Abstract
OBJECTIVE
To determine if temporal glucose profiles differed between 1) women who were randomized to real-time continuous glucose monitoring (RT-CGM) or self-monitored blood glucose (SMBG), 2) women who used insulin pumps or multiple daily insulin injections (MDIs), and 3) women whose infants were born large for gestational age (LGA) or not, by assessing CGM data obtained from the Continuous Glucose Monitoring in Women With Type 1 Diabetes in Pregnancy Trial (CONCEPTT).
RESEARCH DESIGN AND METHODS
Standard summary metrics and functional data analysis (FDA) were applied to CGM data from the CONCEPTT trial (RT-CGM, n = 100; SMBG, n = 100) taken at baseline and at 24- and 34-weeks’ gestation. Multivariable regression analysis determined if temporal differences in 24-h glucose profiles occurred between comparators in each of the three groups.
RESULTS
FDA revealed that women using RT-CGM had significantly lower glucose (0.4–0.8 mmol/L [7–14 mg/dL]) for 7 h/day (0800 h to 1200 h and 1600 h to 1900 h) compared with those with SMBG. Women using pumps had significantly higher glucose (0.4–0.9 mmol/L [7–16 mg/dL]) for 12 h/day (0300 h to 0600 h, 1300 h to 1800 h, and 2030 h to 0030 h) at 24 weeks with no difference at 34 weeks compared with MDI. Women who had an LGA infant ran a significantly higher glucose by 0.4–0.7 mmol/L (7–13 mg/dL) for 4.5 h/day at baseline, by 0.4–0.9 mmol/L (7–16 mg/dL) for 16 h/day at 24 weeks, and by 0.4–0.7 mmol/L (7–13 mg/dL) for 14 h/day at 34 weeks.
CONCLUSIONS
FDA of temporal glucose profiles gives important information about differences in glucose control and its timing, which are undetectable by standard summary metrics. Women using RT-CGM were able to achieve better daytime glucose control, reducing fetal exposure to maternal glucose.
Introduction
Maternal glucose is the major determinant of fetal growth, predicting large for gestational age (LGA) infants and neonatal outcomes (1). However, maternal glucose is dynamic, with glucose tolerance and insulin sensitivity varying across the 24-h day with a circadian rhythmicity (2,3). Superimposed upon this, there are the peaks and troughs in glucose that are determined by the balance between insulin resistance and lifestyle/behavioral factors, including diet, physical activity, energy expenditure, stress, sleep, and shift work. Insulin sensitivity also varies across pregnancy, with insulin resistance increasing with gestation (4). It is this dynamic glucose signal to which the fetus is exposed in pregnancy. Continuous glucose monitoring (CGM) provides the most objective method of assessing this dynamic glucose signal in daily life (5). With up to 288 interstitial fluid glucose measurements per day, CGM accurately reflects blood glucose variations (5). Although standard summary metrics are recommended for the reporting of CGM (5,6), they do not give dynamic information about the timing of glucose excursions, thereby losing much of the detailed temporal glycemic information generated. We have pioneered the application of functional data analysis (FDA) to CGM data to extract shape information and to identify glucose dysregulation that is undetectable by summary statistical measures (7,8). We found that FDA is sensitive at detecting shorter periods of relative hyperglycemia that may not be detectable by summary metrics and enables accurate definition of time periods across the 24-h day where differences in temporal glucose control occurs between groups and in relation to clinical outcomes (7,8). Detecting this variation is particularly important in the context of pregnancy where even small increases in maternal glucose are related to poorer clinical outcomes (1).
