Abstract
The pathogenesis of viral infections involves an immune response by cytokines, causing a deleterious effect on organ function, in addition to tissue destruction due to viral replication. Clinical symptoms and laboratory findings of the human coronavirus disease COVID-19, caused by the novel coronavirus SARS CoV-2, indicate cytokine involvement. Our laboratory showed that an experimental murine coronavirus (MHV-A59) can be transmitted into the brain by intranasal or intracerebral exposure and that neurovirulence is mediated by cytokine secretion. In this study we investigated which cells in the brain produce cytokines, thus functioning as the brain's innate immune system. Using tissue cultures of microglia, and clonal populations of astrocytes, we found that microglia and type I astrocytes (but not types II and III), produced pro-inflammatory cytokines in response to MHV-A59 infection. A molecularly closely related, non-encephalitic strain of the virus (MHV-2) caused in vitro infection, but without cytokine induction. Furthermore, immunofluorescence and immunohistochemistry revealed that type I astrocytes and microglia have perivascular foot processes necessary for the formation of the perivascular glymphatic system, the anatomical site of the brain's innate immune system. Cytokine secretion by type I astrocytes and microglia, as part of the brain's glymphatic and innate immune system, contributes to the pathogenesis of an encephalitic coronavirus infection, and indicates the rationale for anti-cytokine therapies for COVID-19.
Keywords: Coronavirus, Mouse hepatitis virus, Cytokines, Astrocytes, Microglia, COVID-19
Highlights
-
•
Cytokine induction mediates the neurologic pathogenesis of coronavirus infection.
-
•
Type I astrocytes and microglia send foot-processes around blood vessels in the brain, forming the glymphatic system.
-
•
The glymphatic system is the site of the brain’s innate immune system.
-
•
The brain’s innate immune system functions during coronavirus infection by the induction of pro-inflammatory cytokines.
-
•
This experimental coronavirus model system sheds light on the neurologic manifestations of the human disease COVID-19.
1. Introduction
Unlike other organs, the central nervous system (CNS) does not have a traditional lymphatic system or native lymphocytes. Thus, a viral invasion of the CNS requires signaling from the CNS's innate immune system in order to recruit inflammatory cells from the circulation. This signaling typically involves the secretion of pro-inflammatory cytokines into the circulation. The identity of these CNS immune cells that are capable of signaling the immune system is not entirely clear. Microglial cells are CNS immune cells (Nelson et al., 2002), but astrocytes may also have immune functions in the CNS (Farina et al., 2007). Astrocytes, the most abundant cells in the CNS, are heterogeneous and multifunctional, and were recently discovered to be part of the glial lymphatic system (glymphatic) of the CNS (Jessen et al., 2015; Negi and Das, 2018). Astrocytes are divided into subgroups based on morphology and function (Seidman et al., 1997; ffrench-Constant and Raff, 1986), but which subtype of astrocytes forms the glymphatic system and induces CNS innate immune functions is unknown.
A murine tissue culture model system of a CNS infection, that invokes an immune reaction, was used to identify the CNS glymphatic and innate immune cells and to test their ability to induce pro-inflammatory signaling. Because of their potential immune functions, microglia, and clonal populations of three subtypes of astrocytes (type I, II and III), were infected with two murine coronaviruses. Mouse hepatitis virus strain A59 (MHV- A59), is a neurotropic virus that, with intracerebral or intranasal injection, causes encephalitis followed by an inflammatory demyelinating disease in mice similar to the human demyelinating disease multiple sclerosis (Lavi et al., 1984a; Lavi et al., 1984b; Lavi et al., 1988; Lavi et al., 1999). For comparison, similar cells were infected with another closely related strain of coronavirus (MHV-2) that lacks the ability to cause encephalitis or inflammatory demyelination in mouse brains, despite infection of the same cells in vitro (Das Sarma et al., 2001b).
In order to correlate functional activities of astrocytes and microglia with their anatomical structure in the brain, we used immunohistochemically stained random sections of human brain to illustrate a proposed structure of the glymphatic innate immune system of the CNS.
2. Materials and methods
2.1. Viruses and viral growth curves
Plaque-purified MHV-A59 and MHV-2 were propagated and titrated on murine L2 cells in DMEM with 10% FBS. Stock viruses had titers of 107–108 pfu/ml. For viral growth curves L2 cell cultures were prepared in 12 well plates in DMEM with 10% FBS. Confluent monolayers were infected with each virus (M.O.I. = 1) in duplicate wells and incubated for 1 h at 37 °C. After absorption, the cells were washed three times with PBS and then fed with 1.5 ml medium. At the indicated times (0,5,10,15,20,25 h P.I.), the cells were lysed by three cycles of freeze-thawing, and the supernatants were removed and viral titer was determined by plaque assay on L2 cells as previously described (Lavi et al., 1984b).
