Abstract
Pathology departments must rise to new staffing challenges caused by the coronavirus disease-19 pandemic and may need to work more flexibly for the foreseeable future. In light of this, many pathologists and departments are considering the merits of remote or home reporting of digital cases. While some individuals have experience of this, little work has been done to determine optimum conditions for home reporting, including technical and training considerations. In this publication produced in response to the pandemic, we provide information regarding risk assessment of home reporting of digital slides, summarize available information on specifications for home reporting computing equipment, and share access to a novel point-of-use quality assurance tool for assessing the suitability of home reporting screens for digital slide diagnosis. We hope this study provides a useful starting point and some practical guidance in a difficult time. This study forms the basis of the guidance issued by the Royal College of Pathologists, available at: https://www.rcpath.org/uploads/assets/626ead77-d7dd-42e1-949988e43dc84c97/RCPath-guidance-for-remote-digital-pathology.pdf.
Keywords: Digital pathology, home reporting, patient safety, remote reporting, technical specifications
PURPOSE OF THIS GUIDANCE
This guidance document outlines the recommendations of the Royal College of Pathologists' Digital Pathology Committee regarding temporary remote reporting of digital slides in times of clinical and service necessity.[1] This is UK guidance and other jurisdictions may be able to adopt similar principles, but should assess the suitability and safety of remote digital pathology diagnosis themselves on a case-by-case basis.
It is intended as a practical guide to support the safe use of digital pathology until further evidence and practical evaluation is undertaken. It does not replace existing guidance on training, validation, and reporting of digital cases in the department under normal circumstances. The scope of the guidance includes preliminary assessment and primary diagnosis of standard histology cases, including hematoxylin and eosin-stained sections, special stains, and immunohistochemistry. Robotic telepathology is not covered in this document, although similar principles of risk assessment could be applied in this area.
In light of the evolving coronavirus pandemic, the General Medical Council (the body which regulates doctors in the UK) has issued guidance[2] recognizing that primary and secondary care and public health services in the United Kingdom will be put under extreme pressure, exacerbated by staff shortages. The Council acknowledges that this will require temporary changes to practice, and that regulator will take this into account. In particular, it emphasizes that health-care professionals will need to be flexible and may need to work in unfamiliar circumstances or surroundings.
The key messages from the Royal College of Pathologists are:
Existing College Guidance affirms that it is safe to use digital pathology with appropriate experience, risk assessment, and risk reduction
Validation is a self-directed learning process by which pathologists learn how to diagnose digitally, based on comparison with the glass slides
Pathologists who have fully validated already will be confident in working remotely, possibly on lower specification equipment, and be very comfortable with assessing risk and making decisions on digital, sometimes in suboptimal conditions
Pathologists who have limited or no validation, or who have not used digital pathology before will find that they can confidently report some or many cases digitally, without undertaking a formal 1–2 month validation comparing glass and digital, but should be aware of the risks and mitigate this risk where possible
In exceptional circumstances, they may decide to report cases digitally, using a risk mitigation approach – this does not remove the need for validation or quality assurance (QA) once normal services are being provided.
BACKGROUND
Adoption of digital pathology for clinical use is novel, with only a handful of departments across the world currently using digital pathology for primary diagnosis.[3,4,5] In all pathology diagnosis, there is a need to maintain clinical standards and patient safety, and patients deserve the highest standards of diagnosis in all situations.
In some circumstances, the pathologists may need to make diagnoses in a less ideal setting, such as with reduced clinical information or using different equipment. Many pathologists are familiar with using microscopes at home which are not as highly specified as those at work. Evaluating and balancing risks is a routine part of a pathologist's job – deciding when to get a second opinion or order further work from the laboratory, for example. These same practical principles of risk assessment and risk reduction can be applied to remote use of digital pathology.
A combination of departmental policy and standard operating procedures can provide a method for the safe introduction of digital pathology. This includes a few key principles including basing decisions on the evidence available in the literature, a risk assessment of digital reporting, training in using the system, validation with the glass slide to develop confidence in reporting (and provide evidence of this), and risk reduction strategies.
This general approach is detailed in the Royal College of Pathologists guidance for digital pathology implementation.[3] In some departments, digital pathology reporting is further standardized through the use of uniform workstations including (at some sites) “medical grade displays.” This approach was developed based on several years of work and now underpins the national guidance in the UK and Sweden.
