Abstract
OBJECTIVE
To determine the effect of tirzepatide, a dual agonist of glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 receptors, on biomarkers of nonalcoholic steatohepatitis (NASH) and fibrosis in patients with type 2 diabetes mellitus (T2DM).
RESEARCH DESIGN AND METHODS
Patients with T2DM received either once weekly tirzepatide (1, 5, 10, or 15 mg), dulaglutide (1.5 mg), or placebo for 26 weeks. Changes from baseline in alanine aminotransferase (ALT), aspartate aminotransferase (AST), keratin-18 (K-18), procollagen III (Pro-C3), and adiponectin were analyzed in a modified intention-to-treat population.
RESULTS
Significant (P < 0.05) reductions from baseline in ALT (all groups), AST (all groups except tirzepatide 10 mg), K-18 (tirzepatide 5, 10, 15 mg), and Pro-C3 (tirzepatide 15 mg) were observed at 26 weeks. Decreases with tirzepatide were significant compared with placebo for K-18 (10 mg) and Pro-C3 (15 mg) and with dulaglutide for ALT (10, 15 mg). Adiponectin significantly increased from baseline with tirzepatide compared with placebo (10, 15 mg).
CONCLUSIONS
In post hoc analyses, higher tirzepatide doses significantly decreased NASH-related biomarkers and increased adiponectin in patients with T2DM.
Introduction
The prevalence of nonalcoholic fatty liver disease (NAFLD) is ∼25% globally and ∼60–75% in patients with type 2 diabetes mellitus (T2DM) (1,2). Nonalcoholic steatohepatitis (NASH) (NAFLD with inflammation and hepatocyte injury, with or without fibrosis) can progress to cirrhosis, liver failure, hepatocellular carcinoma, and increased cardiovascular risk (3,4). T2DM increases the risk of NASH twofold (5). Weight loss through lifestyle modification reduces liver fat; weight reductions ≥10% can induce NASH resolution in most patients (6).
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) promote weight loss and may have efficacy in NASH (7). Tirzepatide, a 39-amino acid synthetic peptide, has agonist activity at both glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptors (8). In a phase 2 T2DM trial, tirzepatide significantly reduced HbA1c and body weight at 26 weeks, with greater effects than dulaglutide (tirzepatide 15 mg vs. dulaglutide 1.5 mg mean differences 1.3% and 8.6 kg, respectively). More patients treated with tirzepatide 15 mg (37.7%) achieved ≥10% weight loss than with dulaglutide 1.5 mg (9.3%) (9). These weight loss findings suggest that dual GIP and GLP-1 RAs may have greater efficacy for patients with NASH than selective GLP-1 RAs. Therefore, we explored the effect of tirzepatide on biomarkers of NASH and fibrosis in the T2DM phase 2 trial.
Research Design and Methods
Study Design and Participants
Patients from a phase 2 trial (N = 316) were included in these post hoc analyses. Full study details are published (9). Patients with T2DM (HbA1c 7.0–10.5%, inclusive; with or without stable metformin therapy) were randomized (1:1:1:1:1:1) to receive once-weekly subcutaneous tirzepatide (1, 5, 10, or 15 mg), dulaglutide (1.5 mg), or placebo for 26 weeks (Supplementary Data).
Biomarkers
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), keratin-18 (K-18) M30 fragment (hepatocyte apoptosis biomarker; VLVbio, Nacka, Sweden), procollagen III (Pro-C3) (fibrosis biomarker; Nordic Bioscience A/S, Herlev, Denmark), and total adiponectin (adipokine with antifibrogenic and antisteatogenic effects in the liver; Pacific Biomarkers Inc., Seattle, WA) were measured while fasting at baseline and after 12 and/or 26 weeks of treatment (Supplementary Data).
Statistical Methods
Statistical analyses were performed using SAS 9.4 and GraphPad Prism 8 software. Results were analyzed in a modified intention-to-treat population (excludes data after study drug discontinuation or use of glucose-lowering rescue medication) using mixed-effects model repeated-measures analysis (9). Changes from baseline data are presented as least squares mean (LSM) and SE. Treatment differences are presented as LSM and 95% CI. Multiplicity control adjustment was not performed because this was not an efficacy analysis. The contribution of relevant confounding variables to the changes in NASH-related biomarkers was assessed by stepwise variable selection followed by multiple regression analysis (Supplementary Data).
Results
Baseline demographics and clinical characteristics were similar across treatment groups (9) (Supplementary Table 1). Mean baseline concentrations of the biomarkers varied (coefficients of variation 5.4–17.7%) across treatment groups (Supplementary Table 2).
