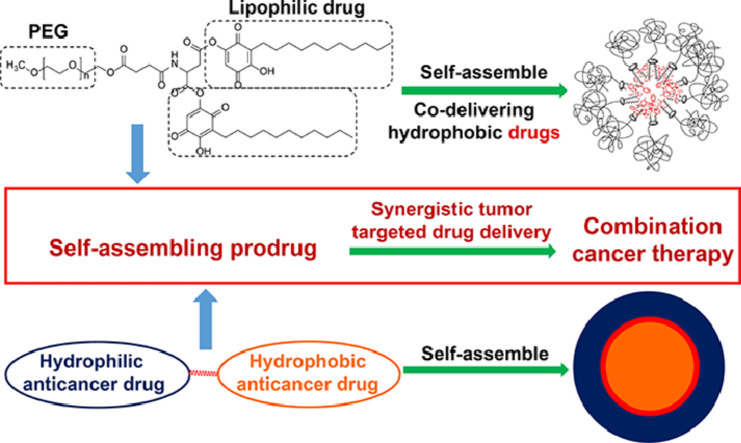
Abstract
Self-assembling prodrugs represents a robust and effective nanotherapeutic approach for delivering poorly soluble anticancer drugs. With numerous intrinsic advantages, self-assembling prodrugs possess the maximum drug loading capacity, controlled drug release kinetics, prolonged blood circulation, and preferential tumor accumulation based on the enhanced permeability and retention (EPR) effect. These prodrug conjugates allow for efficient self-assembly into nanodrugs with the potential of encapsulating other therapeutic agents that have different molecular targets, enabling simultaneous temporal-spatial release of drugs for synergistic antitumor efficacy with reduced systemic side effects. The aim of this review is to summarize the recent progress of self-assembling prodrug cancer nanotherapeutics that are made through conjugating therapeutically active agents to Polyethylene glycol, Vitamin E, or drugs with different physicochemical properties via rational design, for synergistic tumor targeted drug delivery.
Statement of Significance
All current FDA-approved nanomedicines use inert biomaterials as drug delivery carriers. These biomaterials lack any therapeutic potential, contributing not only to the cost, but may also elicit severe unfavorable adverse effects. Despite the reduction in toxicity associated with the payload, these nanotherapeutics have been met with limited clinical success, likely due to the monotherapy regimen. The self-assembling prodrug (SAP) has been emerging as a powerful platform for enhancing efficacy through co-delivering other therapeutic modalities with distinct molecular targets. Herein, we opportunely present a comprehensive review article summarizing three unique approaches of making SAP for synergistic drug delivery: pegylation, vitamin E-derivatization, and drug-drug conjugation. These SAPs may inevitably pave the way for developing more efficacious, clinically translatable, combination cancer nanotherapies.
Keywords: Self-assembling prodrug, Nanotherapeutics, Synergistic drug delivery, Enhanced Cancer Therapy
Graphical abstract
1. Introduction
Clinical application of small molecule drugs such as chemotherapeutics are greatly impeded by their poor solubility in aqueous solutions, short blood circulation times, inadequate efficacy, lack of tissue-specificity and severe systemic adverse effects [1], [2], [3], [4]. Tremendous efforts have been made to develop various drug delivery systems to address these limitations and strengthen tumor targeted delivery with an aim to enhance therapeutic efficacy while improving drug tolerance [5], [6], [7]. Over the past several decades, the self-assembling prodrugs (SAP) approach has emerged as a powerful therapeutic platform for enhancing tumor targeted therapy [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]. Compared to free drugs, well-designed SAP nanotherapeutics (SAPNS) are equipped with a number of advantages in addressing these unmet clinic needs: 1) strengthened physicochemical properties such as enhanced solubility and chemical stability, 2) improved pharmacodynamic profiles and attenuated side effects by favorably altering their pharmacokinetics (PK) and tumor uptake and 3) as effective carriers for delivering poorly water-soluble drugs in vivo in addition to ameliorating their systemic non-specific toxicities as well as boosting their tumor targeted accumulation for more efficacious combination cancer therapy[45,46]. By improving the aqueous solubility, promoting the formation of pertinent nano-sized therapeutics and controlling the release at the same time, the conjugated prodrug and its encapsulated payloads can passively target and enhance the accumulation at tumor sites via the EPR effect [47], [48], [49].
Most of the existing carriers from conventional drug delivery systems utilize “inert” excipients which have no pharmacological activity, but impose additional safety concerns and add to the overall cost [50], [51], [52]. Conversely, synergistic or additive anticancer effects have been discovered between the therapeutic prodrug nanocarriers and delivered anticancer drugs following the intracellular uptake of the drug-laden SAP and the release of the biologically functional therapeutics from the conjugates [7,[53], [54], [55]].
The SAP can be divided into several categories, including polymer-drug, lipid-drug, or drug-drug conjugates [56]. Owing to its high hydrophilicity, ease of formulation, low potential for toxicity, and biocompatibility, polyethylene glycol (PEG), has been extensively used as the hydrophilic segment for conjugation to lipophilic drugs (Embelin: EB; Vitamin E: VE; trans-farnesylthiosalicylic acid: FTS) to address solubility issues and bioavailability [57,58]. Moreover, these PEG-derived prodrugs are not only able to self-assemble to nanoparticles e.g. polymeric nanomicelles, but can also co-deliver other water-insoluble chemotherapeutics in their hydrophobic core for synergistic anticancer activity [1,59,60]. Because of the steric hindrance provided by PEG hydrophilic corona, PEG-drug conjugates significantly improve a drug's PK with prolonged half-life during blood circulation by avoiding the opsonization effect [61,62]. Furthermore, the PEG-drug nanotherapeutics revealed significantly improved tumor targeting efficiency through the leaky vasculature and impaired lymphatic drainage system via the EPR effect [5,61,63].
Recently, lipid-dativization appears to be another robust strategy for formulating hard-to-formulate drugs and addressing their intrinsic instability issues through facilitating their self-assembly into nanoparticles of various shapes [64], [65], [66]. It has been demonstrated that the doxorubicin (DOX)-derivatized α-d-tocopherol succinate prodrug (N-DOX-TOS) can readily form a 250 nm nano-assembly in aqueous solution upon stabilization by d-a-tocopherol poly(ethylene glycol) 2000 succinate and showed greater antitumor efficacy than free unmodified DOX [67]. Our own research has also shown that camptothecin-drived VE can form a nanofiber nanomedicine upon stabilization by a VE-based PEG carrier [9].
In addition, it has been reported that, through rational design based on the unique physicochemical properties of different drugs, amphiphilic hydrophobic drug-hydrophilic drug conjugates can self-assemble into nanotherapeutics with enhanced bioavailability, improved PK and enhanced antitumor efficacy [68]. The merit of this design is that instead of physically loading other drugs inside various nanocarriers for combination cancer therapy, the drug-drug conjugate already contains two distinctly pharmaceutically active agents. Additionally, this amphiphilic drug-drug conjugation approach circumvents the nonuniform biodistribution of individual anticancer agents delivered through cocktail administration and ensures well-controlled temporal and spatial dual drug delivery vital for synergistic cancer elimination [45,46,69,70].
In this review, we will emphasize the progress regarding the PEG-drug, VE-drug, and drug-drug-based SAP conjugates for targeted drug delivery for combination cancer therapy.
2. PEG-derived SAP nanocarriers for synergistic drug delivery
Most of the nanocarrier components used in various drug delivery systems utilize “inert” excipients lacking any therapeutic activity [46,71]. The presence of large amounts of carrier materials not only increases the overall therapy cost, but also gives rise to unwanted side effects [72]. One of the most sophisticated designs of drug delivery systems is that the components forming the carriers can also have therapeutic potential. Following the intracellular delivery, the bioactive component of the delivery carriers can be liberated and cooperate with co-delivered drugs for improved synergistic cancer therapy [10]. Combination therapy with two or more drugs in a single nanocarrier working simultaneously at different molecular signaling pathways can not only maximize the anticancer efficacy, but can also be conducive to overcome drug resistance and counteract the side effects developed by monotherapy. Herein, some of the recently developed SAP amphiphilic nanocarriers with hydrophobic backbone composed of lipophilic anticancer therapeutics are discussed.
2.1. PEG-based embelin SAP as a dual functional nanocarrier for combination cancer therapy
Embelin (EB) (Fig. 1 A) is a naturally occurring alkyl-substituted hydroxyl benzoquinone compound and a major constituent of Embelia ribes BURM. It has been shown to possess hepatoprotective, anti-inflammatory, and antidiabetic effects [73]. In addition, EB also exhibits notable antitumor activity in various types of cancers by inhibiting the activity of X-linked inhibitor of apoptosis protein (XIAP) [74,75]. EB has negligible toxicity in normal cells and thereby presents a good safety profile, as XIAP is particularly overexpressed in various drug-resistant cancer cells with a minimal role in healthy cells. Hence, XIAP has been shown to be an effective target for selectively inhibiting cancer cell growth [76,77]. Additionally through suppressing the NF-κB pathway, EB also has effects of downregulating the expression of several significant cancer promoting factors, such as XIAP, IAP1/2, survivin, cFLIP, TRAF1, Bcl-2 and Bcl-L [75]. However, owing to its long lipophilic acyl chain, EB is extremely hydrophobic, which renders poor aqueous solubility/bioavailability, unfavorable PK and unsatisfactory efficacy.