“Validation” to practice with digital pathology is a learning process which typically takes a number of weeks or months using the technology, and comparing with glass slides, to complete. A full validation procedure may be difficult to complete in times of emergency. We have tried to provide all the information we can to allow pathologists and departments to perform a risk assessment and make their own decisions regarding home reporting.
More work is also needed to develop a formal process for remote reporting, including the development of validation processes for remote reporting, and establishing the minimum specifications for the workstation and display required. Some of this needs to be addressed by a program of work to examine home working; some may require basic research to answer. When working remotely, few pathology departments have provided “home workstations” of similar specification to those used on-site, which may be a short-term impediment to full remote digital reporting. In other specialties, provision of home workstations to enable flexible or off-site review of radiology images is accepted practice.
However, it is recognized that there are occasions on which remote reporting of cases may be necessary for clinical or practical reasons. Worldwide, several pathologists report successful use of workstations of various specifications, both on-site and remotely. Access to digital images during urgent or unusual circumstances offers significant clinical value, e.g., maintaining a service or providing an urgent second opinion.
The authors recognize that while the evidence and experience is still accumulating with appropriate precautions and risk assessment/risk reduction, it is possible to use the technology to facilitate these clinical or practical needs.
The existing Royal College of Guidelines for digital pathology and guidelines on working remotely also provide some guidance in this area.[6,7]
PRINCIPLE AND METHOD
Where there is demonstrable clinical and service necessity, and the agreement of the Clinical Lead (departmental medical management) has been obtained, pathologists may elect to report digital slides remotely. Key points of the procedure are summarized in Box 1. They will need to ensure they have read any local standard operating procedures or guidance available and be familiar with national guidance.
Box 1.
Key steps for emergency remote reporting
| Seek approval of clinical lead/departmental manager |
| Access available local and national guidance on digital pathology and remote reporting |
| Ensure you have VPN connection to the departmental slide archive, and phone contact with the laboratory |
| Consider the need for a validation procedure, if the pathology has not already validated their digital pathology diagnosis |
| Be aware of the technical limitations of your home display |
| Risk assess digital diagnosis on a case by case basis |
| Mitigate and explain risk where necessary eg. seek second opinion, inform clinician of risk |
| Review the need for remote diagnosis regularly |
VPN: Virtual private network
The pathologist will need to access the departmental slide archive/image management software using secure remote access (e.g., a virtual private network [VPN]). Ideally, they should also have access to the Laboratory Information System, so they can review relevant clinical information and report directly from home. Ideally, for large-scale home reporting, cases will need to be tracked through the laboratory. The pathologist will need to be able to contact the office and laboratory directly by phone or E-mail as appropriate, as well as the requesting clinician.
The pathologist will need to assess the risk of making a digital diagnosis on a case-by-case basis and should exercise caution based on their assessment of risk. They may consider a remote diagnosis to be a preliminary or interim diagnosis, deferring definitive reporting until they have access to digital or glass slides on-site.
Pathologists should be aware that the technical specifications of the display (including luminance and resolution and contrast ratio) can affect the quality of the image and ease of use. More challenging diagnoses can be difficult on lower specification displays. The environment should also be considered, with the positioning and degree of natural light impacting image assessment.
The scope of remote/home digital reporting should be clearly defined, with particular differentiation made between primary diagnosis, secondary review/multi-disciplinary team meeting (MDT) review, and immunohistochemistry/auxiliary test review, which bear different levels of risk.
A risk evaluation should be performed to determine the types of case suitable for remote reporting, and those that should be reviewed again on-site, reported on-site, or deferred to glass.
The pathologist may consider lowering their threshold for requesting second/consensus opinion from colleagues, who may be working in the department, or remotely.
Depending on their risk assessment of a case, the pathologist may wish to convey this risk to the requesting clinician, either verbally, or within the report. For example:
“This diagnosis was made on a nonclinical system at a remote site, to expedite giving a rapid opinion, but this diagnosis is provisional and will be confirmed on second review on site.”
The need for remote reporting of digital slides should be reviewed on a regular basis with the clinical lead or medical manager of the department.
Workflows and systems appropriate to the laboratory in question need to be established such that pathologists are aware of which cases are awaiting reporting and which are urgent, for example. In addition, a mechanism for enabling the pathologist to be able to view the request form where appropriate, e.g., for authorization needs to be established.
