Abstract
We describe the implementation of a COVID-19 Autopsy Programme in our Hospital, report the main findings from the first autopsy of the programme and briefly review the reports of lung pathology of these patients.
Keywords: COVID-19, autopsy, Pathology, Diffuse alveolar damage, Vascular thrombosis
Resumen
En este artículo presentamos el proceso de implementación de un Programa de Autopsias COVID-19 en nuestro hospital, presentamos los principales hallazgos de la primera autopsia realizada y revisamos brevemente la enfermedad pulmonar publicada previamente en estos pacientes.
Palabras clave: COVID-19, Autopsia, Anatomía patológica, Daño alveolar difuso, Trombosis vascular
Introduction
The World Health Organization declared the COVID-19 pandemic on the 11th of March, 2020. Previously, on the 14th of February, the first COVID-19 autopsy in Spain had been performed in the Hospital Arnau de Vilanova-Lliria, Valencia; however, the virological diagnosis had been made subsequent to the post-mortem examination1. On March 13th a state of emergency was declared in Spain and the Ministry of Health recommended that autopsies should only be performed under conditions that fulfilled security measures and that appropriate personal protective equipment (PPE) should be obligatory. Due to the initial difficult situation in the majority of hospitals, no autopsies of COVID-19 patients were carried out during March or the beginning of April. Not only was there a shortage of PPE, many autopsy rooms were being used as morgues. However, the situation improved and by mid-April clinicians began requesting autopsy studies. We describe the implementation of a COVID-19 Autopsy program in the Ramón y Cajal University Hospital in Madrid and the results of the first autopsy. As the main target organ in severe COVID-19 disease is the lung, we also reviewed the reports published to date of lung pathology in these patients.
Case Report
Logistic aspects
Several measures were taken in order to implement a COVID-19 autopsy programme in the University Hospital Ramón y Cajal in Madrid. An ad-hoc medical committee (COVID-19 Committee) selected autopsies from possible cases and the coordinator of the committee requested the post-mortem. The number of autopsies, when possible, should be limited to 1 or 2 per day. The autopsy room ventilation was adapted (Fig. 1 A and B) in order to guarantee more than 12 air changes per hour and confirm negative pressure in the room. In addition, the aspiration system of the autopsy table was checked. Air was expelled from the autopsy room to the exterior through a High Efficiency Particulate Air filter. Appropriate PPE were obtained (Table 1 ), according to the recommendations of the hospital Occupational Risk Prevention Service (Fig. 1C). The personnel involved in the autopsy procedure attended a special two-hour course given by the Preventive Medicine Service.
Figure 1.
Autopsy room with dirty (a) and clean (b) areas delimited by a red line. (c) An examiner fully equipped with the PPE: disposable waterproof coverall, goggles, FFP3 mask covered with a surgical mask, nitrile gloves covered with cut-resistant gloves and protected with latex gloves and waterproof shoes covers. In addition, a plastic apron and a face shield, which can be quickly removed during disrobing, provide protection from splashes.
Table 1.
Personal protective equipment required for autopsy.
| PPE | UNE standards |
|---|---|
| Disposable waterproof long-sleeved gown with cuffs or fully disposable coveralls. If these are not available, another option is a disposable fluid resistant gown / coverall with waterproof/chemical resistant apron. |
Biological protection clothing UNE-EN 14126:2004 Chemical protection clothing: UNE-EN 14605:2005 + A1:2009 UNE-EN 13034:2005 + A1:2009 (*) Waterproof chemical apron must comply with the UNE EN 14605 standard, called Types PB [3] and PB [4] of biological protection |
| FFP2 Mask FFP3 Mask (only when danger of aerosols being generated) |
UNE-EN 149:2001 + A1:2009 NIOSH (42 CFR 84): N95 or higher China (GB2626): KN95 or higher |
| Double nitrile glove | UNE-EN ISO 374-5:2016 (virus) |
| Waterproof integral frame protective glasses | UNE-EN 166:2002 Frame marking: field of use 3, 4 or 5 |
| Face shield | UNE-EN 166:2002 Frame marking: field of use 3 |
| Waterproof hood and leggings |
UNE (Spanish Standard (Una Norma Española))
UNE-EN: Adoption of the European Standard (Norma Europea, NE)
Clinical data
The deceased was a 54 year-old-man with hypertension, gout, migraine and obstructive sleep apnea treated with continuous positive airway pressure oxygenotherapy. His body index mass was 30.9. He attended the casualty department complaining of dyspnoea and an eight-day history of chills, fever and cough. On admission, a nasopharyngeal swab PCR was positive for SARS-CoV-2 and an X-ray showed bilateral lung opacities; he had an 88% O2 saturation. The patient was found to have elevated D-Dimer, lymphopenia and raised levels of lactate dehydrogenase, interleukin-6, ferritin and C-reactive protein.
