78 ± 2.17 | >50 | >8.65 |
| Tilorone (dihydrochloride) | 11.25 ± 1.25 | 9.38 ± 0.00 | 0.84 | 26.0 ± 2.24 | 35.9 ± 2.97 | 1.38 |
| Nitazoxanide | NA | 4.69 ± 0.00 | NA | 28.6 ± 7.44 | >50 | >1.75 |
| Gemcitabine (Hydrochloride) | 1.08 ± 0.35 | >100 | 92.60 | 38.6 ± 11.4 | >50 | >1.30 |
| Vismodegib | 32.5 ± 2.50 | >100 | 3.08 | 47.6 ± 3.29 | >50 | >1.05 |
| GS-441524 | 3.5 ± 0.0 | >100 | 28.58 | 6.77 ± 0.71 | >50 | >7.39 |
Abbreviations: EC50: The values of 50% maximal effective concentration; CC50: The values of 50% maximal cytotoxic concentration; NA: Not available.
Data are means ± S.D. from three rounds of experiments, each in triplicate (FIPV); and means ± S.D. from three independent experiments, each in duplicate (HCoV-OC43).
Fig. 1.
The cytopathic effects of 15 drugs and GS-441524 against FIPV. Fcwf-4 cells infected with FIPV (NTU156) showed typical cytopathic effects by crystal violet staining compared to uninfected cells. FIPV infected cells were treated with a series of 7 concentrations at different dilution of the testing compounds. The cytotoxicity of the compounds being tested was also investigated. The 50% maximal effective concentration (EC50) and cytotoxicity concentration (CC50) of each compound were calculated by visual assays. Shown are means ± S.D. from three rounds of experiments, each in triplicate.
Fig. 2.
Immunofluorescent assay of 15 drugs and GS-441524 against HCoV-OC43. Indirect immunofluorescent assay (IFAs) with the antibody against HCoV-OC43 nucleocapsid protein (in green) and Hoechst dye staining (in blue) for the DNA of the host live cells in HCoV-OC43 (0.05 MOI) infected HCT-8 cells at 72 h.p.i. were performed shown here are the representative images of the cells with mock infection (MOCK), the infected cells treated with vehicle (0.5% DMSO), and the infected cells treated with drugs as indicated from 3 independent experiments. Nuclei of live HCT-8 cells in blue were stained with Hoechst dye. The treated concentrations of each drug are labelled with the corresponding images (200×).
Emetine, a drug used as an anti-protozoal and previously reported to have anti-coronaviral activity potential [28], exhibited EC50 values of 11 nM against FIPV and 0.21 μM against HCoV-OC43. Homoharringtonine, a natural plant alkaloid derived from Cephalotaxus fortunei and indicated for the treatment of chronic myeloid leukemia (CML) [29], exhibited EC50 values of 31 nM against FIPV and 0.29 μM against HCoV-OC43. Niclosamide, an anthelmintic indicated for the treatment of tapeworm infections, exhibited EC50 values of 0.29 μM against FIPV and 1.36 μM against HCoV-OC43. Interestingly, niclosamide also demonstrated efficacy against drug-resistant Staphylococcus aureus [30] and showed in vitro activity against SARS-CoV [11].
Atovaquone, a hydroxy-1,4-naphthoquinone with antipneumocystic activity [31], exhibited EC50 values of 4.78 μM against FIPV and 6.78 μM against HCoV-OC43. Conivaptan, a non-peptide inhibitor of the receptor vasopressin and originally approved for hyponatremia [32], exhibited EC50 values of 16.9 μM against FIPV and 12.2 μM against HCoV-OC43. Atovaquone is predicted to inhibit SARS-CoV-2 through targeting of the viral RNA-dependent RNA polymerase or 3C like protease [33].
Chloroquine is being intensively studied in the clinical setting for treating COVID-19 and in the preclinical setting for efficacies against SARS-CoV-2 both in vitro and in vivo [22], but its EC50 values against FIPV (27.9 μM) and HCoV-OC43 (27.4 μM) were higher than of the compounds mentioned above. Nonetheless, this identified EC50 of ∼27 μM is comparable or better than the clinically used effective dosages of chloroquine, 200 ∼ 1000 mg in qd or bid [[34], [35], [36]]. In addition, we also tested the nucleotide analogue GS-441524, the active metabolite of remdesivir [23,24], and it exhibited an EC50 of 3.5 μM against FIPV, compared to an EC50 of 6.77 μM against HCoV-OC43; GS-441524 has been reported to exhibit good in vivo efficacy against FIPV in cats [23] and against SARS-CoV in vitro [37], but it is not an FDA approved drug yet. Remdesivir (GS-5734), the prodrug of GS-441524, has also shown efficacy for the treatment of COVID-19 patients [24,25]. Moreover, this identified EC50 (3 ∼7 μM) of GS-441524 is also equivalent or better than the clinically used effective dosages of remdesivir, 100 ∼ 200 mg [24,25]. Thus the concentrations tested in this particular study are of clinical significance.