RISK ASSESSMENT AND RISK REDUCTION
Equipment
Displays
Digital primary diagnosis at hospitals is usually completed using workstations on a fast network connection and high-quality displays, sometimes “medical grade,” which are high-contrast, high–resolution, and bright displays, which are calibrated and quality controlled. Food and Drug Administration-approved digital pathology systems include a specified display as part of the system, for example, the Philips digital pathology approval specifies a 4-megapixel color medical-grade display.[8]
It is not known what the minimum specification of display screens should be for digital pathology, or how remote/home IT systems should be quality assessed for this purpose. In contrast, in radiology, there has been extensive research on image quality and display specifications required for safe reporting – the UK Royal College of Radiologists has several evidence-based guidelines on display technology.[9] Further research is needed in this area in pathology.
with Home computers and laptops may have lower resolution, less contrast, and less consistent illumination than departmental digital pathology screens, and pathologists may find that their ability to assess certain pathological features is compromised (examples are illustrated below). This particularly applies to older machines. This lower capability of certain displays is sometimes, but not always, readily apparent to the pathologists without side-by-side comparison of some images.
Paradoxically, some modern consumer-grade displays and portable/mobile displays on high-end laptops, tablets, and phones have very high specifications and may be as good as if not better than some medical-grade displays, although they may not have the same level of quality control and calibration as a medical-grade display.
For reference, typical specifications for the medical-grade displays used at Leeds Teaching Hospitals NHS Trust and a “consumer off the shelf” display evaluated in recent research are shown in Table 1 for reference. Please note that similar specifications do not mean equivalence of the displays in terms of diagnostic accuracy – this will require further validation and/or experimental work to establish. Similarly, for long-term use, some process for calibration and QA of displays is necessary.
Table 1.
Key properties of medical and consumer grade screens compared
| Medical grade (e.g., at Leeds Teaching Hospitals NHS trust) | Consumer grade (highest preference score of nonmedical grade displays in testing)[10] | Consumer grade more suited to home working due to lower resolution and physical size | |
|---|---|---|---|
| Size (diagonal) (inches) | 31 | 32 | 24-27 |
| Resolution (Megapixels) | 6 | 8 | 3-4 |
| Contrast ratio | 1500:1 | 1000:1 | 1000:1 |
| Luminance (max) (cd/m2) | 1000 | 350 | 300 |
| Luminance (setting) (cd/m2) | 400 | 300 | 300 |
| Colour calibration | Automatic full colour calibration (sRGB <20%) | Regular calibration should be considered | Regular calibration should be considered |
| Example | Jusha C61 | Philips BDM3275UP | e.g. Dell Ultrasharp U2719D |
| Comment | High end calibrated medical grade display | Consumer grade display which got highest scores in testing[10] | Typical consumer grade display practical and affordable for remote use |
sRGB: Standard red green blue
Pathologists should be aware that reporting on a home computer or laptop with a lower specification display may represent a higher risk than reporting using the departmental digital pathology system and display.
Further guidance on home reporting and recommended minimum specifications is shown in Appendix B, which includes a link to access to a point-of-use QA tool for pathology.[11] The tool tests color accuracy, not diagnostic accuracy, and may be a useful indicator of the suitability of a particular screen for digital pathology diagnostics, but more work is needed to establish this. Figure 1 is an illustrative image of the QA tool.
Figure 1.

A Point of Use QA for pathology (POUQA).[11] The image above is for illustrative purposes only – the live link in appendix B should be used to test displays
HUMAN INTERFACE DEVICES AND SOFTWARE
Pathologists should be aware that the software and “human computer interaction devices” (i.e., mouse/trackball) used remotely may be different and less responsive from that used on-site, and this may present a challenge in navigating digital slides (e.g., screening a whole slide for rare objects such as lymphovascular invasion, or precise control of navigation along or between tissue pieces).
NETWORK CONNECTION
A digital pathology system comprises several elements from the image stored on the digital pathology storage system, through the network connection, to the user workstation and display. In hospitals, network connections are typically 100 Mbit/s to 1000 Mbit/s. This network capacity can support multiple users on high-resolution (6–8 megapixel) displays.