After 3 days of steroid treatment and bed rest, his condition worsened and he was transferred to the Intensive Care Unit where mechanical ventilation and posterior orotracheal intubation was implemented. He was treated with lopinavir/ritonavir, dolquine, azitromicine, metilprednisone, tocilizumab and enoxaparine. However, the patient's condition progressively deteriorated and he developed renal failure and sudden desaturations, making tracheostomy and haemodialysis necessary. He suffered a pulmonary thromboembolism and died after 25 days in Intensive Care. His next of kin authorized the necropsy.
Autopsy procedure workflow
Two clear areas were delimited inside the autopsy room, a dirty area containing the dissection table and a clean area (Figure 1A). Personnel entered via the clean area and left via the dirty area. The corpse was brought in through the dirty area. All personnel dressed in an adjacent room and entered the autopsy room fully clad in PPE (figure 1B). The autopsy was performed by two pathologists with the assistance of one technician. A pathology resident, working in the clean area, was in charge of collecting samples and also helped to disrobe participating personnel after the procedure.
Following inspection of the cadaver, the thoraco-abdominal cavities were opened and carefully explored. In order to reduce aerosol spreading, samples were taken in situ, without removing organs from body. Samples from all lobes of both lungs, heart, liver, spleen, kidneys, costal bone marrow and skin were taken and immediately immersed in 4% formaldehyde. No frozen material was bio-banked. Following safety guidelines, after body closure, the autopsy table was cleaned with bleach and the participating personnel removed their PPE in a specially reserved area at the exit door. Immediately after leaving, they washed and changed their surgical scrub in a bathroom adjacent to the autopsy room, independent from the disrobing area previously described.
Tissue Processing
Conventional histological and histochemical (PAS, Grocott, von-Kossa) processing were performed. Immunohistochemistry using pancytokeratin (Agilent; clone AE1/AE3), macrophage (CD68, clone PG-M1), lymphoid (CD4, clone 4B12; CD8, clone C8/144B), platelet lineage (CD61, clone 2f2) and viral (herpes1, clone 10A3; Herpes2, clone DBM 15.69; cytomegalovirus, clone CCH2) antibodies was requested after H&E evaluation.
Pathological Results
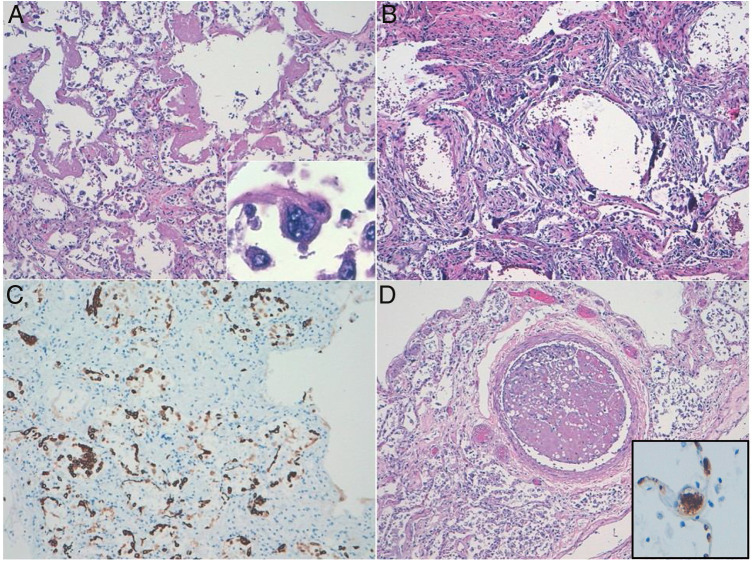
The most striking lesions were observed in both lungs. Macroscopically, they were heavy, firm and condensed. The cut surface was red and rubbery. Microscopically, samples from two different areas of each lobe were studied. Airspaces were reduced due to an increase in the interstitial connective tissue. Histological changes varied, in the best-preserved areas, which represented about 10% of the parenchyma studied, there was minimal septal thickening with capillary congestion, occasional mononuclear inflammatory infiltration and evident alveolar cell desquamation. AE1/AE3 and CD68 immunostaining demonstrated that most desquamated cells were epithelial, although some macrophages were also present. Pneumocytes were hyperplastic and showed cytopathic-like changes, such as