The recent Continuous Glucose Monitoring in Women With Type 1 Diabetes in Pregnancy Trial (CONCEPTT) showed that use of real-time (RT)-CGM during pregnancy in women with type 1 diabetes was associated with improved neonatal outcomes, including a lower incidence of LGA, neonatal hypoglycemia, and neonatal intensive care unit admission (9) compared with women who used only self-monitored blood glucose (SMBG). While these improvements are likely to be attributable to improved glucose control, standard CGM metrics showed no differences in mean glucose, and they showed only that pregnant RT-CGM users spent more time in the pregnancy glucose target range (3.5–7.8 mmol/L or 63–140 mg/dL) and less time hyperglycemic (9). The effect of using pumps or multiple daily insulin injections (MDIs) was also explored and unexpectedly showed that women using pumps had poorer pregnancy outcomes, with significantly more neonatal hypoglycemia and neonatal intensive care admissions (10). Standard CGM metrics showed only that pump users spent 5% more time above the glucose target range at 24 weeks’ gestation and 5% less time in the range at 24 weeks than women on MDI (10). The lack of comprehensive differences in standard CGM metrics while showing differences in neonatal outcomes suggests that there may be differences in temporal glucose profiles that were not detected by the standard CGM metrics.
The objective of the current study was therefore to perform FDA on the CGM data obtained in the CONCEPTT trial to determine if temporal differences in 24-h glucose profiles occurred between 1) women who were randomized to RT-CGM or SMBG, 2) women who used insulin pumps or MDI, and 3) women whose infants had LGA or not.
Research Design and Methods
Study Design
Full details of the CONCEPTT clinical trial protocol have previously been published (9,11). Women with type 1 diabetes were eligible if they were aged 18–40 years, had 12 months’ duration of diabetes, and were on an intensive insulin regimen using either a pump or MDI. Pregnant women had to have a live singleton fetus confirmed by ultrasound before 14 weeks’ gestational age and an HbA1c level between 6.5% and 10% (48–86 mmol/mol). After a run-in period where eligible women wore a masked CGM (iPro2 Professional CGM; Medtronic, Northridge, CA) for at least 96 h, women were randomized to the intervention, where they received an RT-CGM (Guardian REAL-Time or MiniMed MiniLink system; both Medtronic) that required calibration by SMBG, or to the control group, where they were instructed to continue with their usual SMBG testing at least seven times per day (before meals and 1 h after meals, plus before bed). The women were reviewed as per standard clinical care every 1 to 2 weeks, and algorithms were used to help patients and their teams decide on treatment adjustments in both arms. Randomization was stratified by insulin delivery system (pump or MDI) and by baseline HbA1c level (<7.5% vs. ≥7.5% or 58 mmol/mol during pregnancy). Women in the SMBG pregnant group were asked to wear a masked CGM on two further occasions at 24 and 34 weeks. RT-CGM data were obtained at 24 and 34 weeks’ gestation from the RT-CGM group for comparison. LGA was defined as birth weight ≥90th percentile using Gestation-Related Optimal Weight (GROW) software (12), which adjusts for infant sex and gestational age and maternal height, weight, parity, and ethnicity. This current analysis includes data from women who were in the pregnant arm of the original study who had complete birth weight data (n = 200) and where we had >96 h of continuous data.
Study Oversight
The study was approved by the Health Research Authority, East of England Research Ethics Committee (12/EE/0310) for all U.K. sites and at each individual center for all other sites. Participants provided written informed consent.
Standard CGM Metrics
The standard range of summary metrics was calculated for each CGM measurement period (baseline and 24 and 34 weeks’ gestation) including the following: mean CGM glucose levels, the percentage of time spent within the pregnancy glucose target range (3.5–7.8 mmol/L [63–140 mg/dL]), and time spent above (>7.8 mmol/L [>140 mg/dL]) and below (<3.5 mmol/L [<63 mg/dL]) the target range. Measures of glycemic variability (SD and coefficient of variation [CV]) of mean CGM glucose levels were calculated. Comparisons of means between groups were made using a Student t test.