Remote connections can be much slower, especially if running over an encrypted “virtual private network” for security. Hospitals may also have limited bandwidth on their remote access connections, so may choose not to prioritize digital pathology as a service. At the remote site or home environment, additional barriers to the connection speed could include the performance of the internal wireless network, reducing the overall speed of the connection.
If the overall connection speed is too slow, making the use of remote digital pathology difficult for anything but a small number of cases, some sites may prefer to ship glass slides to the home site.
In the experience of the authors, a typical home broadband connection of 15–20 Mbits/s in the UK is acceptable with a lower resolution display (e.g., 2–4 megapixels); a higher resolution screen may suffer from lower performance as the connection to the digital pathology server in the hospital is insufficient to stream a higher resolution image, leading to a slower viewing experience or increased “pixelation”.
VALIDATION AND TRAINING
The Royal College of Pathologists' supports the use of digital slides to make primary diagnosis and recommends a period of training and validation.[3] Digital primary diagnosis has been individually validated by a number of pathologists at many sites worldwide.
In general, following a completed validation procedure, pathologists feel that 1%–2% of digital cases[12,13,14] require a secondary safety check on glass. Those that have not completed a validation procedure and have less experience of digital diagnosis may find more cases for which they are not confident to provide a definitive diagnosis.
Areas of diagnostic difficulty common to all specialties include, but are not limited to:
Assessment of dysplasia
Detection of metastasis and micrometastasis
Identification and assessment of mitotic figures
Identification and classification of granulocytes, particularly eosinophils
Assessment of fine nuclear detail.
A detailed list of areas of diagnostic difficult arranged by topography in presented in appendix A.[9]
Given the emergency circumstances of the coronavirus disease-19 (COVID-19) pandemic, formal prolonged training may not be possible.
In the experience of the authors, pathologists can become familiar enough to use most of the functions of a digital pathology system with minimal training, such as reading the documentation or a short “how to” document provided by the department. Some formal acknowledgment from the department that the individual is working under different circumstances would be useful.
Remote reporting of digital slides represents a higher level of risk for pathologists who have not completed and signed off a full validation procedure using the on-site system. This is because those pathologists who are “fully digital” in the department will likely be better placed to assess the risk of an individual case, based on their greater experience of comparing digital and microscope images, both during and after their validation.
DIGITAL SLIDE QUALITY
Digital slides produced in the laboratory are subject to quality control steps, but occasionally suboptimal slides are issued for pathologist review. This may be especially true with frozen sections or hand-stained sections, which may be thicker and harder to scan.
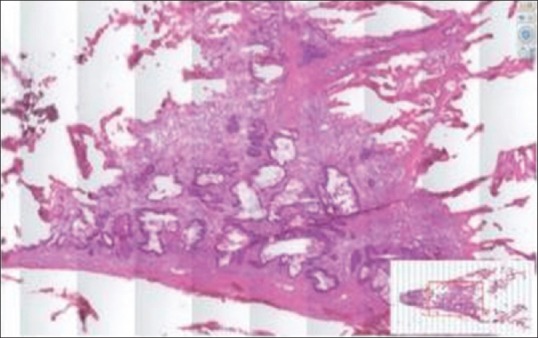
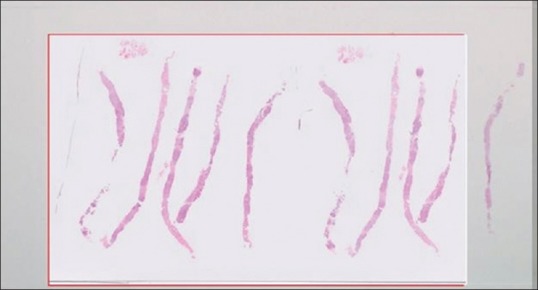
Glass slide artifacts including bubbles and tissue folds will be replicated on the digital slide, but in addition, digital slides may have “digitization artifacts” such as focal areas which are out of focus, or “striping artifact” [Figure 2]. Other artifacts include missing tissue (i.e., not all of the tissue on the slide is in the field of view on the digital image). This can affect pale slides such as those that are mainly adipose tissue more than other types of slides, but could affect other slides such as biopsies where tissue pieces are accidentally left out of the scan area [Figure 3]. This is particularly likely to occur if automated tissue detection algorithms are utilized without additional human QA steps. Pathologists need to be aware of this.
Figure 2.