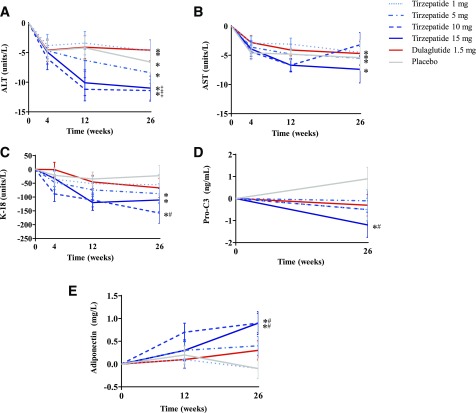
Serum ALT decreased significantly from baseline in all groups at 26 weeks (P ≤ 0.010) (Fig. 1A). Decreases with tirzepatide 10 and 15 mg were significantly greater than with dulaglutide (−6.8 units/L [95% CI −11.8, −1.8] and −6.4 units/L [−11.7, −1.1]; P = 0.008 and P = 0.018, respectively) but not compared with placebo. Serum AST decreased significantly from baseline in all groups (except tirzepatide 10 mg) at 26 weeks (P ≤ 0.033) (Fig. 1B), with no significant differences compared with placebo or dulaglutide.
Figure 1.
Change from baseline in NASH-related biomarkers ALT (A), AST (B), K-18 (C), Pro-C3 (D), and adiponectin (E) through 26 weeks in patients with T2DM. Data are LSM (SE). Modified intention-to-treat population excludes data after study drug discontinuation or use of glucose-lowering rescue medication. *P < 0.05 change from baseline; #P < 0.05 vs. placebo; ‡P < 0.05 vs. dulaglutide.
K-18 decreased significantly from baseline with tirzepatide 5, 10, and 15 mg (P ≤ 0.015) (Fig. 1C). The decrease with tirzepatide 10 mg differed significantly from placebo (−135.2 units/L [95% CI −239.0, −31.3]; P = 0.011) but not dulaglutide. Pro-C3 decreased significantly from baseline with tirzepatide 15 mg at 26 weeks (P = 0.041) (Fig. 1D); this decrease differed significantly compared with placebo (−2.1 ng/mL [−3.6, −0.6]; P = 0.007) but not dulaglutide. Total adiponectin increased significantly from baseline with tirzepatide 10 and 15 mg at 26 weeks (P < 0.001) (Fig. 1E); these increases differed significantly compared with placebo (0.9 mg/L [0.3, 1.5] and 1.0 mg/L [0.3, 1.6]; P = 0.003 and P = 0.004, respectively) but not dulaglutide.
The contributions of relevant confounding variables to the changes in NASH-related biomarkers are presented in Supplementary Tables 3–7. In patients treated with tirzepatide 10 and 15 mg, the baseline value of the biomarker explained 29–62% of the variability in changes for ALT, AST, K-18, and Pro-C3. Change from baseline in body weight was an important variable for ALT, AST, Pro-C3, and adiponectin, explaining 0.18–25.92% of the variability, and change from baseline in HbA1c was an important variable for K-18 and Pro-C3, explaining 0–7.48% of the variability in changes of biomarkers. Safety data from this study are published (9).
Conclusions
These post hoc analyses demonstrated that higher doses of the dual GIP and GLP-1 RA tirzepatide improved K-18, Pro-C3, and adiponectin compared with placebo in a T2DM population. Although patients with NASH may have normal ALT and AST levels, higher ALT levels are associated with higher grades of inflammation and steatosis (10). Dose-dependent decreases from baseline in ALT and AST were observed with tirzepatide, but these decreases were not greater than with placebo. This may reflect the trial’s sample size and the fact that only a minority of patients with T2DM have NASH (11,12). In a large pooled analysis of four clinical trials (N = 1,499), dulaglutide treatment in patients with T2DM and probable NAFLD was associated with significant decreases in ALT and AST compared with placebo (13).
Caspases cleave K-18 during hepatocyte apoptosis, leading to elevated plasma levels of K-18 in patients with NASH. For the diagnosis of NASH, the K-18 M30 fragment has pooled sensitivity and specificity of 83% and 71%, respectively; diagnostic cutoffs range from 122 to 380 units/L, with an average of ∼250 units/L (14). In this study, mean baseline K-18 levels ranged from 363.3 to 478.3 units/L. Tirzepatide 10 mg treatment significantly decreased serum K-18 levels by 135.2 units/L compared with placebo, an effect that may be clinically meaningful for the treatment of NASH. In the PIVENS trial (Pioglitazone vs. Vitamin E vs. Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis), a reduction in K-18 of 150 units/L was associated with a 1.5-fold greater chance of NASH resolution (15).