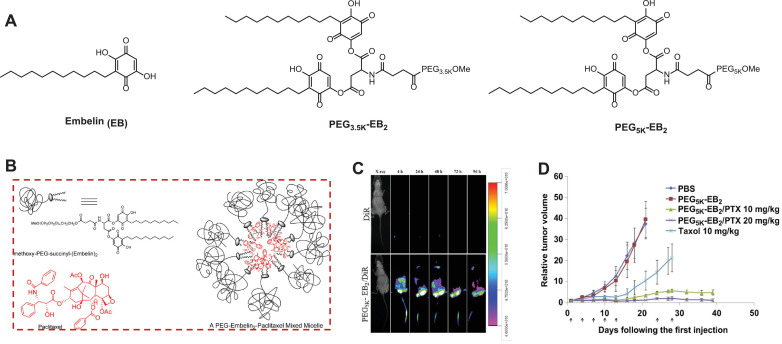
Fig. 1.
(A) Chemical structures of Embelin (EB), PEG3.5K-EB2, and PEG5K-EB2. (B) Schematic illustration of self-assembling PEG-EB2 prodrug conjugate with Paclitaxel loaded inside. (C) In vivo NIRF optical imaging of CL1 lung tumor-bearing SCID mice after intravenous (IV) injection of free DiR dye and DiR-loaded PEG5K-EB2 micelles. (D) Improved anticancer efficacy of PTX formulated in PEG5K-EB2 micelles in nude mice-bearing PC-3 prostate tumors. A week after injecting 2 × 106 cells/mouse subcutaneously, mice were IV administered with different treatments on days 1, 3, 7, 10, 13, 24, and 28, and relative tumor volume was plotted (N = 6). P < 0.005 (20 mg/kg PTX/PEG5K-EB2 vs. Taxol), P < 0.01(10 mg/kg PTX/PEG5K-EB2 vs. Taxol), P < 0.05 (20 mg/kg PTX/PEG5K-EB2 VS 10 mg/kg PTX/PEG5K-EB2). Reproduced with permission from [6,10,58] . (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To address the limitations associated with EB, we developed a series of PEG-dativized EB conjugates, which differ in PEG length and amount of EB, bridging through the aspartic acid-mediated labile ester bond (Fig. 1A). Upon pegylation, the solubility of EB has been drastically increased from less than 1 mg/mL to more than 750 mg/mL. Equally important, PEG-EB conjugates retain similar cytotoxicity as the unmodified EB in three breast and prostate cancer cell lines. This effect is unlikely due to the polymer-drug conjugate surface activity as PEG-EB showed negligible hemolysis against red blood cells. More strikingly, PEG-EB prodrug not only self-assembles into spherical nanomicelles (~20 nm) in aqueous medium, but can also efficiently encapsulate other hydrophobic drugs such as PTX (Fig. 1B) within its hydrophobic pocket to achieve synergistic cancer cell killing effect in four different breast and prostate cancer cell lines. This could be attributed to the efficient EB release from the conjugate following intracellular delivery, and consequently synergizing antitumor activity with PTX, which stabilizes the microtubes during mitosis. Moreover, we have demonstrated that the prodrug with two EB molecules and a longer PEG chain is markedly more effective for encapsulating PTX than the counterpart with a 1:1 PEG/EB molar ratio and a shorter PEG chain [6,58]. This is upheld by the fact that PEG5K-EB2 (0.35 μM) had a much lower critical micelle concentration (CMC) that of PEG3.5K-EB2 (4.9 μM) and at the same carrier/PTX molar ratios, PEG5K-EB2 showed smaller particle size with lower polydispersity, higher drug loading, and improved formulation stability than PEG3.5K-EB2. The strengthened formulation stability and drug loading in PEG5K-EB2 micelles is likely due to the improved PEG stealth effect, which exhibits greater steric hindrance. This prevents the ester linkage from being hydrolyzed under aqueous conditions, resulting in further stabilized micellar nanotherapeutics. Compared to the clinical PTX formulation, TaxolⓇ, the PTX/PEG5K-EB2 micellar formulation displayed a much slower and controlled PTX release as evidenced by the T1/2 = 34.1 h vs T1/2 = 6.57 h in TaxolⓇ. This delayed and more sustained PTX release from PEG5K-EB2 could be attributed to the strong interaction between the carriers and PTX. Additionally, EB has a benzoquinone ring and a long alkyl chain, which can form π-π stacking interactions and hydrogen bonding with PTX in addition to hydrophobic interactions, rendering enhanced overall carrier/PTX interaction. The close proximity of two EB lipidic chain in PEG5K-EB2 conjugate can facilitate the interaction of the carrier with PTX. Furthermore, PTX/PEG5K-EB2 presented a considerably higher maximum tolerated dose (MTD) as compared to Taxol (100 mg/kg vs 15 mg/kg), which is linked to slow release kinetics for PTX, lower nonspecific uptake by healthy organs, as well as the remarkable safety profile, in addition to the selectivity, anti-inflammatory and hepatoprotective activity of EB [6]. The increased MTD and enhanced safety allowed for maximal therapeutic efficacy to be achieved. In addition, PEG5K-EB2 was found to be capable of overcoming multi-drug resistance in NCI/ADR-RES cells through inhibiting the p-glycoprotein (P-gp) efflux pump [10]. Furthermore, near-infrared fluorescence (NIRF) imaging in prostate and lung cancer xenograft-bearing mice showed preferential tumor targeting for PEG5K-EB2 micelles (Fig. 1C) [6,10]. Most importantly, when compared to TaxolⓇ, the co-delivery of PTX by SAP PEG5K-EB2 micelles significantly enhanced tumor reduction in murine models of breast and prostate cancers with no noticeable systemic toxicities (Fig. 1D). Consistent to literature [64,78,79], through anchoring the folic acid (FA), a tumor specific targeting ligand, onto the surface of PEG5K-EB2 nanomicelles, the intratumoral accumulation was greatly enhanced, which led to further boosted tumor growth suppression [10].
2.2. PEG-based vitamin E SAP for synergistic drug delivery
The pegylated vitamin E (VE, Fig. 2 A), d-a-tocopheryl polyethylene glycol 1000 (PEG1K) succinate (TPGS) system is another example of a dual functional nanocarrier [80], [81], [82]. VE, a lipophilic molecule with a long alkyl tail, is conjugated to PEG1K through a biodegradable ester bond, allowing it to self-assemble into micelles with a hydrophobic pocket where water-insoluble drugs can be incorporated. It has been demonstrated that VE has an antitumor effect against a wide spectrum of cancers via various mechanisms including induction of apoptosis, inhibition of tumor cell proliferation and differentiation, suppression of nuclear factor-kappa B (NF-κB) activation, and so forth [76,[82], [83], [84], [85]]. Due to its laudable functions as an emulsifier, solubilizer as well as a permeability and absorption enhancer, TPGS has been approved by the FDA as a safe pharmaceutical adjuvant for drug formulation [81,84,86,87]. Additionally, based on its ability to inhibit P-glycoprotein (P-gp) drug efflux pumps, formulations with TPGS can reverse multidrug resistance (MDR) and improve the bioavailability of anticancer nanocarriers [80,82,83,88]. Moreover, TPGS-based nanoformulations delivering PTX or other therapeutics exhibited potentiated antitumor activity in several animal cancer models [81,82].
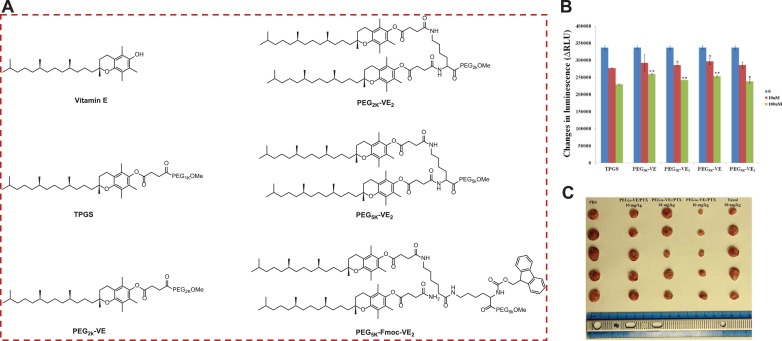
Fig. 2.
(A) Chemical structures of Vitamin E (VE), TPGS, PEG2K-VE, PEG2K-VE2, and PEG5K-VE2, PEG5K-Fmoc-VE2. (B) Inhibition on verapamil-stimulated P-gp ATPase activity for PEG-derived VE conjugates. *p < 0.05 and **p < 0.001 (vs TPGS of equivalent concentration). (C) The tumor images excised at the completion of the anticancer efficacy study in 4T1.2 breast tumor-bearing mice (n = 5), which were IV treated with various PEG-VE/PTX nanomicelles. P < 0.02 (PEG5K–VE2/PTX vs Taxol PEG2K–VE/PTX or FEG2K–VE2/PTX). Reproduced with permission from [7].