2.2. Cell lines and virus infections
Clonal permanent cell lines were derived from the American Type Culture Collection (ATCC, Manassas, VA 20108 USA) based on work previously described (Alliot and Pessac, 1984). The following cells were used: type I Astrocytes (ATCC CRL 2541), type II astrocytes (ATCC CRL-2535), type III astrocytes (ATCC CRL-2534), and microglia cells (ATCC: CRL 2540). Cells were propagated in culture in Dulbecco's modified Eagle's medium supplemented with fetal calf serum (FCS; 10% v/v), 4 mM l-glutamine, 100 U penicillin G ml−1 and 100 mg streptomycin ml−1. The cells were plated on 100 mm plates and cultured at 37 °C with 5% CO2. When the cells reached approximately 80% confluence, they were infected by MHV-A59 or MHV-2 (M.O.I. = 2) for 24 h. For each viral infection, mock-infected cells with virus-free medium were included as controls.
2.3. Immunofluorescence of cell cultures
Immunofluorescence procedures were performed as previously described (Suzumura et al., 1986). Astrocyte cultures were placed on coverslips, pretreated with 0.01% poly-l-lysine solution (Sigma), in 12-well plates, at a concentration of 100,000 cells per well. The cell cultures were infected with MHVs (M.O.I. = 2), or sham-infected (virus free medium) and incubated for 24 h. Cells were rinsed with PBS and fixed in 4% paraformaldehyde in PBS at 4 °C for 1 h. After rinsing 3 times in PBS for 5 min intervals, cells were permeabilized for 10 min with 1% TritonX-100 in PBS and washed 3 times with PBS. Cells were then incubated for 10 min with a blocking solution (10% BSA in PBS) and washed 3 times in PBS. Cells were incubated with the primary antibody (anti-GFAP polyclonal antibody from Novus Bio Inc., at 1: 200 dilution) for 1 h at 37 °C or 4 °C over night and then washed with PBS. Cells were incubated with the secondary antibody (goat anti-rabbit IgG-555 from Molecular Probes, at 1: 500 dilution) for 1 h at room temperature and washed with PBS 3 times for 5 min intervals. The immunofluorescence experiment was performed with one antibody (GFAP) bound to two chromogens (fluorescein for green and rhodamine for red). The results were best with green for type II and III astrocytes and red for type I. The coverslips were inverted onto slides containing 10 μl of mounting media (Vector laboratories). Slides were viewed with an Olympus BX60 fluorescence microscope.
2.4. Cytokine mRNA detection
Total RNA was isolated from cultured cells using StrataPrep Total RNA Miniprep kit (Stratagene, La Jolla, CA). The cytokine mRNA expression levels of the MHVs infected cell cultures were detected using mouse common cytokine GEArray kit (Super Array Inc. Bethesda, MD). Each array consists of 96 genes including interferons and interleukins and tumor necrosis factor as well as control sequences (PUC18 as negative control; beta-actin and glyceraldehydes 3 phosphate dehydrogenase (GAPDH) for loading). Bound biotinylated cDNA probe was detected with alkaline phosphatase-conjugated streptavidin and CDP-Star chemiluminescent substrate (SuperArray). Images of the membranes were recorded on x-ray film and were digitally recorded on a personal densitometer scanner (Molecular Dynamics, Sunnyvale, CA). Data were further processed with GEArray Analyzer software, correcting for background noise by subtraction of the minimum value and normalizing to the maximum value of each individual array. Genes were considered present if the expression level was greater than two times that of the blank negative control. The signal from expression of each cytokine gene was normalized to the signal derived from beta-actin on the same membrane and expressed as cytokine mRNA arbitrary units. Each group was repeated 3 times with different RNA from separate infections.
2.5. Immunohistochemistry
For anatomical correlation, randomly selected sections of human brain were analyzed by immunohistochemistry with antibodies against GFAP and CD68 (Vendor: Dako, Agilent technologies Company, Santa Clara, CA). The GFAP product is a 6F2 clone used at 1:500 dilution and the CD68 product is a KP1 clone used in 1:4000 dilution. Sections were incubated with antibodies and a brown chromogen, and counterstained with hematoxylin by conventional immunohistochemical automated procedures.
