Abstract
Background
COVID-19 is a worldwide public health concern. Disruptions in the drug market are expected and shortages might worsen. Community pharmacies can contribute to early identification and report of medicines’ supply and demand issues.
Objective
The aim of this study is to characterize the impact of the COVID-19 outbreak on outpatient medicines’ sales and shortages.
Methods
A retrospective, time-trend analysis of medicine sales, shortages and laboratory-confirmed COVID-19 cases was performed from February 1st to April 30th, 2020, and its homologous period (regarding sales only). A detailed analysis of 6 pharmaceutical substances was performed. All data were subjected to rescaling using the min-max normalization method, in order to become comparable. Data analysis was performed using Microsoft® Excel.
Results
The pandemic resulted in an increase in medicines’ demand and reported shortages during the early stage of the outbreak. The maximum proportion of medicine sales was registered on March 13th, 2020, 4 days after the WHO declared COVID-19 a pandemic. By the end of March, sales have already dropped to proportions similar to those of 2019. The maximum proportion of drug shortages was reached about one week after the sales peak and by the end of the study period were below those recorded in the pre-COVID-19 period. The analyzed drugs were paracetamol, ascorbic acid, dapagliflozin plus metformin, rosuvastatin plus ezetimibe, formoterol, and hydroxychloroquine, as these pharmaceutical substances registered the highest growth rate in sales and shortages when compared to the same period in the previous year. Hydroxychloroquine showed the most different pattern trends on sales and shortages of these medicines.
Conclusions
Pharmacies can provide timely and real-world data regarding sales and shortages. The adopted measures to guarantee the continuous supply of the medicine market seem to have worked. The long-term impacts of this pandemic are unknown and should continue to be closely monitored.
Keywords: COVID-19, Medicine, Demand, Shortages, Pharmacies
Introduction
On January 30th, 2020, the World Health Organization (WHO) designated the COVID-19 outbreak a public health emergency of international concern and on March 11th it was declared a global pandemic.1 By May 19th, 216 countries, areas or territories worldwide had reported almost 5 million cases of coronavirus disease.2 Preventive measures to flatten the epidemic curve have been implemented, thereby avoiding a demand surge on the healthcare system. Alongside the need to guarantee the supply of key medical and personal protective equipment to control the disease, there is a general concern regarding the preservation of the continued supply of medicines for all citizens.3, 4, 5 Although no major disruptions in pharmaceutical access have been reported thus far, the existing issue of medicine shortages6, 7, 8, 9 may increase, due to the worldwide state of public health emergency. Expected constraints in the manufacturing process (e.g. lockdown of factories due to quarantine) and supply chain disruptions can affect countries' ability to ensure patients' access to pharmacological therapies. Moreover, people's behavior is known to change during an epidemic.10 Its constraints can lead to states of anxiety, hoarding of medical supplies and rioting.11 As a result, an increase in the demand for medicines is anticipated, which may enhance the issue of drug shortages, acknowledged for having a negative impact on population welfare, leading to higher demand in healthcare resources and increased morbidity and mortality.12
Community pharmacies play an important public health role by ensuring the continuous and rational supply of medicines, contributing to patients' education, managing stockpiling and early identification and report of unavailable medicines.13 , 14 This study aims to characterize the impact of the COVID-19 outbreak on the outpatient medicines’ sales and shortages.
Methods
A retrospective, time-trend analysis of total medicine sales, shortages and new laboratory-confirmed COVID-19 cases was performed from February 1st to April 30th, 2020 and its homologous period (regarding sales only). To get a better insight of the market trends’ specificities, a detailed analysis of 6 pharmaceutical substances was performed. The selection took in consideration the substances from different therapeutic classes with the highest growth rates simultaneously in sales and shortages, when compared to the homologous period. These substances were identified by International Non-proprietary Name (INN) and Anatomical Therapeutic Chemical (ATC/WHO) classification code.