Nevertheless, the high CMC value (0.2 mg/mL), renders it unstable in vivo, and unable to serve as an effective nanocarrier to encapsulate therapeutics [85]. In order to improve this drug delivery system, we have developed a number of improved TPGS conjugates by varying the PEG length and altering the ratio of PEG and VE (Fig. 2A). Systemic structure and activity relationship study (SAR) demonstrated that all of the optimized prodrugs (PEG2K-VE, PEG2K-VE2, PEG5K-VE, and PEG5K-VE2) retained the intrinsic activity of TPGS in inhibiting P-gp drug efflux pump [80]. Interestingly, PEG5K-VE2 outperformed other conjugates in terms of PTX drug loading and formulation stability, leading to the highest level of delaying tumor growth even in comparison to TaxolⓇ (FDA-approved PTX formulation). While PEG5K-VE2 performed best among the four systems, the drug loading capacity (DLC) is still not adequate and within a single digit. To further optimize this SAP system, a fluorenylmethyloxycarbonyl (Fmoc) drug-interactive motif was installed at the interfacial region of PEG5K-VE2 [7]. Fmoc has been shown to provide strong π−π stacking and hydrophobic interactions with other aromatic moieties, including itself (Fig. 2B) [89], [90], [91], [92]. To test this hypothesis, Doxorubicin (DOX) was chosen as the model drug since it has several aromatic rings. Upon Fmoc addition, PEG5K-Fmoc-VE2 can physically entrap up to 39.9% DOX, which was a drastic increase over the 2.9% DOX DLC for the PEG5K-VE2, and also higher than most of the existing DOX nanoformulations [7,10,60]. Because of reinforced carrier/carrier and drug/carrier interactions, the Fmoc-embedded formulation improved the PK and MTD when delivering DOX in vivo through controlled and sustained drug release, resulting in a prominently increased tumor growth reduction over DOX/PEG5K-VE2 and DoxilⓇ (FDA-approved DOX nanoformulation) in breast, prostate, and drug-resistant murine cancer models (Fig. 2C).
2.3. PEG-derived S-trans Trans-farnesylthiosalicylic acid SAP micellar carrier
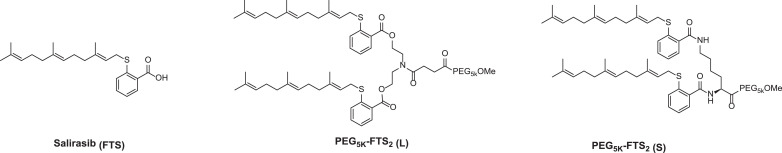
In addition to EB and VE-based PEG SAP nanotherapeutic carriers, PEG-derived trans-farnesylthiosalicylic acid (FTS, Fig. 3 ) has been developed. FTS is a synthetic first-in-class direct Ras antagonist designed to inhibit overactive cell growth in cancers by interfering the anchorage of Ras on cell membrane [93,94]. Nearly one-third of human cancers have constitutive Ras expression [95,96]. By targeting Ras, FTS has shown a noticeable antitumor effect with high selectivity in a wide range of cancers [97]. Similar to EB and VE, FTS contains a long lipophilic acyl chain that gives rise to poor water-solubility/bioavailability. Pegylation not only greatly ameliorates FTS’ aqueous solubility, but also enables the formation of nanomicelles that are capable of encapsulating other hydrophobic drugs, allowing for synergistic antitumor efficacy [98].
Fig. 3.
Chemical structures of Salirasib (FTS), PEG5K-FTS2 (L, labile), and PEG5K-FTS2 (S, Stable). Reproduced with permission from [98].
3. Vitamin E-based SAP cancer nanotherapeutics
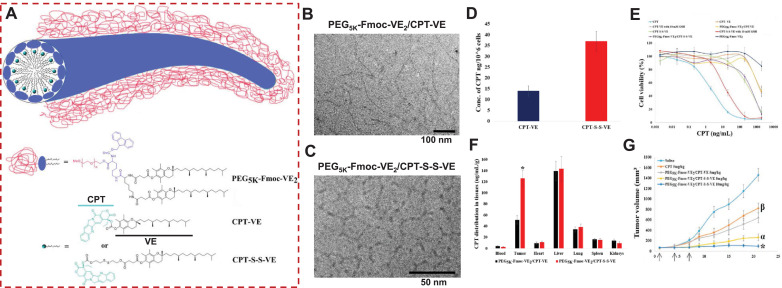
Most of the current approaches are centered on improving the nanocarriers in order to augment their compatibility with the therapeutic payloads. These strategies cannot accommodate some agents with complex chemical structures, such as camptothecin (CPT), despite working well for a number of anticancer drugs that have relatively simple structures. CPT, a potent chemotherapeutic, showed remarkable antineoplastic activity against a wide array of cancers by suppressing the topoisomerase I in the S-phase of the cell cycle during DNA replication [99]. However, owing to its extremely poor solubility, intrinsic instability (under physiologic conditions due to the involuntary conversion of the active lactone form to the inactive carboxylate form) and systemic toxicities, its clinical application has not been granted. Moreover, few of the existing nanocarriers can be formulated with this drug. Polymer derivatization strategy has been attempted to address these shortcomings, but have been met with limited success. This is likely attributed to ineffective drug release because of the considerable steric hindrance posed by macromolecular polymer [100,101]. Instead of optimizing the nanocarrier component, we derivatized CPT with one molecule of VE, using either an ester bond or a disulfide linkage (Fig. 4 A). Surprisingly, both CPT-VE and CPT-S-S-VE prodrugs enabled efficient self-assembly into nanofibers with the stabilization of the PEG5K-Fmoc-VE2-based nanomicellar carrier (Figs. 4B and 4C). The formation of nanofibers of PEG5K-Fmoc-VE2/CPT-VE and PEG5K-Fmoc-VE2/CPT-S-S-VE, as compared to the spherical structures for PEG5K-VE2/CPT, PEG5K-Fmoc-VE2/CPT, PEG5K-VE2/CPT-VE, PEG5K-VE2/CPT-S-S-VE, and spherical shapes of the self-assembling prodrug described above, are likely due to strong π-π stacking interaction arisen from Fmoc moieties among carriers, Fmoc and aromatic rings in CPT and VE, and hydrophobic interactions between alkane chains among VEs between/among carriers and/or prodrugs as evidenced by the fluorescence quenching, UV absorbance, FT-IR, and NMR analysis [9].
Fig. 4.
(A) Mechanistic representation of self-assembly of CPT-VE or CPT-S-S-VE to nanofibers upon stabilization by PEG5K-Fmoc-VE2. Cryo-electron microscopy (cryoEM) imaging of PEG5K-Fmoc-VE2/CPT-S-S-VE (B) and PEG5K-Fmoc-VE2/CPT-VE (C). (D) Intracellular release of CPT in 4T1.2 cells treated with CPT-VE and CPT-S-S-VE (100 ng/mL in terms of CPT) for 24 h. (E) The cytotoxicity of different CPT formulations in 4T1.2 cancer cells treated with indicated concentrations for 72 h and was then assessed by MTT assay. (F) Tissue biodistribution of PEG5K-Fmoc-VE2/CPT-VE and PEG5K-Fmoc-VE2/CPT-S-S-VE (5 mg CPT/kg) in 4T1.2-tumor bearing mice. *p < 0.001, compared to PEG5K-Fmoc-VE2/CPT-VE. (G) In vivo therapeutic efficacy of various VE-derived CPT prodrug nanotherapeutics in breast tumor mouse model (n = 5). Arrows stand for the IV injection. *p < 0.01, compared to PEG5K-Fmoc-VE2/CPT-S-S-VE (5 mg/kg); αp < 0.001, compared to PEG5K-Fmoc-VE2/CPT-VE (5 mg/kg) and CPT (5 mg/kg); βp < 0.001, compared to saline. Reproduced with permission from [9] . (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
CPT-S-S-VE showed significantly higher intracellular (Fig. 4D) and intratumoral drug release (Fig. 4F) as compared to ester-bonded prodrug (CPT-VE) due to the high glutathione levels inside cancer cells and tumor tissue [9,46,71,102], which led to the higher anticancer activity both in vitro (Fig. 4E) and in vivo (Fig. 4G). Both CPT prodrug nanofibers demonstrated greater efficacy over free CPT in curbing breast tumor development in the syngeneic mouse cancer models (Fig. 4G). While the DLC for free CPT was only 0.65% when using the PEG5K-Fmoc-VE2 carrier, it was markedly increased to 9.2% for CPT-S-S-VE with improved formulation stability. This could be due to the fact that: 1) VE in prodrugs can function as a lipid anchor to facilitate its insertion/incorporation into the hydrophobic pocket formed by VE chains in nanocarrier; 2) the increased drug/carrier interactions such as π−π stacking between the Fmoc motif and the aromatic rings of CPT [9].
