Abstract
Background
As COVID-19 cases continue to rise globally, evidence from large randomized controlled trials is still lacking. Currently, numerous trials testing potential treatment and preventative options are being undertaken all over the world.
Objectives
We summarized all registered clinical trials examining treatment and prevention options for COVID-19. Additionally, we evaluated the quality of the retrieved studies.
Data sources
Clinicaltrials.gov, the Chinese Clinical Trial Registry and the European Union Clinical Trials Register were systematically searched.
Study eligibility criteria
Registered clinical trials examining treatment and/or prevention options for COVID-19 were included. No language, country or study design restrictions were applied. We excluded withdrawn or cancelled studies and trials not reporting therapeutic or preventative strategies for COVID-19.
Participants and interventions
No restrictions in terms of participants' age and medical background or type of intervention were enforced.
Methods
The registries were searched using the term ‘coronavirus’ or ‘COVID-19’ from their inception until 26 March 2020. Additional manual search of the registries was also performed. Eligible studies were summarized and tabulated. Interventional trials were methodologically analysed, excluding expanded access studies and trials testing traditional Chinese medicine.
Results
In total, 309 trials evaluating therapeutic management options, 23 studies assessing preventive strategies and three studies examining both were retrieved. Finally, 214 studies were methodologically reviewed. Interventional treatment studies were mostly randomized (n = 150/198, 76%) and open label (n = 73/198, 37%) with a median number of planned inclusions of 90 (interquartile range 40–200). Major categories of interventions that are currently being investigated are discussed.
Conclusions
Numerous clinical trials have been registered since the onset of the COVID-19 pandemic. Summarized data on these trials will assist physicians and researchers to promote patient care and guide future research efforts for COVID-19 pandemic containment.
Keywords: Anti-inflammatory, Antivirals, Clinical trials, COVID-19, Immunomodulators, Novel coronavirus, Pneumonia, Prevention, SARS-CoV-2, Treatment
Graphical abstract
Introduction
Given the steep upsurge of COVID-19 cases worldwide within an unprecedented short period of time, we are still waiting for solid evidence from large randomized controlled trials regarding targeted antiviral treatments. In this systematic review, we aim firstly to summarize the data on all currently tested treatment and prevention options for the COVID-19 disease, and secondly to methodologically analyse and evaluate the quality of the registered interventional studies.
Methods
Registered clinical trials were systematically searched at the ClinicalTrials.gov database [1], the Chinese Clinical Trial Registry [2] and the European Union Clinical Trials Register [3] from their inception up to 26 March 2020 using the search terms ‘coronavirus’ or ‘COVID-19’. Additional manual search of the registries was performed for possibly unidentified studies. No language, country or study design restrictions were applied. Participants of any age and medical background with or at risk for COVID-19 were included, as were any currently tested intervention related to the treatment or prevention of COVID-19. The eligibility criteria were developed using the Patient Intervention Comparison Outcomes Study type (PICOS) framework [4]. Inclusion criteria were.
-
•
population: patients with or at risk for COVID-19 disease (for treatment and prevention studies respectively);
-
•
intervention/comparator: any intervention/comparator related to the treatment or prevention of COVID-19 disease;
-
•
outcomes: any outcomes;
-
•
study type: interventional or observational clinical trials.
We excluded withdrawn or cancelled studies and trials not reporting therapeutic or preventative measures for COVID-19. Eligible studies were summarized and tabulated.
Methodological review of the interventional studies was performed. Traditional Chinese medicine (TCM) and homeopathy were excluded from the in-depth qualitative assessment, as we have no expertise to analyse clinical trials testing these agents that rely on controversial scientific rationale [5,6].
We evaluated the study design, number of planned inclusions and primary outcomes of interventional studies, excluding retrospective and ‘expanded access’ studies. Studies were also analysed according to their primary endpoint, i.e. clinical, virological (viral excretion in clinical samples), radiological (imaging results such as computed tomography scans or X-rays) or biological/immunological (complete blood count, CD8+/CD4+ T cells count, etc.). This review was conducted according to the PRISMA guidelines [7].
Results
General data retrieved
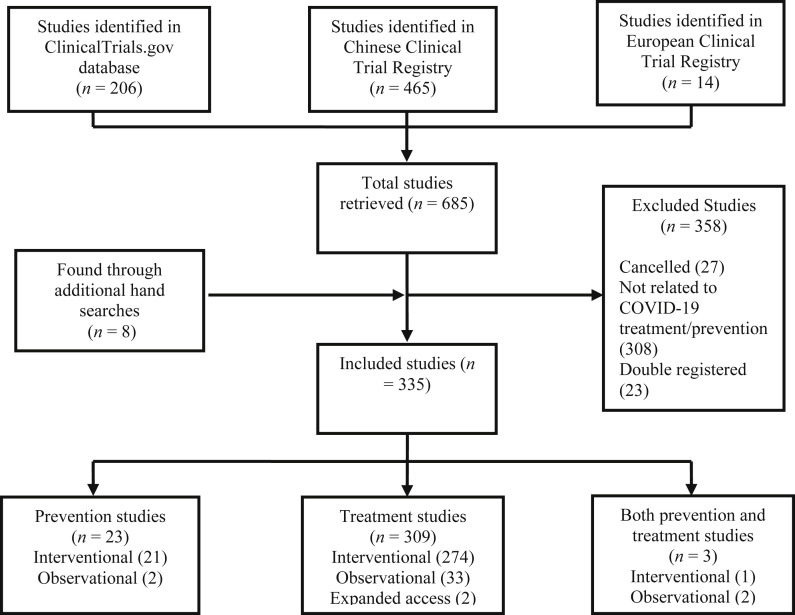
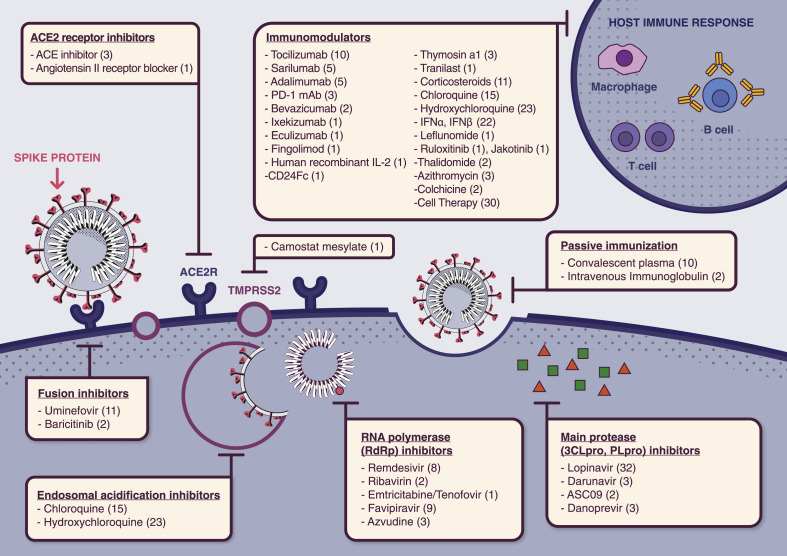
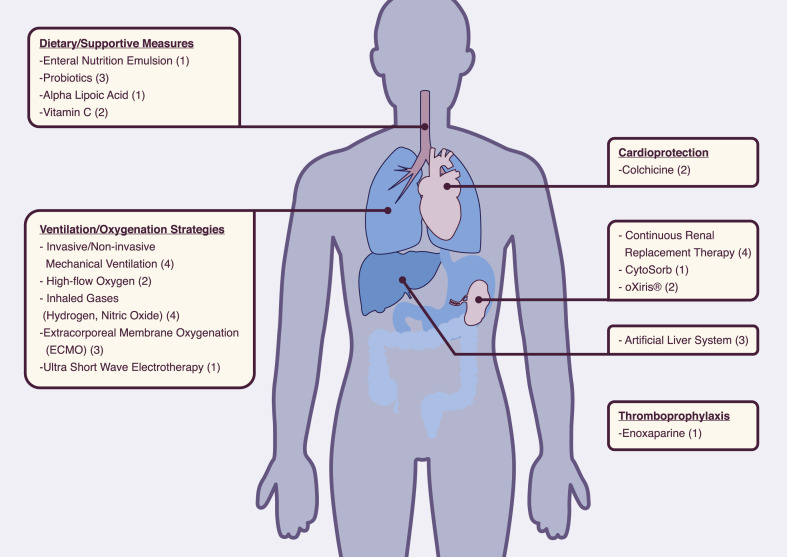
In total, 335 studies were retrieved, including 309 trials evaluating therapeutic molecules, devices and other management options, and 23 studies assessing preventive strategies such as medications and vaccines under development, as well as three studies examining both (Fig. 1 , and Tables S1 and S2). Regarding the methodological review, 214 studies were qualitatively assessed: among the 335 studies initially included, 80 evaluating TCM and five retrospective or ‘expanded access’ studies were excluded. An overview of currently tested therapeutic interventions is presented in Fig. 2, Fig. 3 .