Medicine sales
Medicine sales were retrieved from a national representative panel of community pharmacies with Sifarma®-dispensing software (n ≈ 2400, 83% of the total number of Portuguese pharmacies). Data are collected daily and stored in a data warehouse since 2002.
Medicine shortages
In this study, the term ‘medicine shortages’ is used to describe the pharmacy medicine orders that were unfulfilled by the wholesale distributors. All pharmacies with Sifarma® have an application installed that allows them to, voluntarily and daily, report the ordered prescription-only-medicines (POMs) and over-the-counter (OTC) pharmaceutical products that were not delivered to the pharmacy.
COVID-19 laboratory-confirmed diagnosis
The number of new daily laboratory-confirmed cases of COVID-19 in Portugal was collected from the General Directorate of Health's official website.15 Confirmed cases are those that tested positive for SARS-CoV-2 in a laboratory-confirmed analysis. Daily cases of COVID-19 refer to the number of positive test results until the midnight of the previous day.
Data analysis
For this analysis, the data were subjected to rescaling using the min-max normalization method,16 in order to become comparable. Data analysis was performed using Microsoft® Excel 2016.
Results
The first infections by SARS-CoV-2 in Portugal were reported on March 2nd, 2020. Up until the end of this week, when comparing 2020 medicine sales with the homologous period (Fig. 1 ), a similar pattern was registered. However, on the week of March 9th, when WHO declared COVID-19 a pandemic, medicine sales started to increase. This trend went as far as March 13th (+60% vs the previous week), when the sales peak was reached. Then, on March 16th, the first death was announced in Portugal and the volume of medicines sold in 2020 began to decrease. As of March 22nd, sales stabilized and approached those of 2019, although they were slightly lower than in the previous year. The number of new confirmed cases presented an exponential increase followed by a stabilization and reduction during the study period.
Fig. 1.
Normalized time series of community pharmacies' medicine sales in 2019 and 2020, new COVID-19 cases and major events regarding COVID-19 in Portugal.