The human brain sections were derived from cortical areas of non-diseased adult human brains that were stained for trainee teaching material of the anatomy and morphology of brain cells. The sections presented here are representative sections of multiple sections that showed similar finding in both genders and different age groups. The sections are anonymous and cannot be traced to specific individuals. Sections were derived from brains obtained at autopsy and comported with ethical protocols of the hospital.
3. Results
3.1. Cell cultures
The clonal astrocytic cell cultures, analyzed by immunofluorescence with GFAP antibodies showed the following features: type I astrocytes were cells with both thick and thin processes, moderate amount of cytoplasm and occasional foot processes; type II astrocytes were cells with small amount of cytoplasm, only thin and long processes and without foot processes; type III astrocytes were flat with abundant cytoplasm and no processes (Fig. 1 ). The morphology of the cells was consistent with the previously described morphology of the original cell cultures (Alliot and Pessac, 1984).
Fig. 1.
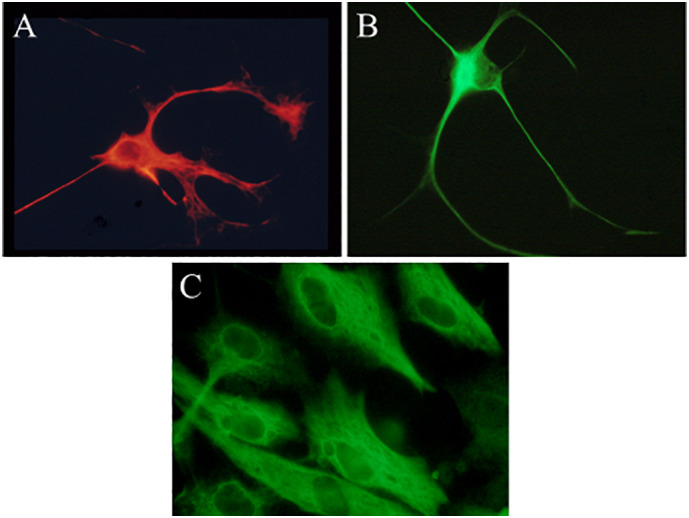
Murine astrocyte types in tissue cultures. Type I (A), type II (B) and type III astrocytes (C). Cell cultures were analyzed by immunofluorescence, stained with GFAP specific antibodies with red chromogen for type I and green chromogen for type II and III. Original magnification x400. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Viral infections
Infection of type I and type II astrocytes with MHV-A59 and MHV-2 was productive and reached titers of 4–5 Log10PFU/ml. Infection with both viruses of type III astrocytes was negative. Microglial cells were infected with both MHV-A59 and MHV-2 and reached a level of 3–4 Log10PFU/ml.
3.3. Cytokine induction
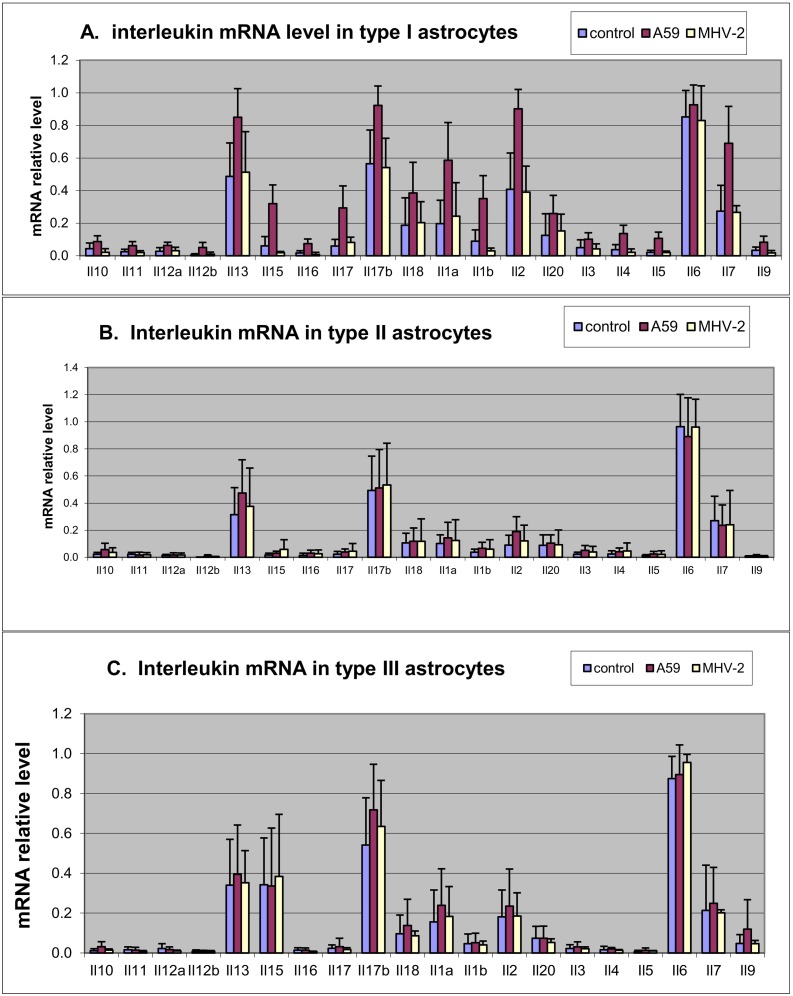
Following infection with MHV-A59, upregulation of proinflammatory cytokines was seen in type I astrocytes (Fig. 2A) but not in type II (Fig. 2B) or type III astrocytes (Fig. 2C). There was no upregulation of cytokine induction in MHV-2 infection compared to sham-infected cultures in all three astrocytic cultures. The significant upregulated interleukin cytokines in MHV-A59 infection of type I astrocytes were IL1alpha and beta, IL7, IL13, IL15, IL16, IL17, IL17beta, IL18. There was no significant upregulation of IL3, IL4, IL5, IL6, IL9, IL10, IL11, IL12 and IL20.