Functional Data Analysis
For each individual, the mean of the four or more days of temporal CGM data obtained at each glucose time point across the 24-h day was taken. In this way, there was no missing data for performing the FDA. Each of the glucose values recorded during the measurement episodes (at baseline and at 24 and 34 weeks’ gestation) was assumed to be dependent upon (rather than independent of) the preceding glucose levels. Changes in glucose over time were therefore assumed to be progressive, occurring in a trend or sequence that could be considered smooth (in a mathematical sense) without step changes from one measurement to the next. For this reason, sequential glucose measurements from each measurement episode were modeled as trajectories by calculating continuous mathematical functions of CGM-derived glucose measurements collected every 5 min throughout that measurement episode. These trajectories were modeled using the technique of fitting B-splines to the repeated measures (7,8,13). This technique generates a polynomial function that describes the curve (or spline) used to model changes in glucose levels over time for each participant, with splines required to pass though measured glucose values at discrete time points (called knots) during each 24-h period. At each of these knots, the spline function was required to be continuous (i.e., with no breaks or step changes) so that the function remained mathematically smooth. Knots were placed at 30-min intervals over each 24-h measurement period, with data from measurements recorded during the 4 h on either side of midnight (i.e., from 2000 h to 0400 h) repeated at the beginning and end to eliminate artifactual edge effects. In this way, the splines provided a smooth mathematical function describing glucose levels recorded across each measurement episode.
Multivariable regression analysis was used for the FDA-generated glucose function to establish the relationship between maternal glucose levels in 1) women who were randomized to RT-CGM compared with those on SMBG (combining the 24- and 34-weeks data), 2) women who used insulin pumps compared with MDI (at baseline and at 24 and 34 weeks’ gestation), and 3) women whose infants had LGA compared with those that did not (at baseline and at 24 and 34 weeks’ gestation). No adjustment was made for multiple comparisons. These specific questions were defined prior to performing FDA, and CIs were used to assess the significance of the relationship. All statistical analyses were conducted in Stata (14) and R (15).
Results
CGM and neonatal outcome data were available from 200 women in the pregnant arm of the CONCEPTT trial (RT-CGM, n = 100; SMBG, n = 100). The participant characteristics are shown in Table 1.
Table 1.
Participant characteristics
| Total | Intervention | Treatment | Birth weight | ||||
|---|---|---|---|---|---|---|---|
| RT-CGM | SMBG | Pumps | MDI | LGA | Non-LGA | ||
| Number | 200 | 100 | 100 | 90 | 110 | 122 | 78 |
| BMI, kg/m2 | 25.7 ± 4.6 | 26.2 ± 5.1 | 25.2 ± 3.9 | 26.0 ± 4.8 | 25.4 ± 4.4 | 25.5 ± 4.4 | 26.0 ± 4.8 |
| Primiparous | 98 (49) | 49 (49) | 49 (49) | 42 (47) | 56 (51) | 61 (50) | 37 (47) |
| Mean gestation at birth, weeks | 36.9 ± 1.7 | 37.2 ± 1.4 | 36.8 ± 1.9 | 36.8 ± 1.8 | 37.1 ± 1.6 | 36.9 ± 1.6 | 37.1 ± 1.9 |
| Birth weight, kg | 3.56 ± 0.71 | 3.55 ± 0.65 | 3.58 ± 0.78 | 3.53 ± 0.75 | 3.59 ± 0.69 | 3.91 ± 0.58 | 3.03 ± 0.56 |
| GROW birth weight centile | 82.0 ± 25.8 | 78.4 ± 26.8 | 85.5 ± 24.4 | 79.4 ± 28.4 | 84.1 ± 23.4 | 97.8 ± 28.2 | 57.2 ± 26.2 |
Data are expressed as means ± SD or n (%). GROW, Gestation-Related Optimal Weight.
RT-CGM Versus SMBG
Standard CGM Metrics
The results of the CGM metrics are shown in Table 2A. There were no differences in mean glucose between groups at any time point across pregnancy. However, when mean glucose was calculated separately for day and night, there was a significantly higher glucose overnight at 24 weeks, with a significantly lower glucose during the day at 24 weeks. There were no differences in any other standard measures at 24 weeks. At 34 weeks, women randomized to the RT-CGM group had significantly more time in the pregnancy glucose target range and less time spent above the target compared with SMBG control subjects. Women using RT-CGM had significantly less glucose variability at 34 weeks with lower SD and CV glucose.
