Abstract
Several studies have demonstrated the advantages of environmental surveillance through the monitoring of sewage for the assessment of viruses circulating in a given community (wastewater-based epidemiology, WBE).
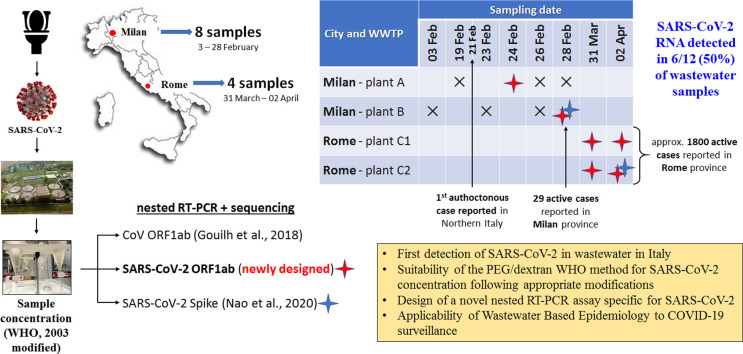
During the COVID-19 public health emergency, many reports have described the presence of SARS-CoV-2 RNA in stools from COVID-19 patients, and a few studies reported the occurrence of SARS-CoV-2 in wastewaters worldwide. Italy is among the world's worst-affected countries in the COVID-19 pandemic, but so far there are no studies assessing the presence of SARS-CoV-2 in Italian wastewaters. To this aim, twelve influent sewage samples, collected between February and April 2020 from Wastewater Treatment Plants in Milan and Rome, were tested adapting, for concentration, the standard WHO procedure for Poliovirus surveillance. Molecular analysis was undertaken with three nested protocols, including a newly designed SARS-CoV-2 specific primer set.
SARS-CoV-2 RNA detection was accomplished in volumes of 250 ml of wastewaters collected in areas of high (Milan) and low (Rome) epidemic circulation, according to clinical data. Overall, 6 out of 12 samples were positive. One of the positive results was obtained in a Milan wastewater sample collected a few days after the first notified Italian case of autochthonous SARS-CoV-2.
The study confirms that WBE has the potential to be applied to SARS-CoV-2 as a sensitive tool to study spatial and temporal trends of virus circulation in the population.
Keywords: SARS-CoV-2, Coronavirus, COVID-19, Sewage, Wastewater, Surveillance
Graphical abstract
Highlights
-
•
Detection of SARS-CoV-2 in wastewater in Italy is described for the first time.
-
•
Use of the PEG/dextran concentration method for SARS-CoV-2 is reported.
-
•
A novel nested PCR assay specific for SARS-CoV-2 was designed.
-
•
Wastewater-based epidemiology can be applied for COVID-19 surveillance.
1. Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is responsible for the coronavirus disease COVID-19, a public health emergency worldwide. On March 11th 2020, the World Health Organization declared COVID-19 a pandemic. Italy is among the world's most affected countries in the COVID-19 pandemic. Indeed, after entering Italy, COVID-19 has been spreading fast. As of April 20th 2020, the total number of cases reported by the authorities reached 181,228, with 108,237 active cases (Dipartimento della Protezione Civile, 2020c), mainly located in Northern Italy (Lombardy, and its neighbouring regions of Emilia-Romagna and Piedmont).
Presymptomatic and paucisymptomatic carriers, mostly undetected in clinical and laboratory surveillance systems, contribute to the spread of the disease (Bai et al., 2020; Nicastri et al., 2020; Rothe et al., 2020; WHO, 2020) and hamper the efforts made to assess the extent of SARS-CoV-2 circulation in the population and to control efficiently virus transmission. Analytical regular investigation of wastewaters provides valuable information to measure viral circulation in the population as Wastewater Treatment Plants (WWTPs), collecting and concentrating human excreta, are useful sampling points receiving discharges from the entire community.