Fig. 1.
Systematic review flow chart.
Fig. 2.
Currently tested therapeutic molecules targeting different steps of SARS-CoV-2 life cycle. Numbers in parentheses refer to the number of the registered trials per intervention.
Fig. 3.
General and supportive therapeutic interventions tested for novel coronavirus disease (COVID-19). Numbers in parentheses refer to the number of the registered trials per intervention.
Main treatment interventions
Protease inhibitors
Lopinavir is a protease inhibitor (PI) active against human immunodeficiency virus 1 (HIV-1) infection. The main coronavirus proteinase (3C-like proteinase or 3CLpro) plays a key role in processing viral polyproteins [8,9]. PIs effectively inhibit the 3CLpro enzyme, thus posing a possibly potent therapeutic agent against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
PIs have shown effectiveness against severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV) and SARS-CoV-2 viruses in in vitro susceptibility models [[10], [11], [12], [13]]. During the SARS-CoV epidemic, lopinavir boosted by ritonavir (a cytochrome P450-3A4 inhibitor), with or without ribavirin, significantly reduced adverse outcomes, including mortality, in a controlled interventional study with historical controls [14]. The MIRACLE trial that examines the efficacy of ritonavir-boosted lopinavir combined with recombinant interferon-beta 1b (IFN-β1b) in the treatment of MERS, is currently undertaken in Saudi Arabia and results are pending [15].
For COVID-19, lopinavir/ritonavir combined with or without other agents has been reported to successfully reduce adverse outcomes in sporadic cases from China [[16], [17], [18], [19]]. These promising reports have set the ground for numerous trials addressing the safety and efficacy of PIs in SARS-CoV-2 infection (Table 1 ). Other PIs that are currently being assessed are ritonavir boosted ASC09 (a novel PI), cobicistat boosted darunavir as well as the NS3/4A protease inhibitor danoprevir combined with ritonavir (Table 1 and Table S1).
Table 1.
Treatment interventions currently being evaluated for the novel coronavirus disease (COVID-19) globally
| Therapeutic intervention | Countries | No. of trials | No. of RCTs (% of trials per intervention) | Additional agents tested in the same interventional arm |
|---|---|---|---|---|
| TCMs | China | 92 | 61 (66.3) | IFNα, LPV/r, ribavirin, chloroquine, umifenovir, ulinastatin |
| Antimalaria drugs (hydroxychloroquine, chloroquine, dihydroartemisinin piperaquine) | China, France, Germany, Mexico, Norway, Spain, Brazil, Canada | 35 | 30 (85.7) | Azithromycin, LPV/r, DRV/r or c, favipiravir, oseltamivir, umifenovir, IFNα |
| Lopinavir ± ritonavir (LPV ± r) | China, Thailand, Hong Kong, UK, Europe, South Korea, Canada | 32 | 30 (93.8) | Chloroquine, IFNα, IFNβ, novaferon, thymosin a1, FTC/TAF, ribavirin, ebastine, oseltamivir, favipiravir, TCM |
| Cell therapy | China, USA, Brazil, Jordan | 30 | 19 (63.3) | Ruxolitinib |
| Interferon (IFNα, IFNα2β, IFNβ, rSIFN-co) | China, UK, Hong Kong, Europe | 22 | 21 (95.4) | Umifenovir, dihydroartemisinine piperaquine, TCM, bromhexine, favipiravir, LPV/r, ribavirin, ebastine, danoprevir/r, thalidomide, methylprednisolone, TTF2 |
| Anti-IL-6 mAb (tocilizumab, sarilumab) | China, Italy, France, Switzerland, Denmark, USA, Canada, Global | 13 | 8 (61.5) | Favipiravir, adalimumab |
| Dietary/Supportive | China, Egypt | 14 | 12 (85.7) | N/A |
| Corticosteroids | China, UK, Italy | 11 | 9 (81.8) | IFNα umifenovir, thalidomide |
| Umifenovir | China | 11 | 11 (100) | novaferon, IFNα, IFNα2β, dihydroartemisinine piperaquine, bromhexine, favipiravir, thalidomide, methylprednisolone |
| Convalescent plasma | China, Italy | 10 | 6 (60) | N/A |
| Adjuvant device | China | 10 | 3 (30) | N/A |
| Favipiravir | China, Thailand | 9 | 9 (100) | Tocilizumab, bromhexine, IFNα, umifenovir, (hydroxy)chloroquine, LPV/r, DRV/r |
| Remdesivir | Global, Europe, USA, China | 8 | 7 (87.5) | Hydroxychloroquine |
| Ventilation/Oxygenation strategies | China | 7 | 0 (0) | N/A |
| Macrolides (azithromycin, carrimycin) | China, Brazil, Denmark | 4 | 4 (100) | Hydroxychloroquine |
| Inhaled gases | China, USA, Italy | 4 | 4 (100) | N/A |
| JAK inhibitors (jakotinib, ruxolitinib, baricitinib) | China, Italy, Canada | 4 | 2 (50) | Cell therapy, ritonavir |
| ACE inhibitor/ARBs | China, USA | 3 | 2 (66.7) | N/A |
| Azvudine | China | 3 | 1 (33.3) | N/A |
| Danoprevir/ritonavir | China | 3 | 3 (100) | IFN |
| Darunavir/cobicistat (DRV/r) | China, Thailand | 3 | 3 (100) | Thymosin a1, oseltamivir, hydroxychloroquine, favipiravir |
| Pirfenidone | China | 3 | 3 (100) | N/A |
| Anti-PD-1 mAb | China | 3 | 3 (100) | N/A |
| Oseltamivir | Thailand, China | 3 | 3 (100) | Hydroxychloroquine, DRV/r, LPV/r |
| Thymosin a1 | China | 3 | 3 (100) | DRV/c, LPV/r |
| Adalimumab | China | 2 | 2 (100) | Tocilizumab |
| Bevazicumab | China, Italy | 2 | 1 (50) | N/A |
| Colchicine | Italy, Canada | 2 | 2 (100) | N/A |
| Novaferon | China | 2 | 2 (100) | LPV/r, umifenovir |
| Ribavirin | China, Hong Kong | 2 | 2 (100) | IFN, LPV/r |
| Thalidomide | China | 2 | 2 (100) | IFNα, umifenovir, methylprednisolone |
| Ulinastatin | China | 2 | 1 (50) | TCM |
| ASC09/ritonavir | China | 2 | 2 (100) | N/A |
| Intravenous Immunoglobulin | China | 2 | 2 (100) | N/A |
| Acetylcysteine | China | 1 | 1 (100) | N/A |
| Antiviral peptide LL-37 | China | 1 | 0 (0) | N/A |
| Baloxavir marboxil | China | 1 | 1 (100) | N/A |
| Bismuth potassium | China | 1 | 1 (100) | N/A |
| Bronchoscopic alveolar lavage | China | 1 | 0 (0) | N/A |
| CD24Fc | USA | 1 | 1 (100) | N/A |
| Dipyridamole | China | 1 | 1 (100) | N/A |
| Ebastine | China | 1 | 1 (100) | IFNα, LPV/r |
| Eculizumab | N/A | 1 | 0 (0) | N/A |
| Enoxaparin | China | 1 | 1 (100) | N/A |
| Escin | Italy | 1 | 0 (0) | N/A |
| Fingolimod | China | 1 | 0 (0) | N/A |
| FTC/TAF | China | 1 | 1 (100) | LPV/r |
| hrIL-2 | China | 1 | 1 (100) | N/A |
| Inactivated mycobacterial vaccine | China | 1 | 1 (100) | N/A |
| Itraconazole | Belgium | 1 | 1 (100) | N/A |
| Ixekizumab | China | 1 | 1 (100) | IFNα, ribavirin, chloroquine, LPV/r, umifenovir |
