Abstract
During the COVID-19 outbreak, personal protective equipment is widely used to limit infection of caregivers. Innovative solutions have been described to overcome supply shortage. The adaptation of the EasyBreath® surface snorkeling mask by the Prakash team has benefited from outstanding media coverage. We present four 3D-printed devices that we have modified from the initial innovative design in order to adapt to local constraints. We tested the mask during surgery. The modifications that we made provide better ergonomics, visibility and communication capacities, but that have no official approval for use and can therefore only be recommended in the absence of a validated alternative solution. 3D printing is a tool of prime importance in the production of devices for medical use in health crisis situations.
Keywords: COVID-19, SARS-CoV-2, Tracheostomy, Personal protective equipment, 3D printing
1. Introduction
There is no validated prevention or treatment against COVID-19 infection. During the current pandemic, the use of personal protective equipment is essential to reduce the risks of contamination of healthcare providers. There is thus a global demand for such devices, leading to supply issues and shortages, even in large university hospital trusts such as Assistance Publique–Hôpitaux de Paris. Furthermore, the management of COVID-19 patients involves procedures such as intubation, extubation and tracheostomy that are at high risk of viral contamination [1], [2], [3].
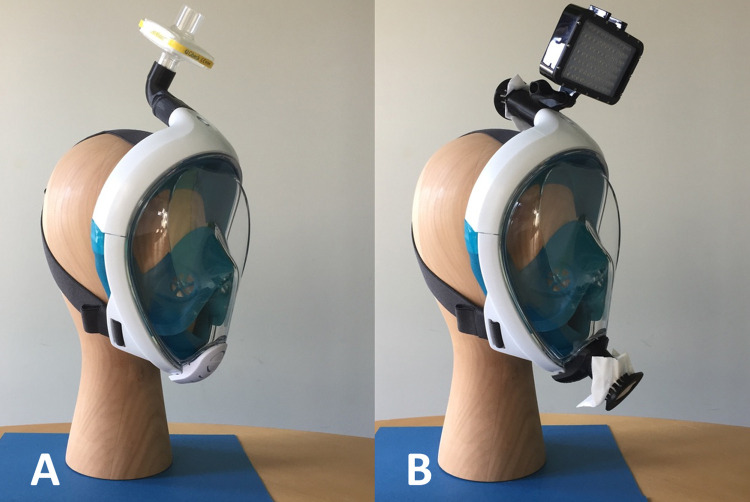
Many teams have proposed custom-made alternative protection devices, without formal safety assessments. Favero's team in Italy and Prakash's team at Stanford University, USA, (http://web.stanford.edu/group/prakash-lab/cgi-bin/labsite/) used surface snorkeling masks with specific adaptors plugged with bacterial/viral filters as facial masks. Their innovative approach benefited from an outstanding media coverage (Fig. 1A) and a formal authorization for medical use by local authorities (FDA-2020-D-1138). This design was secondarily adapted by a French industrial consortium (https://adaptateur-masque.planktonplanet.org) involving the Bic® company (Clichy, France).
Fig. 1.
(A) EasyBreath® mask with an upper adaptor connected to a filter, as designed by Prakash. This option does not include an inferior adaptor. (B) EasyBreath® mask with (1) an upper adaptor with a FFP2/N95 mask piece fixed by a clip and a GoPRo® adaptator for dive lights and (2) a lower adaptor with a FFP2/N95 mask piece fixed by a clip.
We considered using the design offered by Prakash with an EasyBreath® Décathlon surface snorkeling mask but encountered three practical issues:
-
•
the trust of Paris University Hospitals (Assistance Publique–Hôpitaux de Paris) did not recommend the use of respirator filters for personal protection to avoid a shortage of these filters for COVID-positive patients ventilated in intensive care units;
-
•
the EasyBreath® masks available in France do not have an outlet for the fixation of a surgical light source, which was a serious issue when performing bedside tracheostomies in intensive care units;
-
•
the caregiver wearing the mask could not be heard, which induced issues during potentially life-threatening procedures such as bedside tracheostomies in overweight patients.