The VE-derivation has also been investigated for improving the compatibility of other common difficult-to-load drugs with existing carriers. It has been shown that DOX conjugated with d-a-tocopherol succinate (NDOX-TOS) formed a nanoassembly in aqueous solution after mixing with d-a-tocopherol poly(ethylene glycol) 2000 succinate with a much higher DOX DLC (34%) and led to superior tumor growth delay to free DOX [67]. Moreover, a recent discovery has demonstrated that PTX-conjugated VE, bridged via a disulfide linkage, led to spherical nanoparticles with improved PK and enhanced tumor killing effect in vivo as compared to TaxolⓇ [103]. This approach was realized by the hypothesis that the insertion of a single disulfide bond into hydrophobic molecules can balance the competition between intermolecular forces, resulting in the consequent nanotherapeutic self-assembly.
4. Drug-Drug self-assembling nanomedicines for combination cancer therapy
In another approach to enhance the delivery of anticancer agents to tumor sites in the form of self-assembled nanotherapeutics, amphiphilic drug-drug conjugates (ADDCs) have been developed by conjugating a hydrophilic drug and a hydrophobic drug with labile linkage [27,68,[104], [105], [106], [107]]. The advantages of this ADDC design is multi-faceted: 1) with coupling of a hydrophilic drug molecule, the hydrophobic drug solubility issue will be addressed; 2) since the conjugates are solely composed of drugs, ADDC alleviates the limited drug loading seen in traditional nanocarriers; 3) self-assembling ADDC can prolong the blood circulation of free drugs that are normally prone to rapid clearance mediated by opsonization;[108] 4) improved tumor targeted accumulation with reduced non-specific toxicity to normal tissues based on the EPR effect; 5) decreased safety concern frequently encountered through the use of synthetic nanomaterials; [109] 6) can release drugs that have different molecular targets in a simultaneously temporal-spatial controlled fashion for synergistic anticancer activity; 7) can bypass drug efflux-pump-mediated drug resistance (eg., P-gp), as ADCC SAPs are taken up by cells via endocytosis, which is independent of the transporter-facilitated intracellular delivery.
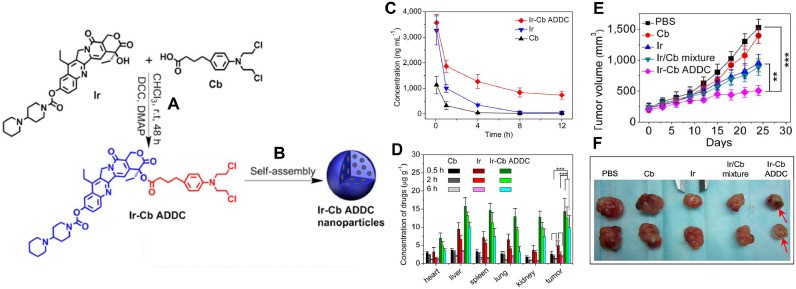
Huang P. et al. constructed an ADDC using the water-soluble drug, Irinotecan (Ir) and the water insoluble drug, Chlorambucil (Cb) that self-assembled into spherical nanoparticle (Figs. 5 A and 5B). The authors demonstrated that the release of free Ir and free Cb was accelerated at weakly acidic environment, indicating potential hydrolysis in the tumor microenvironment. It was shown that the in vitro cytotoxicity of Ir-Cb ADCC was dependent on the critical aggregation concentration (CAC). When the concentration of Ir-Cb ADCC was lower than CAC, it did not produce any significant cytotoxicity. However, at concentration higher than CAC, Ir-Cb ADCC could produce significantly higher cytotoxicity compared to free Ir and free Cb. The authors attributed this observation to higher uptake of nanoparticles by the cells and subsequent intracellular release of Ir and Cb elicited synergistic effect. The Ir-Cb ADCC nanoparticles were also shown to accumulate to a greater extent in drug-resistant MCF-7/ADR cells by overcoming P-gp efflux pump. The blood retention time and biodistribution studies demonstrated that Ir-Cb ADCC nanoparticle could circulate for longer time in blood and achieved higher tumor accumulation via EPR effect compared to Ir and Cb (Figs. 5C and 5D). The in vivo antitumor efficacy study in MCF-7 tumor bearing nude mice showed that Ir-Cb ADCC nanoparticles produce highest reduction in tumor volume in comparison to Cb, Ir and Cb/Ir mixture (Figs. 5E and 5F) [27]. In addition, Liang et al. reported a Janus CPT-floxuridine (FUDR, a hydrophilic chemotherapeutics) conjugate (JCFC) where two molecules of CPT were conjugated with two molecules of FUDR to produce a liposome-like nanocapsules (JCFC—NCs) around 115 nm in size with spherical morphology [68]. The CPT and FUDR were efficiently released at acidic pH and in the presence of esterase. JCFC—NCs can be internalized into a prostate carcinoma cell line rapidly with a significantly higher antiproliferative effect than free drugs. Moreover, when compared with free drugs and CPT and FUDR mixtures, JCFC—NCs elicited longer blood circulation time, increased tumor uptake, as well as enhanced tumor growth inhibition in prostate cancer [68]. In addition, Xu et al., has developed a supramolecular cisplatin-vorinostat nanodrug that can overcome drug resistance in synergistic cancer therapy [105]. Furthermore, Chlorambucil-Gemcitabine, Methotrexate-CPT, and CPT-Cytarabine ADDC self-assembling nanotherapeutics have been reported for efficacious combination cancer therapy [106], [107], [108].
Fig. 5.
(A) Synthetic route of Irinotecan-Chlorambucil (Ir-Cb) amphiphilic drug-drug conjugates (ADDC). (B) The Ir–Cb ADDC self-assembles into spherical nanoparticles. (C) Representative plasma concentration–time profiles of free Cb, Ir, and Ir–Cb ADDC after IV injection into rats (a dose of 8 mg/kg) (n = 4). (D) Tissue distribution of Cb, Ir, and Ir–Cb ADDC after IV administration of free Cb (3.5 mg/kg), Ir (6.7 mg/kg), and Ir–Cb ADDC nanoparticles (10 mg/kg) in MCF-7 tumor-bearing nude mice (n = 4). (E) Changes of tumor volume post IV injection of PBS, Cb, Ir, Ir/Cb mixture, and Ir–Cb ADDC nanoparticles in MCF-7 tumor-bearing nude mice (n = 6). (F) Representative tumors separated from animals after IV injection of PBS, Cb, Ir, Ir/Cb mixture, and Ir–Cb ADDC nanoparticles. Data are represented as average ± standard error. Statistical significance: **P < 0.005; ***P < 0.001. Reproduced with permission from [27].
5. Concluding remarks
A prodrug approach, particularly when they can self-assemble to form nano-sized drug delivery systems, is equipped with many advantages over parental drugs, such as 1) improved aqueous solubility that is more suitable for systemic administration; 2) enhanced intrinsic chemical stability through proper functionalization; 3) ameliorated bioavailability and pharmacokinetics; 4) boosted therapeutic efficacy (eg., anticancer activity); 5) reduced non-specific systemic toxicities to healthy tissues. In addition, since a considerable portion of if not all the nanocarrier materials are comprised of therapeutically active prodrugs, SAP can constitutionally and drastically augment drug loading capacity and minimize the potential long-term adverse effects that could have been caused by using conventional nanomaterials. Furthermore, with the ability to concurrently encapsulate other therapeutic modalities, SAP can fulfill the precise and simultaneous temporal-spatial drug release, enabling the synergistic efficacy in combatting cancers. Rather than improving the nanocarrier system for better drug packaging efficiency and formulation stability, SAP addresses formulation issues by having the drug anchored in carrier molecules during spontaneous self-assembly process. Moreover, by making drugs into SAP, they are able to circumvent the multi-drug resistance that often encountered by many free drugs, as which can be readily pumped out of cells by drug exporting transporters (e.g., P-gp), through nanoparticle-mediated endocytosis cellular uptake mechanism which can bypass the drug efflux transporters. Owing to these merits, a wide variety of SAPs have been developed, which has revolutionized the cancer treatment paradigm in the recent decades. Noteworthily, several aspects that are still needed to be taken into consideration when taking advantage of the SAP systems. First, prodrugs are not biologically active unless the active parent drugs are released. To boost the drug release, conjugation chemistry to anchor suitable linkers (e.g., ester bonds, disulfide linkages, and hydrazone bonds, etc.) should be carefully considered in order to utilize the distinct microenvironment in tumors (e.h., high hydrolase and glutathione levels, and low pH) [67,110]. Additionally, the anticancer agents that either physically or covalently encapsulated to SAP systems ought to have different cancer molecular targets than those of the SAP's in order to realize the synergistic anticancer activity. Last but not least, to allow the efficient self-assembly into nanodrugs, the constructed prodrug conjugates must be amphiphilic. Apart from chemotherapeutic agents, the SAP platform can also be applied to other therapeutic modalities that have similar functional groups, such as tyrosine kinase inhibitors, corticosteroids, anti-inflammatory agents, immunomodulators, and drugs that target infectious diseases for improved therapeutic efficacy and reduced side effects.