| Leflunomide | China | 1 | 1 (100) | N/A |
| M1 | China | 1 | 1 (100) | N/A |
| Polyinosinic/polycytidylic acid | China | 1 | 1 (100) | N/A |
| rhG-CSF | China | 1 | 1 (100) | N/A |
| Sargramostin (GM-CSF) | Belgium | 1 | 1 (100) | N/A |
| Sildenafil | China | 1 | 0 (0) | N/A |
| Siltuximab | Italy | 1 | 0 (0) | N/A |
| Suramin | China | 1 | 0 (0) | N/A |
| T89 | N/A | 1 | 1 (100) | N/A |
| TFF-2 | China | 1 | 1 (100) | IFN-κ |
| TMPRSS2 inhibitor (camostat mesylate) | Denmark | 1 | 1 (100) | N/A |
| Tranilast | China | 1 | 1 (100) | N/A |
| VIP | USA, Israel | 1 | 1 (100) | N/A |
| vMIP | China | 1 | 0 (0) | N/A |
| Meplazumab (anti-CD147) | China | 1 | 1 (100) | N/A |
| Sodium Aescinate | China | 1 | 1 (100) | N/A |
| Triazavirin | China | 1 | 1 (100) | N/A |
ACE, angiotensin converting enzyme; anti-PD-1 mAb, Anti Program Cell Death Protein-1 monoclonal antibody; ARBs, angiotensin II receptor blockers; CD24Fc, CD24 extracellular domain-IgG1 Fc domain recombinant fusion protein; DRV/r or c, darunavir/ritonavir or cobicistat; FTC/TAF, emtricitabine/tenofovir alafenamide; GM-CSF, granulocyte macrophage-colony stimulating factor; hrIL-2, human recombinant interleukin 2; IFΝα, interferon alpha; IFNβ, interferon beta; IFNκ, interferon kappa; IL, interleukin; JAK, Janus kinases; LPV/r, lopinavir/ritonavir; M1, type i macrophage; N/A, not applicable; RCTs, randomized controlled trials; rhG-CSF, recombinant human granulocyte-colony stimulating factor; TCMs, traditional Chinese medicines; TMPRSS2, transmembrane protease serine 2; TTF-2, transcription termination factor 2; VIP, vasoactive intestinal peptide; vMIP, viral macrophage inflammatory protein.
RNA polymerase inhibitors
SARS-CoV-2 and SARS-CoV RNA-dependent RNA polymerase (RdRp) share 96% sequence identity; this has justified the assumption that inhibitors effective against SARS-CoV could have similar inhibitory effects against SARS-CoV-2 [20]. Nucleoside analogues compete with natural nucleosides for the RdRp active site, thus inhibiting the viral genome replication [21]. Current research efforts focus on repurposing older molecules in COVID-19 treatment, as their safety profile has already been documented [22].
Remdesivir (GS-5734™) is an adenosine analogue with broad-spectrum antiviral properties [23]. Preclinical data demonstrated the efficacy of remdesivir against SARS-CoV and MERS-CoV, as it has the potential to outcompete the proofreading ability of coronavirus exonuclease, and carries a high genetic resistance barrier [13,20,22,24]. Wang et al. confirmed its in vitro efficacy against SARS-CoV-2 [25]. Currently, it is investigated in seven randomized, controlled trials (Table 1 and Table S1).
Favipiravir is a nucleoside analogue inhibiting the RNA polymerase, initially approved for the treatment of novel influenza viruses [26]. It is also effective against a broad range of viruses, including positive-sense single-stranded RNA viruses [26]. Since there have been some promising in vitro results for its efficacy against SARS-CoV-2, favipiravir is now being investigated in nine clinical trials.
Ribavirin is a guanosine analogue that inhibits inosine monophosphate dehydrogenase required for the synthesis of guanosine triphosphate, leading to lethal mutagenesis of RNA genome [27]. Ribavirin was used in the SARS epidemic in combination with either lopinavir/ritonavir or IFN alpha (IFN-α), and these combinations are currently recommended by the China National Practice Guidelines for the treatment of severe COVID-19 [14,28].
Azvudine, an azidocytidine analogue that inhibits viral reverse transcriptase, has been effective against HIV and hepatitis B and C viruses [29]. Its efficacy against SARS-CoV-2 is being tested in three ongoing clinical trials (Table 1 and Table S1). Another nucleoside analogue undergoing investigation for COVID-19 pneumonia is emtricitabine/tenofovir alafenamide.
Antimalaria drugs
Chloroquine and hydroxychloroquine are currently licensed for the treatment of malaria and autoimmune diseases [30]. However, they have also been studied against several viruses with promising in vitro results, but never confirmed in humans [[31], [32], [33]]. As weak bases, they are concentrated in acidic intracellular organelles, leading to alkalization and disruption of the low pH-dependent steps of viral replication, including viral cell fusion and uncoating [30,32]. Moreover, they impair the terminal glycosylation of the angiotensin-converting enzyme 2 (ACE2) receptor in the Golgi apparatus, thus inhibiting viral penetration into the host cells [34].
As they are accumulated in lymphocytes and macrophages, these drugs reduce secretion of proinflammatory cytokines, and particularly of tumour necrosis factor alpha (TNF-α) [33]. Experimental data demonstrated that chloroquine is highly effective in vitro against SARS-CoV-2 in an estimated effective concentration that is easily achievable with standard dosing regimens [25]. However, the efficacy of antimalaria drugs in clinical practice is still much debated. Some preliminary reports from ongoing trials supporting their effectiveness, alone or in combination with azithromycin [35,36], have been called into question on the basis of their methodology. Moreover, these results were challenged by new trials that did not find any substantial benefit from hydroxychloroquine administration [[37], [38], [39]]. Therefore, clinical trials with a control group are needed to provide reliable answers for clinicians; antimalaria drugs are being tested in 30 randomized controlled trials (Table 1 and Table S1).
Immunomodulators and anti-inflammatory drugs
Virus-induced immune response leading to cytokine storm syndrome (CSS) and secondary haemophagocytic lymphohistiocytosis (HLH) is probably the underlying pathogenetic mechanism that leads to critical and often fatal COVID-19 infection [40,41]. Hyperinflammation is associated with acute respiratory distress syndrome (ARDS) and fulminant multi-organ failure that are fatal if left untreated. In this context, immunosuppressors (along with antivirals) may play a key role in counteracting severe SARS-CoV-2 infection [40].