Example of a digital slide with prominent striping artefact. (Note the vertical stripes cutting through the tissue image
Figure 3.
Example of a digital slide in which not all the tissue has been scanned, but the “overview image” shows the missing area
Risk may be mitigated using the following strategies:
Emphasizing importance of tissue placement of glass slides to ensure they are within the scannable margins of the slide
Default scanning of the entire scannable area of the slide for certain scenarios where missed tissue is more likely, e.g., breast biopsies, fatty tumors, smear preparations, and friable specimens
Introducing QA steps, such as human checks and adjustments of automatic scanning windows before final image acquisition
Referring to macro images of glass slides[15]
In all cases of artifact, the pathologist will need to exercise professional judgment as to whether slide quality precludes diagnosis or initial assessment of a slide. They will need to contact the laboratory to arrange re-scanning of affected slides as appropriate.
Remote reporting of suboptimal digital slides represents a higher level of risk.
REPORTING ENVIRONMENT
Environmental factors can impact upon your performance at the digital microscope. Bright ambient lighting can negatively impact on ability to use digital slides, especially if the display being used is less bright. Natural light sources are potentially more impactful than most artificial light sources, particularly on bright days. Positioning of the display in front of a window (so the user is looking at the screen and out of the window simultaneously) can inhibit performance more than other positions. A suitable blind or curtain can reduce ambient light and increase the relative luminance and contrast of the display.
The pathologists should also consider the privacy of their reporting setting and the need to maintain patient confidentiality. This guidance is intended for use in a secure home environment, the setting most likely to be utilized during the COVID-19 pandemic. Ideally, the pathologist should try to find a quiet place to work, separated from human and other distractions. Further guidance on working from home can be found in the preexisting Royal College of Pathologists' guideline.[7]
Prolonged use of display monitors can result in fatigue, and remote reporting pathologists should exercise their judgment in when to take “screen breaks.”
Pathologists reporting digitally remotely should consider the effects of ambient lighting and take regular screen breaks to avoid fatigue.
CONCLUSIONS
During periods of service need and clinical necessity, pathologists may request, or be requested, to work remotely. Digital slide reporting may help expedite assessment of urgent cases and help maintain pathology services. Pathologists should ensure they abide by any local, state, or national laws when performing home diagnosis, and ensure they have the support of their department or institution.
It is important that patients still deserve the best quality diagnosis, and the correct diagnosis, notwithstanding the crisis. Pathologists should decide if they can do that. If they cannot, and cannot issue a useful provisional diagnosis, they should not use digital pathology for that case.
If a pathologist wishes to provide diagnoses remotely using digital slides, they will need to assess the level of risk of doing so on a case-by-case basis, considering the factors outlined in this document. The scope of cases and scenarios suitable for remote reporting should be discussed beforehand, and the pathologist should use the following risk mitigating strategies where appropriate:
Deferral to glass slides
Referral for a second opinion
Request for rescanning of suboptimal slides
Informing requesting clinician of the relative risk of the assessment.
As with on-site digital reporting, ongoing QA of the process is recommended, for example, by recording any discordances noted between the remote diagnosis and the subsequent final diagnosis.
Funding acknowledgement
BW, DT, and DB are funded as part of the Northern Pathology Imaging Cooperative (NPIC). NPIC (Project no. 104687) is supported by a £50m investment from the Data to Early Diagnosis and Precision Medicine strand of the government's Industrial Strategy Challenge Fund, managed and delivered by UK Research and Innovation (UKRI). Other authors funded by this investment are GB (iCAIRD, Project no. 104690), and CV and DS (PathLAKE, Project no. 104689).
AW is funded by Yorkshire Cancer Research.
The research was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The views expressed are those of the author (s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
APPENDIXES
Appendix A.