Environmental microbiologists have studied pathogens in sewage for decades (La Rosa and Muscillo, 2013; Sinclair et al., 2008). The screening of wastewater, as a public health surveillance tool, defined as wastewater-based epidemiology (WBE), is currently well recognized (Daughton, 2018; Xagoraraki and O'Brien, 2020). In the recent years, scientists have applied WBE to a wide range of waterborne, foodborne and fecal-oral viruses, which infected individuals usually excrete in high concentration with faeces (Katayama et al., 2008; Iaconelli et al., 2017; Bisseux et al., 2018). However, the concept of WBE can also be applied to viruses beyond those commonly associated with the fecal-oral route (i.e. enteric viruses), since viral shedding may involve different body fluids ultimately discharged into urban sewage.
Some studies have reported the presence of viral RNA in the stools of COVID-19 patients in percentages ranging from 16.5% to 100% at a concentration up to 6.8 log10 genome copies/g of stool (Chen et al., 2020; Lo et al., 2020; Han et al., 2020; Lescure et al., 2020). Furthermore, preliminary studies have reported the detection of SARS-CoV-2 RNA in wastewater in The Netherlands (Medema et al., 2020), France (Wurtzer et al., 2020), USA (Wu et al., 2020), and Australia (Ahmed et al., 2020). To date, no study has yet provided insights into the presence of SARS-CoV-2 in wastewaters in Italy.
Herein we report the results of the screening for SARS-CoV-2 presence in sewage samples collected between the end of February and the beginning of April 2020 from WWTPs in Milan (Northern Italy) and Rome (Central Italy).
2. Material and methods
Twelve raw sewage samples were collected between the 3rd of February and the 2nd of April 2020 from three WWTPs, located in Milan (two distinct plants, reported as A and B) and in Rome (one plant receiving two different pipelines, C1 and C2, from different districts of the town), respectively. Total numbers of inhabitants served by these WWTPs (expressed as population equivalents) were 1.050.000, 1.050.000, and 900.000, for Plant A, B, and C, respectively. Composite samples, representing 24-hour period were collected from the WWTP influent, immediately stored at −20 °C, and dispatched frozen to the National Institute of Health for analysis. Before viral concentration, samples underwent a 30 min treatment at 56 °C to increase the safety of the analytical protocol for the laboratory personnel and environment. After heat treatment, samples were processed using Class II biological safety cabinets, and standard precautions were applied (hand hygiene products and personal protective equipment e.g., gloves, gowns, face and eye protection).
Sample concentration took place using a two-phase (PEG-dextran method) separation as detailed in the 2003 WHO Guidelines for Environmental Surveillance of Poliovirus protocol (World Health Organization, 2003a), with modifications to adapt the protocol to enveloped viruses. In brief, the wastewater sample (250 ml) was centrifuged to pellet the wastewater solids, retaining the pellet for further processing. The clarified wastewater was mixed with dextran and polyethylene glycol (PEG), and the mixture was left to stand overnight at 4 °C in a separation funnel. The bottom layer and the interphase were then collected drop-wise, and this concentrate was added to the pellet from the initial centrifugation. The chloroform treatment that the WHO protocol envisages at this stage was omitted to preserve the integrity of the enveloped viruses object of this study. The extraction of viral RNA was done using the NucliSENS miniMAG semi-automated extraction system with magnetic silica carried out following manufacturer's instructions (bioMerieux, Marcy l'Etoile, France) with however slight modifications. The lysis phase was prolonged to 20 min, and brief centrifugation (2000 ×g, 1 min) was used to pellet the sediment; subsequently, magnetic silica beads were added to the cleaned supernatant. Before molecular tests, the extracted nucleic acids were further purified from potential PCR inhibitors using the OneStep PCR Inhibitor Removal Kit (Zymo Research, CA, USA).
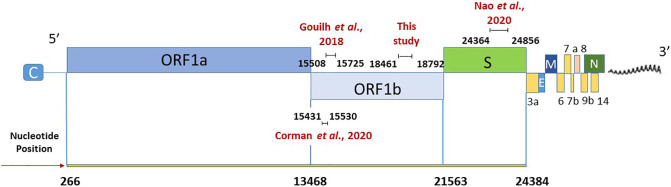
In the absence of a standardized method for SARS-CoV-2 detection in environmental samples, RNAs were tested for the presence of SARS-CoV-2 using three different nested RT-PCR assays and one real-time qPCR assay (Table 1 and Fig. 1 ):
-
a)
A broad range Coronavirus assay targeting the ORF1ab (Ar Gouilh et al., 2018). Primers were previously designed targeting a highly conserved region (nsp12) among all Coronavirinae sequences to detect a broad range of coronaviruses by a semi-nested PCR producing a fragment of 218 bp.