Preliminary data from COVID-19 patients in China reported significantly higher interleukin (IL)-6 levels in patients with critical COVID-19 disease than those with severe or mild disease [42]. Tocilizumab and sarilumab, both humanized monoclonal antibodies (mAbs) against the IL-6 receptor, are currently evaluated in 13 clinical trials (Table 1 and Table S1); tocilizumab, in particular, improved symptoms and laboratory parameters in a small retrospective study in China [43]. Moreover, a multicentre non-randomised clinical trial examining the efficacy of personalised immunotherapy with tocilizumab or anakinra (an IL-1 receptor antagonist) in COVID-19 patients, based on laboratory biomarkers indicating either macrophage activation syndrome or immune dysregulation, has been recently commenced in Greece (ESCAPE trial, NCT04339712). Various other molecules oriented against different cytokines, as well as intra- and extracellular inflammatory pathways, are currently being tested in COVID-19 including adalimumab, anti-programmed cell death protein 1 mAbs, bevacizumab, ixekizumab, eculizumab, human recombinant IL-2, inhibitors of NLRP3 inflammasome activation (tranilast), Janus kinase inhibitors, fingolimod and a recombinant fusion protein targeting an immune pathway checkpoint (CD24Fc).
Immunomodulators licensed for haematological and rheumatological conditions (such as leflunomide and thalidomide) as well as colchicine, which counteracts the assembly of the NLRP3 inflammasome, are also being studied for their therapeutic use against SARS-CoV-2 (Table 1 and Table S1) [44]. Colchicine in particular, is being tested in randomised controlled trials in Canada (COLCORONA trial, NCT04322682), in Italy (ColCOVID-19 trial, NCT04322565) and, more recently, in Greece (GRECCO-19 trial, NCT04326790).
The immunomodulatory effects of macrolide antibiotics, as well as their pharmacodynamic property to achieve at least tenfold higher concentrations in epithelial lung fluid than in serum, have led researchers to repurpose them against SARS-CoV-2 (Table 1, Table S1) [45,46].
Corticosteroids are major anti-inflammatory drugs, with a conflicting safety profile in severe viral infections [47]. However, their use is currently recommended by the European Society of Intensive Care Medicine in COVID-19 cases with shock and/or evidence of CSS/HLH and/or mechanically ventilated patients with ARDS [48]. Corticosteroids are being tested in 11 clinical trials (Table 1 and Table S1).
Progression to pulmonary fibrosis has been observed in survivors of severe COVID-19 disease, although the exact mechanisms remain unclear [49,50]. The role of cytokines in the cascade of events that lead to lung fibrosis has been elucidated in the pathogenesis of idiopathic pulmonary fibrosis [51]. In COVID-19 disease, pro-inflammatory cytokines mediate an aberrant immune response leading to a persistent lung injury [52]. Based on these observations the antifibrotic agent pirfenidone is being evaluated in at least three randomized clinical trials for its efficacy in the prevention of post-COVID-19 pneumonia fibrosis (Table 1 and Table S1). Pirfenidone targets collagen synthesis by inhibiting transforming growth factor beta, diminishing extracellular matrix deposition and reducing the activity of lung fibroblasts in vitro [53].
Finally, immunostimulatory molecules that enhance the hosts' immune response against the invading pathogen, like IFN-α, IFN-β, the recombinant protein produced by DNA-shuffling of IFN-α (novaferon), the inactivated Mycobacterium vaccine and thymosin a1, are evaluated as viable therapeutic options in various combinations against SARS-CoV-2 [54].
Membrane fusion inhibitors and inhibitors of ACE2 receptor connection
SARS-CoV-2 spike protein (S-protein) binds the ACE2 receptor on the epithelial cells' membrane [55]. The host's type II transmembrane serine protease (TMPRSS2) facilitates membrane fusion and augments viral internalization by cleaving and activating the S-protein [56,57]. These proteins, being an integral part of the viral life cycle, can be used as possible therapeutic targets.
Renin–angiotensin system (RAS) inhibition conceivably ameliorates the overaccumulation of angiotensin II induced by the downregulation of ACE2, as noted in other CoV infections; therefore, the inhibition of RAS system may be protective against the development of fulminant myocarditis and ARDS [58]. Currently, two randomized controlled trials testing losartan are ongoing in the United States.
Camostat mesylate is a potent serine protease inhibitor, inhibiting the TMPRSS2 and is approved for chronic pancreatitis [59]. Umifenovir is a small indole derivative with a broad-spectrum antiviral activity developed about 30 years ago [60,61]. Although it is currently used in Russia and China to combat influenza, it has shown in vitro activity against numerous DNA and RNA viruses including SARS-CoV [60,62,63]. It primarily targets the interaction between viral capsid and the membrane of the host cell by inhibiting the attachment, the fusion and the internalization of the virus [60]. Moreover, umifenovir has direct virucidal effects against enveloped viruses through interaction with the viral lipid envelope or with key residues within structural proteins of virions [60,63,64]. Limited data support that umifenovir may exhibit immunomodulatory effects by stimulating hosts' humoral immune response, IFN production and activation of phagocytes [65]. A small retrospective study in China demonstrated some promising results regarding its effectiveness against SARS-CoV-2 when used in combination with lopinavir/ritonavir [66]. Currently 11 relevant clinical trials are in progress globally (Table 1 and Table S1).
Passive immunization
The use of convalescent plasma (CP) to passively immunize patients against viral pathogens has been previously reported, especially when no other treatment options have been available [67]. CP obtained from recovering patients was used during the SARS epidemic; two retrospective studies demonstrated that CP administration in SARS patients reduced duration of hospitalisation and mortality rates, when treatment with ribavirin and corticosteroids failed [[68], [69], [70]].
A meta-analysis of patients with severe respiratory infection induced by various viruses showed a statistically significant reduction of 75% in mortality odds in those who received CP across all viral aetiologies, including the influenza A 2009 pandemic strain (H1N1pdm09) and SARS-CoV [71]. Evidence of survival benefit was noted after early administration, while no serious adverse effects were reported. Although results are pending from ten relevant clinical trials, the potential short-lasting immunity after CoV infection has raised uncertainty regarding the clinical efficacy of CP antibodies in clinical practice [72,73].
Cell therapies
Mesenchymal stromal cells (MSCs) exhibit immunomodulating qualities, may skew immune cell differentiation and have shown promise in H5N1-associated acute lung injury in preclinical models [74]. A pilot study involving MSC transplantation in seven patients with COVID-19 resulted in cure or significant improvement of pulmonary function and symptoms without adverse effects [75]. MSCs may partially accumulate in lungs and improve the pulmonary microenvironment. A significant induction of regulatory dendritic cells, along with a shift from Th1 towards Th2 immune responses, was also observed [75]. Other clinical trials utilizing MSCs for curing COVID-19 are ongoing (Table 1 and Table S1).
MSC-derived exosomes are extracellular bodies mediating intercellular communication, containing mRNAs, miRNAs, lipids, growth factors and cytokines, that possibly exert the paracrine immunoregulatory effects of MSC [76]. Furthermore, MSC exosome administration is associated with lower risks (e.g. tumour formation, immunogenicity) than the intravenous injection of MSCs [77].
Natural killer cells have shown promising results as adoptive immunotherapy in various cancers, and may also be potent effector cells in the SARS-CoV-2 infection. A phase 1 clinical trial has been launched to determine their efficacy (Table S1). Lastly, ozone therapy may inactivate viruses, among other pathogens, and activate the immune system via upregulation of Th1 cytokines including IFN and TNF; as such, three trials have been launched to evaluate ozone autohaemotherapy efficacy in COVID-19 [78].