The aim of this report was to provide details on the modifications that we performed on the original device designed by Prakash in order to overcome the technical difficulties that we encountered using it in our daily clinical practice. We do not provide any kind of formal validation for the use of the EasyBreath® mask as a protection device and do not discuss the issues related to the decontamination of the mask after performing a high-risk procedure.
Technical note
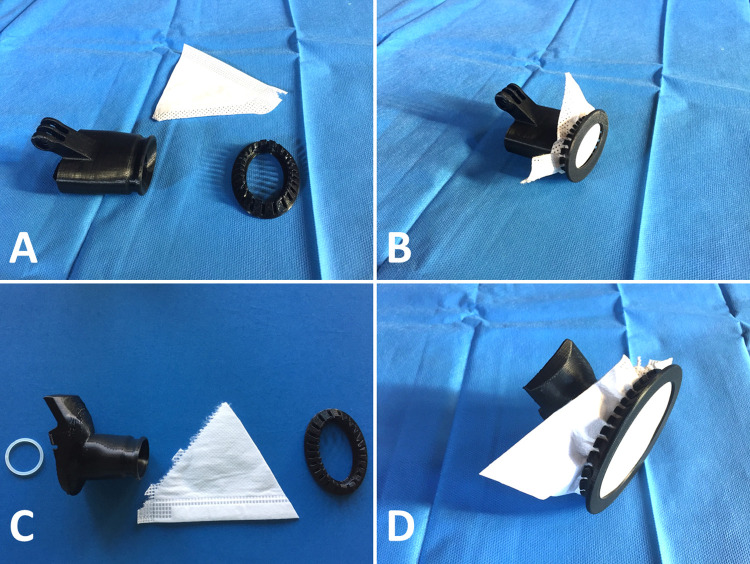
We report four single-use designs: 2 adaptors, 1 clip and 1 joint (Fig. 2 ), resulting from the open-access work of a large team of independent engineers and medical caregivers. The adaptors and the clip were printed using fused deposition modeling (FDM) Stratasys (Eden Prairie, Minnesota, USA) F120 professional devices and acrylonitrile butadiene styrene (ABS) with 0.25 mm layers and an 80% density. The upper adaptor was printed in 1 h 46 min, the lower adaptor in 2 h10 min and the clip in 32 min. The joint was printed using a Stratasys J750 polyjet device and Agilus30 rubber-like photopolymer. This joint was printed in 18 min.
Fig. 2.
(A) Upper adaptor, FFP2/N95 mask piece and clip. (B) Final montage before positioning on the mask. (C) Lower adaptor, O-ring joint, FFP2/N95 mask piece and clip. (D) Final montage before positioning on the mask.
The upper adaptor was designed with a spout allowing the clipping of a fragment of FFP2/N95 face mask, in single or double layers. This upper adaptor also carried a specific GoPro (San Mateo, California, USA) adaptor for fixing a surgical light. Video 1 explains how to use the upper adaptor.
Similarly, one or two layers of FFP2/N95 mask fragments could be clipped on the lower adaptor that was used in replacement of the one-way valve of the original snorkeling mask. The sealing of this lower adaptor was improved by the polyjet-printed O-ring joint. Video 2 explains how to use the lower adaptor. The clips used for the upper and lower adaptors were the same and their positioning is shown in Video 3.
In order to assess our prototype, we used EasyBreath® surface snorkeling masks provided by Decathlon (Villeneuve-d’Ascq, France) with the 2 adaptors, the 2 clips and the joint as reported earlier (Fig. 1B), with prior approval of our local hygiene team. We furthermore used a 5000 lumen portable dive light (Suptig®, waterproof dive light, amazon.com) fixed on the upper adaptor. The snorkeling masks were used by two surgeons performing a bed-side tracheostomy procedure for a COVID-19 patient in an intensive care unit. Infection control rules were otherwise respected during the procedure [4]. The filters clipped on the upper and lower adaptors and the dive light were not displaced during the procedure. The lower adaptor allowed easy communication between surgeons and with the team around them during the procedure, and the light provided enough visibility during tracheostomy. One FFP2/N95 mask was used for the four adaptors of the two masks. Compared to the combined use of a headshield and a FFP2/N95 facemask, surgeons felt safer and had better vision of the surgical field thanks to the portable light. Of note, there is no formal proof of better protection against SARS-CoV-2 using the snorkeling mask compared to a headshield and an FFP2/N95 face mask.