When it comes to comparing 2020 medicine sales and shortages (Fig. 2 ), it is apparent that, before the confirmation of the first COVID-19 cases in Portugal, these trends behaved similarly, with the proportion of normalized sales being slightly higher than the normalized shortages (February 1st to March 8th). From March 9th these patterns started to change, with the proportion of shortages recording a peak (+62% vs the previous week) just about a week after the sales peak occurred. At this stage, the number of new infections by SARS-CoV-2 was also increasing. By the end of April, 1.5 months after the pandemic and first national state of emergency declarations, the proportion of total medicine sales and shortages were below those recorded in the pre-COVID-19 period.
Fig. 2.
Normalized time series of the new COVID-19 cases and community pharmacies' medicine sales and shortages in Portugal.
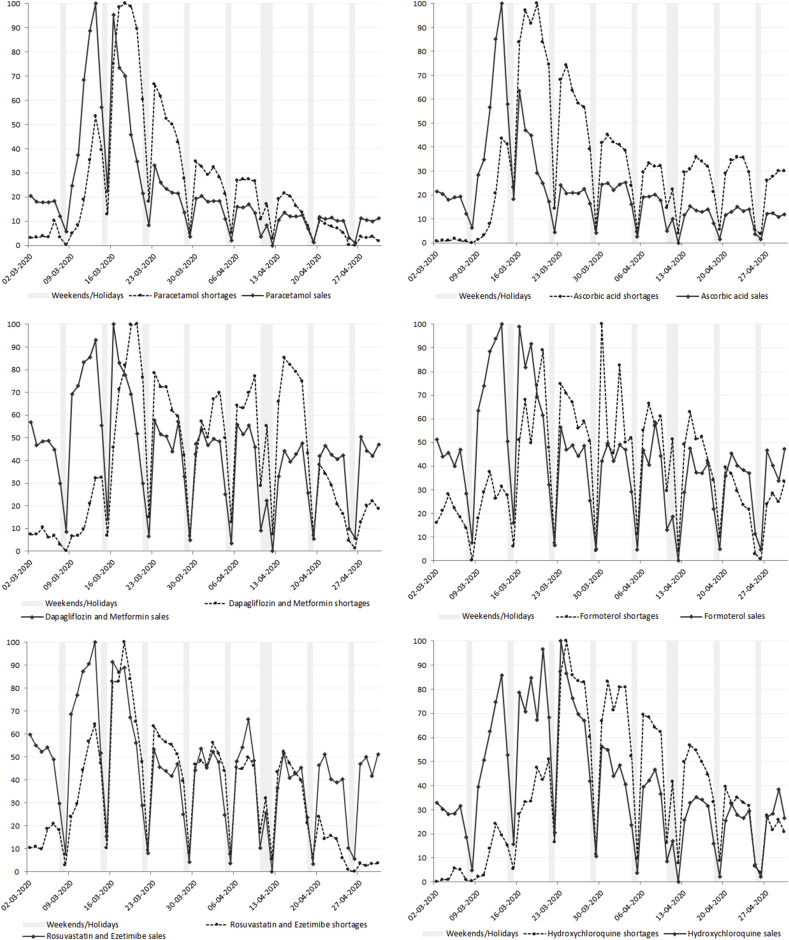
Fig. 3 shows the trend of medicine shortages and sales for the six identified pharmaceutical substances: 1) paracetamol (N02); 2) ascorbic acid (vitamin C) (A11); 3) dapagliflozin plus metformin (A10); 4) formoterol (R03); 5) rosuvastatin plus ezetimibe (C10) and 6) hydroxychloroquine (P01).
Fig. 3.
Normalized time series of community pharmacies' medicine sales and shortages in Portugal, regarding six INNs: paracetamol (N02); ascorbic acid (vitamin C) (A11); dapagliflozin plus metformin (A10); formoterol (R03); rosuvastatin plus ezetimibe (C10) and hydroxychloroquine (P01).
For these drugs, some patterns stand out. Paracetamol and ascorbic acid showed similar trends. There was a very substantial increase in supply compared to the first week of data collection. The maximum proportion of sales for these drugs, as for the total market, occurred in the week WHO declared COVID-19 a pandemic. Since then, shortages and sales started to decrease and have stabilized by the end of April, although ascorbic acid's shortages have not returned to normal values.
Dapagliflozin plus metformin, formoterol and rosuvastatin plus ezetimibe, all used in chronic conditions, also showed similar sales and shortages’ trends. Sales registered the highest proportions during the second and third weeks of March, while shortages reached their maximum values up to three weeks after the first peak in sales. From that point on, the proportion of shortages and sales has decreased and reached values close to those in the pre-COVID-19 period.
Out of the 6 INNs, hydroxychloroquine presents the most different pattern, registering a high proportion of sales for the longest period and reaching its peak on the last week of March. As for shortages, an increase is observed from March 9th to March 25th, when the maximum value is reached. In April, these proportions slowly began to diminish, but shortages have not yet reached the values verified before the COVID-19 outbreak.