Main preventative measures
The World Health Organization (WHO) and the European Centre for Disease Prevention and Control emphasize the role of screening, precaution measures, exposure prevention and environmental disinfection as the mainstay of COVID-19 prevention [79,80]. As no effective preventative options are available yet, several clinical trials are under way to explore the efficacy of various prevention strategies (Table 2 and Table S2).
Table 2.
Prevention interventions currently being evaluated for the novel coronavirus disease (COVID-19) globally
| Prevention intervention | Countries | No. of trials | No. of RCTs (% of trials per intervention) | Additional agents tested in the same interventional arm |
|---|---|---|---|---|
| TCMs | China | 10 | 3 (30) | N/A |
| Antimalaria Drugs | China, USA, Mexico, UK, Spain | 6 | 6 (100) | Umifenovir, DRV/c |
| Vaccine | USA, China | 4 | 0 (0) | N/A |
| Health education | China, Hungary | 3 | 2 (66.7) | TCM |
| IFNα1β | China | 2 | 0 (0) | Thymosin a1 |
| Darunavir/cobicistat | Spain | 1 | 1 (100) | Chloroquine |
| Lopinavir/ritonavir | Canada | 1 | 1 (100) | N/A |
| Mask | Canada | 1 | 1 (100) | N/A |
| PUL-042 | USA | 1 | 1 (100) | N/A |
| Thymosin a1 | China | 1 | 0 (0) | IFNα1β |
| Umifenovir | China | 1 | 1 (100) | Hydroxychloroquine |
DRV/c, darunavir/cobicistat; IFΝα, interferon alpha; LPV/r, lopinavir/ritonavir; N/A, not applicable; RCTs, randomized controlled trials; TCMs, traditional Chinese medicines.
Many studies are currently evaluating the efficacy of TCM in COVID-19 prevention in China. Importantly, at least four vaccines are under development. Among them, an mRNA-based vaccine encoding the S-protein is being assessed for its safety, reactogenicity and efficacy against SARS-CoV-2 (Table 2 and Table S2). Besides the registered trials, other large companies have also announced the initiation of vaccine development [81,82].
Other preventative molecules include hydroxychloroquine and the recombinant human IFN-α1b spray. In the United States, exposed individuals are randomized to hydroxychloroquine or placebo, evaluating the agent's potential as post-exposure prophylaxis (NCT04308668, Table S2). Furthermore, another randomized clinical trial evaluates the efficacy of a 3-month course of chloroquine in at-risk healthcare personnel (NCT04303507, Table S2). Finally, the live attenuated strain of Mycobacterium bovis is expected to be tested as a preventative strategy against COVID-19 among healthcare professionals in Australia and France.
Methodological analysis of interventional studies
Study populations
In total, 198 interventional treatment and 16 prevention trials were included in the methodological analysis respectively (Table 3 ). Among the eligible treatment studies, recruitment of children (i.e. <14 years old) was reported in seven clinical trials in total: one testing darunavir with cobicistat (NCT04252274); two on human stem cells transfusion (ChiCTR2000029606, ChiCTR2000030944); one testing hydroxycholoroquine (EudraCT Number: 2020-000890-25); one using tocilizumab (NCT04317092); and one assessing nutritional supplements (NCT04323345) (Table S1). With respect to relevant prevention studies, children were included in two vaccine trials (NCT04276896, NCT04299724) as shown in Table S2.
Table 3.
Description of the clinical trials registered for the treatment and prevention of COVID-19
| Study characteristics | Treatment studies n = 198 (%) | Prevention studies n = 16 (%) | |
|---|---|---|---|
| Study phase | |||
| Phase I | 9 (5) | 3 (19) | |
| Phase IIa | 31 (16) | 2 (13) | |
| Phase IIIb | 35 (18) | 6 (38) | |
| Phase IV | 40 (20) | 1 (6) | |
| Unspecified | 83 (42) | 4 (25) | |
| Study design | |||
| Randomised | 150 (76) | 10 (63) | |
| Non-randomised | 21 (11) | 3 (19) | |
| Single-arm | 27 (14) | 3 (19) | |
| Blinding | |||
| Double-blind | 31 (16) | 6 (38) | |
| Single-blind | 16 (8) | 2 (13) | |
| Open-label | 73 (37) | 5 (31) | |
| Non-applicablec | 29 (15) | 3 (19) | |
| Unspecified | 49 (25) | 0 (0) | |
| Total number of planned inclusions | |||
| <50 | 62 (31) | 1 (6) | |
| 50-100 | 41 (21) | 0 (0) | |
| 100-150 | 32 (16) | 3 (19) | |
| 150-200 | 9 (5) | 0 (0) | |
| 200-250 | 15 (8) | 1 (6) | |
| ≥250 | 39 (20) | 11 (69) | |
| Primary endpoint | |||
| Clinical | 128 (65) | 13 (81) | |
| Virological | 39 (20) | 2 (13) | |
| Radiological | 19 (10) | 0 (0) | |
| Immunological/biological | 12 (6) | 1 (6) | |
Including phase I/II trials.
Including phase II/III trials.
Single-arm or factorial trials.
Study designs
Phase IV and phase III treatment trials were the most commonly reported interventional study types (n = 40, 20% and n = 35, 18% respectively) as demonstrated in Table 3. Nonetheless, the majority of registered trials do not disclose the study phase (n = 83, 42%).
In terms of blinding, 73 open-label (37%), 31 double-blinded (16%), and 16 single-blinded (8%) studies were retrieved. Most trials were randomized (n = 150, 76%) with a parallel assignment between arms. The median (interquartile range (IQR)) number of planned inclusions is 90 (40–200) with a range of five to 6000 participants.
Phase III and phase I prevention studies were the most commonly reported ones (n = 6, 38% and n = 3, 19% respectively, Table 3). As with treatment trials, many prevention trials do not report the study phase (n = 4, 25%).
Regarding prevention studies' blinding, six double-blinded (38%), five open-label (31%) and two single-blinded (13%) were found. Most studies were randomized (n = 10, 63%) with a parallel assignment design. The median (IQR) number of planned inclusions is 513 (177–2958) ranging from 45 to7576 participants.
