Abstract
Background:
Cervical screening could potentially be improved by better stratifying individual risk for the development of cervical cancer or precancer, possibly even allowing follow-up of individual patients differently than proposed under current guidelines that focus primarily on recent screening test results. We explore the use of a Bayesian decision science model to quantitatively stratify individual risk for the development of cervical squamous neoplasia.
Materials and Methods:
We previously developed a dynamic multivariate Bayesian network model that uses cervical screening and histopathologic data collected over 13 years in our system to quantitatively estimate the risk of individuals for the development of cervical precancer or invasive cervical cancer. The database includes 1,126,048 liquid-based cytology test results belonging to 389,929 women. From-the-vial, high risk human papilloma virus (HPV) test results and follow-up gynecological surgical procedures were available on 33.6% and 12% of these results (378,896 and 134,727), respectively.
Results:
Historical data impacted 5-year cumulative risk for both histopathologic cervical intraepithelial neoplasia 3 (CIN3) and squamous cell carcinoma (SCC) diagnoses. The risk was highest in patients with prior high grade squamous intraepithelial lesion cytology results. Persistent abnormal cervical screening test results, either cytologic or HPV results, were associated with variable increasing risk for squamous neoplasia. Risk also increased with prior histopathologic diagnoses of precancer, including CIN2, CIN3, and adenocarcinoma in situ.
Conclusions:
Bayesian modeling allows for individualized quantitative risk assessments of system patients for histopathologic diagnoses of significant cervical squamous neoplasia, including very rare outcomes such as SCC.
Keywords: Bayesian network modeling, cervical cancer screening, risk stratification
INTRODUCTION
Cervical screening strives to prevent cervical cancer and minimize morbidity and mortality while also limiting harms and controlling costs.[1] Despite success primarily in preventing cervical squamous carcinoma (SCC), cervical cancer has never been completely eradicated in any system with an estimated lifetime risk of developing cervical cancer of 0.6% based on 2013–2015 data from the Surveillance, Epidemiology, and End Results program.[2]
In August 2018, the US Preventive Services Task Force published their latest recommendations on cervical screening.[1] These recommendations were based on a literature review of the current evidence including clinical trials and cohort studies in addition to a decision analysis model to evaluate the benefits and harms of various cervical screening strategies. In the microsimulation model, the variables included screening start and stop ages, frequency, coverage, and triage testing based on recent test results.[3]
More recently, preliminary American Society for Colposcopy and Cervical Pathology (ASCCP) Risk-Based Management Consensus Guidelines have been released for public comment (http://www.asccp.org/consensus-guidelines). The new guidelines use both current and past results to estimate risk for histopathologic cervical intraepithelial neoplasia 3 (CIN3) or cervical cancer (CIN3+) as the endpoint for risk. Prior high-risk human papilloma virus (HPV) test results and/ or history of precancer (defined as histopathologic CIN2/3) are the most important risk stratifiers, but prior cytology results are not included.
The Pittsburgh Cervical Cancer Screening Model (PCCSM) is a dynamic Bayesian network able to assess patient risk for cervical precancer and cervical invasive cancer.[4,5,6,7] Unlike most current screening guideline formulations, risk stratifications utilizing the PCCSM are able to take into consideration extended cervical screening histories, including multiple cytology and HPV test results, earlier cervical biopsy findings, HPV vaccination history, and other clinical variables extending well beyond recent screening test results. The PCCSM is capable of quantitative risk assessment for critically important but uncommon histopathologic outcomes such as invasive cervical cancer.[7]
Cervical screening and follow-up could potentially be enhanced by better stratifying individual risk for the development of cervical cancer or precancer, possibly even allowing follow-up of individual patients differently than proposed under guidelines that focus on recent screening test results. In this study, we explore the use of a Bayesian decision science model to quantitatively stratify individual risk of patients in our system for the development of cervical squamous neoplasia.