Other drugs which have already been repurposed in the context of COVID-19 include ivermectin, a type of avermectin used to treat many types of parasite infestations [38], and the combination of hydroxychloroquine and the antibiotic azithromycin [39]. Therefore, all of these above-mentioned drugs are therefore proposed as potential treatments for COVID-19. In conclusion, several existing drugs were identified as having good inhibitory activities against FIPV and HCoV-OC43, and the doses at which they are currently used for their respective disease indications could be referenced when contemplating their application against SARS-CoV-2 in patients of COVID-19.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgements
The authors gratefully acknowledge Dr. Kung-Yee Liang, National Health Research Institutes, for his full support and supervision on this work. This work was funded by the National Health Research Institutes, Taiwan, R.O.C. and the Ministry of Science and Technology, Taiwan, R.O.C. (grants of MOST 106-2320-B-400-009-MY3 and MOST 108-2811-B-400-511) as well as an emergent grant from the Ministry of Health and Welfare, Taiwan, R.O.C.
Footnotes
Peer review under responsibility of Chang Gung University.
Contributor Information
Chiung-Tong Chen, Email: ctchen@nhri.edu.tw.
Shiow-Ju Lee, Email: slee@nhri.org.tw.
References
- 1.Velavan T.P., Meyer C.G. The COVID-19 epidemic. Trop Med Int Health. 2020;25:278–280. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duffy S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018;16 doi: 10.1371/journal.pbio.3000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kautz T.F., Forrester N.L. RNA virus fidelity mutants: a useful tool for evolutionary biology or a complex challenge? Viruses. 2018;10:600. doi: 10.3390/v10110600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gralinski L.E., Baric R.S. Molecular pathology of emerging coronavirus infections. J Pathol. 2015;235:185–195. doi: 10.1002/path.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen L., Niu J., Wang C., Huang B., Wang W., Zhu N. High-throughput screening and identification of potent broad-spectrum inhibitors of coronaviruses. J Virol. 2019;93 doi: 10.1128/JVI.00023-19. e00023–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang C.W., Lee Y.Z., Kang I.J., Barnard D.L., Jan J.T., Lin D. Identification of phenanthroindolizines and phenanthroquinolizidines as novel potent anti-coronaviral agents for porcine enteropathogenic coronavirus transmissible gastroenteritis virus and human severe acute respiratory syndrome coronavirus. Antivir Res. 2010;88:160–168. doi: 10.1016/j.antiviral.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang C.W., Chang H.Y., Hsu H.Y., Lee Y.Z., Chang H.S., Chen I.S. Identification of anti-viral activity of the cardenolides, Na(+)/K(+)-ATPase inhibitors, against porcine transmissible gastroenteritis virus. Toxicol Appl Pharmacol. 2017;332:129–137. doi: 10.1016/j.taap.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang C.W., Chang H.Y., Lee Y.Z., Hsu H.Y., Lee S.J. The cardenolide ouabain suppresses coronaviral replication via augmenting a Na(+)/K(+)-ATPase-dependent PI3K_PDK1 axis signaling. Toxicol Appl Pharmacol. 2018;356:90–97. doi: 10.1016/j.taap.2018.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y.Z., Yang C.W., Hsu H.Y., Qiu Y.Q., Yeh T.K., Chang H.Y. Synthesis and biological evaluation of tylophorine-derived dibenzoquinolines as orally active agents: exploration of the role of tylophorine e ring on biological activity. J Med Chem. 2012;55:10363–10377. doi: 10.1021/jm300705j. [DOI] [PubMed] [Google Scholar]
- 10.Liang R., Wang L., Zhang N., Deng X., Su M., Su Y. Development of small-molecule MERS-CoV inhibitors. Viruses. 2018;10:721. doi: 10.3390/v10120721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu C.J., Jan J.T., Chen C.M., Hsieh H.P., Hwang D.R., Liu H.W. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob Agents Chemother. 2004;48:2693–2696. doi: 10.1128/AAC.48.7.2693-2696.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto N., Yang R., Yoshinaka Y., Amari S., Nakano T., Cinatl J. HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem Biophys Res Commun. 2004;318:719–725. doi: 10.1016/j.bbrc.2004.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C.N., Lin C.P., Huang K.K., Chen W.C., Hsieh H.P., Liang P.H. Inhibition of SARS-CoV 3C-like protease activity by theaflavin-3,3'-digallate (TF3). Evid Based Complement Alternat Med. 2005;2:209–215. doi: 10.1093/ecam/neh081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnard D.L., Kumaki Y. Recent developments in anti-severe acute respiratory syndrome coronavirus chemotherapy. Future Virol. 2011;6:615–631. doi: 10.2217/fvl.11.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Momattin H., Al-Ali A.Y., Al-Tawfiq J.A. A systematic review of therapeutic agents for the treatment of the Middle East respiratory syndrome coronavirus (MERS-CoV) Trav Med Infect Dis. 2019;30:9–18. doi: 10.1016/j.tmaid.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan J.F., Yao Y., Yeung M.L., Deng W., Bao L., Jia L. Treatment with lopinavir/ritonavir or interferon-beta1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212:1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hohdatsu T., Okada S., Ishizuka Y., Yamada H., Koyama H. The prevalence of types I and II feline coronavirus infections in cats. J Vet Med Sci. 1992;54:557–562. doi: 10.1292/jvms.54.557. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen N.C. Serologic studies of naturally occurring feline infectious peritonitis. Am J Vet Res. 1976;37:1449–1453. [PubMed] [Google Scholar]