Discussion
To the best of our knowledge, this is the first study to characterize the outpatient sell-out medicines and shortages' trend changes during the novel corona virus outbreak in Portugal. Community pharmacies are the last player of this supply chain with direct contact with the suppliers and the patients. Therefore, they are in a privileged position to detect and provide data on medicine supply disruptions and changes in demand behavior. Results show that in the week of the first confirmed COVID-19 cases in Portugal (March 2nd), the demand for medicines remained unchanged (Fig. 1). The greatest growth in volume sales was registered just after WHO declared COVID-19 a global pandemic. This behavior may have resulted from citizens’ unfamiliarity with the pandemic consequences and fear of entry into lockdown mode, which was already in place in the closest EU countries,17 , 18 as people started to stock up in anticipation of possible drug shortages and hampered access. On March 18th, the first emergency state was declared, and 4 days later Portugal entered lockdown period. In this week, total sales dropped into a normal range. The maximum value of new daily COVID-19 cases was registered on April 10th. This peak was reached not only because of new infections but also due to an increase in the number of analyzed samples during that week.13
In light of the initial increased demand for medicines and to prevent stockpiling, national authorities have taken several emergency responses.4 For example, it has been recommended that users should only buy one package of OTCs, with special emphasis for paracetamol presentations. As for POMs, guidelines and later legislation imposed restrictions on the quantities that can be purchased by citizens. Pharmacies can no longer fully dispense 6-month renewal prescriptions at once. The maximum amount allowed to avoid inequities and ensure that all patients receive the medicines they need is the number of packages required for treatment for up to two months.19 Also, in line with recent EU guidelines,20 pharmacies were permitted to deliver POMs to peoples’ homes. This measure intended to prevent patients from hoarding medicines, going to the pharmacy as frequently and be exposed to the coronavirus.21 In order to aid pharmacies in providing this service to the population, the Portuguese National Association of Pharmacies, which represents about 94% of community pharmacies, established a partnership with the Portuguese Post Office. These immediate measures seem to be successful in helping to control high demand, as up to date sales have dropped below pre-outbreak levels (Fig. 1).
As for drug shortages, and despite the registered increment in the two weeks following the sales peak, their proportions dropped to lower values than those reported before the outbreak (Fig. 2). The initial increase in medicine shortages could have been caused by a variety of reasons: a) an increment in the volume of orders as pharmacies tried to stock up due to the increased demand and emptying of security stocks; b) a supply chain rupture, related or not to COVID-19 constraints, or c) wholesalers prorating drugs to prevent future shortages and equalize access to medicines classified as essential or with short-supply on the national market. Besides the measures taken by suppliers and in order to reduce the risk of medicine shortages in the COVID-19 context, the Government and the National Authority of Medicines and Health Products (INFARMED) took coordinated actions with the European Union.20 To help preserve the integrity of the national market, the list of medications that need prior notification to be exported or distributed to other member states was updated with the strategic national stockpile medicines.22 It must be considered that exportation problems and parallel trade mechanisms within the EU members are often identified as possible causes for shortages in countries with lower prices such as Portugal.23 , 24 So far, data suggest that the medicines' unavailability situation is stable. However, some manufacturers seem to be struggling to ensure a continuous production and/or distribution of their products to the Portuguese market. For instance, since January 2020, paracetamol's, rosuvastatin plus ezetimibe's and formoterol's marketing authorization holders (MAH) have reported temporary medicines' unavailability to Portuguese health authorities.25
Tackling drug shortages is essential. Medicines are effective technology, and, if used correctly, can provide major benefits to patients. At this stage, there is a concerning evidence of underuse of healthcare due to COVID-19 and increased mortality for all causes compared to the homologous period, especially among older people.26 , 27 It is crucial to ensure that patients maintain safe and timely access to their medicines in order to avoid additional distress, disease progression and/or worsening of symptoms due to delay in treatment, potential dose reduction or even treatment interruption.
Insights from the six pharmaceutical substances
The demand for paracetamol rocked up just after the declaration of the first official positive case in Portugal (Fig. 3), which may be explained by the fact it is used to treat fever, one of the most common symptoms of COVID-19.28 An increased demand for OTC ascorbic acid's oral forms was also recorded after the media highlighted the possible benefits of vitamin C intake as a prophylactic measure, given its effect on immune system reinforcement.29 However, despite a recent published RCT carried out in USA patients with sepsis related to acute respiratory distress syndrome, which indicated that high daily intra venous dosage (~15 g/day) of vitamin C for 4 days may decrease mortality,30 there is no evidence that vitamin C can prevent COVID-19.31 Although sales have already fallen to normal values, shortages remain high, which may indicate a lack of product in the market to meet demand needs.