MATERIALS AND METHODS
The database used in this study is cervical screening data collected at our institution over 13 years (between January 2005 and August 2017). It includes 1,126,048 liquid-based cytology (LBC) ThinPrep Pap test[8] (Hologic Corp., Bedford, MA, USA) results from 389,929 women. The Food and Drug Administration (FDA) approved from the PreservCyt vial high risk (hr) HPV test results and follow-up gynecological surgical procedures were available on 33.6% and 12% of these results (378,896 and 134,727), respectively. hrHPV tests used included the Digene Hybrid Capture 2 HPV test[9] (through May 2013) (Qiagen Corp., Gaithersburg, Maryland, USA), the Cervista HR HPV test[10] (June 2013 to May 2015) (Hologic Corp., Madison, Wisconsin, USA), and the Aptima HPV test[11] (since June 2015) (Hologic/Gen-Probe, San Diego, CA, USA). The 2001 Bethesda System[12] was used to report cytology results, and screening utilized the ThinPrep Imaging System (Hologic Corp., Bedford, MA, USA).[13]
In addition to cervical cancer screening test results and subsequent diagnostic or therapeutic procedures, the database also includes clinical information such as the history of sexually transmitted infections, cancers, excisional procedures, use of contraception, menstrual history, and HPV vaccine status, as well as demographic variables such as age and race. The data were analyzed utilizing the PCCSM, a dynamic multivariate Bayesian network model that was constructed in GeNIe and Structural Modeling, Inference, and Learning Engine, a development environment for creating and reasoning in graphical probabilistic models developed at the Decision Systems Laboratory, University of Pittsburgh (http://www. bayesfusion.com). The model’s prospective risk projections are based not only on recent test results but also take into account historical and demographic data and the impact of system treatments to allow a personalized risk assessment over varying periods of follow-up time for selected diagnostic endpoints.[7] In this study, the PCCSM used cervical screening test results, clinical history, and histopathologic data collected over 13 years in our system to quantitatively estimate the risk of individuals for the development of squamous cervical precancer (CIN3) or invasive cervical cancer (SCC).
RESULTS
Using the PCCSM model, cumulative 5-year risk assessments with varying patient histories were determined for histopathologic outcomes of CIN3 and SCC [Figures 1 and 2]. Historical data affected 5-year cumulative risk for both CIN3 and SCC; however, the levels of risk were as expected consistently much lower for SCC than for CIN3. The greatest risk for subsequent histopathologic diagnoses of both CIN3 and SCC were in patients with prior high-grade squamous intraepithelial lesion cytology results (23% and 2.16%, respectively). Persistent abnormal cervical screening test results, either abnormal cytology results and/or HPV-positive results, were associated with increasing risk for subsequent documentation of squamous neoplasia. For example, patients with three persistently negative cytology results and occasional positive HPV (“Pap(-) x3, HPV(+), HPV(+), HPV(−)”) constituted a lower risk group than patients with persistent abnormal cytology and positive HPV results (“AGCx3, HPV(+) x3”). Similarly, patients with persistent ASCUS constituted a higher risk group than patients with only an isolated ASCUS result. Varying extended screening histories were associated with different risk estimates. For example, patients with persistent ASCUS and additional HPV information (“ASCUSx3, HPV(+) x2, HPV (NA)”) constituted a higher risk group than patients with persistent ASCUS and no HPV result available (“ASCUSx3”). Different increased quantitative risks were also documented with prior histopathologic diagnoses of precancer, including CIN2, CIN3, and adenocarcinoma in situ (AIS). The PCCSM model showed that a patient history of AGC associated with a positive HPV result (e.g. “AGCx3, HPV(+) x3” or “AGC, HPV(-) x2, HPV(+)”) has a significant impact on increasing the risk of both SCC and CIN3.
Figure 1.
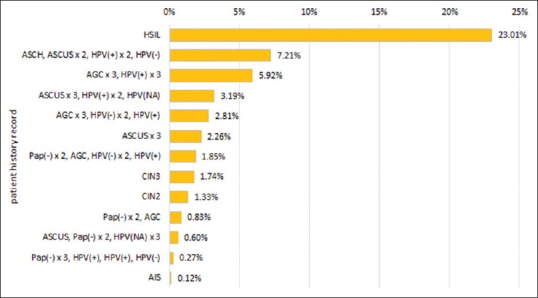
5-year cumulative risk of cervical intraepithelial neoplasia 3
Figure 2.