- 19.Paltrinieri S. Human severe acute respiratory syndrome (SARS) and feline coronaviruses. J Feline Med Surg. 2004;6:131–132. doi: 10.1016/j.jfms.2003.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau S.K., Lee P., Tsang A.K., Yip C.C., Tse H., Lee R.A. Molecular epidemiology of human coronavirus OC43 reveals evolution of different genotypes over time and recent emergence of a novel genotype due to natural recombination. J Virol. 2011;85:11325–11337. doi: 10.1128/JVI.05512-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim Y.X., Ng Y.L., Tam J.P., Liu D.X. Human coronaviruses: a review of virus-host interactions. Diseases. 2016;4:26. doi: 10.3390/diseases4030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Touret F., de Lamballerie X. Of chloroquine and COVID-19. Antivir Res. 2020;177:104762. doi: 10.1016/j.antiviral.2020.104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy B.G., Perron M., Murakami E., Bauer K., Park Y., Eckstrand C. The nucleoside analog GS-441524 strongly inhibits feline infectious peritonitis (FIP) virus in tissue culture and experimental cat infection studies. Vet Microbiol. 2018;219:226–233. doi: 10.1016/j.vetmic.2018.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amirian E., Levy J. Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses. One health. 2020;9:100128. doi: 10.1016/j.onehlt.2020.100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin C.N., Su B.L., Wu C.W., Hsieh L.E., Chueh L.L. Isolation and identification of a novel feline coronavirus from a kitten with naturally occurring feline infectious peritonitis in Taiwan. Taiwan Vet J. 2009;35:145–152. [Google Scholar]
- 27.Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938;27:493–497. [Google Scholar]
- 28.Bleasel M.D., Peterson G.M. Emetine, ipecac, ipecac alkaloids and analogues as potential antiviral agents for coronaviruses. Pharmaceuticals. 2020;13:51. doi: 10.3390/ph13030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kantarjian H.M., O'Brien S., Cortes J. Homoharringtonine/omacetaxine mepesuccinate: the long and winding road to food and drug administration approval. Clin Lymphoma Myeloma Leuk. 2013;13:530–533. doi: 10.1016/j.clml.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajamuthiah R., Fuchs B.B., Conery A.L., Kim W., Jayamani E., Kwon B. Repurposing salicylanilide anthelmintic drugs to combat drug resistant Staphylococcus aureus. PloS One. 2015;10 doi: 10.1371/journal.pone.0124595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ball M.D., Bartlett M.S., Shaw M., Smith J.W., Nasr M., Meshnick S.R. Activities and conformational fitting of 1,4-naphthoquinone derivatives and other cyclic 1,4-diones tested in vitro against Pneumocystis carinii. Antimicrob Agents Chemother. 2001;45:1473–1479. doi: 10.1128/AAC.45.5.1473-1479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Udelson J.E., Smith W.B., Hendrix G.H., Painchaud C.A., Ghazzi M., Thomas I. Acute hemodynamic effects of conivaptan, a dual V(1A) and V(2) vasopressin receptor antagonist, in patients with advanced heart failure. Circulation. 2001;104:2417–2423. doi: 10.1161/hc4501.099313. [DOI] [PubMed] [Google Scholar]
- 33.Odhar H.A., Ahjel S.W., Albeer A., Hashim A.F., Rayshan A.M., Humadi S.S. Molecular docking and dynamics simulation of FDA approved drugs with the main protease from 2019 novel coronavirus. Bioinformation. 2020;16:236–244. doi: 10.6026/97320630016236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh A.K., Singh A., Shaikh A., Singh R., Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metabol Syndrome. 2020;14:241–246. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao J., Hu S. Update on use of chloroquine/hydroxychloroquine to treat coronavirus disease 2019 (COVID-19) Biosci Trends. 2020;14:156–158. doi: 10.5582/bst.2020.03072. [DOI] [PubMed] [Google Scholar]
- 37.Cho A., Saunders O.L., Butler T., Zhang L., Xu J., Vela J.E. Synthesis and antiviral activity of a series of 1'-substituted 4-aza-7,9-dideazaadenosine C-nucleosides. Bioorg Med Chem Lett. 2012;22:2705–2707. doi: 10.1016/j.bmcl.2012.02.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang S.N.Y., Atkinson S.C., Wang C., Lee A., Bogoyevitch M.A., Borg N.A. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antivir Res. 2020;177:104760. doi: 10.1016/j.antiviral.2020.104760. [DOI] [PubMed] [Google Scholar]
- 39.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]