Fig. 2.
Interleukin (Il) levels in the three types of astrocytes following infections with MHV-A59 (red)), MHV-2 (yellow) and control (purple). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
All interferons (alpha, beta and gamma), and some of the molecules in the TNF family were upregulated in response to MHV-A59 infection but not following MHV-2 infection in type I astrocytes. There was no upregulation of interferons and TNF family molecules in type II and III astrocytes following infections with MHV-A59 or MHV-2 (data not shown).
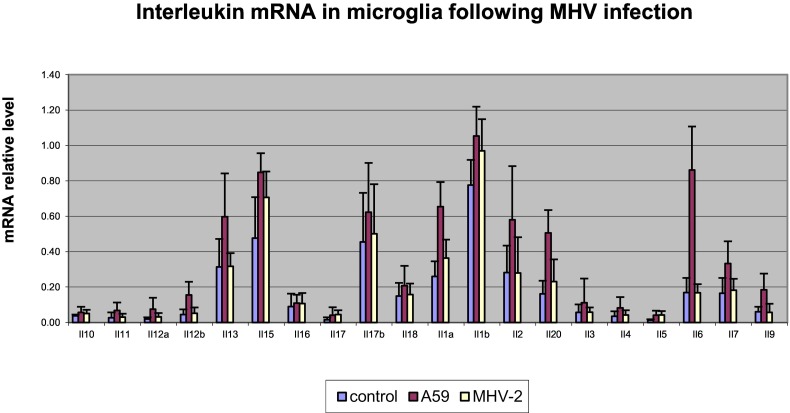
Following MHV-A59 infection of microglia the main interleukin upregulated was IL6, while all interferons and members of the TNF molecules cytokines were upregulated compared to sham infection. None of the cytokines were upregulated following MHV-2 infection of microglia (Fig. 3 ).
Fig. 3.
Interleukin (Il) levels in microglia following infections with MHV-A59 (red), MHV-2 (yellow) and control (purple). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Immunohistochemistry on human brain sections
The results in murine tissue culture cells suggested that two types of cells: microglia and a subtype of astrocytes, participate in immune functions. This indicates that both murine and human brains have an anatomical structure designed to provide these immune functions. The ideal site for these functions is a structure composed by cell types that have direct relationship with endothelial cells, thus, forming the perivascular glymphatic system. We therefore investigated whether, in human brain, perivascular astrocytes have the morphology of type I astrocytes and whether microglial cells also send processes to a perivascular location and participate in the brain glymphatic system. We selected random images of human brain sections stained with antibodies that identify glial and microglial cells (GFAP and CD68 respectively). These sections, representing the same pattern of anatomical findings in any human brain, were used to present anatomical structures that exist in all human brains. We identified small caliber and capillary blood vessels completely surrounded by GFAP astrocytic foot processes. Also, intermittent processes of CD68 positive microglial cells were identified in the perivascular space. Cells of the perivascular distribution expressed morphological features of type I astrocytes, but not type II or III (Fig. 4 ).