Areas of digital diagnostic difficulty by topography[12]
| Histopathology subspecialty | Potential pitfalls |
|---|---|
| General | Identification and grading of dysplasia |
| Identification of lymph node metastasis and micrometastasis | |
| Identification and quantification of mitotic figures | |
| Identification of granulation tissue | |
| Identification of microorganisms | |
| Breast | Identification and grading of nuclear atypia |
| Identifying microinvasion and lymphovascular space invasion | |
| Identification of lobular carcinoma | |
| Grading invasive cancers (mitotic count component) | |
| Identification of Weddellite calcification Identification of sentinel lymph node metastasis/micrometastasis | |
| Skin and soft tissue | Identification and grading of squamous dysplasia |
| Microorganism detection | |
| Granulomatous inflammation | |
| Melanocytic lesions | |
| Granulocyte identification and classification | |
| Identification of sentinel node metastasis | |
| Identification of amyloid | |
| Identification of lymphoproliferative disease/malignancy | |
| Endocrine | Identification of granulomata |
| Identification of lymph node metastasis | |
| Identification of amyloid in medullary carcinoma of the thyroid classification of thyroid neoplasms- | |
| identification of cellular papillary features | |
| Identification of mitoses and atypical mitoses | |
| Genitourinary | Identification and grading of urothelial dysplasia |
| Identification of microorganisms | |
| Identification of granulomatous inflammation | |
| Identification and classification of inflammatory cells (especially granulocytes) | |
| Identification of amyloid | |
| Identification of lymphoproliferative disease/malignancy grading renal carcinoma (nuclear features) | |
| Gastrointestinal | Identification and grading of esophageal dysplasia Identification of focal activity in inflammatory bowel disease |
| Identification of eosinophils in esophageal biopsies | |
| Identification of granulomata | |
| Identification of microorganisms - particularly Helicobacter pylori | |
| Gynecological | Identifying and grading cervical dysplasia |
| Identifying metastasis/micrometastasis | |
| Assessing endometrial atypia | |
| Identifying mitotic figures (particularly in soft tissue uterine lesions) | |
| Identifying mucin | |
| Head and neck | Identification and grading of squamous dysplasia |
| Identification of microorganisms including fungal forms | |
| Identification of granulomata | |
| Identification and typing of inflammatory cells | |
| Hepatobiliary/pancreatic | Interpretation of liver special stains |
| Identification of dysplastic epithelium (particularly gall bladder) | |
| Identification and typing of inflammatory cells | |
| Neuropathology | Identification of granulomata |
| Identification and assessment of mitotic figures | |
| Identification of necrosis | |
| Identification of eosinophilic granular bodies | |
| Assessment of nuclear features | |
| Cardiothoracic | Identification of dysplasia/malignancy in small biopsy specimens |
| Identification of microorganisms including mycobacteria | |
| Identification of granulomatous inflammation | |
| Identification of micrometastasis in EBUS specimens |
APPENDIX B
SUGGESTED DISPLAY REQUIREMENTS FOR REMOTE REPORTING OF DIGITAL PATHOLOGY
In the absence of sufficient experimental work to evaluate the minimum specifications for digital pathology displays, we recommend the following display specifications as a minimum, based on a pragmatic approach and results of initial testing.[10]
Display requirements
Maximum luminance (brightness) of 350 cd/m2 or greater.
Resolution 3 Megapixels or greater (a typical microscope is an equivalent of 10 Megapixels, approximately)
A size of 24 inches or more for a desktop display provides most comfortable experience.
Display adjustment
Ideally use display curve gamma 2.2
If you can change the color space and are using a web browser to view images select sRGB
Adjust contrast and brightness using the monitor on-screen display so you can simultaneously see both the 5% black and 5% white squares
Select brightness to a comfortable level while still being able to see the 5% squares
Avoid reflections from windows/lamps, angle the screen to avoid these, preferably view in dimmed lighting conditions.
Display quality assurance
-
Check you can see all four letters on the point-of-use QA tool provided by the Northern Pathology Imaging Co-Operative[11] (http://www.virtualpathology.leeds.ac.uk/research/systems/pouqa) this should be checked regularly – e.g. every few weeks, or for each reporting session if the viewing environment has changed
If you are unable to cannot pass the POUQA test:
- Recheck the display adjustment
- If possible try another display
- Consider altering the environmental lighting (e.g. draw the blinds)
- If urgent proceed with reporting but be aware you might miss some features and you can use the software brightness and contrast controls to see into areas of concern. (On a LCD display try moving your head to the sides to increase contrast).
Use a commercial screen wipe to clean the display regularly.
(If you have no commercial screen wipes get three soft paper towels. Moisten one towel (you should not be able to easily squeeze out any drops of water) and put on a very small drop of washing up liquid, wrap this in another towel and clean the display in circular patterns, buff off with the third towel).