As for formoterol, a medicine used to control moderate to severe forms of asthma and chronic pulmonary obstructive disease,32 the demand surge is in line with the virus outbreak (Fig. 3). Rosuvastatin plus ezetimibe, an association of lipid lowering drugs used to treat patients with high cardiovascular risk,33 and dapagliflozin plus metformin,34 an association of antidiabetic agent, present very similar sales’ patterns (Fig. 3). They are expensive medicines and quite recent in the Portuguese market (2018 and 2016, respectively), with scarcely available therapeutic equivalents.25 The increased demand for these medicines may be due to a fear of being infected, since chronic patients are at risk of more severe clinical outcomes if they contract the coronavirus. Furthermore, it is possible that patients have already experienced shortages of these brand medicines as this has been a constant problem in the last years.7 , 35 Formoterol and dapagliflozin plus metformin remain with a high proportion of shortages, which may indicate an undersupply issue that can compromise access to these therapeutics in the long run.
Although currently there is no known effective treatment or vaccine to fight the virus, hydroxychloroquine has showed some effectiveness in COVID-19 treatment.36, 37, 38 Despite the positive results, EMA recommended the use of this drug only on clinical trials and according to national protocols.39 However, the use of hydroxychloroquine in patients with coronavirus pneumonia was widespread37 and the medical prescription and demand for this medicine tripled (Fig. 3). About two-months after the first confirmed cases in Portugal, hydroxychloroquine shortages remain high, which suggests pharmacies still struggle to fulfill these orders. As this medicine is used in the treatment of rheumatoid arthritis and lupus and also as an antimalaria drug, the off-label use demand and market unavailability can impact patients’ welfare.40
At this moment, no major supply disruptions are recorded. However, the long-term impacts of this pandemic are unknown and should be monitored, especially bearing in mind the concerning historical problem of medicine shortages in Europe.23 , 35
Limitations
Some limitations to this paper should be noted. When it comes to data collection, as monthly medicine sales are validated in the second half of the following month, the presented values for April are provisional estimates. However, the amount of sales removed by the validation process is extremely low, which allows the use of these estimates for analyses without the risk of major differences in data. Unfulfilled pharmacy orders were used as a proxy of medicine shortages, thus do not represent a direct report from patients, but can be used to study trends as all pharmacies report them the same way. Also, since medicine shortages are dependent on the pharmacists' direct report, it is important to consider that, in this period, pharmacies are working under very controlled conditions (e.g. reduced teams) and therefore may not report all their unfulfilled orders, i.e., this registry may suffer from underreporting. Regarding data analysis, namely the analysis of the INNs, it was assumed that the medicine sales and shortages registered on the first week of March were representative of the pre-COVID-19 period values, as this can be observed for the total medications’ market (Fig. 2).
Conclusions
Pharmacies are able to provide timely and real-world outpatient market data to monitor medicines' trends, which in turn can help national and European regulatory and policy entities take coordinated actions and propose guidelines regarding preventive management of medicines in response to public health crisis, such as COVID-19. Data suggest that medicines’ sales and shortages were impacted by the COVID-19 lockdown in Portugal, although measures taken seem to have led to the stabilization of the market. The long-term impacts of this pandemic are unknown and should continue to be closely monitored.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author on reasonable request.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
As no personal data were collected for this study, requirements regarding compliance with General Data Protection Regulation (GDPR), including those established in Article 89, were not addressed, nor was ethical approval required.
Declaration of competing interest
The authors declare that they have no known competing personal or financial interests that could influence this research.
Acknowledgements
We want to gratefully acknowledge all community pharmacies who voluntarily agreed to share their sell-out and unfulfilled wholesale orders' data.
References
- 1.WHO Director-General’s opening remarks at the media briefing on-COVID-19. 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 11 March. Accessed April 10, 2020.
- 2.WHO Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Accessed May 19, 2020.