Fig. 4.
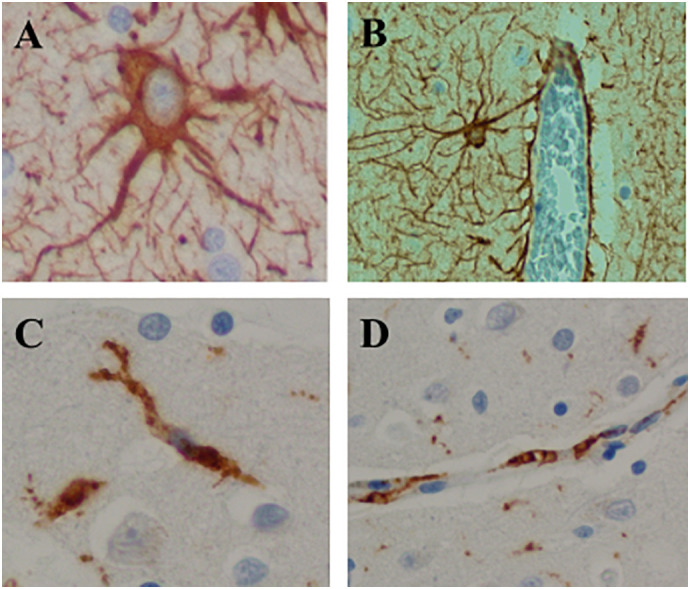
Human brain sections depicting components of the CNS innate immune system by immunohistochemistry, GFAP antibody (A, B) and CD68 antibody (C, D), brown chromogen and hematoxylin counterstain. A. Type 1 astrocyte. x400. B. Astrocytes surrounding a blood vessel illustrating the relationship between type I astrocytic foot processes and brain blood vessels. x100. C. Microglial cells with bipolar cytoplasmic processes. x400. D. Microglial foot processes in close proximity to the endothelial cells of a brain blood vessel. x200. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
This study investigated immune mediated aspects of the pathogenesis of an experimental model of coronaviruses. The coronavirus genus of viruses belongs to the coronaviridae family of the nidovirales order that produce a variety of hepatic, enteric, and neurologic diseases in animals, and upper respiratory and enteric diseases in humans (Cavanagh, 1997; Lai and Cavanagh, 1997; Lavi et al., 1999; Lavi and Weiss, 1989). In 2003 a virulent coronavirus infection caused an epidemic of severe acute respiratory syndrome (SARS), followed by middle eastern respiratory syndrome (MERS) (Peiris et al., 2003). In 2019, a new human coronavirus emerged, named SARS CoV-2, causing the pandemic of COVID-19. Both the original SARS virus and SARS CoV-2 induce cytokine release mainly IL-6, contributing to a “cytokine storm,” that exacerbates viral pathogenesis, resulting in a high mortality rate (Cheung et al., 2005; Channappanavar and Perlman, 2017; Mehta et al., 2020). The understanding of the pathogenesis of human coronaviruses is greatly enhanced by studies of experimental animal models of coronaviruses.
MHV-A59 is a member of the coronavirus genus that with intracerebral or intranasal exposure in mice produces a bi-phasic disease with acute hepatitis, meningitis, and encephalitis followed by chronic inflammatory demyelinating disease. While the acute phase of the disease is associated with direct viral replication and antigen expression in the brain and liver, the chronic, immune-mediated, demyelinating disease is associated with rare RNA persistence in the brain, without evidence of direct viral replication or antigen expression. Demyelination was found to be molecularly controlled by a region in the S1 portion of the spike glycoprotein gene of MHV-A59, downstream from the receptor binding domain, based on mutational analysis and the finding that a single point mutation (Asparagine to Serine in position 345 of S gene) abolished demyelination completely (Das Sarma et al., 2000; Das Sarma et al., 2001a; Fu et al., 2004; Fu and Lavi, 2005). MHV-2 in comparison, is a hepatotropic virus causing hepatitis, and mild meningitis without encephalitis, even when inoculated intracerebrally. Neurovirulence and the ability of MHV to cause encephalitis is directly related to its ability to induce pro-inflammatory cytokines, both in vivo and in vitro (Li et al., 2004). The present investigation determined that infection with the encephalitic strain MHV-A59 produced a pro-inflammatory cytokine reaction of the innate immune system mediated by microglia and type I astrocytes. This is in contrast to the non-encephalitic strain (MHV-2) which did not produce a cytokine response. It is therefore concluded that a cytokine response determines the ability of the virus to cause encephalitis.