Declaration of Competing Interest
There are no conflicts of interest to declare.
Acknowledgements
This work was supported in part by a Startup Fund from the College of Pharmacy at The University of Arizona and two COVID-19 Rapid Turn-Around Seed Grants from the BIO5 Institute and the Technology and Research Initiative Fund (TRIF), and by NIH grants P30 ES006694 and R35 ES031575.
References
- 1.Zhang Y., Huang Y., Li S. Polymeric micelles: nanocarriers for cancer-targeted drug delivery. AAPS Pharm. Sci. Tech. 2014;15(4):862–871. doi: 10.1208/s12249-014-0113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peer D., Karp J.M., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007;2(12):751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 3.Senapati S., Mahanta A.K., Kumar S., Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Trans. Targeted Ther. 2018;3:7. doi: 10.1038/s41392-017-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yingchoncharoen P., Kalinowski D.S., Richardson D.R. Lipid-based drug delivery systems in cancer therapy: what is available and what is yet to come. Pharmacol. Rev. 2016;68(3):701–787. doi: 10.1124/pr.115.012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torchilin V.P. Micellar nanocarriers: pharmaceutical perspectives. Pharm. Res. 2007;24(1):1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 6.Lu J., Huang Y., Zhao W., Marquez R.T., Meng X., Li J., Gao X., Venkataramanan R., Wang Z., Li S. PEG-derivatized embelin as a nanomicellar carrier for delivery of paclitaxel to breast and prostate cancers. Biomaterials. 2013;34(5):1591–1600. doi: 10.1016/j.biomaterials.2012.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu J., Zhao W., Liu H., Marquez R., Huang Y., Zhang Y., Li J., Xie W., Venkataramanan R., Xu L., Li S. An improved d-alpha-tocopherol-based nanocarrier for targeted delivery of doxorubicin with reversal of multidrug resistance, J. Controlled Release. 2014;196:272–286. doi: 10.1016/j.jconrel.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sofias A.M., Dunne M., Storm G., Allen C. The battle of "nano" paclitaxel. Adv. Drug Delivery Rev. 2017;122:20–30. doi: 10.1016/j.addr.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Lu J., Liu C., Wang P., Ghazwani M., Xu J., Huang Y., Ma X., Zhang P., Li S. The self-assembling camptothecin-tocopherol prodrug: an effective approach for formulating camptothecin. Biomaterials. 2015;62:176–187. doi: 10.1016/j.biomaterials.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J., Zhao W., Huang Y., Liu H., Marquez R., Gibbs R.B., Li J., Venkataramanan R., Xu L., Li S., Li S. Targeted delivery of Doxorubicin by folic acid-decorated dual functional nanocarrier. Mol. Pharm. 2014;11(11):4164–4178. doi: 10.1021/mp500389v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheetham A.G., Chakroun R.W., Ma W., Cui H., prodrugs Self-assembling. Chem. Soc. Rev. 2017;46(21):6638–6663. doi: 10.1039/c7cs00521k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doi T., Hamaguchi T., Shitara K., Iwasa S., Shimada Y., Harada M., Naito K., Hayashi N., Masada A., Ohtsu A. NC-6004 Phase I study in combination with gemcitabine for advanced solid tumors and population PK/PD analysis. Cancer Chemother. Pharmacol. 2017;79(3):569–578. doi: 10.1007/s00280-017-3254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senanayake T.H., Warren G., Wei X., Vinogradov S.V. Application of activated nucleoside analogs for the treatment of drug-resistant tumors by oral delivery of nanogel-drug conjugates. J. Controlled Release. 2013;167(2):200–209. doi: 10.1016/j.jconrel.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao L., Liu J., Dreaden E.C., Morton S.W., Shopsowitz K.E., Hammond P.T., Johnson J.A. A convergent synthetic platform for single-nanoparticle combination cancer therapy: ratiometric loading and controlled release of cisplatin, doxorubicin, and camptothecin. J. Am. Chem. Soc. 2014;136(16):5896–5899. doi: 10.1021/ja502011g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J., Liu W., Weitzhandler I., Bhattacharyya J., Li X., Wang J., Qi Y., Bhattacharjee S., Chilkoti A. Ring-opening polymerization of prodrugs: a versatile approach to prepare well-defined drug-loaded nanoparticles. Angew. Chem. Int. Ed. Engl. 2015;54(3):1002–1006. doi: 10.1002/anie.201409293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S., Zou J., Elsabahy M., Karwa A., Li A., Moore D.A., Dorshow R.B., Wooley K.L. Poly(ethylene oxide)-block-polyphosphester-based Paclitaxel Conjugates as a Platform for Ultra-high Paclitaxel-loaded Multifunctional Nanoparticles. Chem. Sci. 2013;4(5):2122–2126. doi: 10.1039/C3SC50252J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y., Pan D., Luo K., Li L., Gu Z. Biodegradable and amphiphilic block copolymer-doxorubicin conjugate as polymeric nanoscale drug delivery vehicle for breast cancer therapy. Biomaterials. 2013;34(33):8430–8443. doi: 10.1016/j.biomaterials.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 18.Mai Y., Eisenberg A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012;41(18):5969–5985. doi: 10.1039/c2cs35115c. [DOI] [PubMed] [Google Scholar]
- 19.McDaniel J.R., MacEwan S.R., Li X., Radford D.C., Landon C.D., Dewhirst M., Chilkoti A. Rational design of "heat seeking" drug loaded polypeptide nanoparticles that thermally target solid tumors. Nano Lett. 2014;14(5):2890–2895. doi: 10.1021/nl5009376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mastria E.M., Chen M., McDaniel J.R., Li X., Hyun J., Dewhirst M.W., Chilkoti A. Doxorubicin-conjugated polypeptide nanoparticles inhibit metastasis in two murine models of carcinoma. J. Controlled Release. 2015;208:52–58. doi: 10.1016/j.jconrel.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattacharyya J., Bellucci J.J., Weitzhandler I., McDaniel J.R., Spasojevic I., Li X., Lin C.C., Chi J.T., Chilkoti A. A paclitaxel-loaded recombinant polypeptide nanoparticle outperforms Abraxane in multiple murine cancer models. Nat. Commun. 2015;6:7939. doi: 10.1038/ncomms8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Couvreur P., Stella B., Reddy L.H., Hillaireau H., Dubernet C., Desmaele D., Lepetre-Mouelhi S., Rocco F., Dereuddre-Bosquet N., Clayette P., Rosilio V., Marsaud V., Renoir J.M., Cattel L. Squalenoyl nanomedicines as potential therapeutics. Nano Lett. 2006;6(11):2544–2548. doi: 10.1021/nl061942q. [DOI] [PubMed] [Google Scholar]
- 23.Yan Y., Huang J., Tang B.Z. Kinetic trapping - a strategy for directing the self-assembly of unique functional nanostructures. Chem. Commun. 2016;52(80):11870–11884. doi: 10.1039/c6cc03620a. [DOI] [PubMed] [Google Scholar]
- 24.Semiramoth N., Di Meo C., Zouhiri F., Said-Hassane F., Valetti S., Gorges R., Nicolas V., Poupaert J.H., Chollet-Martin S., Desmaele D., Gref R., Couvreur P. Self-assembled squalenoylated penicillin bioconjugates: an original approach for the treatment of intracellular infections. ACS Nano. 2012;6(5):3820–3831. doi: 10.1021/nn204928v. [DOI] [PubMed] [Google Scholar]
- 25.Maksimenko A., Dosio F., Mougin J., Ferrero A., Wack S., Reddy L.H., Weyn A.A., Lepeltier E., Bourgaux C., Stella B., Cattel L., Couvreur P. A unique squalenoylated and nonpegylated doxorubicin nanomedicine with systemic long-circulating properties and anticancer activity. Proc. Natl. Acad. Sci. U. S. A. 2014;111(2):E217–E226. doi: 10.1073/pnas.1313459110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen Y., Jin E., Zhang B., Murphy C.J., Sui M., Zhao J., Wang J., Tang J., Fan M., Van Kirk E., Murdoch W.J. Prodrugs forming high drug loading multifunctional nanocapsules for intracellular cancer drug delivery. J. Am. Chem. Soc. 2010;132(12):4259–4265. doi: 10.1021/ja909475m. [DOI] [PubMed] [Google Scholar]