- 3.Infarmed (National Authority of Medicines and Health Products, I.P.) 2020. Circular Normativa N.o 002/CD/100.20.200. Orientações Para a Gestão Responsável de Medicamentos No Actual Contexto de Pandemia COVID-19. (in Portuguese). Lisboa, Portugal. [Google Scholar]
- 4.Infarmed (National Authority of Medicines and Health Products, I.P.) and DGS (General Directorate of Health) 2020. NORMA 003/2020. Infeção Por SARS-Cov-2 (COVID-19) (in Portuguese). Lisboa, Portugal. [Google Scholar]
- 5.European Medicines Agency (EMA) Addressing the potential impact of novel coronavirus disease (COVID-19) on medicines supply in the EU. 2020. https://www.ema.europa.eu/en/news/addressing-potential-impact-novel-coronavirus-disease-covid-19-medicines-supply-eu Published. Accessed April 29, 2020.
- 6.Rinaldi F., de Denus S., Nguyen A., Nattel S., Bussières J.F. Drug shortages: patients and health care providers are all drawing the short straw. Can J Cardiol. 2017;33(2):283–286. doi: 10.1016/j.cjca.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 7.De Weerdt E., Simoens S., Casteels M., Huys I. Clinical, economic and policy implications of drug shortages in the European union. Appl Health Econ Health Pol. 2017;15(4):441–445. doi: 10.1007/s40258-016-0264-z. [DOI] [PubMed] [Google Scholar]
- 8.Fox E.R., Sweet B.V., Jensen V. Drug shortages: a complex health care crisis. Mayo Found Med Educ Res n Mayo Clin Proc. 2014;89(3):361–373. doi: 10.1016/j.mayocp.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Said A., Goebel R., Ganso M., Zagermann-Muncke P., Schulz M. Drug shortages may compromise patient safety: results of a survey of the reference pharmacies of the Drug Commission of German Pharmacists. Health Pol. 2018;122(12):1302–1309. doi: 10.1016/j.healthpol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Cadogan C.A., Hughes C.M. On the frontline against COVID-19 : community pharmacists ’ contribution during a public health crisis. Res Soc Adm Pharm. 2020:1–4. doi: 10.1016/j.sapharm.2020.03.015. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fast S.M., González M.C., Wilson J.M., Markuzon N. Modelling the propagation of social response during a disease outbreak. J R Soc Interface. 2015;12(104) doi: 10.1098/rsif.2014.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO . 2020. COVID-19: Operational Guidance for Maintaining Essential Health Services during an Outbreak; pp. 1–14. March. [Google Scholar]
- 13.International Pharmaceutical Federation (FIP) Updated 26 March.; 2020. Coronavirus SARS-CoV-2/COVID-19 Pandemic: Information and Interim Guidelines for Pharmacists and the Pharmacy Workforce. [Google Scholar]
- 14.Infarmed (National Authority of Medicines and Health Products, I.P.) 2019. Regulamento Gestão Da Disponibilidade Do Medicamento, Em Anexo à Deliberação n.o 93/CD/2019. Lisboa, Portugal. [Google Scholar]
- 15.DGS (General Directorate of Health) Ponto de Situação atual em Portugal - COVID-19. https://covid19.min-saude.pt/ponto-de-situacao-atual-em-portugal/ (in portuguese) Accessed April 8, 2020.
- 16.Patro S.G.K., sahu K.K. Normalization: a preprocessing stage. Iarjset. 2015:20–22. doi: 10.17148/iarjset.2015.2305. [DOI] [Google Scholar]
- 17.WHO coronavirus disease (COVID-19) dashboard. 2020. https://covid19.who.int/ Published. Accessed April 23, 2020.
- 18.World Economic Forum Coronavirus – this is how the world is responding. https://www.weforum.org/agenda/2020/03/coronavirus-this-is-how-the-world-is-responding/ Accessed May 14, 2020.