Type III collagen is synthesized and deposited during fibrogenesis. The Pro-C3 assay measures a fragment of the NH2-terminal propeptide of Pro-C3 that is cleaved during type III collagen maturation and deposition (16,17). For detection of advanced fibrosis, proposed Pro-C3 cutoffs are lower in patients with T2DM than in those with NASH (13.2 vs. 20.9 ng/mL) (16,17). In this study, mean baseline Pro-C3 ranged from 8.6 to 9.9 ng/mL, consistent with early stages of liver fibrosis. In a longitudinal NASH study, mean Pro-C3 increased with fibrosis worsening and decreased with fibrosis improvement (16). In a study of pioglitazone and vitamin E in patients with T2DM and NASH, changes in Pro-C3 and fibrosis stage were correlated; decreases in Pro-C3 associated with a one-stage improvement in fibrosis were similar to those observed with tirzepatide 15 mg (2.1 ng/mL vs. placebo) in this study (17).
Serum adiponectin levels are typically lower in patients with NAFLD compared with healthy control subjects and may decrease as simple steatosis progresses to NASH (18). In this study, baseline adiponectin values ranging from 4.0 to 5.4 mg/L were comparable to levels in patients with NASH (18). Treatment with higher doses of tirzepatide was associated with significant increases in adiponectin (21.8–26.4%), possibly because of weight loss. A 1-year lifestyle intervention study in patients with T2DM reported that 13% weight loss was associated with a 36% increase in adiponectin (19).
Although greater weight loss was achieved with higher doses of tirzepatide than with dulaglutide 1.5 mg, the improvements in K-18, Pro-C3, and adiponectin levels did not differ between treatments. However, greater decreases in ALT occurred with tirzepatide 10 and 15 mg than with dulaglutide. Clinical trials assessing liver histology will be needed to compare dual GIP and GLP-1 RAs and selective GLP-1 RAs in NASH.
This work has limitations because it is a post hoc analysis that is exploratory in nature and hypothesis generating. An unknown percentage of patients in this study had NASH, and liver fat content was not assessed. Therefore, the magnitude of the treatment effect should not be compared with studies of patients with biopsy-confirmed NASH. Baseline NASH biomarker values were not matched across treatment groups (likely because of unequal distribution of patients with NASH across groups), resulting in some inconsistency of the magnitude of changes from baseline. This may explain why the changes in the biomarkers were not consistently dose responsive. The baseline value of the biomarker was a more important determinant of the change from baseline of the biomarker than the amount of weight loss or the change in HbA1c. This may reflect the fact that this population of patients with T2DM likely included many patients who did not have NASH.
In conclusion, higher doses of the dual GIP and GLP-1 RA tirzepatide significantly improved NASH-related biomarkers in a T2DM population. Because of the limitations of this study, these results should not be interpreted as providing evidence of efficacy for tirzepatide in patients with NASH. Nonetheless, these NASH biomarker data, along with the weight loss findings (9), support further evaluation of tirzepatide in patients with NASH.
Article Information
Duality of Interest. Funding of this study is from Eli Lilly and Company. M.L.H., J.M.W., A.N., R.B., C.A.K., K.L.D., D.A.R., and A.H. are employees and shareholders of Eli Lilly and Company. A.J.S. has received consulting fees from Eli Lilly and Company. He has served as a consultant to AstraZeneca, Bristol-Myers Squibb, Nitto Denko, Ardelyx, Conatus, Nimbus, Amarin, Salix, Tobira, Takeda, Merck, Genentech, Fibrogen, Janssen, Bird Rock Bio, 89Bio, Poxel, Surrozen, Path AI, Histoindex, Gilead, Boehringer Ingelheim, Siemens, Sanofi, Lipocine, Metacrine, Cirius, Madrigal, Galectin, Blade, Pliant, Biocellvia, Glympse Bio, Fortress Diagnostics, Artham, Terns, Fractyl, Eli Lilly and Company, Zafgen, Novartis, Novo Nordisk, and Pfizer. He also has served as an unpaid consultant to Exalenz, Intercept, Echosense, Immuron, Galectin, Fractyl, NorthSea Therapeutics, Gencia, Synlogic, Afimune, ChemomAb, Nordic Bioscience, Zydus, and Bristol-Myers Squibb. His institution has received grant support from Gilead, Salix, Tobira, Bristol-Myers Squibb, Shire, Intercept, Merck, AstraZeneca, Mallinckrodt, Cumberland, and Novartis. He receives royalties from Elsevier and UptoDate. He is the president of Sanyal Biotechnology and owns stock in Genfit, Akarna, Tiziana, Indalo, and Durect. R.L. serves as a consultant or advisory board member for Arrowhead Pharmaceuticals, AstraZeneca, Bird Rock Bio, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Cirius, CohBar, Conatus, Eli Lilly and Company, Galmed, Gemphire, Gilead, Glympse Bio, GNI, GRI Bio, Intercept, Ionis, Janssen, Merck, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Prometheus, Sanofi, Siemens, and Viking Therapeutics. In addition, his institution has received grant support from Allergan, Boehringer Ingelheim, Bristol-Myers Squibb, Cirius, Eli Lilly and Company, Galectin Therapeutics, Galmed Pharmaceuticals, GE, Genfit, Gilead, Intercept, Grail, Janssen, Madrigal, Merck, NGM Biopharmaceuticals, NuSirt, Pfizer, pH Pharma, Prometheus, and Siemens. He is also co-founder of Liponexus. No other potential conflicts of interest relevant to the article were reported.
