Diabetes is a serious health challenge for the aging population, contributing to morbidity and mortality primarily through increased risk for ischemic heart disease, heart failure, and stroke (1). Approximately 25–30% of adults over the age of 65 years have diabetes (2,3), and this proportion is expected to increase rapidly in the coming decades (4). Older individuals with diabetes also have higher rates of comorbidities including functional disability, accelerated muscle loss, chronic kidney disease, and coexisting cardiovascular risk factors compared with those without diabetes (3). Since multiple comorbidity among older adults with diabetes is common, individualized risk assessment to guide therapy using standard risk stratification tools has proven to be challenging. Therefore, new strategies are needed to improve risk assessment and inform use of novel and emerging therapies for patients with diabetes given important risk/benefit trade-offs between efficacy and adverse effects in the older adult population.
Incorporation of high-sensitivity cardiac troponin (hs-cTn) into risk stratification and treatment decision algorithms is one such strategy that has the potential to improve personalized diabetes care. hs-cTn–based risk stratification (using both the hs-cTnT and hs-cTnI isoforms) has been extensively investigated in multiple population settings and for multiple outcomes including coronary artery disease, heart failure, stroke, and mortality (5–8). The association is strongest for heart failure and mortality and weakest for ischemic events (6). When measured in ambulatory individuals without cardiac symptoms, hs-cTn is a measure of subclinical myocardial injury and provides independent, additional prognostic information beyond other cardiac biomarkers and diagnostic tests such as electrocardiographic left ventricular hypertrophy, N-terminal pro B-type natriuretic peptide, and coronary artery calcium (9,10). Importantly, although hs-cTn is commonly elevated beyond the detectable threshold in older adults (∼2/3 will have a level of hs-cTnT ≥3 pg/mL), in the Cardiovascular Health Study (mean age ∼73 years), prevalent diabetes was associated with a greater likelihood of elevated hs-cTnT and participants with detectable hs-cTnT had a greater comorbidity burden. Moreover, both baseline and changes in hs-cTn levels over time were linked with excess risk for incident heart failure and cardiovascular death (11). These data clearly demonstrate a pathophysiological link between subclinical myocardial injury represented by hs-cTn and medical comorbidity in older adults; however, the joint associations between cardiac troponin and comorbidity and their potential interaction regarding mortality risk among older adults with diabetes remain unanswered.
In this issue of Diabetes Care, Tang et al. (12) directly address these questions using the Atherosclerosis Risk in Communities (ARIC) study cohort visit 5, which included 1,835 older adults aged 67–89 years with self-reported or laboratory-diagnosed diabetes, self-reported or administrative assessment of medical comorbidities specified by the American Diabetes Association (2,13), and cardiac troponin T and I measured with highly sensitive assays (12). The cohort was a broad representation of older adults in the U.S.: ∼57% female, ∼31% black race, and 61% with a “high” (≥3) comorbidity burden. As hypothesized, hs-cTns were associated with greater comorbidity, and both hs-cTnT and hs-cTnI and comorbidity index were associated with higher all-cause mortality, improving discrimination of mortality risk beyond traditional risk factor models. Notably, the utility of hs-cTn was predominantly realized through downclassification of risk for mortality among those with undetectable levels. When considered jointly, both hs-cTn and comorbidity burden contributed independent prognostic risk information for mortality such that participants with high hs-cTn (≥85th percentile in the ARIC population at visit 5) and high comorbidity were at the highest risk for death, those with either high hs-cTn or high comorbidity represented an intermediate risk group, and those with low hs-cTn and low comorbidity had the lowest mortality risk.