- 27.Huang P., Wang D., Su Y., Huang W., Zhou Y., Cui D., Zhu X., Yan D. Combination of small molecule prodrug and nanodrug delivery: amphiphilic drug-drug conjugate for cancer therapy. J. Am. Chem. Soc. 2014;136(33):11748–11756. doi: 10.1021/ja505212y. [DOI] [PubMed] [Google Scholar]
- 28.Gao Y., Kuang Y., Guo Z.F., Guo Z., Krauss I.J., Xu B. Enzyme-instructed molecular self-assembly confers nanofibers and a supramolecular hydrogel of taxol derivative. J. Am. Chem. Soc. 2009;131(38):13576–13577. doi: 10.1021/ja904411z. [DOI] [PubMed] [Google Scholar]
- 29.Du X., Zhou J., Shi J., Xu B. Supramolecular Hydrogelators and Hydrogels: from Soft Matter to Molecular Biomaterials. Chem. Rev. 2015;115(24):13165–13307. doi: 10.1021/acs.chemrev.5b00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugahara K.N., Teesalu T., Karmali P.P., Kotamraju V.R., Agemy L., Greenwald D.R., Ruoslahti E. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science. 2010;328(5981):1031–1035. doi: 10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J., Kuang Y., Shi J., Zhou J., Medina J.E., Zhou R., Yuan D., Yang C., Wang H., Yang Z., Liu J., Dinulescu D.M., Xu B. Enzyme-instructed intracellular molecular self-assembly to boost activity of cisplatin against drug-resistant ovarian cancer cells. Angew. Chem. Int. Ed. Eng. 2015;54(45):13307–13311. doi: 10.1002/anie.201507157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma W., Cheetham A.G., Cui H. Building nanostructures with drugs. Nano Today. 2016;11(1):13–30. doi: 10.1016/j.nantod.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheetham A.G., Zhang P., Lin Y.A., Lock L.L., Cui H. Supramolecular nanostructures formed by anticancer drug assembly. J. Am. Chem. Soc. 2013;135(8):2907–2910. doi: 10.1021/ja3115983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su H., Koo J.M., Cui H. One-component nanomedicine. J. Controlled Release. 2015;219:383–395. doi: 10.1016/j.jconrel.2015.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lock L.L., Reyes C.D., Zhang P., Cui H. Tuning cellular uptake of molecular probes by rational design of their assembly into supramolecular nanoprobes. J. Am. Chem. Soc. 2016;138(10):3533–3540. doi: 10.1021/jacs.6b00073. [DOI] [PubMed] [Google Scholar]
- 36.Lin Y.A., Cheetham A.G., Zhang P., Ou Y.C., Li Y., Liu G., Hermida-Merino D., Hamley I.W., Cui H. Multiwalled nanotubes formed by catanionic mixtures of drug amphiphiles. ACS Nano. 2014;8(12):12690–12700. doi: 10.1021/nn505688b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan X., Li B.B., Lu X., Jia F., Santori C., Menon P., Li H., Zhang B., Zhao J.J., Zhang K. Light-triggered, self-immolative nucleic Acid-drug nanostructures. J. Am. Chem. Soc. 2015;137(19):6112–6115. doi: 10.1021/jacs.5b00795. [DOI] [PubMed] [Google Scholar]
- 38.Cui H., Cheetham A.G., Pashuck E.T., Stupp S.I. Amino acid sequence in constitutionally isomeric tetrapeptide amphiphiles dictates architecture of one-dimensional nanostructures. J. Am. Chem. Soc. 2014;136(35):12461–12468. doi: 10.1021/ja507051w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lock L.L., Li Y., Mao X., Chen H., Staedtke V., Bai R., Ma W., Lin R., Li Y., Liu G., Cui H. One-component supramolecular filament hydrogels as theranostic label-free magnetic resonance imaging agents. ACS Nano. 2017;11(1):797–805. doi: 10.1021/acsnano.6b07196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang P., Cheetham A.G., Lin Y.A., Cui H. Self-assembled Tat nanofibers as effective drug carrier and transporter. ACS Nano. 2013;7(7):5965–5977. doi: 10.1021/nn401667z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang P., Lock L.L., Cheetham A.G., Cui H. Enhanced cellular entry and efficacy of tat conjugates by rational design of the auxiliary segment. Mol. Pharmacol. 2014;11(3):964–973. doi: 10.1021/mp400619v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lang T., Liu Y., Zheng Z., Ran W., Zhai Y., Yin Q., Zhang P., Li Y. Cocktail strategy based on spatio-temporally controlled nano device improves therapy of breast cancer. Adv. Mater. 2019;31(5) doi: 10.1002/adma.201806202. [DOI] [PubMed] [Google Scholar]
- 43.Le Q.V., Suh J., Choi J.J., Park G.T., Lee J.W., Shim G., Oh Y.K. In situ nanoadjuvant-assembled tumor vaccine for preventing long-term recurrence. ACS Nano. 2019;13(7):7442–7462. doi: 10.1021/acsnano.9b02071. [DOI] [PubMed] [Google Scholar]
- 44.Duan X., Chan C., Han W., Guo N., Weichselbaum R.R., Lin W. Immunostimulatory nanomedicines synergize with checkpoint blockade immunotherapy to eradicate colorectal tumors. Nat Commun. 2019;10(1):1899. doi: 10.1038/s41467-019-09221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wen X., Zheng P., Ma Y., Ou Y., Huang W., Li S., Liu S., Zhang X., Wang Z., Zhang Q., Cheng W., Lin R., Li H., Cai Y., Hu C., Wu N., Wan L., Pan T., Rao J., Bei X., Wu W., Jin J., Yan J., Liu G. Salutaxel, a conjugate of docetaxel and a muramyl dipeptide (MDP) analogue, acts as multifunctional prodrug that inhibits tumor growth and metastasis. J. Med. Chem. 2018;61(4):1519–1540. doi: 10.1021/acs.jmedchem.7b01407. [DOI] [PubMed] [Google Scholar]
- 46.Zhang F., Zhu G., Jacobson O., Liu Y., Chen K., Yu G., Ni Q., Fan J., Yang Z., Xu F., Fu X., Wang Z., Ma Y., Niu G., Zhao X., Chen X. Transformative nanomedicine of an amphiphilic camptothecin prodrug for long circulation and high tumor uptake in cancer therapy. ACS Nano. 2017;11(9):8838–8848. doi: 10.1021/acsnano.7b03003. [DOI] [PubMed] [Google Scholar]
- 47.Zhang P., Huang Y., Liu H., Marquez R.T., Lu J., Zhao W., Zhang X., Gao X., Li J., Venkataramanan R., Xu L., Li S. A PEG-Fmoc conjugate as a nanocarrier for paclitaxel. Biomaterials. 2014;35(25):7146–7156. doi: 10.1016/j.biomaterials.2014.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001;41(1):189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 49.Maeda H., Wu J., Sawa T., Matsumura Y., Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Controlled Release. 2000;65(1):271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 50.Croy S.R., Kwon G.S. Polymeric micelles for drug delivery. Curr. Pharm. Des. 2006;12(36):4669–4684. doi: 10.2174/138161206779026245. [DOI] [PubMed] [Google Scholar]
- 51.Tang N., Du G., Wang N., Liu C., Hang H., Liang W. Improving penetration in tumors with nanoassemblies of phospholipids and doxorubicin. J. Natl. Cancer. Inst. 2007;99(13):1004–1015. doi: 10.1093/jnci/djm027. [DOI] [PubMed] [Google Scholar]
- 52.Li G., Liu J., Pang Y., Wang R., Mao L., Yan D., Zhu X., Sun J. Polymeric micelles with water-insoluble drug as hydrophobic moiety for drug delivery. Biomacromolecules. 2011;12(6):2016–2026. doi: 10.1021/bm200372s. [DOI] [PubMed] [Google Scholar]
- 53.Dietis N., Guerrini R., Calo G., Salvadori S., Rowbotham D.J., Lambert D.G. Simultaneous targeting of multiple opioid receptors: a strategy to improve side-effect profile. Br. J. Anaesth. 2009;103(1):38–49. doi: 10.1093/bja/aep129. [DOI] [PubMed] [Google Scholar]
- 54.Verschraegen C.F., Skubitz K., Daud A., Kudelka A.P., Rabinowitz I., Allievi C., Eisenfeld A., Singer J.W., Oldham F.B. A phase I and pharmacokinetic study of paclitaxel poliglumex and cisplatin in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2009;63(5):903–910. doi: 10.1007/s00280-008-0813-8. [DOI] [PubMed] [Google Scholar]
- 55.Langer C.J., O'Byrne K.J., Socinski M.A., Mikhailov S.M., Lesniewski-Kmak K., Smakal M., Ciuleanu T.E., Orlov S.V., Dediu M., Heigener D., Eisenfeld A.J., Sandalic L., Oldham F.B., Singer J.W., Ross H.J. Phase III trial comparing paclitaxel poliglumex (CT-2103, PPX) in combination with carboplatin versus standard paclitaxel and carboplatin in the treatment of PS 2 patients with chemotherapy-naive advanced non-small cell lung cancer. J. Thorac. Oncol. 2008;3(6):623–630. doi: 10.1097/JTO.0b013e3181753b4b. [DOI] [PubMed] [Google Scholar]