-
b)
A newly designed primer set specific for SARS-CoV-2. Novel nested primers, amplifying a 332 bp fragment of ORF1ab, were designed using Primer3 software (http://primer3.ut.ee/).
For the assays a) and b) first-strand cDNA was synthesized using Super Script IV Reverse Transcriptase (ThermoFisher Scientific) with the reverse primer. PCR reaction was performed using 2.5 μl of cDNA in a final volume of 25 μl (Kit Platinum™ SuperFi™ Green PCR Master Mix, Thermo), using 1 μl of primers (10 μM). The PCR conditions were as follows: 98 °C for 30 s; 35 cycles of 98 °C for 10 s, 50 °C and 54 °C for 10 s for assay a) and b), respectively, and 72 °C for 30 s; final extension 72 °C for 5 min. After the first round PCR, nested PCR was performed using 2 μl of first PCR product and under the same reaction composition and thermal profile conditions. A synthetic DNA (Biofab Research, Italy) including the PCR target region, was used to set up PCR conditions before experiments with study samples, but was not amplified along with samples to avoid risks of PCR contamination. Molecular grade water was used as the negative control.
-
c)
A published nested RT-PCR for SARS-CoV-2 targeting the spike region (Nao et al., 2020). cDNA was synthesized from 5 μl of sample RNA, using SuperScript III Reverse Transcriptase (ThermoFisher Scientific), 0.5 μM of the reverse primer (WuhanCoV-spk2-r, Table 1) and a 50 min reaction at 50 °C (20 μl final volume). First PCR reaction was performed by adding the reaction mix (Dream Taq polymerase and buffer from ThermoFisher Scientific, 0.4 μM of primers WuhanCoV-spk2-r and WuhanCoV-spk1-f directly to the whole volume of synthesized cDNA. The used PCR conditions were as follows: 95 °C for 1 min; 35 cycles of 95 °C for 30 s, 56 °C for 30 s, and 72 °C for 40 s; final extension 72 °C for 5 min. Nested PCR (primers NIID_WH-1_F24381 and NIID_WH-1_R24873) was performed in a total volume of 50 μl using 5 μl of first PCR product, with the same conditions applied for the first PCR and 45 cycles.
-
d)
A published real-time RT-qPCR assay targeting the RdRP gene, as described by Corman et al. (2020), using the probe specific for SARS-CoV-2. RT-qPCR mix (25 μl total volume) was prepared using the UltraSense one-step qRT-PCR System (Life Technologies, CA, USA), and 5 μl aliquots of sample RNA were analysed in reactions containing 1× buffer, 0.1× ROX reference dye, 1.25 μl of RNA UltraSense enzyme mix, and 600 nM, 800 nM, and 250 nM of primer RdRp-SARSr-F2, primer RdRp-SARSr-F2, and probe RdRp-SARSr-P2, respectively. Amplification conditions were as follows: reverse transcription for 30 min at 50 °C, inactivation for 5 min at 95 °C and 45 cycles of 15 s at 95 °C and 1 min at 58 °C. All reactions were performed in duplicate. For standard curve construction, the targeted region, coupled with a T7 promoter, was synthetized and quantified by Eurofins Genomics (Germany), and tenfold dilutions were used for curve construction. In vitro synthetized RNA using the standard curve DNA as a template was used as an external amplification control to check for PCR inhibition. All amplifications were conducted on a QuantStudio 12K instrument (Thermo Scientific). Molecular biology water served as a non-template control.
Table 1.