Table 2.
Standard summary metrics of CGM data across pregnancy comparing RT-CGM group to SMBG control group (A), pump to MDI (B), and LGA to non-LGA (C)
| A: RT-CGM group to SMBG control group | Baseline | 24 weeks | 34 weeks | |||
|---|---|---|---|---|---|---|
| CGM | SMBG | CGM | SMBG | CGM | SMBG | |
| Number | 100 | 100 | 89 | 90 | 77 | 76 |
| Glucose, mmol/L | 7.3 ± 1.2 | 7.6 ± 1.1 | 7.6 ± 1.2 | 7.8 ± 1.3 | 6.7 ± 0.9 | 7.0 ± 1.1 |
| 0001–0600 h glucose, mmol/L | 6.7 ± 1.5 | 7.1 ± 1.4 | 7.2 ± 1.4 | 7.0 ± 1.4 | 6.2 ± 1.0 | 6.3 ± 1.2 |
| 0601–0000 h glucose, mmol/L | 7.5 ± 1.3 | 7.8 ± 1.2 | 7.7 ± 1.3 | 8.1 ± 1.4 | 7.0 ± 1.0 | 7.3 ± 1.2 |
| Percentage of time 3.5–7.8 mmol/L | 51.7 ± 13.0 | 51.5 ± 13.7 | 53.0 ± 15.5 | 49.8 ± 15.0 | 67.6 ± 12.6 | 61.3 ± 15.5 |
| Percentage of time below 3.5 mmol/L | 10.0 ± 7.7 | 7.8 ± 6.4 | 4.8 ± 4.8 | 5.5 ± 5.7 | 4.6 ± 4.9 | 5.7 ± 5.2 |
| Percentage of time above 7.8 mmol/L | 38.4 ± 14.9 | 40.6 ± 13.8 | 42.3 ± 17.6 | 44.7 ± 16.0 | 27.9 ± 13.4 | 33.1 ± 15.0 |
| Individual SD | 3.1 ± 0.8 | 3.2 ± 0.8 | 2.7 ± 0.6 | 2.9 ± 0.7 | 2.2 ± 0.5 | 2.5 ± 0.7 |
| Individual CV, % | 42.2 ± 8.7 | 42.4 ± 8.1 | 35.6 ± 5.9 | 36.9 ± 7.2 | 32.5 ± 5.8 | 34.9 ± 7.6 |
| B: Pump to MDI | Baseline | 24 weeks | 34 weeks | |||
|---|---|---|---|---|---|---|
| Pump | MDI | Pump | MDI | Pump | MDI | |
| Number | 90 | 110 | 81 | 98 | 71 | 82 |
| Glucose, mmol/L | 7.4 ± 1.2 | 7.5 ± 1.1 | 7.9 ± 1.3 | 7.5 ± 1.1 | 6.9 ± 0.9 | 6.8 ± 1.1 |
| 0001–0600 h glucose, mmol/L | 7.6 ± 1.3 | 7.8 ± 1.2 | 7.4 ± 1.4 | 6.9 ± 1.4 | 6.3 ± 1.1 | 6.2 ± 1.1 |
| 0601–0000 h glucose, mmol/L | 6.9 ± 1.6 | 6.9 ± 1.4 | 8.1 ± 1.4 | 7.7 ± 1.3 | 7.1 ± 1.0 | 7.1 ± 1.2 |
| Percentage of time 3.5–7.8 mmol/L | 53.6 ± 13.4 | 50.0 ± 13.1 | 48.8 ± 16.5 | 53.6 ± 13.9 | 64.1 ± 13.3 | 64.8 ± 15.4 |
| Percentage of time below 3.5 mmol/L | 8.1 ± 6.3 | 9.5 ± 7.8 | 4.5 ± 4.5 | 5.7 ± 5.7 | 5.0 ± 5.2 | 5.2 ± 4.9 |