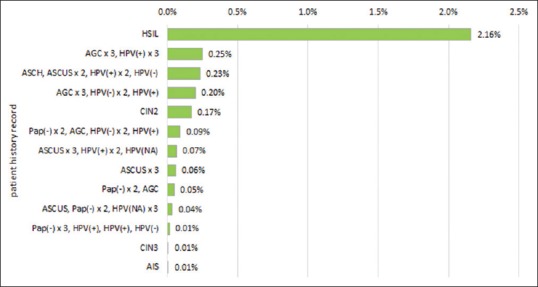
5-year cumulative risk of squamous cell carcinoma
The PCCSM model is flexible in terms of choosing a patient history. Figures 1 and 2 present only selected patient history. For example, we have not reported in Figures 1 and 2 a history of LSIL since it does not increase much the risk of CIN3 or SCC.
DISCUSSION
The quantitative effects of diverse patient historical data on future risk for cervical neoplasia have received limited attention in published cervical screening guidelines. The American College of Obstetricians and Gynecologists (ACOG) 2016 Cervical Cancer Screening and Prevention Practice Bulletin does acknowledge that its guidelines are “intended for average-risk women” and that women with other risk factors may require more frequent screening.[14] The only risk factors specifically mentioned by the ACOG Practice Bulletin include HIV infection, immunocompromised status, in utero diethylstilbestrol exposure, and previous treatment for CIN2, CIN3, or cancer.[14] The American Cancer Society, ASCCP, and American Society for Clinical Pathology guidelines also acknowledge that some subsets of women with a history of prior diagnoses of CIN2 or worse lesions (CIN2+) may need to be screened differently than women without this history.[15,16] Otherwise, the focus of published guidelines has been on current cervical screening test results utilizing cytology and, when available, adjunctive HPV reflex testing or co-testing. The identification of patients with complex cervical screening and treatment histories who may require more intensive screening or follow-up than advised by guidelines “intended for average risk women” is left to the clinical judgment of individual treating physicians. The Bayesian modeling tool allows us to compare projected risk levels for cervical precancer or cancer as part of individualized patient assessments as an aid in judging whether or not a specific patient may exceed average risk levels that could merit more intense screening or follow-up. The modeled individualized risk assessments also have the ability to take into account complex long-term screening and treatment histories that are beyond the scope of current guidelines.
All published US guidelines have been based on broad literature reviews and previously published studies. International datasets, however, often reflect screening methods and organized screening system protocols that differ significantly from what occurs in the US, making their application to the US setting challenging. The ASCCP group has in the past largely focused on accumulating data from the large US Kaiser Permanente Northern California (KPNC) Medical Care Plan.[16,17,18] The prior reliance of ASCCP guidelines developers on KPNC risk projections has now been recognized as potentially misleading, and recently released preliminary ASCCP guidelines have at least acknowledged that data from more diverse sources need to be taken into consideration. In addition to representing a relatively affluent well-screened population, KPNC has also long followed a number of cervical screening practices that are unusual in the US. First, in relying exclusively on the conventional Pap smear until 2009, KPNC may have been the last large US system to adopt LBC.[19] Second, KPNC has collected Pap and HPV specimens as two separate specimens rather than utilizing from-the-vial HPV testing as recommended by the FDA in clinical trials.[20,21] The Bayesian risk assessments generated by the PCCSM model reflect the use of the most widely utilized FDA-approved LBC methods and FDA-approved from-the-vial HPV testing. Furthermore, the risk assessments reflect follow-up and treatment protocols most commonly followed in our own integrated health system. Any system interested in employing this modelling approach can also have its own data entered into the model for system-specific adjustment of risk projections.