Primers and amplification protocols used in the study.
| Target | Region | Primer name | Nucleotide sequence | Orientation | Usage | Amplicon size (bp) | Reference |
|---|---|---|---|---|---|---|---|
| Broad-range coronavirus | ORF1ab | Bat-CoV pol 15197 | GGTTGGGAYTAYCCWAARTGTGA | + | First PCR | 440 | Ar Gouilh et al. (2018) |
| Bat-CoV pol 15635 | CCATCRTCMGAHARAATCATCATA | − | |||||
| Bat-CoV pol 15419 | GTGCTAAACCACCGCCTG | + | Nested PCR | 218 | |||
| Bat-CoV pol 15635 | CCATCRTCMGAHARAATCATCATA | − | |||||
| SARS-CoV-2 | ORF1ab | 2274 - CO-FW1 | GTGCTAAACCACCGCCTG | + | First PCR | 368 | This study |
| 2275 - CO-REV1 | CAGATCATGGTTGCTTTGTAGGT | − | |||||
| 2276 - CO-FW2 | CGCCTGGAGATCAATTTAAACAC | + | Nested PCR | 332 | |||
| 2277 - CO-REV2 | ACCTGTAAAACCCCATTGTTGA | − | |||||
| SARS-CoV-2 | S | WuhanCoV-spk1-f | TTGGCAAAATTCAAGACTCACTTT | + | First PCR | 547 | Nao et al. (2020) |
| WuhanCoV-spk2-r | TGTGGTTCATAAAAATTCCTTTGTG | − | |||||
| NIID_WH-1_F24381 | TCAAGACTCACTTTCTTCCAC | + | Nested PCR | 493 | |||
| NIID_WH-1_R24873 | ATTTGAAACAAAGACACCTTCAC | − | |||||
| SARS-CoV-2 | RdRP | RdRP_SARSr-F2 | GTGARATGGTCATGTGTGGCGG | + | Real-time RT-qPCR | − | Corman et al. (2020) |
| RdRP_SARSr-R1 | CARATGTTAAASACACTATTAGCATA | − | |||||
| RdRP_SARSr-P2 | FAM-CAGGTGGAACCTCATCAGGAGATGC- BHQ1 |
Fig. 1.
SARS-CoV-2 genome, modified from Viralzone (https://viralzone.expasy.org/9076). Positions of the primers used in the study are related to sequence NC_045512.
All samples were retested for confirmation of results obtained with methods a), b), and c). The PCR products were revealed by electrophoresis on 2% agarose gels and were purified using a Montage PCRm96 Microwell Filter Plate (Millipore, Billerica, MA, USA) and then direct sequenced on both strands (BioFab Research, Rome, Italy). Sequences were identified in terms of the closest homology sequence using BLAST https://blast.ncbi.nlm.nih.gov/Blast.cgi. All Italian SARS-CoV-2 genome sequences available at the time of analysis were retrieved from Gisaid (https://www.gisaid.org/) for comparison with study sequences, using the MEGA X software (Kumar et al., 2018).
Sequences were submitted to NCBI GenBank with the accession numbers: MT373156–MT373163.
3. Results and discussion
The 50% (6/12) of the wastewater samples showed positive results for SARS-CoV-2 RNA, and the newly designed assay in the RdRp gene showed a higher sensitivity compared to the assay targeting the spike gene (Table 2 ). Both the published and newly designed SARS-CoV-2 specific primer sets detected bands of the expected size and were confirmed by sequencing. In contrast, only unspecific products were detected with a broad range assay for coronavirus. Upon comparison of broad range primers with SARS-CoV-2 genome, we noted that they showed only 77.1 to 91.3% nt identity, which explain why these were not able to amplify the novel coronavirus. No positive results were obtained by real-time RT-qPCR, therefore no quantitative data could be provided for the positive samples. This may be related to the sensitivity of the RdRp assay used in this study. Indeed, in recent comparative studies, the sensitivity of this assay was shown to be low compared to others developed by WHO referral laboratories (Etievant et al., 2020). In particular, the limit of detection (LOD) of this assay was estimated at 316 viral genomic equivalents per reaction by Nalla et al. (2020) and above 500 genome copies per reaction by Vogels et al. (2020) as well as in our hands (data not shown), suggesting that virus concentration was below the LOD of the assay. However, the external inhibition control associated to this assay was useful to confirm the acceptable levels of PCR inhibitors, all samples being below the acceptability criterion (median inhibition 29.1%, range 8.7%–51.4%).
Table 2.