Treatments and interventions
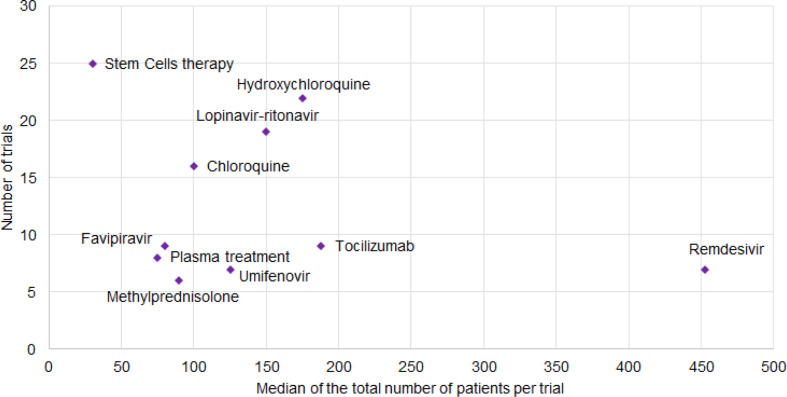
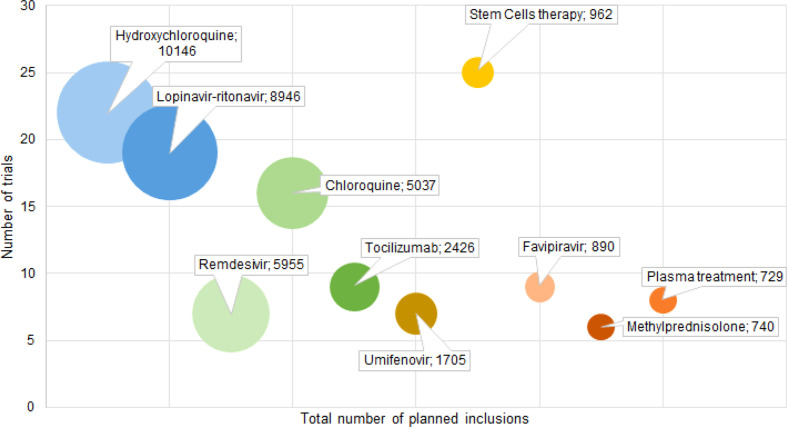
Various types of interventions are currently evaluated; however, their appraisal is limited by the lack of reported data concerning the treatment dose and duration. Fig. 4 demonstrates the number of trials by the median of planned inclusions per trial for the ten most frequently encountered therapies. Although remdesivir was tested in only seven trials, these studies have the highest median number of planned inclusions per trial (453, IQR 397–650). On the other hand, cell therapies were assessed by the highest number of trials (n = 25), but with a disproportionately low median number of planned inclusions (30, IQR 20–60). Fig. 5 shows the total number of planned inclusions and the number of clinical trials for the ten most frequently studied treatments, with hydroxychloroquine being the treatment associated with the highest total number (10 146 planned inclusions).
Fig. 4.
Number of trials reported by the median of the total number of planned inclusions per trial for the most common treatments.
Fig. 5.
Number of trials per total number of planned inclusions for the ten most frequently assessed treatments. The size of the circle corresponds to the addition of the total numbers of planned inclusions for all trials evaluating one of the treatments.
Primary endpoints and outcomes
A clinical primary outcome was defined in 128 out of 198 therapeutic trials (65%; Table 3). Among them, 75 (58.6%) studies focused on a composite outcome for aggravation or recovery, 25 (19.5%) studies on the ordinal scale proposed by the WHO master protocol [83], 20 (15.6%) studies on mortality, five (3.9%) studies on a clinical score (e.g. SOFA, lung injury score or pneumonia severity index) and the remaining three (2.3%) studies focused on other clinical outcomes such as hospital admission or temperature. Virological, radiological and biological/immunological primary endpoints were reported in 39 (20%), 19 (10%) and 12 (6%) studies respectively (Table 3).
Regarding prevention studies, 10/16 (62%) disclosed a clinical primary outcome, such as confirmed symptomatic COVID-19 for three studies, severe COVID-19 for two studies, confirmed or suspected COVID-19 for one study and safety for four (studies evaluating vaccines). The other prevention studies had a virological outcome (confirmed SARS-CoV-2 infection with or without symptoms, n = 5) or a biological outcome (n = 1, routine blood tests) (Table 3).
Discussion
The SARS-CoV-2 is the third emerging coronavirus of the last 20 years, but the one that has led to an evolving pandemic, a fact that urgently requires the implementation of novel therapeutics. However, repurposing of existing drugs is a viable and less time-consuming alternative; licensed broad-spectrum antiviral agents with a well-documented safety profile are currently tested against SARS-CoV-2. Alternatively, screening of libraries of chemical compounds may be useful in the discovery of an efficacious treatment against COVID-19 disease [84]. In this review, we have summarized and methodologically appraised the ongoing therapeutic and preventive trials for COVID-19.
Therapeutic strategies should follow the two-phased immune response to COVID-19. Initially, treatment should aim at strengthening the host's immune response against the virus, and inhibiting viral replication [85]. Early initiation of antiviral therapy has been proved beneficial for the prognosis of patients [86]. As advanced age correlates with higher viral loads, older patients could strongly benefit from the prompt initiation of antivirals [87]. Moreover, high inflammatory cytokine levels have been correlated with disease severity and the extent of lung damage [88]. Thus, in the later phase of the infection, patients could be availed by anti-inflammatory therapeutic approaches such as the Janus kinase inhibitors, blood purification or tocilizumab [89,90].
Prevention is a key player in combating pandemics. The mRNA 1273 vaccine, is currently in phase I, but is not expected to be commercially available this year [91]. The prophylactic potential of hydroxychloroquine/chloroquine is also actively being examined among healthcare professionals [92]. Moreover, prompt testing and isolation are of utmost importance for disease prevention. At the moment, real-time quantitative polymerase chain reaction is the reference standard in diagnostics [93]. The FDA has recently announced the authorisation of rapid molecular tests that are capable of delivering results within minutes, thus facilitating early treatment initiation (when viral loads are highest) and timely isolation [87,94,95].
Based on the retrieved data, cell therapies and hydroxychloroquine were the most frequently evaluated candidate therapies (25 and 22 trials respectively), while remdesivir was associated with the highest median number of planned inclusions per trial (453, IQR 397–650). Although TCMs and homeopathy represent a large proportion of the identified interventional studies, we excluded them from our methodological analysis as we do not have the expertise to analyse clinical trials testing these agents.
This review shows the considerable amount of clinical trials that are currently registered. Although the number of identified trials was high, there were several methodological caveats. Firstly, study design data and details on the interventions being assessed were often lacking. This hampers the available information to researchers and relevant stakeholders, and potentially influences the discovery of successful treatments.
Secondly, most trials, and especially those registered at the beginning of the pandemic, disclosed low participant numbers, which may impact the robustness of their future results. However, these numbers should be cautiously interpreted, as they represent the anticipated and not the actual number of inclusions. Thirdly, reported primary endpoints were highly heterogeneous among studies. The use of clinical and uniform primary endpoints should be encouraged in an infection where the association between the clinical status and viral clearance, radiological or immunological evolution is obscure.
Our study underlines the need to meticulously register as many study details as possible on international registries during outbreaks, in order to guide the development and enhance the consistency of future trials. Reporting as much detail as possible is instrumental in having consistent clinical trials and enhancing the reproducibility of the results, especially as studies are more often associated with a low number of planned inclusions, and composite or weak outcomes that can limit the treatments' efficacy assessment. That is why transparency and consistency are crucial when reporting clinical trials in order to improve statistical power by conducting, for example, meta-analyses.
The development of clinical trials during an outbreak is an adaptive process and new evidence emerges at an impressive rate. A review of the early registered clinical trials is an important asset for researchers and methodologists alike. These results might encourage transparency when developing and registering future clinical trials and help improve their methodology, hence the robustness of their results.
Transparency declaration
Conflict of interest: For Y.Y.: Chair of the Global Research Collaboration for Infectious Disease Preparedness (GloPID-R) and the coordinator of REsearch and ACTion targeting emerging infectious diseases (REACTing). For C.S.: Consultancy and research funding, Hycor Biomedical and Thermo Fisher Scientific; Consultancy, Bencard Allergie; Research Funding, Mead Johnson Nutrition. Supported by Universities Giessen and Marburg Lung Centre (UGMLC), the German Center for Lung Research, University Hospital Giessen and Marburg research funding according to article 2, section 3 cooperation agreement, and the Deutsche Forschungsgemeinschaft-funded-SFB 1021 (C04), -KFO 309 (P10), and SK 317/1-1 (Project number 428518790). All grants are outside the submitted work. No external funding was received for this study.