multinucleation, prominent nucleolus and granular cytoplasm (Fig. 2 A). In addition to previous described changes, approximately 10% of the lung parenchyma analysed showed hyaline membranes, indicating the exudative stage of diffuse alveolar damage (DAD) (Fig. 2A). The most extensive pattern of lung lesions was consistent with DAD in proliferative (organizing) (70%) and fibrotic (10%) stages. There was intense septal thickening with abundant reactive fibroblasts (Fig. 2B and C). Interstitial inflammatory infiltrates were scarce and mainly composed of CD8 + lymphocytes with inconspicuous neutrophils. There were abundant thrombi in both medium- and small-sized blood vessels, which were observed in all 10 histological sections analysed. Thrombi in all locations showed CD61 expression indicating their platelet composition (Fig. 2D). Histochemical and immunohistochemical studies did not reveal the presence of any further infectious pathogen. Some Von Kossa positive calcium deposits were found in both alveolar spaces and septum.
Figure 2.
Diffuse alveolar damage (DAD) pattern of lung acute injury in COVID19. (a) Exudative phase showing hyaline membranes. Multinucleated pneumocyte with cytopathic-like changes. (b) Organizing-fibrotic phase of DAD, note the presence of calcification. (c) Desquamative pneumocytes highlighted with pankeratin stain. (d) Organized thrombus. Inset: CD61 expression in a septal capillary thrombus.
The kidneys showed cortical necrosis and oxalate calcium crystalluria. No other relevant histological alterations were noted.
Discussion
The COVID-19 pandemic had an enormous impact on the Spanish Health System during March and the two first weeks of April, especially in areas such as Madrid. In most hospitals, more than 90% of the hospital beds were occupied by COVID-19 patients, the number of patients attending the emergency department was two to three times higher than usual, there was a marked shortage of PPE and more than 20% of hospital staff were infected by COVID-19. In such an urgent situation, autopsies were not considered a priority, at least in our centre.
The Ministry of Health, advised by the SEAP-IAP, and in agreement with most international scientific institutions, recommended performing autopsies only if all security issues regarding the autopsy rooms and PPEs were taken into account. Rigorous technical verification of the autopsy room is mandatory in order to guarantee the safety of personnel and the environment. As high-risk autopsies are not frequently performed in most Pathology Departments in Spain, training in the use of specific PPE is highly recommended previous to carrying out this type of autopsy. In our experience, appointing a single physician to be in charge of requesting all post mortems facilitated administrative issues and was a positive factor in the implementation of the autopsy programme.
In the autopsy reported here, the main pathological findings were observed, as expected, in the lung. There were extensive lesions of DAD, in addition to cytopathic-like changes in the pneumocytes, probably attributed to COVID-19. Although there were some areas of exudative DAD, the most generalized pattern was consistent with an organizing/proliferative phase.