Results of SARS-CoV-2 detection in the study period.
| City and WWTP | Date of sampling |
|||||||
|---|---|---|---|---|---|---|---|---|
| 03 Feb | 19 Feb | 23 Feb | 24 Feb | 26 Feb | 28 Feb | 31 Mar | 02 Apr | |
| Milan – plant A | × | ○a | × | × | ||||
| Milan – plant B | × | × | × | ○● | ||||
| Rome – plant C1 | ○a | ○ | ||||||
| Rome – plant C2 | ○ | ○● | ||||||
× SARS-CoV-2 not detected; ○ SARS-CoV-2 detected (ORF1ab); ● SARS-CoV-2 detected (spike).
Weak positive.
In this study, a thermal treatment of samples (30 min at 56 °C) was included before concentration to increase the safety for the laboratory personnel during sample manipulation. These conditions were reported to reduce the virulence of SARS-CoV-2 by over 5 log without affecting RNA integrity (Pastorino et al., 2020). Similar results were obtained by Batéjat et al. (2020) and by Wang et al. (2020). Moreover, the effectiveness of thermal treatment at 56 °C for virus inactivation was reported for SARS-CoV (World Health Organization, 2003b). To confirm that heat inactivation did not negatively affect virus detection, seeded experiments were performed using a surrogate virus (Mengovirus), confirming that no significant loss of RNA signal was recorded in heat-treated samples (data not shown).
With the used protocols, SARS-CoV-2 RNA was first detected with a weak amplification signal in an influent sample from Milan WWTPs (Lombardy, North Italy) collected on February 24th from the plant A with the ORF1ab assay. A positive result for SARS-CoV-2 was also detected with an intense amplification signal, on February 28th from the plant B, with both targets (ORF1ab and spike genes). In the influent samples taken from the WWTP in Rome (Latium, Central Italy), SARS-CoV-2 was detected in both sampling dates (31st of March and 2nd of April) and both pipelines, C1 and C2, using the newly designed primer sets specific for SARS-CoV-2. The analysed sequences showed, for both ORF1ab and S partial gene regions, 100% identity with the first SARS-CoV-2 sequence detected in Italy (MT066156), isolated on 30th January 2020 from a Chinese tourist by the Institute “Lazzaro Spallanzani” (INMI, Rome). Given the high level of conservation of the two analysed regions, 100% identity was also detected with several sequences in GenBank and with all the other Italian SARS-CoV-2 genomes deposited in Gisaid.
Significantly, on February 24th and 28th, when the samples positive for SARS-CoV-2 were collected in Milan, COVID-19 infections were still limited in Italy, the first Italian autochthonous SARS-CoV-2 positive case having been reported only a few days earlier, on February 21st. On February 28th, the total number of SARS-CoV-2 positive patients reported in all Italy was only 888, with 531 (57%) in Lombardy, the most affected region in the country. However, at that time, the vast majority of cases in Lombardy were recorded in the provinces of Lodi, Cremona and Bergamo (182, 123, and 103 cases, respectively). In comparison, in the province of Milan (an even larger area compared to the metropolitan area served by the selected WWTPs) only 29 cases had been reported (Dipartimento della Protezione Civile, 2020a).
These results provide evidence of the sensitivity of environmental surveillance for the detection of ongoing outbreaks in the population. Virus detection in sewage, despite the low incidence of reported human infections, may be associated with the ability of sewage surveillance to estimate after careful epidemiological models, mild, subclinical, or asymptomatic cases. These infected individuals shed viruses into local sewage systems and contribute to virus circulation while remaining substantially undetectable by clinical surveillance, a phenomenon known as the “surveillance pyramid” (Martinez Wassaf et al., 2014). Clinical surveillance, indeed, only captures the tip of the iceberg of viral diseases (hospitalized patients or laboratory diagnosed cases). In contrast, monitoring of urban wastewaters makes it possible to capture the full extent of the diseases at a community level.
As regards to the influent samples collected in Rome, SARS-CoV-2 was detected on March 31, when the epidemic had spread considerably in Italy. In that date, a total of 77.635 SARS-CoV-2 infections had been reported in Italy, of which 3.095 in Latium Region and 2.186 in the province of Rome (Dipartimento della Protezione Civile, 2020b), with about 85% of them being active cases (Assessorato alla Sanità e all'Integrazione Socio Sanitaria della Regione Lazio, 2020). Given the spread of the virus, with such several excreting patients (symptomatic and asymptomatic), the detection of the viral RNA in the tested samples is not surprising and, consistently, the samples taken two days later, on the 2nd April, at the same WWTP were still positive for SARS-CoV-2 RNA.