Author contributions
Conception and design: P.C.F., S.T., D.B., N.P.S., F.M., C.L. Acquisition of data: P.C.F., E.K., C.D.M., H.J., C.S., D.B., N.P.S., Y.Y. Interpretation of data: all authors. Drafting the article: P.C.F., E.K., C.D.M., H.J., C.S., D.B., N.P.S., F.M., C.L. and Y.Y.. Revising critically the manuscript for important intellectual content: P.C.F., C.S., S.T. P.C.F. and D.B. contributed equally as first authors, C.D.M. and N.P.S. contributed equally as second authors, C.L .and S.T. contributed equally as senior authors. All authors approved the final version of the manuscript submitted.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.05.019.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Home – ClinicalTrials.gov. https://clinicaltrials.gov/ n.d.
- 2.Chinese Clinical Trial Register (ChiCTR) The world health organization international clinical trials registered organization registered platform. http://www.chictr.org.cn/searchprojen.aspx n.d.
- 3.EU clinical trials register. https://www.clinicaltrialsregister.eu/ Update n.d.
- 4.Schardt C., Adams M.B., Owens T., Keitz S., Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hard to swallow. Nature. 2007;448:105–106. doi: 10.1038/448106a. [DOI] [PubMed] [Google Scholar]
- 6.Ernst E. Homeopathy, a “helpful placebo” or an unethical intervention? Trends Pharmacol Sci. 2010;31:1. doi: 10.1016/j.tips.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Moher D., Liberati A., Tetzlaff J., Altman D.G., Altman D., Antes G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L., Lin D., Kusov Y., Nian Y., Ma Q., Wang J. α-Ketoamides as broad-spectrum inhibitors of coronavirus and enterovirus replication: structure-based design, synthesis, and activity assessment. J Med Chem. 2020;63:4562–4578. doi: 10.1021/acs.jmedchem.9b01828. [DOI] [PubMed] [Google Scholar]
- 9.Fan K., Wei P., Feng Q., Chen S., Huang C., Ma L. Biosynthesis, purification, and substrate specificity of severe acute respiratory syndrome coronavirus 3C-like proteinase. J Biol Chem. 2004;279:1637–1642. doi: 10.1074/jbc.M310875200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nukoolkarn V., Lee V.S., Malaisree M., Aruksakulwong O., Hannongbua S. Molecular dynamic simulations analysis of ritonavir and lopinavir as SARS-CoV 3CL(pro) inhibitors. J Theor Biol. 2008;254:861–867. doi: 10.1016/j.jtbi.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen F., Chan K.H., Jiang Y., Kao R.Y.T., Lu H.T., Fan K.W. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31:69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., van Nieuwkoop S., Bestebroer T.M. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu C.M., Cheng V.C.C., Hung I.F.N., Wong M.M.L., Chan K.H., Chan K.S. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arabi Y.M., Alothman A., Balkhy H.H., Al-Dawood A., AlJohani S., Al Harbi S. Treatment of Middle East respiratory syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19:81. doi: 10.1186/s13063-017-2427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu K., Cai H., Shen Y., Ni Q., Chen Y., Hu S. [Management of corona virus disease-19 (COVID-19): the Zhejiang experience] Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49 doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han W., Quan B., Guo Y., Zhang J., Lu Y., Feng G. The course of clinical diagnosis and treatment of a case infected with coronavirus disease 2019. J Med Virol. 2020;92:461–463. doi: 10.1002/jmv.25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim J., Jeon S., Shin H.Y., Kim M.J., Seong Y.M., Lee W.J. Case of the index patient who caused tertiary transmission of coronavirus disease 2019 in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 pneumonia monitored by quantitative RT-PCR. J Korean Med Sci. 2020;35:e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z., Chen X., Lu Y., Chen F., Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14:64–68. doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- 20.Morse J.S., Lalonde T., Xu S., Liu W.R. Learning from the Past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem. 2020;21:730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez M.A. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob Agents Chemother. 2020;64:1–18. doi: 10.1128/AAC.00399-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018;9 doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delang L., Abdelnabi R., Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antivir Res. 2018;153:85–94. doi: 10.1016/j.antiviral.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Koren G., King S., Knowles S., Phillips E. Ribavirin in the treatment of SARS: a new trick for an old drug? CMAJ. 2003;168:1289–1292. [PMC free article] [PubMed] [Google Scholar]
- 28.Chan K.W., Wong V.T., Tang S.C.W. COVID-19: an update on the epidemiological, clinical, preventive and therapeutic evidence and guidelines of integrative Chinese–Western medicine for the management of 2019 novel coronavirus disease. Am J Chin Med. 2020;48:737–762. doi: 10.1142/S0192415X20500378. [DOI] [PubMed] [Google Scholar]
- 29.Wang R.-R., Yang Q.-H., Luo R.-H., Peng Y.-M., Dai S.-X., Zhang X.-J. Azvudine, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro. PLoS One. 2014;9 doi: 10.1371/journal.pone.0105617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rolain J.-M., Colson P., Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents. 2007;30:297–308. doi: 10.1016/j.ijantimicag.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Touret F., de Lamballerie X. Of chloroquine and COVID-19. Antivir Res. 2020;177:104762. doi: 10.1016/j.antiviral.2020.104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colson P., Rolain J.-M., Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J Antimicrob Agents. 2020;55:105923. doi: 10.1016/j.ijantimicag.2020.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Antiviral effects of chloroquine Effects of chloroquine on viral infections : an old drug against today ’ s diseases ? Lancet. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savarino A., Di Trani L., Donatelli I., Cauda R., Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 37.Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W. Hydroxychloroquine in patients mainly with mild to moderate COVID-19: an open-label, randomized, controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenberg E.S., Dufort E.M., Udo T., Wilberschied L.A., Kumar J., Tesoriero J. Association of treatment with hydroxychloroquine or azithromycin with In-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020 doi: 10.1001/jama.2020.8630. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G. Observational study of hydroxychloroquine in hospitalized patients with covid-19. N Engl J Med. 2020;NEJMoa2012410 doi: 10.1056/NEJMoa2012410. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen C., Zhang X.R., Ju Z.Y., He W.F. Advances in the research of cytokine storm mechanism induced by Corona Virus Disease 2019 and the corresponding immunotherapies. Zhonghua Shao Shang Za Zhi. 2020;36:E005. doi: 10.3760/cma.j.cn501120-20200224-00088. [DOI] [PubMed] [Google Scholar]
- 42.Chen L., Liu H.G., Liu W., Liu J., Liu K., Shang J. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:203–208. doi: 10.3760/cma.j.issn.1001-0939.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Xu X., Han M., Li T., Sun W., Wang D., Fu B. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demidowich A.P., Davis A.I., Dedhia N., Yanovski J.A. Colchicine to decrease NLRP3-activated inflammation and improve obesity-related metabolic dysregulation. Med Hypotheses. 2016;92:67–73. doi: 10.1016/j.mehy.2016.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Min J.-Y., Jang Y.J. Macrolide therapy in respiratory viral infections. Mediators Inflamm. 2012;2012:649570. doi: 10.1155/2012/649570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanoh S., Rubin B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010;23:590–615. doi: 10.1128/CMR.00078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Important announcement of new SSC guidelines – COVID-19 – ESICM. https://www.esicm.org/ssc-covid19-guidelines/ n.d.