- 19.Ordinance No 90-A/2020 of April 9 . Ministry of Health; Lisbon, Portugal: 2020. Cria Um Regime Excecional e Temporário Relativo à Prescrição Eletrónica de Medicamentos e Respetiva Receita Médica, Durante a Vigência Do Estado de Emergência. (in Portuguese) [Google Scholar]
- 20.European Commission . 2020. Communication from the Commission. Guidelines on the Optimal and Rational Supply of Medicines to Avoid Shortages during the COVID-19 Outbreak. 8.4.2020, COM. 2272 Final. Brussels, Belgium; 2020. [Google Scholar]
- 21.Infarmed (National Authority of Medicines and Health Products, I.P.) 2020. Circular Normativa 001/CD/100.20.200. Orientações Técnicas Para Farmácias No Âmbito Da Pandemia COVID-19. (in Portuguese). Lisboa, Portugal. [Google Scholar]
- 22.Infarmed (National Authority of Medicines and Health Products, I.P.) 2020. Circular Informativa 066/CD/100.2.200 Atualização Da Lista de Medicamentos Abrangidos Pela Notificação Prévia de Exportação Ou Distribuição Para Outros Estados Membros. (in Portuguese). Lisboa, Portugal. [Google Scholar]
- 23.Hyde R. Europe faces worsening medicine shortages. Lancet. 2020;395(10223):481–482. doi: 10.1016/S0140-6736(20)30354-8. [DOI] [PubMed] [Google Scholar]
- 24.Joint stakeholder paper . 2019. Addressing the Root Causes of Medicines Shortages. Supply Chain Stakeholders' Views on Root Causes and Solutions. [Google Scholar]
- 25.Infarmed (National Authority of Medicines and Health Products, I.P.). InfoMed - Medicinal Products Database.
- 26.Nogueira P.J., Nobre M.D.A., Nicola P.J., Furtado C., Vaz Carneiro A. Excess mortality estimation during the COVID-19 pandemic: preliminary data from Portugal. Acta Med Port. 2020;33(13) doi: 10.20344/amp.13928. [DOI] [PubMed] [Google Scholar]
- 27.DGS (General Directorate of Health) Mortality surveillance. 2020. https://evm.min-saude.pt/ Accessed May 23, 2020.
- 28.Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). J Gen Intern Med March 2020:1-5. doi:10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed]
- 29.Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017;9(11):1–25. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fowler A.A., Truwit J.D., Hite R.D. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. J Am Med Assoc. 2019;322:1261–1270. doi: 10.1001/jama.2019.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.European Medicines Agency (EMA) Update on treatments and vaccines against COVID-19 under development. https://www.ema.europa.eu/en/news/update-treatments-vaccines-against-covid-19-under-development Press release 31/03/2020. Accessed May 10, 2020.
- 32.Bartow R.A., Brogden R.N. Formoterol. An update of its pharmacological properties and therapeutic efficacy in the management of asthma. Drugs. 1998;55(2):303–322. doi: 10.2165/00003495-199855020-00016. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y.J., Lee S.H., Kim B.S. Combination therapy of rosuvastatin and ezetimibe in patients with high cardiovascular risk. Clin Therapeut. 2017;39(1):107–117. doi: 10.1016/j.clinthera.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Tan X., Hu J. Combination therapy for type 2 diabetes: dapagliflozin plus metformin. Expet Opin Pharmacother. 2016;17(1):117–126. doi: 10.1517/14656566.2016.1121235. [DOI] [PubMed] [Google Scholar]
- 35.Musazzi U.M., Di Giorgio D., Minghetti P. New regulatory strategies to manage medicines shortages in Europe. Int J Pharm. 2020;579(February) doi: 10.1016/j.ijpharm.2020.119171. 119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gautret P., Lagier J.-C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. 105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 38.Colson P., Rolain J.-M., Lagier J.-C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55(4) doi: 10.1016/j.ijantimicag.2020.105932. 105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.European Medicines Agency (EMA) COVID-19: chloroquine and hydroxychloroquine only to be used in clinical trials or emergency use programmes. https://www.ema.europa.eu/en/news/covid-19-chloroquine-hydroxychloroquine-only-be-used-clinical-trials-emergency-use-programmes News 01/04/2020. Accessed April 30, 2020.
- 40.LiverTox . National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD)https://www.ncbi.nlm.nih.gov/books/NBK548738/ Hydroxychloroquine. [Updated 2018 Mar 25] Accessed May 10, 2020. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.