Our results are consistent with broad literature indicating the effect of selected prior history factors on cervical squamous neoplasia risk, such as different screening intervals which may be employed and prior diagnoses and treatment for CIN3.[22,23] Some risk estimates associated with prior preinvasive histopathologic diagnosis were lower than reported in the literature (0.01% 5-year cumulative risk of developing SCC after CIN3 diagnosis). We believe the best explanation is that the Bayesian risk projections reflected both the extent to which patients are lost to follow-up in our system and also the combined risk of both disease progression and the success or failure of ablative treatments. The worldwide success of cervical cytologic screening to date has been largely due to the ability of cervical screening to detect and ablate squamous precancerous lesions associated with the development of cervical squamous carcinoma.[24,25,26] This success in preventing cervical squamous carcinoma has largely dominated assessments of the value of different screening techniques and intervals and the development of screening policies derived from these assessments. Therefore, we believe it is an advantage of the Bayesian modelling tool that we can easily consider separately risk for squamous and glandular cervical neoplasia. Observations on cervical glandular neoplasia are the subject of other ongoing Bayesian investigations. Cervical screening has been significantly less able to prevent the development of cervical adenocarcinoma, and the chief clinical benefit of cervical screening in the area of glandular neoplasia has been the ability of cervical screening to detect early stage treatable cervical adenocarcinomas when they can still be cured.[26,27,28]
The clinical relevance of projected relative risk levels varies with different clinical endpoints. As we note in this study and have previously reported,[7] the lowest projected Bayesian risk estimates are for invasive cervical carcinoma while the clinical significance of invasive cancer risk to both the treating physician and the patient is higher than for other endpoints such as CIN2, CIN3, or even CIN3+. Since the individualized risk projections generated by our model are based on our own system data, the risk projections will be considered by system clinicians in light of experience in our system, their general clinical experience, and available clinical guidelines.
Several widely cited simulation modeling approaches have attempted to estimate the natural history of cervical cancer and have been used in developing screening guidelines.[3,15,16,29] It is not widely appreciated, however, that these simulation models have largely relied on the detection in clinical trials of prevalent CIN3 (detection sensitivity) as the key measure of effective screening test performance. The effectiveness of any screening method in preventing invasive cancer, however, is dependent on the ability of screening to detect the much smaller subset of potentially precancerous changes that will actually progress to cervical cancer (screening sensitivity).[30] Detection of this subset can only be measured indirectly using the interval cancer method. The only randomized controlled clinical cervical screening trial designed to measure screening sensitivity came to different conclusions than all other trials,[31] but this difference has not been emphasized in any of the widely used simulation models which have focused on trial data measuring detection sensitivity. The Bayesian model is able to focus on the subset of screened patients in our system who have actually progressed to cervical cancer and identify prior history findings and risk factors within this unique subset.
Another limitation of classical statistical approaches is the inability to handle incomplete data. In the clinical scenario, extended patient history is often either not available or incomplete at the time of diagnosis. With the Bayesian network model, not all information on a patient needs to be observed to calculate a risk value. This is a property that distinguishes Bayesian network analysis from classical statistical approaches where no missing values among covariates are allowed.[7]
CONCLUSIONS