Upregulated cytokines in type I astrocytes following MHV-A59 infection were IL1 alpha and beta, IL2, IL15, IL13, IL17, all three interferons and TNF. All of these cytokines are known to be pro-inflammatory and some have known innate immune functions, including maintenance of the blood brain barrier integrity. In general, the cytokine response in type I astrocytes was consistent with a TH1and TH17 response (role in pro-inflammation and autoimmunity). The cytokine response profile in microglia was primarily involving IL-6, interferons and TNF. IL-6 in its initial response is also pro-inflammatory; although, it can also serve at later stages of infection as inducer of IL-10 and anti-inflammatory reaction. Thus, the profiles of the cytokine response in both cell types were complementary and aimed at inducing a pro-inflammatory reaction that would enable a cellular inflammatory reaction to enter the CNS and be directed against the invading virus. The lack of induction of anti-inflammatory cytokines, such as IL-4 and IL-10, due to coronavirus infection may explain the enhanced inflammatory response that occurs during a “cytokine storm”.
The finding of a cytokine response originating in type I astrocytes and microglia suggests that these two cell types form an anatomic structure responsible for the innate immune response in the CNS. It is well known that astrocytic foot processes surround brain capillaries and during development induce endothelial cells to form tight junctions which are the basis for the blood brain barrier. The immune reaction in the brain originates in a perivascular structure, known as the glymphatic system, because of its similarity to the systemic lymphatic system. The morphology of the cultured type I astrocytes appears to be identical to the morphology of human perivascular astrocytes that send their foot processes to the blood vessels. The morphology of these perivascular astrocytes is clearly different from the cultured type II or type III. In addition, we found immunohistochemical evidence in human tissue that microglia also send processes that are in close proximity to endothelial cells, likely participating with astrocytes type I in the perivascular structure. The combination of the morphological study with the function of cytokine production suggests that type I astrocytes and microglia form the perivascular glymphatic system that is involved in an innate immune reaction by the secretion of cytokines following a neurotropic encephalitic viral infection. A graphic illustration of the perivascular role of type I astrocytes and microglia in the blood brain barrier and glymphatic system is shown in Fig. 5 .
Fig. 5.
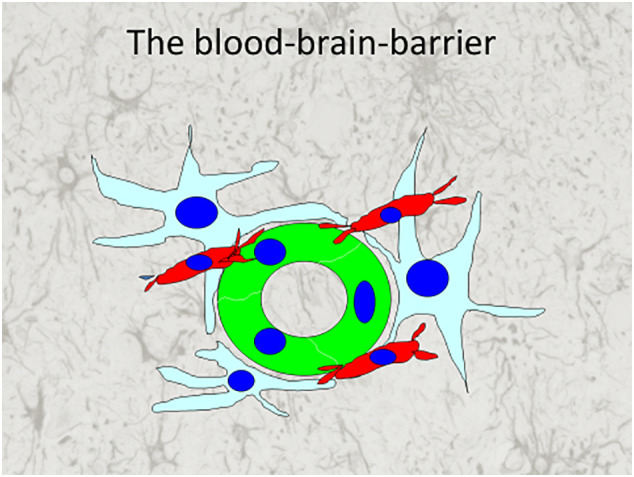
An illustration of the cellular components of the blood brain barrier. In green are endothelial cells of a blood vessel surrounded by the foot processes of type I astrocytes (light blue). In red are microglia cells also extending foot processes to the endothelial cells. The glymphatic system is the virtual space between endothelial cells and foot processes of astrocytes and microglia. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The experimental murine coronavirus MHV-A59 and the pandemic human coronavirus SARS CoV-2 have multiple similarities. Infection with both murine and human coronaviruses induce a proinflammatory cytokine reaction, most notably involving IL-6. Inhalation of MHV-A59 causes limbic encephalitis (Lavi et al., 1988) and anosmia (loss of smell and taste) is common in COVID-19 suggesting involvement of the olfactory nerves with both viral infections. Central nervous system infection is seen with both murine and human coronaviruses. Acute and subacute neurologic manifestations of COVID-19 include change in mental status, amnesia, delirium, encephalitis, stroke and a GBS like illness (Asadi-Pooya and Simani, 2020; Toscano et al., 2020). Cerebrovascular disease occurs in about 5% of patients with COVID-19 including in those younger than 50 years old. Strokes involve both large and small vessels, in arterial and venous circulations, in multiple vascular territories. The pathophysiology of the varied cerebrovascular symptomatology is presumed to be due to cytokine upregulation, coagulopathy and endothelial cell dysfunction (Oxley et al., 2020; Bevrouti et al., 2020). The RNA viral persistence in patients with COVID-19 (Wang et al., 2020), was similar to rare RNA persistence described in MHV-A59 infection (Lavi et al., 1984a). Due to differences in organ affinity, COVID-19 had RNA persistence in the gastrointestinal tract while MHV-A59 persistence was in the brain. Infection with the coronavirus MHV-A59 is a murine model for the CNS demyelinating disease, multiple sclerosis. Infection with the coronavirus SARS CoV-2 is recently recognized and its long-term neurological consequences, if any, are as yet unknown. Long term monitoring of patients who survived COVID-19 is needed to determine if there are any remote neurological effects of the SARS CoV-2 infection.