- 56.Gaucher G., Dufresne M.H., Sant V.P., Kang N., Maysinger D., Leroux J.C. Block copolymer micelles: preparation, characterization and application in drug delivery. J. Controlled Release. 2005;109(1–3):169–188. doi: 10.1016/j.jconrel.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 57.Zhang P., Xu J.N., Gao S.E., Li S. Dual-function nanocarriers with interfacial drug-interactive motifs for improved delivery of chemotherapeutic agents. Appl. Nanobiomater. 2016:367–394. [Google Scholar]
- 58.Huang Y., Lu J., Gao X., Li J., Zhao W., Sun M., Stolz D.B., Venkataramanan R., Rohan L.C., Li S. PEG-derivatized embelin as a dual functional carrier for the delivery of paclitaxel. Bioconjugate Chem. 2012;23(7):1443–1451. doi: 10.1021/bc3000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pan A., Zhang H., Li Y., Lin T.Y., Wang F., Lee J., Cheng M., Dall’Era M., Li T., deVere White R., Pan C.X., Lam K.S. Disulfide-crosslinked nanomicelles confer cancer-specific drug delivery and improve efficacy of paclitaxel in bladder cancer. Nanotechnol. 2016;27(42) doi: 10.1088/0957-4484/27/42/425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y., Huang Y., Zhao W., Lu J., Zhang P., Zhang X., Li J., Gao X., Venkataramanan R., Li S. Fmoc-conjugated PEG-vitamin E2 micelles for tumor-targeted delivery of paclitaxel: enhanced drug-carrier interaction and loading capacity. AAPS J. 2014;16(6):1282–1291. doi: 10.1208/s12248-014-9651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mi Y., Liu Y., Feng S.S. Formulation of docetaxel by folic acid-conjugated d-alpha-tocopheryl polyethylene glycol succinate 2000 (Vitamin E TPGS(2k)) micelles for targeted and synergistic chemotherapy. Biomaterials. 2011;32(16):4058–4066. doi: 10.1016/j.biomaterials.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 62.Sutton D., Nasongkla N., Blanco E., Gao J. Functionalized micellar systems for cancer targeted drug delivery. Pharm. Res. 2007;24(6):1029–1046. doi: 10.1007/s11095-006-9223-y. [DOI] [PubMed] [Google Scholar]
- 63.Gao Z., Lukyanov A.N., Singhal A., Torchilin V.P. Diacyllipid-polymer micelles as nanocarriers for poorly soluble anticancer drugs. Nano Lett. 2002;2(9):979–982. [Google Scholar]
- 64.Lovell J.F., Jin C.S., Huynh E., Jin H., Kim C., Rubinstein J.L., Chan W.C., Cao W., Wang L.V., Zheng G. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater. 2011;10(4):324–332. doi: 10.1038/nmat2986. [DOI] [PubMed] [Google Scholar]
- 65.Lu J., Liu X., Liao Y.P., Salazar F., Sun B., Jiang W., Chang C.H., Jiang J., Wang X., Wu A.M., Meng H., Nel A.E. Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat. Commun. 2017;8(1):1811. doi: 10.1038/s41467-017-01651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu J., Liu X., Liao Y.P., Wang X., Ahmed A., Jiang W., Ji Y., Meng H., Nel A.E. Breast cancer chemo-immunotherapy through liposomal delivery of an immunogenic cell death stimulus plus interference in the IDO-1 pathway. ACS Nano. 2018;12(11):11041–11061. doi: 10.1021/acsnano.8b05189. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Duhem N., Danhier F., Pourcelle V., Schumers J.M., Bertrand O., Leduff C.S., Hoeppener S., Schubert U.S., Gohy J.F., Marchand-Brynaert J., Preat V. Self-assembling doxorubicin-tocopherol succinate prodrug as a new drug delivery system: synthesis, characterization, and in vitro and in vivo anticancer activity. Bioconjugate Chem. 2014;25(1):72–81. doi: 10.1021/bc400326y. [DOI] [PubMed] [Google Scholar]
- 68.Liang X., Gao C., Cui L., Wang S., Wang J., Dai Z. Self-assembly of an amphiphilic janus camptothecin-floxuridine conjugate into liposome-like nanocapsules for more efficacious combination chemotherapy in cancer. Adv. Mater. 2017;29(40) doi: 10.1002/adma.201703135. [DOI] [PubMed] [Google Scholar]
- 69.Hu M., Huang P., Wang Y., Su Y., Zhou L., Zhu X., Yan D. Synergistic combination chemotherapy of camptothecin and floxuridine through self-assembly of amphiphilic drug-drug conjugate. Bioconjugate Chem. 2015;26(12):2497–2506. doi: 10.1021/acs.bioconjchem.5b00513. [DOI] [PubMed] [Google Scholar]
- 70.Yu G., Zhao X., Zhou J., Mao Z., Huang X., Wang Z., Hua B., Liu Y., Zhang F., He Z., Jacobson O., Gao C., Wang W., Yu C., Zhu X., Huang F., Chen X. Supramolecular polymer-based nanomedicine: high therapeutic performance and negligible long-term immunotoxicity. J. Am. Chem. Soc. 2018;140(25):8005–8019. doi: 10.1021/jacs.8b04400. [DOI] [PubMed] [Google Scholar]
- 71.Zhou Q., Shao S.Q., Wang J.Q., Xu C.H., Xiang J.J., Piao Y., Zhou Z.X., Yu Q.S., Tang J.B., Liu X.R., Gan Z.H., Mo R., Gu Z., Shen Y.Q. Enzyme-activatable polymer-drug conjugate augments tumour penetration and treatment efficacy. Nat. Nanotechnol. 2019;14(8):799–809. doi: 10.1038/s41565-019-0485-z. [DOI] [PubMed] [Google Scholar]
- 72.Schluep T., Hwang J., Cheng J., Heidel J.D., Bartlett D.W., Hollister B., Davis M.E. Preclinical efficacy of the camptothecin-polymer conjugate IT-101 in multiple cancer models. Clin. Cancer Res. 2006;12(5):1606–1614. doi: 10.1158/1078-0432.CCR-05-1566. [DOI] [PubMed] [Google Scholar]
- 73.Chitra M., Sukumar E., Suja V., Devi C.S. Antitumor, anti-inflammatory and analgesic property of embelin, a plant product. Chemotherapy. 1994;40(2):109–113. doi: 10.1159/000239181. [DOI] [PubMed] [Google Scholar]
- 74.Nikolovska-Coleska Z., Xu L., Hu Z., Tomita Y., Li P., Roller P.P., Wang R., Fang X., Guo R., Zhang M., Lippman M.E., Yang D., Wang S. Discovery of embelin as a cell-permeable, small-molecular weight inhibitor of XIAP through structure-based computational screening of a traditional herbal medicine three-dimensional structure database. J. Med. Chem. 2004;47(10):2430–2440. doi: 10.1021/jm030420+. [DOI] [PubMed] [Google Scholar]
- 75.Ahn K.S., Sethi G., Aggarwal B.B. Embelin, an inhibitor of X chromosome-linked inhibitor-of-apoptosis protein, blocks nuclear factor-kappaB (NF-kappaB) signaling pathway leading to suppression of NF-kappaB-regulated antiapoptotic and metastatic gene products. Mol. Pharmacol. 2007;71(1):209–219. doi: 10.1124/mol.106.028787. [DOI] [PubMed] [Google Scholar]
- 76.Tamm I., Kornblau S.M., Segall H., Krajewski S., Welsh K., Kitada S., Scudiero D.A., Tudor G., Qui Y.H., Monks A., Andreeff M., Reed J.C. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin. Cancer Res. 2000;6(5):1796–1803. [PubMed] [Google Scholar]
- 77.Holcik M., Gibson H., Korneluk R.G. XIAP: apoptotic brake and promising therapeutic target. Apoptosis. 2001;6(4):253–261. doi: 10.1023/a:1011379307472. [DOI] [PubMed] [Google Scholar]
- 78.van Dongen M.A., Silpe J.E., Dougherty C.A., Kanduluru A.K., Choi S.K., Orr B.G., Low P.S., Banaszak Holl M.M. Avidity mechanism of dendrimer-folic acid conjugates. Mol. Pharmacol. 2014;11(5):1696–1706. doi: 10.1021/mp5000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee R.J., Low P.S. Delivery of liposomes into cultured KB cells via folate receptor-mediated endocytosis. J. Biol. Chem. 1994;269(5):3198–3204. [PubMed] [Google Scholar]
- 80.Collnot E.M., Baldes C., Schaefer U.F., Edgar K.J., Wempe M.F., Lehr C.M. Vitamin E TPGS P-glycoprotein inhibition mechanism: influence on conformational flexibility, intracellular ATP levels, and role of time and site of access. Mol. Pharm. 2010;7(3):642–651. doi: 10.1021/mp900191s. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Z., Feng S.S. Nanoparticles of poly(lactide)/vitamin E TPGS copolymer for cancer chemotherapy: synthesis, formulation, characterization and in vitro drug release. Biomaterials. 2006;27(2):262–270. doi: 10.1016/j.biomaterials.2005.05.104. [DOI] [PubMed] [Google Scholar]