The PCCSM allows for individualized quantitative risk assessments of system patients for histopathologic diagnoses of significant cervical squamous neoplasia, including very rare outcomes such as SCC. Bayesian risk modeling could prove to be of use as a personalized aid in identifying patients at above average risk and in considering patient follow-up and treatment options. We encourage its wider use in the analysis of medical data specifically while developing cervical screening guidelines.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Available FREE in open access from: http://www.jpathinformatics.org/text.asp?2020/11/1/9/281607
REFERENCES
- 1.Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, et al. US Preventive Services Task Force. Screening for cervical cancer: US preventive services task force recommendation statement. JAMA. 2018;320:674–86. doi: 10.1001/jama.2018.10897. [DOI] [PubMed] [Google Scholar]
- 2.Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, et al. Bethesda, MD: National Cancer Institute; 2018. SEER Cancer Statistics Review, 1975-2015. https://seercancergov/csr/1975_2015/ based on November 2017 SEER data submission, posted to the SEER web site, April. [Google Scholar]
- 3.Kim JJ, Burger EA, Regan C, Sy S. Screening for cervical cancer in primary care: A decision analysis for the us preventive services task force. JAMA. 2018;320:706–14. doi: 10.1001/jama.2017.19872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austin RM, Onisko A, Druzdzel MJ. The Pittsburgh cervical cancer screening model: A risk assessment tool. Arch Pathol Lab Med. 2010;134:744–50. doi: 10.5858/134.5.744. [DOI] [PubMed] [Google Scholar]
- 5.Onisko A, Druzdzel MJ, Austin RM. How to interpret the results of medical time series data analysis: Classical statistical approaches versus dynamic Bayesian network modeling. J Pathol Inform. 2016;7:50. doi: 10.4103/2153-3539.197191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin RM, Onisko A. Increased cervical cancer risk associated with extended screening intervals after negative human papillomavirus test results: Bayesian risk estimates using the Pittsburgh cervical cancer screening model. J Am Soc Cytopathol. 2016;5:9–14. doi: 10.1016/j.jasc.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Onisko A, Druzdzel MJ, Austin RM. Application of Bayesian network modeling to pathology informatics. Diagn Cytopathol. 2019;47:41–7. doi: 10.1002/dc.23993. [DOI] [PubMed] [Google Scholar]
- 8.Lee KR, Ashfaq R, Birdsong GG, Corkill ME, McIntosh KM, Inhorn SL. Comparison of conventional Papanicolaou smears and a fluid-based, thin-layer system for cervical cancer screening. Obstet Gynecol. 1997;90:278–84. doi: 10.1016/S0029-7844(97)00228-7. [DOI] [PubMed] [Google Scholar]
- 9.Terry G, Ho L, Londesborough P, Cuzick J, Mielzynska-Lohnas I, Lorincz A. Detection of highrisk HPV types by the hybrid capture 2 test. J Med Virol. 2001;65:155–62. [PubMed] [Google Scholar]
- 10.Einstein MH, Martens MG, Garcia FA, Ferris DG, Mitchell AL, Day SP, et al. Clinical validation of the Cervista HPV HR and 16/18 genotyping tests for use in women with ASC-US cytology. Gynecol Oncol. 2010;118:116–22. doi: 10.1016/j.ygyno.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Iftner T, Becker S, Neis KJ, Castanon A, Iftner A, Holz B, et al. Head-to-head comparison of the rna-based aptima human papillomavirus (HPV) assay and the DNA-based hybrid capture 2 HPV test in a routine screening population of women aged 30 to 60 years in Germany. J Clin Microbiol. 2015;53:2509–16. doi: 10.1128/JCM.01013-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, et al. The 2001 Bethesda system: Terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–9. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 13.Davey E, d’Assuncao J, Irwig L, Macaskill P, Chan SF, Richards A, et al. Accuracy of reading liquid based cytology slides using the ThinPrep Imager compared with conventional cytology: Prospective study. BMJ. 2007;335:31. doi: 10.1136/bmj.39219.645475.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Committee on Practice Bulletins—Gynecology. Practice bulletin no.168: Cervical cancer screening and prevention. Obstet Gynecol. 2016;128:e111–30. doi: 10.1097/AOG.0000000000001708. [DOI] [PubMed] [Google Scholar]
- 15.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147–72. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17:S1–S27. doi: 10.1097/LGT.0b013e318287d329. [DOI] [PubMed] [Google Scholar]