5. Conclusion
Infection of type I astrocytes and microglia with the murine coronavirus, MHV-A59 results in a proinflammatory cytokine mediated encephalitis. Type I astrocytes and microglia form the perivascular glymphatic system, the site of the CNS innate immune system. The pathogenesis of this murine coronavirus infection mirrors that of COVID-19. Similarities between murine infection with MHV-A59 and human infection with SARS CoV-2 include: their affinity for the olfactory system; their spread into the brain causing neurologic symptoms; their high virulence; their pathogenic dependence on cytokine induction; and the persistence of their viral RNA in infected organs.
Author statement
The authors participated in the project design, creation of data, interpretation of the results, and in the writing and reviewing the manuscript. Funding for this project was not provided by external sources. Funding was provided by the Department of Pathology & Laboratory Medicine at Weill Cornell Medicine.
Declaration of Competing Interest
The authors declare that there is no conflict of interest in the publication of this work.
Acknowledgements
The authors participated in the project design, creation of data, interpretation of the results, and in the writing and reviewing the manuscript. Funding for this project was not provided by external sources.
References
- Alliot F., Pessac B. Astrocytic cell clones dervived from established cultures of 8-day postnatal mouse cerebella. Brain Res. 1984;306:283–291. doi: 10.1016/0006-8993(84)90377-9. [DOI] [PubMed] [Google Scholar]
- Asadi-Pooya A.A., Simani L. Central nervous system manifestations of COVID-19: A systematic review. J. Neurol. Sci. 2020:413. doi: 10.1016/j.jns.2020.116832. April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevrouti R., Adams M.E., Benjamin L. Characteristics of ischemic stroke associated with COVID-19. J. Neurol. Neurosurg. Psychychiatry. 2020 doi: 10.1136/jnnp-2020-323586. April 30, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 1997;142:629–633. [PubMed] [Google Scholar]
- Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semmin. Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C.Y., Poon L.L., Ng I.H. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 2005;79(12):7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Sarma J., Fu L., Tsai J.C., Weiss S.R., Lavi E. Demyelination determinants map to the spike glycoprotein gene of coronavirus mouse hepatitis virus. J. Virol. 2000;74:9206–9213. doi: 10.1128/jvi.74.19.9206-9213.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Sarma J., Fu L., Hingley S.T., Lai M.M., Lavi E. Sequence analysis of the S gene of recombinant MHV-2/A59 coronaviruses reveals three candidate mutations associated with demyelination and hepatitis. J. Neurovirol. 2001;7:432–436. doi: 10.1080/135502801753170282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Sarma J., Fu L., Hingley S.T., Lavi E. Mouse hepatitis virus type-2 infection in mice: an experimental model system of acute meningitis and hepatitis. Exp. Mol. Pathol. 2001;71:1–12. doi: 10.1006/exmp.2001.2378. [DOI] [PubMed] [Google Scholar]
- Farina C., Aloisi F., Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:3. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- ffrench-Costant, C, Raff M.C. The oligodendrocyte-type-2 astrocyte cell lineage is specialized for myelination. Nature. 1986;323:335–338. doi: 10.1038/323335a0. [DOI] [PubMed] [Google Scholar]
- Fu L., Lavi E. Molecular determinants in coronavirus- MHV-induced demyelination. In: Lavi E., Constantinescu C.S., editors. Experimental Models of Multiple Sclerosis. Springer Academic Publishers; New York: 2005. pp. 849–858. [Google Scholar]