- 82.Varma M.V., Panchagnula R. Enhanced oral paclitaxel absorption with vitamin E-TPGS: effect on solubility and permeability in vitro, in situ and in vivo. Eur. J. Pharm. Sci. 2005;25(4–5):445–453. doi: 10.1016/j.ejps.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 83.Dintaman J.M., Silverman J.A. Inhibition of P-glycoprotein by d-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS) Pharm. Res. 1999;16(10):1550–1556. doi: 10.1023/a:1015000503629. [DOI] [PubMed] [Google Scholar]
- 84.Mu L., Feng S.S. Vitamin E TPGS used as emulsifier in the solvent evaporation/extraction technique for fabrication of polymeric nanospheres for controlled release of paclitaxel (TaxolⓇ) J. Controlled Release. 2002;80(1):129–144. doi: 10.1016/s0168-3659(02)00025-1. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Z., Tan S., Feng S.S. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials. 2012;33(19):4889–4906. doi: 10.1016/j.biomaterials.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 86.Muthu M.S., Kulkarni S.A., Xiong J., Feng S.S. Vitamin E TPGS coated liposomes enhanced cellular uptake and cytotoxicity of docetaxel in brain cancer cells. Int. J. Pharm. 2011;421(2):332–340. doi: 10.1016/j.ijpharm.2011.09.045. [DOI] [PubMed] [Google Scholar]
- 87.Liu Y., Huang L., Liu F. Paclitaxel nanocrystals for overcoming multidrug resistance in cancer. Mol. Pharmaceutics. 2010;7(3):863–869. doi: 10.1021/mp100012s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Constantinides P.P., Han J., Davis S.S. Advances in the use of tocols as drug delivery vehicles. Pharm. Res. 2006;23(2):243–255. doi: 10.1007/s11095-005-9262-9. [DOI] [PubMed] [Google Scholar]
- 89.Jayawarna V., Ali M., Jowitt T.A., Miller A.F., Saiani A., Gough J.E., Ulijn R.V. Nanostructured hydrogels for three-dimensional cell culture through self-assembly of fluorenylmethoxycarbonyl–dipeptides. Adv. Mater. 2006;18(5):611–614. [Google Scholar]
- 90.Zhang Y., Gu H., Yang Z., Xu B. Supramolecular hydrogels respond to ligand-receptor interaction. J. Am. Chem. Soc. 2003;125(45):13680–13681. doi: 10.1021/ja036817k. [DOI] [PubMed] [Google Scholar]
- 91.Mahler A., Reches M., Rechter M., Cohen S., Gazit E., Rigid Self-Assembled Hydrogel Composed of a Modified Aromatic Dipeptide. Adv. Mater. 2006;18(11):1365–1370. [Google Scholar]
- 92.Zhang P., Lu J., Huang Y., Zhao W., Zhang Y., Zhang X., Li J., Venkataramanan R., Gao X., Li S. Design and evaluation of a PEGylated lipopeptide equipped with drug-interactive motifs as an improved drug carrier. AAPS J. 2014;16(1):114–124. doi: 10.1208/s12248-013-9536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haklai R., Weisz M.G., Elad G., Paz A., Marciano D., Egozi Y., Ben-Baruch G., Kloog Y. Dislodgment and accelerated degradation of Ras. Biochemistry. 1998;37(5):1306–1314. doi: 10.1021/bi972032d. [DOI] [PubMed] [Google Scholar]
- 94.Marom M., Haklai R., Ben-Baruch G., Marciano D., Egozi Y., Kloog Y. Selective inhibition of Ras-dependent cell growth by farnesylthiosalisylic acid. J. Biol. Chem. 1995;270(38):22263–22270. doi: 10.1074/jbc.270.38.22263. [DOI] [PubMed] [Google Scholar]
- 95.Blum R., Kloog Y. Tailoring Ras-pathway–inhibitor combinations for cancer therapy. Drug Resist. Updates. 2005;8(6):369–380. doi: 10.1016/j.drup.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 96.Kloog Y., Cox A.D. RAS inhibitors: potential for cancer therapeutics. Mol. Med. Today. 2000;6(10):398–402. doi: 10.1016/s1357-4310(00)01789-5. [DOI] [PubMed] [Google Scholar]
- 97.Gana-Weisz M., Halaschek-Wiener J., Jansen B., Elad G., Haklai R., Kloog Y. The Ras inhibitor S-trans,trans-farnesylthiosalicylic acid chemosensitizes human tumor cells without causing resistance. Clin. Cancer Res. 2002;8(2):555–565. [PubMed] [Google Scholar]
- 98.Zhang X., Lu J., Huang Y., Zhao W., Chen Y., Li J., Gao X., Venkataramanan R., Sun M., Stolz D.B., Zhang L., Li S. PEG-farnesylthiosalicylate conjugate as a nanomicellar carrier for delivery of paclitaxel. Bioconjugate Chem. 2013;24(3):464–472. doi: 10.1021/bc300608h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Venditto V.J., Simanek E.E. Cancer therapies utilizing the camptothecins: a review of the in vivo literature. Mol. Pharm. 2010;7(2):307–349. doi: 10.1021/mp900243b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Caiolfa V.R., Zamai M., Fiorino A., Frigerio E., Pellizzoni C., d’Argy R., Ghiglieri A., Castelli M.G., Farao M., Pesenti E., Gigli M., Angelucci F., Suarato A., camptothecin Polymer-bound. initial biodistribution and antitumour activity studies. J. Controlled Release. 2000;65(1–2):105–119. doi: 10.1016/s0168-3659(99)00243-6. [DOI] [PubMed] [Google Scholar]
- 101.McRae Page S., Martorella M., Parelkar S., Kosif I., Emrick T. Disulfide cross-linked phosphorylcholine micelles for triggered release of camptothecin. Mol. Pharmaceutics. 2013;10(7):2684–2692. doi: 10.1021/mp400114n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gamcsik M.P., Kasibhatla M.S., Teeter S.D., Colvin O.M. Glutathione levels in human tumors. Biomarkers. 2012;17(8):671–691. doi: 10.3109/1354750X.2012.715672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Y., Liu D., Zheng Q., Zhao Q., Zhang H., Ma Y., Fallon J.K., Fu Q., Haynes M.T., Lin G., Zhang R., Wang D., Yang X., Zhao L., He Z., Liu F. Disulfide bond bridge insertion turns hydrophobic anticancer prodrugs into self-assembled nanomedicines. Nano Lett. 2014;14(10):5577–5583. doi: 10.1021/nl502044x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.He W., Hu X., Jiang W., Liu R., Zhang D., Zhang J., Li Z., Luan Y. Rational design of a new self-codelivery system from redox-sensitive camptothecin-cytarabine conjugate assembly for effectively synergistic anticancer therapy. Adv. Healthcare Mater. 2017;6(24) doi: 10.1002/adhm.201700829. [DOI] [PubMed] [Google Scholar]
- 105.Xu S., Zhu X., Huang W., Zhou Y., Yan D. Supramolecular cisplatin-vorinostat nanodrug for overcoming drug resistance in cancer synergistic therapy. J. Controlled Release. 2017;266:36–46. doi: 10.1016/j.jconrel.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 106.Fan M., Liang X., Li Z., Wang H., Yang D., Shi B. Chlorambucil gemcitabine conjugate nanomedicine for cancer therapy. Eur. J. Pharm. Sci. 2015;79:20–26. doi: 10.1016/j.ejps.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 107.Li Y., Lin J.J., Ma J.Y., Song L., Lin H.R., Tang B.W., Chen D.Y., Su G.H., Ye S.F., Zhu X., Luo F.H., Hou Z.Q. Methotrexate-camptothecin prodrug nanoassemblies as a versatile nanoplatform for biomodal imaging-guided self-active targeted and synergistic chemotherapy. ACS Appl. Mater. Interfaces. 2017;9(40):34650–34665. doi: 10.1021/acsami.7b10027. [DOI] [PubMed] [Google Scholar]
- 108.Li J.G., Liu P. Self-assembly of drug-drug conjugates as drug self-delivery system for tumor-specific pH-triggered release. Part. Part. Syst. Charact. 2019;36(7) [Google Scholar]
- 109.Song Q., Wang X., Wang Y., Liang Y., Zhou Y., Song X., He B., Zhang H., Dai W., Wang X., Zhang Q. Reduction responsive self-assembled nanoparticles based on disulfide-linked drug-drug conjugate with high drug loading and antitumor efficacy. Mol. Pharm. 2016;13(1):190–201. doi: 10.1021/acs.molpharmaceut.5b00631. [DOI] [PubMed] [Google Scholar]
- 110.Yang X., Grailer J.J., Rowland I.J., Javadi A., Hurley S.A., Matson V.Z., Steeber D.A., Gong S. Multifunctional stable and pH-responsive polymer vesicles formed by heterofunctional triblock copolymer for targeted anticancer drug delivery and ultrasensitive MR imaging. ACS Nano. 2010;4(11):6805–6817. doi: 10.1021/nn101670k. [DOI] [PubMed] [Google Scholar]