| Percentage of time above 7.8 mmol/L | 38.3 ± 15.2 | 40.4 ± 13.6 | 46.7 ± 17.8 | 40.8 ± 15.5 | 31.0 ± 14.2 | 30.0 ± 14.7 |
| Individual SD | 3.1 ± 0.8 | 3.2 ± 0.8 | 2.8 ± 0.7 | 2.8 ± 0.7 | 2.3 ± 0.6 | 2.3 ± 0.7 |
| Individual CV, % | 41.3 ± 7.3 | 43.1 ± 9.1 | 35.6 ± 6.7 | 36.7 ± 6.5 | 33.7 ± 6.8 | 33.7 ± 6.8 |
| C: LGA to non-LGA | Baseline | 24 weeks | 34 weeks | |||
|---|---|---|---|---|---|---|
| LGA | Non-LGA | LGA | Non-LGA | LGA | Non-LGA | |
| Number | 122 | 78 | 111 | 68 | 96 | 57 |
| Glucose, mmol/L | 7.6 ± 1.2 | 7.3 ± 1.2 | 7.9 ± 1.2 | 7.3 ± 1.2 | 7.0 ± 1.1 | 6.6 ± 0.8 |
| 0001–0600 h glucose, mmol/L | 7.0 ± 1.4 | 6.8 ± 1.6 | 7.3 ± 1.4 | 6.9 ± 1.4 | 7.3 ± 1.2 | 6.8 ± 0.9 |
| 0601–0000 h glucose, mmol/L | 7.8 ± 1.3 | 7.5 ± 1.2 | 8.1 ± 1.3 | 7.5 ± 1.3 | 6.4 ± 1.1 | 6.1 ± 1.0 |
| Percentage of time 3.5–7.8 mmol/L | 49.6 ± 13.8 | 54.7 ± 13.6 | 48.2 ± 14.9 | 56.6 ± 14.4 | 62.6 ± 11.8 | 67.6 ± 11.8 |
| Percentage of time below 3.5 mmol/L | 9.2 ± 7.0 | 8.4 ± 7.5 | 5.0 ± 15.3 | 5.4 ± 5.1 | 4.5 ± 4.6 | 6.2 ± 5.6 |
| Percentage of time above 7.8 mmol/L | 41.2 ± 14.4 | 36.8 ± 14.0 | 46.9 ± 16.3 | 38.0 ± 16.2 | 33.0 ± 15.3 | 26.2 ± 11.7 |
| Individual SD | 3.3 ± 0.8 | 3.0 ± 0.9 | 2.9 ± 0.6 | 2.6 ± 0.7 | 2.4 ± 0.7 | 2.2 ± 0.5 |
| Individual CV, % | 43.3 ± 8.5 | 41.1 ± 8.1 | 36.6 ± 6.8 | 35.5 ± 6.3 | 33.6 ± 7.2 | 33.8 ± 6.2 |
Data are expressed as means (SD). Boldface type indicates P < 0.05 in a t test comparing the difference.
Functional Data Analysis
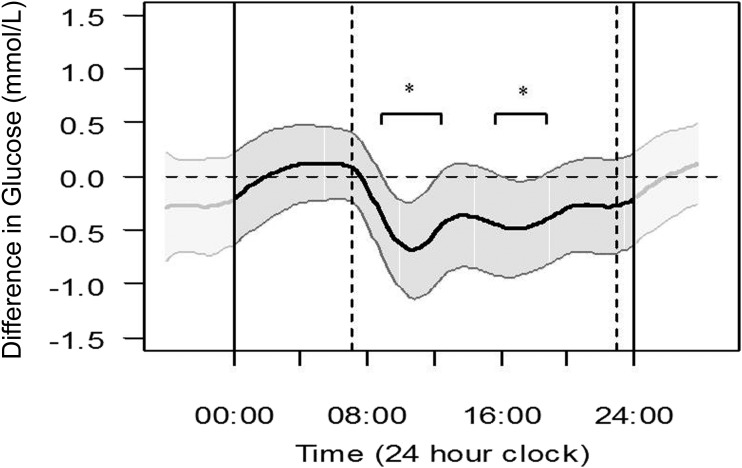
Figure 1 illustrates the difference in CGM glucose across the 24-h day in women who were randomized to RT-CGM compared with SMBG after applying FDA. Women who used RT-CGM ran a significantly lower glucose by 0.4–0.8 mmol/L (7–14 mg/dL) for 7 h during the daytime (0800 h to 1200 h and 1600 h to 1900 h). There were no significant differences in glucose overnight.