Footnotes
Available FREE in open access from: http://www.jpathinformatics.org/text.asp?2020/11/1/12/282725
REFERENCES
- 1.Royal College of Pathologists, United Kingdom. 2020. Mar, [Last accessed on 2020 Mar 30]. Available from: https://www.rcpath.org/uploads/assets/626ead77-d7dd-42e1-949988e43dc84c97/RCPath-guidance-for-remote-digital-pathology.pdf .
- 2.General Medical Council, United Kingdom. 2020. Mar, [Last accessed on 2020 Mar 30]. Available from: https://www.gmc-uk.org/news/newsarchive/supporting-doctors-in-theevent-of-a-covid19-epidemic-in-the-uk .
- 3.Thorstenson S, Molin J, Lundström C. Implementation of large-scale routine diagnostics using whole slide imaging in Sweden: Digital pathology experiences 2006-2013. J Pathol Inform. 2014;5:14. doi: 10.4103/2153-3539.129452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stathonikos N, Veta M, Huisman A, van Diest PJ. Going fully digital: Perspective of a Dutch academic pathology lab. J Pathol Inform. 2013;4:15. doi: 10.4103/2153-3539.114206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans AJ, Chetty R, Clarke BA, Croul S, Ghazarian DM, Kiehl TR, et al. Primary frozen section diagnosis by robotic microscopy and virtual slide telepathology: The University Health Network experience. Semin Diagn Pathol. 2009;26:165–76. doi: 10.1053/j.semdp.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Royal College of Pathologists. Best Practice Recommendations for Implementing Digital Pathology. 2018. [Last accessed on 2020 Mar 30]. Available from: https://www.rcpath.org/uploads/assets/f465d1b3-797b-4297b7fedc00b4d77e51/Best-practice-recommendations-for-implementing-digital-pathology.pdf .
- 7.Royal College of Pathologists. Royal College of Pathologists Guidelines on Working from Home. 3rd ed. United Kingdom: Royal College of Pathologists; 2014. Apr, [Last accessed on 2020 Mar 30]. Available from: https://www.rcpath.org/profession/guidelines/specialty-specific-publications.html . [Google Scholar]
- 8.Food and Drug Administration, US. FDA Allows Marketing of First Whole Slide Imaging System for Digital Pathology. 2017. [Last accessed on 2020 Mar 30]. Available from: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm552742.htm .
- 9.Royal College of Radiologists, United Kingdom. Clinical Radiology IT Guidance. [Last accessed on 2020 Mar 30]. https://www.rcr.ac.uk/clinical-radiology/being-consultant/publications/it-guidance .
- 10.Clarke E, Munnings C, Williams BJ, Brettle D, Treanor D. Display evaluation for primary diagnosis using digital pathology. doi: 10.1117/1.JMI.7.2.027501. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Point of use Quality Assurance (POUQA) for Digital Pathology Display Evaluation. [Last accessed on 2020 Mar 30]. Available from: http://www.virtualpathology.leeds.ac.uk/research/systems/pouqa/pathology/
- 12.Williams BJ, Treanor D. Practical guide to training and validation for primary diagnosis with digital pathology. J Clin Pathol. 2019 doi: 10.1136/jclinpath-2019-206319. Doi: 101136/Jclinpath-2019-206319. [DOI] [PubMed] [Google Scholar]
- 13.Williams BJ, Hanby A, Millican-Slater R, Nijhawan A, Verghese E, Treanor D. Digital pathology for the primary diagnosis of breast histopathological specimens: An innovative validation and concordance study on digital pathology validation and training. Histopathology. 2018;72:662–71. doi: 10.1111/his.13403. [DOI] [PubMed] [Google Scholar]
- 14.Williams BJ, Ismail A, Chakrabarty A, Treanor D. Clinical digital neuropathology: Experience and observations from a departmental digital pathology training programme, validation and deployment. J Clin Pathol. 2020 doi: 10.1136/jclinpath-2019-206343. [DOI] [PubMed] [Google Scholar]
- 15.Fraggetta F, Yagi Y, Garcia-Rojo M, Evans AJ, Tuthill JM, Baidoshvili A, et al. The Importance of eSlide Macro Images for Primary Diagnosis with Whole Slide Imaging. J Pathol Inform. 2018;9:46. doi: 10.4103/jpi.jpi_70_18. [DOI] [PMC free article] [PubMed] [Google Scholar]