Following this investigation on the occurrence of SARS-CoV-2 RNA in sewage, the production of quantitative data on virus concentration in raw sewage will be undertaken with the use of molecular methods optimized for environmental samples. This approach will allow obtaining a rough estimation of the total number of subjects excreting the virus, by integrating - as done by Wu et al. (2020) in samples taken in the United States - the available information on viral shedding rates, WWTPs loads, and virus concentration in wastewaters. Moreover, the environmental surveillance will be extended to the collection of wastewater samples available in the Department of Environment and Health of the Italian National Health Institute, that were collected throughout Italy in the framework of different projects on enteric viruses. Such monitoring will provide a picture of the SARS-CoV-2 circulation across the different regions of Italy and over time, to better understand the virus circulation, as provided by wastewater-based epidemiology (WBE) and compare it to the clinical data. Samples collected before the reporting of the first known Italian case on February 21 will also be tested, to possibly infer when SARS-CoV-2 first appeared in Italy. In a previous study, indeed, wastewater monitoring provided evidence that a novel variant of Norovirus GII.17 (termed Kawasaki 2014) had been circulating in the Italian population before its first appearance and identification in clinical cases, later becoming one of the prevalent variants in the population (Suffredini et al., 2018).
Also and most important, environmental monitoring of SARS-CoV-2 in sewage will continue when the emergency phase will be over, and its circulation in the population will be considered limited. Indeed, sewage surveillance could also serve for the early detection of a possible re-emergence of COVID-19 in urban areas. WHO recommends environmental surveillance for poliovirus as an early warning system. As an example, during 2013, Israel observed the silent reintroduction and transmission of wild poliovirus type 1, detected through routine environmental surveillance performed on sewage samples without the reporting of any clinical cases (Manor et al., 2014). Environmental monitoring, therefore, appears to be an effective measure for proving early warning against pathogen reintroduction.
In conclusions, the main findings of this study are:
-
1)
first detection of SARS-CoV-2 RNA fragments in sewage in Italy;
-
2)
demonstration of the suitability of the WHO protocol for sewage treatment to enveloped viruses after appropriate modifications;
-
3)
design of a novel nested PCR assay specific for SARS-CoV-2, useful for screening purposes.
Further research will clarify the applicability of WBE to SARS-CoV-2 for prompt detection, the study, and the assessment of viral outbreaks.
CRediT authorship contribution statement
Giuseppina La Rosa:Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing.Marcello Iaconelli:Investigation, Formal analysis.Pamela Mancini:Investigation, Formal analysis.Giusy Bonanno Ferraro:Investigation, Formal analysis.Carolina Veneri:Investigation, Formal analysis.Lucia Bonadonna:Conceptualization, Formal analysis.Luca Lucentini:Conceptualization, Formal analysis.Elisabetta Suffredini:Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing.
Declaration of competing interest
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Editor: Damia Barcelo
References
- Ahmed W., Angel N., Edson J. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ar Gouilh M., Puechmaille S.J., Diancourt L., Vandenbogaert M., Serra-Cobo J., Lopez Roïg M. SARS-CoV related betacoronavirus and diverse alphacoronavirus members found in western old-world. Virology. 2018;517:88–97. doi: 10.1016/j.virol.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assessorato alla Sanità e all'Integrazione Socio Sanitaria della Regione Lazio Ministero della Salute – Bulletin. 2020. http://www.salute.gov.it/imgs/C_17_notizie_4370_0_file.pdf 31.03.
- Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L., Wang M. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batéjat C., Grassin Q., Manuguerra J.C., Leclercq I. 2020. Heat Inactivation of the Severe Acute Respiratory Syndrome Coronavirus 2. (bioRxiv preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisseux M., Colombet J., Mirand A., Roque-Afonso A.M., Abravanel F., Izopet J., Archimbaud C. Monitoring human enteric viruses in wastewater and relevance to infections encountered in the clinical setting: a one-year experiment in central France, 2014 to 2015. Euro Surveill. 2018;23(7) doi: 10.2807/1560-7917.ES.2018.23.7.17-00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Chen L., Deng Q., Zhang G., Wu K., Ni L. The presence of SARS-CoV-2 RNA in feces of COVID-19 patients. J. Med. Virol. 2020:1–8. doi: 10.1002/jmv.25825. (Accepted Author Manuscript) [DOI] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. Jan. (PMID: 31992387; PMCID: PMC6988269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C.G. Monitoring wastewater for assessing community health: sewage chemical-information mining (SCIM) Sci. Total Environ. 2018;619–620(2018):748–764. doi: 10.1016/j.scitotenv.2017.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipartimento della Protezione Civile Bulletin 28.02.2020. 2020. https://github.com/pcm-dpc/COVID-19/blob/master/dati-province/dpc-covid19-ita-province-20200228.csv
- Dipartimento della Protezione Civile Bulletin 31.03. 2020. https://github.com/pcm-dpc/COVID-19/blob/master/dati-province/dpc-covid19-ita-province-20200331.csv
- Dipartimento della Protezione Civile Bulletin 20.04. 2020. http://www.protezionecivile.gov.it/media-comunicazione/comunicati-stampa/dettaglio/-/asset_publisher/default/content/coronavirus-la-situazione-dei-contagi-in-ital-2
- Etievant S., Bal A., Escurret V., Brengel-Pesce K., Bouscambert M., Cheynet V., Generenaz L., Oriol G., Destras G., Billaud G., Josset L., Frobert E., Morfin F., Gaymard A. 2020. Sensitivity Assessment of SARS-CoV-2 PCR Assays Developed by WHO Referral Laboratories. medRxiv 2020.05.03.20072207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M.S., Seong M.-W., Heo E.Y., Park J.H., Kim N., Shin S. Sequential analysis of viral load in a neonate and her mother infected with SARS-CoV-2. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa447. ciaa447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaconelli M., Muscillo M., Della Libera S., Fratini M., Meucci L., De Ceglia M., Giacosa D., La Rosa G. One-year surveillance of human enteric viruses in raw and treated wastewaters, downstream river waters, and drinking waters. Food Environ Virol. 2017;9(1):79–88. doi: 10.1007/s12560-016-9263-3. [DOI] [PubMed] [Google Scholar]
- Katayama H., Haramoto E., Oguma K., Yamashita H., Tajima A., Nakajima H., Ohgaki S. One-year monthly quantitative survey of noroviruses, enteroviruses, and adenoviruses in wastewater collected from six plants in Japan. Water Res. 2008;42:1441–1448. doi: 10.1016/j.watres.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Muscillo M. Viruses in Food and Water: Risks, Surveillance and Control. 2013. Molecular detection of viruses in water and sewage; pp. 97–125. [Google Scholar]
- Lescure F.X., Bouadma L., Nguyen D., Parisey M., Wicky P.H., Behillil S. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30200-0. S1473-3099(20)30200-0. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo I.L., Lio C.F., Cheong H.H., Lei C.I., Cheong T.H., Zhong X. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int. J. Biol. Sci. 2020;16(10):1698–1707. doi: 10.7150/ijbs.45357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor Y., Shulman L.M., Kaliner E. Intensified environmental surveillance supporting the response to wild poliovirus type 1 silent circulation in Israel, 2013. Euro Surveill. 2014;19(7):20708. doi: 10.2807/1560-7917.es2014.19.7.20708. Published 2014 Feb 20. [DOI] [PubMed] [Google Scholar]