Figure 1.
Differences in mean temporal glucose levels across the 24-h day, assessed by FDA (at 24 and 34 weeks’ gestation combined) between those women who were randomized to RT-CGM (represented by the dark wavy line) compared with those using SMBG (represented by the horizontal zero dotted line) with 95% pointwise CIs (gray section). Where both of the CIs sit to the same side of 0.0, there is a significant difference. Dashed vertical lines represent daytime at 0700 h and 2300 h. *Significant differences using 95% CIs.
Pumps Versus MDI
Standard CGM Metrics
Standard CGM metrics (Table 2B) showed a significantly higher mean glucose, with higher mean glucose shown both overnight and during the day at 24 weeks’ gestation in those women on pumps, and more time spent above the target. There were no differences in glucose variability measures at any point.
Functional Data Analysis
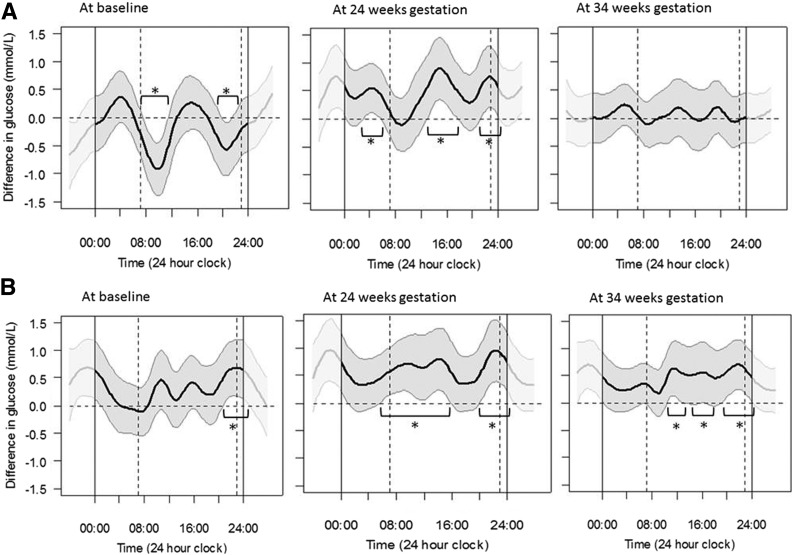
Figure 2A shows that women who used insulin pumps had significantly lower glucose levels by 0.4–0.9 mmol/L (7–16 mg/dL) for 5.5 h of the 24-h day (0730 h to 1130 h and 2000 h to 2130 h) at baseline, but they had significantly higher glucose levels by 0.4–0.9 mmol/L (7–16 mg/dL) for a total of 12 h a day (0300 h to 0600 h, 1300 h to 1800 h, and 2030 h to 0030 h) at 24 weeks’ gestation and no difference in glucose levels at 34 weeks’ gestation. These differences were predominantly seen during daytime hours.
Figure 2.
Differences in mean temporal glucose levels across the 24-h day, assessed by FDA. A: Differences in women who used pumps (represented by dark wavy line) compared with those on MDI (represented by the horizontal zero dotted line) with 95% pointwise CIs (gray section). B: Differences in women who gave birth to an LGA infant (represented by the dark wavy line) compared with those who did not (represented by the horizontal zero dotted line) with 95% pointwise CIs (gray section). Dashed vertical lines represent daytime at 0700 h and 2300 h. *Significant differences using 95% CIs.