There were several strengths of this study over prior work that add to our understanding of mortality risk in older adults with diabetes. First, the cohort is reflective of the contemporary older adult population with diabetes and study findings are likely generalizable to the aging U.S. population. Second, the investigators analyzed both hs-cTnT and hs-cTnI assays, which are known to be nonredundant and provide complementary information (14). Third, the investigators included several noncardiovascular comorbidities essential to mortality prognostication specifically in older adults such as dementia and frailty. Several limitations also merit comment. The assessment of comorbidities was relatively restricted, and other comorbid conditions with potential mortality impact (e.g., bleeding history) were not included in the analysis. Furthermore, use of a simple count of the number of comorbid conditions led to equal weighting of all comorbidities despite the fact that diseases such as stroke, heart failure, and cancer are much more important contributors to mortality compared with incontinence and arthritis. Finally, the focus on all-cause mortality as the study end point does not present a full picture of the outcomes relevant to an aging population. Future studies incorporating exercise capacity and nonfatal cardiac end points would be of additional interest.
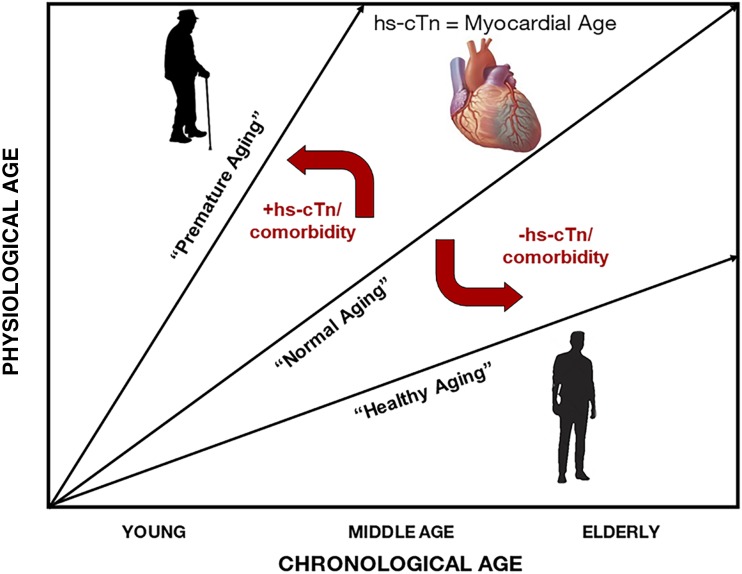
Integrating this work on mortality risk prediction with prior studies of hs-cTn, we can conceptualize a framework through which to interpret hs-cTn among older adults, including those with diabetes. It may be useful to consider hs-cTn as a biomarker of myocardial “age,” whereby individuals with undetectable levels (or levels that are lower than age- and sex-dependent normative values) can be considered “young at heart.” This concept is supported by the observations from Tang et al., where low hs-cTn downgraded risk for mortality even among older individuals with high comorbidity burden. In contrast, those with higher-than-expected hs-cTn levels may be considered to have accelerated myocardial aging. Considering these factors together, higher hs-cTn (representing pathological myocardial aging) and high medical comorbidity burden can be integrated to reflect a state of accelerated physiological aging. The presence of one or both entities can shift the trajectory of “normal” chronological age toward “premature aging” such that adverse outcomes are more likely to occur at a lower chronological age. Conversely, individuals with no significant comorbidity and/or low hs-cTn can be downgraded to lower mortality risk consistent with a more “healthy aging” trajectory (Fig. 1). This paradigm may be utilized to downward classify risk in older adults with negative troponins despite existing medical conditions. Furthermore, as novel diabetes therapies (e.g., sodium–glucose cotransporter 2 inhibitors and glucagon-like peptide 1 agonists) are emerging as important therapies for prevention of adverse cardiovascular outcomes, hs-cTns may prove useful as tools to identify older adults with diabetes likely to derive the most benefit from these risk-reducing medications. Additional study is needed, however, to evaluate this hypothesis.
Figure 1.