After surgery, the two masks and light were decontaminated using a detergent and rinsed with filtered hospital water. Single-use adaptors were binned as biomedical waste. The two surgeons did not have signs of SARS-CoV-2 10 days after the procedure.
The masks (Fig. 1B) were well tolerated for a maximum period of 120 min.
2. Discussion
Thanks to an international team of designers and engineers and to the flexibility of 3D printing, we managed to implement modifications to open-access prototypes and obtained a device that match our local technical requirements. This process took place within the Emergency 3D-Printing Facility of Assistance Publique–Hôpitaux de Paris, which includes 60 professional FDM printers (Stratasys J120, J170 and J370), 3 polyjet printers (Stratasys J735 and J750) and employs 5 full-time engineers during the COVID-19 crisis. Fast manufacturing and validation procedures were implemented in order to allow the accelerated clinical diffusion of a large number of critical devices. The initial design was provided by the Decathlon®’s collaborative design platform (cocreation.decathlon.fr). The design process was performed with the help of a collaborative task solving platform (jogl.io) and the diffusion of the devices benefited from the help of a dedicated platform (emergency.io). Details on the workflow and designs are provided on Covid3D.org.
Our current design for the use of snorkeling masks as protection devices is not officially validated by health authorities. Therefore, it can only be recommended in case of shortage of all validated alternative solutions. As shortage situations may occur in the coming months, we provide all the design files as supplementary materials (Iconosup 1 and 2). These files can also be uploaded on Covid3D.org. Formal validations are in process in order to compare this solution with similar adaptors designed for connecting viral/bacterial filters.
3. Conclusion
During the current pandemic, 3D printing has demonstrated its critical role in the design and manufacture of protection devices and medical devices. The Covid3D initiative is the first attempt ever to provide industrial level manufacture facilities within a hospital. Its straight collaboration with local medical and surgical teams enables rapid clinical tests. We believe that this model will be replicated in future health, environmental or military crises with high risks of issues in supply chains, including in Europe.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgements
Special thanks to the Kering company® (Paris, France) which funded the 3D printing facility at Assistance Publique–Hôpitaux de Paris and to Sandrine Saubanaire from Hermès® (Paris, France).
Juliette Prébot and Jérémy Adam from Bone3D (Paris, France) designed the devices de novo or by using existing open-access STL files.
We are deeply grateful to the international community of designers, engineers and makers who opened access to their work.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.anorl.2020.05.006.
Appendix A. Supplementary data
How to clip the upper adaptor.
How to clip the lower adaptor.
How to clip the FFP2/N95 mask pieces.
References
- 1.Pichi B., Mazzola F., Bonsembiante A., Petruzzi G., Zocchi J., Moretto S. CORONA-steps for tracheotomy in COVID-19 patients: A staff-safe method for airway management. Oral Oncol. 2020;105:104682. doi: 10.1016/j.oraloncology.2020.104682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schultz P., Morvan J.-B., Fakhry N., Morinière S., Vergez S., Lacroix C. French consensus regarding precautions during tracheostomy and post-tracheostomy care in the context of COVID-19 pandemic. Eur Ann Otorhinolaryngol Head Neck Dis. 2020;135:167–169. doi: 10.1016/j.anorl.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tay J.K., Khoo M.L.-C., Loh W.S. Surgical Considerations for Tracheostomy During the COVID-19 Pandemic: Lessons Learned From the Severe Acute Respiratory Syndrome Outbreak. JAMA Otolaryngol Head Neck Surg. 2020 doi: 10.1001/jamaoto.2020.0764. [DOI] [PubMed] [Google Scholar]
- 4.Fakhry N., Schultz P., Morinière S., Breuskin I., Bozec A., Vergez S. French consensus on management of head and neck cancer surgery during COVID-19 pandemic. Eur Ann Otorhinolaryngol Head Neck Dis. 2020;137:159–160. doi: 10.1016/j.anorl.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
How to clip the upper adaptor.
How to clip the lower adaptor.
How to clip the FFP2/N95 mask pieces.