LGA Versus Non-LGA
Standard CGM Metrics
Women who went on to have an LGA infant had significantly higher mean glucose at 24 and 34 weeks’ gestation (Table 2C). Both daytime and nighttime mean glucose levels were significantly higher in the LGA group at 24 weeks, but at 34 weeks, only the nighttime glucose level was significantly higher. Time spent in the pregnancy target range was significantly lower in each trimester in those women who had an LGA infant, with significantly more time spent above the pregnancy target range of 3.5–7.8 mmol/L (63–140 mg/dL) throughout the pregnancy. There was significantly greater glucose variability in the first and second trimesters in those women who went on to have an LGA infant as demonstrated by SD and CV glucose.
Functional Data Analysis
Figure 2B shows that women who had an LGA infant ran a significantly higher glucose by 0.4–0.7 mmol/L (7–13 mg/dL) for 4.5 h from 2100 h at baseline, a significantly higher glucose by 0.4–0.9 mmol/L (7–16 mg/dL) for 16 h/day at 24 weeks’ gestation, and significantly higher glucose by 0.4–0.7 mmol/L (7–13 mg/dL) for 14 h/day at 34 weeks’ gestation. These higher glucose levels were predominantly seen during daytime hours.
Conclusions
By applying FDA to the CGM data obtained in CONCEPTT, we are able to clearly identify differences in maternal glucose and determine when and for how long across the 24-h day this is occurring, even when standard CGM metrics fail to detect a variation. In doing so, this study demonstrates that pregnant women randomized to RT-CGM had lower glucose during the daytime than women using SMBG alone. It shows that although women using insulin pumps started pregnancy with better glucose control, they had a higher glucose for 12 h during the daytime during the middle of the pregnancy, only achieving comparable glucose control to women using MDI in late pregnancy. Finally, it shows that women who delivered an LGA infant ran a higher glucose throughout the pregnancy, which was sustained for up to 16 h/day at 24 weeks’ gestation.
The CONCEPTT trial showed a beneficial effect of using RT-CGM on neonatal outcomes, and its data have supported the adoption of time-in-range targets for using CGM in type 1 diabetes pregnancy (6,9). While improving time in range by 5% improves pregnancy outcomes, it is not clear which periods of the day are best targeted to achieve benefit (9). Our current analysis helps to define these periods. Although there was no difference in mean glucose between RT-CGM and SMBG using standard CGM metrics, it did not mean that there were no significant differences in glucose at certain time points across the day. FDA allows this visualization, showing that using RT-CGM leads to reduced fetal exposure to daytime maternal glucose. This finding suggests that RT-CGM data help women to observe the impact of carbohydrate ingestion on daytime glucose profiles better than SMBG does and that the data allow the women to take appropriate action to prevent/manage glucose level fluctuations. It is worth noting that the women using RT-CGM only had significantly better glucose control for 7 h/day and that although LGA was reduced in the RT-CGM group, LGA rates remained high (9). Given that we showed that women who went on to have LGA infants had higher glucose for 16 h/day, we suggest that there is room for further improvement in daytime glucose control in the RT-CGM group.
It was interesting that contrary to expectations, women using pumps had poorer neonatal outcomes than women using MDIs (10). However, the original analysis was unable to show any significant differences in glucose between the two groups using standard CGM metrics, except that pump users spent significantly less time below 3.5 mmol/L (63 mg/dL) compared with MDI users throughout pregnancy and 5% less time in range at 24 weeks (10). The differences in temporal glucose profiles seen between women using pumps or MDIs that were found using FDA provide new insights into why these outcomes occurred. The FDA clearly shows that women using insulin pumps entered pregnancy with better first trimester glucose control. This advantage is, however, lost as pregnancy progresses, with evidence of substantially worse daytime glucose control at 24 weeks’ gestation. It again suggests that mealtime glucose control is particularly important and that clinicians and patients are possibly less effective at optimizing midtrimester insulin to carbohydrate during pregnancy using insulin pumps. No differences were seen in total insulin doses between pumps and MDI, but data were not available on the insulin-to-carbohydrate or the basal-to-bolus ratios used (10,16).