Author Contributions. M.L.H., A.J.S., R.L., J.M.W., K.L.D., and A.H. contributed to the biomarker analysis plan. M.L.H. and C.A.K. wrote the first draft of the manuscript. A.N. and R.B. were responsible for the statistical analysis. D.A.R. was responsible for the trial design and medical oversight during the trial. All authors participated in the interpretation of the data, critical review of the manuscript, and decision to submit for publication.
M.L.H. and A.H. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019, and the 55th Annual Meeting of the European Association for the Study of Diabetes, Barcelona, Spain, 16–20 September 2019.
Footnotes
Clinical trial reg. no. NCT03131687, clinicaltrials.gov
This article contains Supplementary Data online at https://doi.org/10.2337/dc20-1234/suppl.12014322.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84 [DOI] [PubMed] [Google Scholar]
- 2.Cusi K, Sanyal AJ, Zhang S, et al. Non-alcoholic fatty liver disease (NAFLD) prevalence and its metabolic associations in patients with type 1 diabetes and type 2 diabetes. Diabetes Obes Metab 2017;19:1630–1634 [DOI] [PubMed] [Google Scholar]
- 3.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–357 [DOI] [PubMed] [Google Scholar]
- 4.Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology 2017;65:1557–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loomba R, Abraham M, Unalp A, et al.; Nonalcoholic Steatohepatitis Clinical Research Network . Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology 2012;56:943–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol 2017;67:829–846 [DOI] [PubMed] [Google Scholar]
- 7.Armstrong MJ, Gaunt P, Aithal GP, et al.; LEAN Trial Team . Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016;387:679–690 [DOI] [PubMed] [Google Scholar]
- 8.Coskun T, Sloop KW, Loghin C, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab 2018;18:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 2018;392:2180–2193 [DOI] [PubMed] [Google Scholar]
- 10.Fracanzani AL, Valenti L, Bugianesi E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology 2008;48:792–798 [DOI] [PubMed] [Google Scholar]
- 11.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124–131 [DOI] [PubMed] [Google Scholar]
- 12.Portillo-Sanchez P, Bril F, Maximos M, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab 2015;100:2231–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cusi K, Sattar N, García-Pérez LE, et al. Dulaglutide decreases plasma aminotransferases in people with Type 2 diabetes in a pattern consistent with liver fat reduction: a post hoc analysis of the AWARD programme. Diabet Med 2018;35:1434–1439 [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Zhu Y, Zheng Q, Jiang J. Serum cytokeratin-18 in the diagnosis of non-alcoholic steatohepatitis: a meta-analysis. Hepatol Res 2014;44:854–862 [DOI] [PubMed] [Google Scholar]
- 15.Vuppalanchi R, Jain AK, Deppe R, et al. Relationship between changes in serum levels of keratin 18 and changes in liver histology in children and adults with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2014;12:2121–2130.e1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo Y, Oseini A, Gagnon R, et al. An evaluation of the collagen fragments related to fibrogenesis and fibrolysis in nonalcoholic steatohepatitis. Sci Rep 2018;8:12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bril F, Leeming DJ, Karsdal MA, et al. Use of plasma fragments of propeptides of type III, V, and VI procollagen for the detection of liver fibrosis in type 2 diabetes. Diabetes Care 2019;42:1348–1351 [DOI] [PubMed] [Google Scholar]
- 18.Lemoine M, Ratziu V, Kim M, et al. Serum adipokine levels predictive of liver injury in non-alcoholic fatty liver disease. Liver Int 2009;29:1431–1438 [DOI] [PubMed] [Google Scholar]
- 19.Pasarica M, Tchoukalova YD, Heilbronn LK, et al.; Look AHEAD Adipose Research Group . Differential effect of weight loss on adipocyte size subfractions in patients with type 2 diabetes. Obesity (Silver Spring) 2009;17:1976–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]