- 49.Wang J., Wang B.J., Yang J.C., Wang M.Y., Chen C., Luo G.X. Advances in the research of mechanism of pulmonary fibrosis induced by Corona Virus Disease 2019 and the corresponding therapeutic measures. Zhonghua Shao Shang Za Zhi. 2020;36:E006. doi: 10.3760/cma.j.cn501120-20200307-00132. [DOI] [PubMed] [Google Scholar]
- 50.Lei P., Fan B., Mao J., Wei J., Wang P. The progression of computed tomographic (CT) images in patients with coronavirus disease (COVID-19) pneumonia: running title: the CT progression of COVID-19 pneumonia. J Infect. 2020;80:e30–e31. doi: 10.1016/j.jinf.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wynn T.A. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conti P., Ronconi G., Caraffa A., Gallenga C., Ross R., Frydas I. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34:1. doi: 10.23812/CONTI-E. In press. [DOI] [PubMed] [Google Scholar]
- 53.Jin J., Togo S., Kadoya K., Tulafu M., Namba Y., Iwai M. Pirfenidone attenuates lung fibrotic fibroblast responses to transforming growth factor-β1. Respir Res. 2019;20:119. doi: 10.1186/s12931-019-1093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sallard E., Lescure F.-X., Yazdanpanah Y., Mentre F., Peiffer-Smadja N. C-20-15 DisCoVeRy French Steering Committee. Type 1 interferons as a potential treatment against COVID-19. Antivir Res. 2020;178:104791. doi: 10.1016/j.antiviral.2020.104791. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers in COVID-19 - CEBM. https://www.cebm.net/covid-19/angiotensin-converting-enzyme-ace-inhibitors-and-angiotensin-receptor-blockers-in-covid-19/ n.d.
- 56.Chen Y., Guo Y., Pan Y., Zhao Z.J. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. 2020;525:135–140. doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pohlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88:1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Danser A.H.J., Epstein M., Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension. 2020;75:1382–1385. doi: 10.1161/HYPERTENSIONAHA.120.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamawaki H., Futagami S., Kaneko K., Agawa S., Higuchi K., Murakami M. Camostat mesilate, pancrelipase, and rabeprazole combination therapy improves epigastric pain in early chronic pancreatitis and functional dyspepsia with pancreatic enzyme abnormalities. Digestion. 2019;99:283–292. doi: 10.1159/000492813. [DOI] [PubMed] [Google Scholar]
- 60.Blaising J., Polyak S.J., Pécheur E.-I. Arbidol as a broad-spectrum antiviral: an update. Antivir Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fink S.L., Vojtech L., Wagoner J., Slivinski N.S.J., Jackson K.J., Wang R. The antiviral drug arbidol inhibits zika virus. Sci Rep. 2018;8:8989. doi: 10.1038/s41598-018-27224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hulseberg C.E., Fénéant L., Szymańska-de Wijs K.M., Kessler N.P., Nelson E.A., Shoemaker C.J. Arbidol and other low-molecular-weight drugs that inhibit Lassa and Ebola viruses. J Virol. 2019;93 doi: 10.1128/JVI.02185-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi L., Xiong H., He J., Deng H., Li Q., Zhong Q. Antiviral activity of arbidol against influenza A virus, respiratory syncytial virus, rhinovirus, coxsackie virus and adenovirus in vitro and in vivo. Arch Virol. 2007;152:1447–1455. doi: 10.1007/s00705-007-0974-5. [DOI] [PubMed] [Google Scholar]
- 64.Deng P., Zhong D., Yu K., Zhang Y., Wang T., Chen X. Pharmacokinetics, metabolism, and excretion of the antiviral drug arbidol in humans. Antimicrob Agents Chemother. 2013;57:1743–1755. doi: 10.1128/AAC.02282-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glushkov R.G., Gus’kova T.A., Krylova L.I., Nikolaeva I.S. [Mechanisms of arbidole’s immunomodulating action] Vestn Ross Akad Meditsinskikh Nauk. 1999:36–40. [PubMed] [Google Scholar]
- 66.Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H. Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sahr F., Ansumana R., Massaquoi T.A., Idriss B.R., Sesay F.R., Lamin J.M. Evaluation of convalescent whole blood for treating Ebola virus disease in Freetown, Sierra Leone. J Infect. 2017;74:302–309. doi: 10.1016/j.jinf.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong V.W.S., Dai D., Wu A.K.L., Sung J.J.Y. Treatment of severe acute respiratory syndrome with convalescent plasma. Hong Kong Med J. 2003;9:199–201. [PubMed] [Google Scholar]
- 69.Cheng Y., Wong R., Soo Y.O.Y., Wong W.S., Lee C.K., Ng M.H.L. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soo Y.O.Y., Cheng Y., Wong R., Hui D.S., Lee C.K., Tsang K.K.S. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10:676–678. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Cleary P., Khaw F.-M., Lim W.S. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arabi Y.M., Hajeer A.H., Luke T., Raviprakash K., Balkhy H., Johani S. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg Infect Dis. 2016;22:1554–1561. doi: 10.3201/eid2209.151164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cao W.-C., Liu W., Zhang P.-H., Zhang F., Richardus J.H. Disappearance of antibodies to SARS-associated coronavirus after recovery. N Engl J Med. 2007;357:1162–1163. doi: 10.1056/NEJMc070348. [DOI] [PubMed] [Google Scholar]
- 74.Loy H., Kuok D.I.T., Hui K.P.Y., Choi M.H.L., Yuen W., Nicholls J.M. Therapeutic implications of human umbilical cord mesenchymal stromal cells in attenuating influenza A(H5N1) virus-associated acute lung injury. J Infect Dis. 2019;219:186–196. doi: 10.1093/infdis/jiy478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu B., Zhang X., Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142–4157. doi: 10.3390/ijms15034142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yin K., Wang S., Zhao R.C. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomark Res. 2019;7:8. doi: 10.1186/s40364-019-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Di Paolo N., Bocci V., Gaggiotti E. Ozone therapy. Int J Artif Organs. 2004;27:168–175. doi: 10.1177/039139880402700303. [DOI] [PubMed] [Google Scholar]
- 79.Infection prevention and control. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/infection-prevention-and-control n.d.
- 80.COVID-19. https://www.ecdc.europa.eu/en/covid-19-pandemic n.d.
- 81.Moderna’s COVID-19 vaccine could reach healthcare workers this fall FierceBiotech. https://www.fiercebiotech.com/biotech/moderna-s-covid-19-vaccine-could-reach-healthcare-workers-fall n.d.
- 82.Johnson & Johnson identifies lead COVID-19 vaccine candidate. https://www.drugtargetreview.com/news/58911/johnson-johnson-identifies-lead-covid-19-vaccine-candidate/ n.d.
- 83.WHO R&D Blueprint: novel Coronavirus: outline of trial designs for experimental therapeutics. 2020.4. World Health Organization; Switzerland: 2020. [Google Scholar]
- 84.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 85.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu J., Li W., Shi X., Chen Z., Jiang B., Liu J. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19) J Intern Med. 2020 doi: 10.1111/joim.13063. In press. [DOI] [PubMed] [Google Scholar]
- 87.To K.K.-W., Tsang O.T.-Y., Leung W.-S., Tam A.R., Wu T.-C., Lung D.C. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;3099:1–10. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Diagnosis and treatment protocol for COVID-19 (trial version 7) http://en.nhc.gov.cn/2020-03/29/c_78469.htm n.d.
- 91.NIH clinical trial of investigational vaccine for COVID-19 begins, National Institutes of Health (NIH) https://www.nih.gov/news-events/news-releases/nih-clinical-trial-investigational-vaccine-covid-19-begins n.d.
- 92.Chloroquine/hydroxychloroquine prevention of coronavirus disease (COVID-19) in the healthcare setting – full text view - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04303507 n.d.
- 93.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cepheid Xpert® xpress SARS-CoV-2 has received FDA emergency use authorization. https://www.cepheid.com/coronavirus n.d.
- 95.Detect COVID-19 in as Little as 5 minutes Abbott Newsroom. https://www.abbott.com/corpnewsroom/product-and-innovation/detect-covid-19-in-as-little-as-5-minutes.html n.d.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.