To date, there are few reports regarding the pathological lesions produced by COVID-19 in the lung.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 A summary of the major clinicopathological findings are presented in Table 2 . In some cases, the authors describe pneumonic changes similar to those we observed in less affected areas of the lung in our patient. In this phase, the presence of cells with cytopathic changes was evident. In most previously reported cases, the lesions were consistent with DAD in the exudative and, less frequently, the proliferative/organizing phase. Our case showed all the spectrum of DAD from the early exudative phase to extensive fibrosis, suggesting a continuous viral effect on the lung throughout the clinical course of the disease. One previous, brief report found acute fibrinous organizing pneumonia as the main feature in four out of five biopsies. In a preliminary analysis of 10 autopsies performed in our hospital (in preparation), two patients showed this type of lesion, which had been previously reported in 30% of autopsies from patients with severe acute respiratory syndrome due to SARS-CoV infection.18, 19 Whether or not this is a specific lesion of a pattern of tissue organization in the context of DAD, or its possible clinical relevance, remain to be seen. Another frequent finding in previous reports was the presence of vascular thrombi in blood vessels of different size. The only reports in which this finding is not observed were those based on the study of biopsies, where sampling is more limited. It is controversial whether COVID-19 patients present a higher hypercoagulability status than other critical conditions, which could explain these findings. However, it should be noted that vascular thrombosis is an integral component of DAD and we did not observe vascular thrombosis elsewhere.
Table 2.
Reported cases of lung injury in COVID-19 pandemics.
| Authors | n | Specimen source | Days since admission | Lung pathology | Vascular thrombi |
|---|---|---|---|---|---|
| Tian et al.(2) | 2 | Lung cancer resection | - | Viral pneumonia | No |
| Pernaza (3) | 1 | Lung cancer resection | Viral pneumonia | No | |
| Cai (4) | 1 | Lung cancer resection | Viral pneumonia | No | |
| Zhang et al.(5) | 1 | Biopsy | 7 | DAD (¿P?) | No |
| Xu et al (6) | 1 | PMB | 14 | DAD (E) | No |
| Tian et al (7) | 4 | PMB | 22-52 | DAD (E) (3) DAD (P) (1) |
Yes |
| Copin et al (8) | 5 | PMB | 5 20 |
Viral pneumonia (1) AFOP (4) |
No |
| Yao et al (9) | 1 | PMB | 17 | DAD (E) | Yes |
| Gagiannis et al (10) | 3 | TBB/PMB | - | DAD (O) (1) AFOP (1) |
NR |
| Karami et al (11) | 1 | Autopsy | 1 | DAD (E) | NR |
| Barton et al (12) | 2 | Autopsy | 2-6 | DAD (E) (1) Broncopneumonia (1) |
Yes |
| Fox et al (13) | 3 | Autopsy | < 7 | DAD (exudativa) (3) | Yes |
| Carsana et al (14) | 38 | Autopsy | 16 (5-31) | DAD (E/P) | Yes (33) |
| Bradley et al (15) | 12 | Autopsy | 12 (1-14) | DAD (E/P) | Yes |
| Lovly et al (16) | 1 | Autopsy | 20 | DAD (P) | NR |
| Menter et al (17) | 21 | Autopsy | 5.7 (0-16) | DAD (E/P) | Yes |
| Navarro-Conde et al (1) | 1 | Autopsy | 3 | DAD (E) | Yes |
| Present Case | 1 | Autopsy | 30 | DAD (P/F) | Yes |
PMB: post-mortem biopsy; TBB: transbronchial biopsy; DAD: diffuse alveolar damage; E: exudative phase; P: proliferative phase; F: fibrotic phase; AFOP: acute fibrinous and organizing pneumonia, NR: not reported.
In conclusion, we present the process of implementation of a COVID-19 Autopsy Program in our Hospital, report the main findings from the first autopsy performed and briefly review the lung pathology reported in these patients.
Financiación
Supported by grants from the Instituto de Salud Carlos III (ISCIII): PI19/01331 and CIBERONC (CB16/12/00316), cofinanced by the European Development Regional Fund “A way to achieve Europe” (FEDER).