- Fu L., Gonzales D.M., Sarma J.D., Lavi E. A combination of mutations in the S1 part of the spike glycoprotein gene of coronavirus MHV-A59 abolishes demyelination. J. Neurovirol. 2004;10:41–51. doi: 10.1080/13550280490262229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen N.A., Munk A.S., Nedergaard M. The glymphatic system: a beginner's guide. Neurochem. Res. 2015;40:2583–2599. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.M.C., Cavanagh D. The molecular biology of coronaviruses. Adv. Virus Res. 1997;48:1–100. doi: 10.1016/S0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi E., Weiss S.R. Coronaviruses. In: Gilden D.H., Lipton H.L., editors. Clinical and Molecular Aspects of Neurotropic Viral Infections. Kluwer, Academic Publishers; Boston: 1989. pp. 101–139. (Developments in Medical Virology). [Google Scholar]
- Lavi E., Gilden D.H., Highkin M.K., Weiss S.R. Persistence of MHV-A59 RNA in a slow virus demyelinating infection in mice as detected by in situ hybridization. J. Virol. 1984;51:563–566. doi: 10.1128/jvi.51.2.563-566.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi E., Gilden D.H., Wroblewska Z., Rorke L.B., Weiss S.R. Experimental demyelination produced by the A59 strain of mouse hepatitis virus. Neurology. 1984;34:597–603. doi: 10.1212/wnl.34.5.597. [DOI] [PubMed] [Google Scholar]
- Lavi E., Fishman S.P., Highkin M.K., Weiss S.R. Limbic encephalitis following inhalation of murine coronavirus MHV-A59. Lab. Investig. 1988;58:31–36. [PubMed] [Google Scholar]
- Lavi E., Schwartz T., Jin Y.P., Fu L. Nidovirus infections: experimental model systems of human neurologic diseases. J. Neuropathol. Exp. Neurol. 1999;58:1197–1206. doi: 10.1097/00005072-199912000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Fu L., Gonzales D.M., Lavi E. Coronavirus neurovirulence correlates with the ability of the virus to induce proinflammatory cytokine signals from astrocytes and microglia. J. Virol. 2004;78:3398–3406. doi: 10.1128/JVI.78.7.3398-3406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. March 16, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi N., Das B.K. CNS: not an immunoprivilaged site anymore but a virtual secondary lymphoid organ. Int. Rev. Immunol. 2018;37(1):57–68. doi: 10.1080/08830185.2017.1357719. [DOI] [PubMed] [Google Scholar]
- Nelson P.T., Soma L.A., Lavi E. Microglia in diseases of the central nervous system. Ann. Med. 2002;8:491–500. doi: 10.1080/078538902321117698. [DOI] [PubMed] [Google Scholar]
- Oxley T.J., Mocco J., Majidi S. Large-vessel stroke as a presenting feature of COVID-19 in the young. N. Engl. J. Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. April 28, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S.M., Lai S.T., Poon L.L.M., Guan Y., Yam L.Y.C., Nicholls J., Yee W.K.S., Yan W.W., Cheung M.T., Cheng V.C.C., Chan K.H., Tsang D.N.C., Yung R.W.H., Ng T.K., Yuen K.Y., group motSs Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;1361:1–7. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman K.J.N., Teng A.L., Rosenkopf R., Spilotro P., Weyhenmyer J.A. Isolation, cloning and characterization of a putative type-1 astrocyte cell line. Brain Res. 1997;753:18–26. doi: 10.1016/s0006-8993(96)01481-3. [DOI] [PubMed] [Google Scholar]
- Suzumura A., Lavi E., Weiss S.R., Silberberg D.H. Coronavirus infection induces H-2 antigen expression on oligodendrocytes and astrocytes. Science. 1986;232(4753):991–993. doi: 10.1126/science.3010460. May 23. [DOI] [PubMed] [Google Scholar]
- Toscano G. Guillain-Barre syndrome associated with SARS-CoV-2. NEJM. 2020;2020 doi: 10.1056/NEJMc2009191. April 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhou Y., Jiang N., Zhou Q., Ma W.L. Persistence of intestinal SARS-CoV-2 infection in patients with COVID-19 leads to re-admission after pneumonia resolved. Int. J. Infect. Dis. 2020;2020 doi: 10.1016/j.ijid.2020.04.063. April 27. [DOI] [PMC free article] [PubMed] [Google Scholar]