- Martinez Wassaf G.M., Pisano M.B., Barril P.A., Elbarcha O.C., Pinto M.A., Mendes de O.J. First detection of hepatitis E virus in central Argentina: environmental and serological survey. J. Clin. Virol. 2014;61:334–339. doi: 10.1016/j.jcv.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. 2020. Presence of SARS-Coronavirus-2 in Sewage. (medRxiv preprint) [DOI] [PubMed] [Google Scholar]
- Nalla A.K., Casto A.M., Huang M.W. Comparative performance of SARS-CoV-2 detection assays using seven different primer/probe sets and one assay kit. J. Clin. Microbiol. 2020 doi: 10.1128/JCM.00557-20. published online ahead of print, 2020 Apr 8. JCM.00557-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nao N., Shirato K., Katano H., Matsuyama S., Takeda M. 2020. Detection of Second Case of 2019-nCoV Infection in Japan. [Google Scholar]
- Nicastri E., D'Abramo A., Faggioni G., De Santis R., Mariano A., Lepore L. Coronavirus disease (COVID-19) in a paucisymptomatic patient: epidemiological and clinical challenge in settings with limited community transmission, Italy, February 2020. Euro surveillance: bulletin Europeen sur les maladies transmissibles. 2020;25(11) doi: 10.2807/1560-7917.ES.2020.25.11.2000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino B., Touret F., Gilles M., de Lamballerie X., Charrel R.N. 2020. Evaluation of Heating and Chemical Protocols for Inactivating SARS-CoV-2. bioRxiv prept. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair R.G., Choi C.Y., Riley M.R., Gerba C.P. Pathogen surveillance through monitoring of sewer systems. Adv. Appl. Microbiol. 2008;65:249–269. doi: 10.1016/S0065-2164(08)00609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffredini E., Iaconelli M., Equestre M. Genetic diversity among genogroup II noroviruses and progressive emergence of GII.17 in wastewaters in Italy (2011–2016) revealed by next-generation and sanger sequencing. Food Environ Virol. 2018;10(2):141–150. doi: 10.1007/s12560-017-9328-y. published correction appears in Food Environ Virol. 2018 May 4. [DOI] [PubMed] [Google Scholar]
- Vogels C.B.F., Brito Anderson F., Wyllie Anne Louise, Fauver Joseph R., Ott Isabel M., Kalinich Chaney C., Petrone Mary E., Casanovas-Massana Arnau, Catherine Muenker M., Moore Adam J., Klein Jonathan, Peiwen Lu, Lu-Culligan Alice, Jiang Xiaodong, Kim Daniel J., Kudo Eriko, Mao Tianyang, Moriyama Miyu, Ji Eun Oh., Park Annsea, Silva Julio, Song Eric, Takehashi Takehiro, Taura Manabu, Tokuyama Maria, Venkataraman Arvind, Weizman Orr-El, Wong Patrick, Yang Yexin, Cheemarla Nagarjuna R., White Elizabeth, Lapidus Sarah, Earnest Rebecca, Geng Bertie, Vijayakumar Pavithra, Odio Camila, Fournier John, Bermejo Santos, Farhadian Shelli, Cruz Charles Dela, Iwasaki Akiko, Ko Albert I., Landry Marie-Louise, Foxman Ellen F., Grubaugh Nathan D. 2020. Analytical Sensitivity and Efficiency Comparisons of SARS-COV-2 qRT-PCR Primer-Probe Sets. medRxiv 2020.03.30.20048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Lien C., Liu S., Selveraj P. 2020. Effective Heat Inactivation of SARS-CoV-2. (medRxiv 2020.04.29.20085498) [DOI] [Google Scholar]
- World Health Organization Guidelines for Environmental Surveillance of Poliovirus Circulation. 2003. https://apps.who.int/iris/handle/10665/67854 Available online, accessed on.
- World Health Organization First data on stability and resistance of SARS coronavirus compiled by members of WHO laboratory network. 2003. https://www.who.int/csr/sars/survival_2003_05_04/en/
- World Health Organization World Health Organization; 2020. Advice on the use of masks in the context of COVID-19: interim guidance, 6 April 2020. https://apps.who.int/iris/handle/10665/331693
- Wu F., Xiao A., Zhang J., Gu X., Lee W.L., Kauffman K. 2020. SARS-CoV-2 Titers in Wastewater are Higher Than Expected From Clinically Confirmed Cases. medRxiv prep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.M., Moulin L. 2020. Time Course Quantitative Detection of SARS-CoV-2 in Parisian Wastewaters Correlates With COVID-19 Confirmed Cases. medRxiv prep. [DOI] [Google Scholar]
- Xagoraraki I., O’Brien E. Wastewater-based epidemiology for early detection of viral outbreaks. In: O’Bannon D., editor. Women in Water Quality. Springer; 2020. pp. 75–97. (Women in Water Quality. Women in Engineering and Science). [Google Scholar]