Conflict of interest
The authors declare that they have no conflict of interest
References
- 1.Navarro-Conde P., Alemany-Monraval P., Medina-Medina C., Jiménez-Sánchez A., Andres- Teruel J.C., Ferrando Marco Autopsia clínica en el Síndrome Respiratorio Severo por SARS-CoV-2 Primer fallecido en España. Rev Esp Patol. 2020 doi: 10.1016/j.patol.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients with Lung Cancer. J Thorac Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pernazza A., Mancini M., Rullo E., Bassi M., De Giacomo T., Rocca C.D. Early histologic findings of pulmonary SARS-CoV-2 infection detected in a surgical specimen. Virchows Arch. 2020 doi: 10.1007/s00428-020-02829-1. Epub 2020 Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai Y., Hao Z., Gao Y., Ping W., Wang Q., Peng S. Coronavirus Disease 2019 in the Perioperative Period of Lung Resection: A Brief Report From a Single Thoracic Surgery Department in Wuhan People's Republic of China. J Thorac Oncol. 2020;S1556–0864:30298–30307. doi: 10.1016/j.jtho.2020.04.003. Epub 2020 Apr 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H., Zhou P., Wei Y., Yue H., Wang Y., Hu M. Histopathologic Changes and SARS-CoV-2 Immunostaining in the Lung of a Patient With COVID-19. Ann Intern Med. 2020 doi: 10.7326/L20-0895. Epub 2020 Mar https://doi.org/12. 10.7326/M20-0533. [DOI] [PubMed] [Google Scholar]
- 6.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian S., Xiong Y., Liu H., Niu L., Guo J., Liao M. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020 doi: 10.1038/s41379-020-0536-x. Epub 2020 Apr 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Copin M.C., Parmentier E., Duburcq T., Poissy J., Mathieu D. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 2020. doi: 10.1007/s00134-020-06057-8. Epub 2020 Apr 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao X.H., He Z.C., Li T.Y., Zhang H.R., Wang Y., Mou H. Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-for-discharge patient. Cell Res. 2020 doi: 10.1038/s41422-020-0318-5. Epub 2020 Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gagiannis D., Steinestel J., Hackenbroch C., Hannemann M., Umathum V.G., Gebauer N. COVID-19-induced acute respiratory failure: an exacerbation of organ-specific autoimmunity? MedRxiv preprint. 2020 Epub 2020 May 1 https://doi.org/10.1101/2020.04.27.20077180. [Google Scholar]
- 11.Karami P., Naghavi M., Feyzi A., Aghamohammadi M., Novin M.S., Mobaien A. Mortality of a pregnant patient diagnosed with COVID-19: A case report with clinical, radiological, and histopathological findings. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101665. Epub 2020 Apr 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barton L.M., Duval E.J., Stroberg E., Ghosh S., Mukhopadhyay S. COVID-19 Autopsies, Oklahoma. USA. Am J Clin Pathol. 2020 doi: 10.1093/ajcp/aqaa062. Epub 2020 Apr 10. 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Brown J.Q., Vander Heide R.S. Pulmonary and Cardiac Pathology in Covid-19: The First Autopsy Series from New Orleans. MedRxiv preprint. 2020 doi: 10.1016/S2213-2600(20)30243-5. Epub 2020 Apr 10. https://doi.org/10.1101/2020.04.06.20050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carsana L., Sonzogni A., Nasr A., Rossi R., Pellegrinelli A., Zerbi P. Pulmonary post-mortem findings in a large series of COVID-19 cases from Northern Italy. MedRxiv preprint. 2020 doi: 10.1016/S1473-3099(20)30434-5. Epub 2020 Apr 22 https://doi.org/10.1101/2020.04.19.20054262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradley B.T., Maioli H., Johnston R., Chaudhry I., Fink S.L., Xu H. Histopathology and Ultrastructural Findings of Fatal COVID-19 Infections. MedRxiv preprint. 2020 doi: 10.1016/S0140-6736(20)31305-2. Epub 2020 Apr 21 https://doi.org/10.1101/2020.04.17.20058545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovly C.M., Boyd K.L., Gonzalez-Ericsson P.I., Lowe C.L., Brown H.M., Hoffman R.D. Rapidly fatal pneumonitis from immunotherapy and concurrent SARS-CoV-2 infection in a patient with newly diagnosed lung cancer. MedRxiv preprint. 2020 Epub 2020. May 1. https://doi.org/10.1101/2020.04.29.20085738. [Google Scholar]
- 17.Menter T., Haslbauer J.D., Nienhold R., Savic S., Hopfer H., Deigendesch N. Histpathology. 2020 doi: 10.1111/his.14134. https://doi.org/10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang D.M., Chamberlain D.W., Poutanen S.M., Low D.E., Asa S.L., Butany J. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod Pathol. 2005;18:1–10. doi: 10.1038/modpathol.3800247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu J., Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol. 2007;170:1136–1147. doi: 10.2353/ajpath.2007.061088. https://doi.org/10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]