Relationship between physiological and chronological age and trajectory shifts by elevated hs-cTns and medical comorbidities in older adults with diabetes. The relationship between chronological and physiological age may be modified by considering high hs-cTn (representing pathological myocardial aging) and high medical comorbidity burden. The presence of one or both entities can shift the trajectory of “normal” chronological age toward “premature aging” such that adverse outcomes are more likely to occur at a lower chronological age. Conversely, individuals with low hs-cTn and/or no significant medical comorbidity can be downgraded in mortality risk consistent with a more “healthy aging” trajectory.
As prevailing evidence points to diabetes continuing to be a global chronic illness burden in aging societies (15), practitioners will need to shift focus away from age as a number toward age as a state of health. The study by Tang et al. provides further evidence that the age-risk relationship is modified by a deeper recognition of physiological/myocardial aging and that health trajectories can inform care in older adults with diabetes. Future therapeutic studies in diabetes may utilize risk modifiers such as hs-cTn in refining and selecting the best strategies to prevent and treat morbidity and mortality in the aging population.
Article Information
Funding. I.J.N. is supported by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grant K23DK106520 and by the Dedman Family Scholarship in Clinical Care from UT Southwestern.
Duality of Interest. I.J.N. reports being an investigator on a trial of liraglutide (Novo Nordisk) for obesity and being a former speaker/consultant for Boehringer Ingelheim/Lilly Alliance. J.A.d.L. has received grant support from Roche Diagnostics and Abbott Diagnostics, honoraria from participation in data and safety monitoring board committees from Novo Nordisk and Eli Lilly, and consulting income from Ortho Clinical Diagnostics. He has been named as a co-inventor on a patent issued to the University of Maryland (U.S. patent application number 15/309754) titled “Methods for Assessing Differential Risk for Developing Heart Failure.” No other potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 1200.
References
- 1.Virani SS, Alonso A, Benjamin EJ, et al.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 2020;141:e139–e596 [DOI] [PubMed] [Google Scholar]
- 2.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care 2012;35:2650–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association 11. Older adults: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S119–S125 [DOI] [PubMed] [Google Scholar]
- 4.Rothberg AE, Halter JB. Obesity and diabetes in an aging population: time to rethink definitions and management? Clin Geriatr Med 2015;31:1–15, vii [DOI] [PubMed] [Google Scholar]
- 5.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA 2010;304:2503–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation 2011;123:1367–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seliger SL, Hong SN, Christenson RH, et al. High-sensitive cardiac troponin T as an early biochemical signature for clinical and subclinical heart failure: MESA (Multi-Ethnic Study of Atherosclerosis). Circulation 2017;135:1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia X, Sun W, Hoogeveen RC, et al. High-sensitivity troponin I and incident coronary events, stroke, heart failure hospitalization, and mortality in the ARIC study. Circulation 2019;139:2642–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neeland IJ, Drazner MH, Berry JD, et al. Biomarkers of chronic cardiac injury and hemodynamic stress identify a malignant phenotype of left ventricular hypertrophy in the general population. J Am Coll Cardiol 2013;61:187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lemos JA, Ayers CR, Levine BD, et al. Multimodality strategy for cardiovascular risk assessment: performance in 2 population-based cohorts. Circulation 2017;135:2119–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA 2010;304:2494–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang O, Daya N, Matsushita K, et al. Performance of high-sensitivity cardiac troponin assays to reflect comorbidity burden and improve mortality risk stratification in older adults with diabetes. Diabetes Care 2020;43:1200–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laiteerapong N, Iveniuk J, John PM, Laumann EO, Huang ES. Classification of older adults who have diabetes by comorbid conditions, United States, 2005-2006. Prev Chronic Dis 2012;9:E100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welsh P, Preiss D, Hayward C, et al. Cardiac troponin T and troponin I in the general population. Circulation 2019;139:2754–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinclair A, Saeedi P, Kaundal A, Karuranga S, Malanda B, Williams R. Diabetes and global ageing among 65-99-year-old adults: findings from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 14 February 2020 [Epub ahead of print]. DOI: 10.1016/j.diabres.2020.10807832068097 [DOI] [PubMed] [Google Scholar]