- 17.Castle PE, Fetterman B, Thomas Cox J, Shaber R, Poitras N, Lorey T, et al. The age-specific relationships of abnormal cytology and human papillomavirus DNA results to the risk of cervical precancer and cancer. Obstet Gynecol. 2010;116:76–84. doi: 10.1097/AOG.0b013e3181e3e719. [DOI] [PubMed] [Google Scholar]
- 18.Demarco M, Lorey TS, Fetterman B, Cheung LC, Guido RS, Wentzensen N, et al. Risks of CIN 2+, CIN 3+, and cancer by cytology and human papillomavirus status: The foundation of risk-based cervical screening guidelines. J Low Genit Tract Dis. 2017;21:261–7. doi: 10.1097/LGT.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katki HA, Schiffman M, Castle PE, Fetterman B, Poitras NE, Lorey T, et al. Five-year risks of CIN 3 + and cervical cancer among women with HPV-positive and HPV-negative high-grade Pap results. J Low Genit Tract Dis. 2013;17:S50–5. doi: 10.1097/LGT.0b013e3182854282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiffman M, Kinney WK, Cheung LC, Gage JC, Fetterman B, Poitras NE, et al. Relative performance of HPV and cytology components of cotesting in cervical screening. J Natl Cancer Inst. 2018;110:501–8. doi: 10.1093/jnci/djx225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Department of Health and Human Services Food and Drug Administration. Rockville, MD: Food and Drug Administration; 2011. Guidance for Industry and Food and Drug Administration Staff: Establishing the Performance Characteristics of In vitro Diagnostic Devices for the Detection or Detection and Differentiation of Human Papillomaviruses. [Google Scholar]
- 22.Miller MG, Sung HY, Sawaya GF, Kearney KA, Kinney W, Hiatt RA. Screening interval and risk of invasive squamous cell cervical cancer. Obstet Gynecol. 2003;101:29–37. doi: 10.1016/s0029-7844(02)02454-7. [DOI] [PubMed] [Google Scholar]
- 23.Strander B, Andersson-Ellström A, Milsom I, Sparén P. Long term risk of invasive cancer after treatment for cervical intraepithelial neoplasia grade 3: Population based cohort study. BMJ. 2007;335:1077. doi: 10.1136/bmj.39363.471806.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: A 35-year population-based analysis. J Womens Health (Larchmt) 2012;21:1031–7. doi: 10.1089/jwh.2011.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunnell AS, Ylitalo N, Sandin S, Sparén P, Adami HO, Ripatti S. A longitudinal Swedish study on screening for squamous cell carcinoma and adenocarcinoma: Evidence of effectiveness and overtreatment. Cancer Epidemiol Biomarkers Prev. 2007;16:2641–8. doi: 10.1158/1055-9965.EPI-07-0278. [DOI] [PubMed] [Google Scholar]
- 26.Kinney W, Sawaya GF, Sung HY, Kearney KA, Miller M, Hiatt RA. Stage at diagnosis and mortality in patients with adenocarcinoma and adenosquamous carcinoma of the uterine cervix diagnosed as a consequence of cytologic screening. Acta Cytol. 2003;47:167–71. doi: 10.1159/000326498. [DOI] [PubMed] [Google Scholar]
- 27.Vizcaino AP, Moreno V, Bosch FX, Muñoz N, Barros-Dios XM, Parkin DM. International trends in the incidence of cervical cancer: I. Adenocarcinoma and adenosquamous cell carcinomas. Int J Cancer. 1998;75:536–45. doi: 10.1002/(sici)1097-0215(19980209)75:4<536::aid-ijc8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 28.Sasieni P, Castanon A, Cuzick J. Screening and adenocarcinoma of the cervix. Int J Cancer. 2009;125:525–9. doi: 10.1002/ijc.24410. [DOI] [PubMed] [Google Scholar]
- 29.Lew JB, Simms KT, Smith MA, Hall M, Kang YJ, Xu XM, et al. Primary HPV testing versus cytology-based cervical screening in women in Australia vaccinated for HPV and unvaccinated: Effectiveness and economic assessment for the National Cervical Screening Program. Lancet Public Health. 2017;2:e96–e107. doi: 10.1016/S2468-2667(17)30007-5. [DOI] [PubMed] [Google Scholar]
- 30.Hakama M, Pokhrel M, Malina N, Hakulinen T. Sensitivity effect and overdiagnosis in screening for cancers with detectible preinvasive phase. Int J cancer. 2014;136:928–35. doi: 10.1002/ijc.29053. [DOI] [PubMed] [Google Scholar]
- 31.Malila N, Leinonen M, Kotaniemi-Talonen L, Laurila P, Tarkkanen J, Hakama M. The HPV test has similar sensitivity but more overdiagnosis than the Pap test: A randomised health services study on cervical cancer screening in Finland. Int J Cancer. 2013;132:2141–7. doi: 10.1002/ijc.27850. [DOI] [PubMed] [Google Scholar]