The standard CGM metrics readily showed significant differences when it came to LGA, with a higher mean glucose at 24 and 34 weeks’ gestation: significantly lower time spent in the pregnancy target range in each trimester, significantly higher time spent above the pregnancy target range of 3.5–7.8 mmol/L (63–140 mg/dL) throughout pregnancy, and greater glucose variability in the first and second trimesters in those women who went on to have an LGA infant. This result is consistent with the recent findings of an observational study of 186 pregnant women with type 1 diabetes using CGM in Sweden, which showed that higher mean CGM glucose levels in the second and third trimesters were significantly associated with LGA as well as less time spent in pregnancy target range and greater SD in the second trimester (17). The FDA performed in our study again provides further insights, showing that there are actually periods of relatively higher glucose as early as the first trimester that are associated with LGA and that it is predominantly higher daytime glucose control that is contributing to the higher overall mean glucose observed with standard CGM metrics. This result supports our earlier work on FDA in a much smaller cohort of women with type 1 and type 2 diabetes wherein we showed that a significantly higher glucose across the daytime in middle and late gestation is associated with LGA in women being treated to tight, postprandial glucose targets (7). It seems likely that the length of duration of time exposed to even small amounts of extra glucose is important in the context of fetal growth in pregnancy.
It is interesting that we previously observed a different glucose profile associated with LGA in women being treated for gestational diabetes (8). In that study, we saw that daytime glucose control was achieved but that nocturnal glucose control was suboptimal, with women who went on to have LGA infants running significantly higher glucose for 6 h overnight (8). This difference may reflect the different emphasis in management between the two types of diabetes: the focus of management in gestational diabetes is very much on making significant dietary changes, whereas we do not consider that this is always the case in type 1 diabetes, where the focus is more on adjustment of insulin to accommodate normal eating (18).
Overall, this analysis of temporal glucose profiles shows that women who have poorer pregnancy outcomes (women on SMBG, pumps, and those with LGA infants) run relatively higher glucose levels during the daytime than women who do not. The reason for this is likely to be related to carbohydrate ingestion, indicating that greater attention is needed to improving the management of mealtime and snack hyperglycemia in women with type 1 diabetes during pregnancy. The higher daytime glucose is particularly pronounced at 24 weeks’ gestation, and we hypothesize that this also reflects changes in insulin responsiveness at this stage in pregnancy (16). While there are no changes in glucose bioavailability or postprandial glucose appearance between early and late gestation in type 1 diabetes, there are significant delays in postprandial glucose disposal as pregnancy advances, possibly due to a combination of increased peripheral insulin resistance and a slower achievement of maximum insulin concentration leading to a more prolonged hyperglycemia (19). We know from dietary assessment of women in CONCEPTT that their food choices, especially of between-meal snacks, tended to be of highly processed carbohydrates of low nutritional value (18) and that this leads to a rapid increase in glucose with a lag time for any extra insulin to catch up and bring it down. Going forward, the solutions are to bolus insulin 15 min before the meal, increasing to 40 min later in pregnancy (19); replace rapidly absorbed carbohydrate-rich meals with more slowly absorbed ones; or advise postprandial physical activity to enhance peripheral glucose uptake. It would seem sensible to emphasize making more healthy dietary changes in women with type 1 diabetes while pregnant to help reduce daytime hyperglycemia, given that currently normal eating habits are far from ideal (18).
The strengths of this study are that it used data from a large, multicenter, international, randomized controlled trial. It is thus representative of the women being managed for type 1 diabetes in routine clinical care internationally. CGM provides far more frequent glucose measurements than SMBG and far more information on short- to medium-term trends in glucose levels than either SMBG or HbA1c. CGM nonetheless has recognized imitations, particularly with regard to the quality of glucose readings during rapid blood glucose changes and in situations of hypoglycemia. The measurement of interstitial glucose may also not precisely reflect the levels of blood glucose. CGM data were only obtained at three time points across gestation in this study, which may not be representative of glucose control at other times in pregnancy, and we acknowledge that recently published consensus guidelines suggest that 2 weeks of CGM data are preferred for analysis (although this recommendation is based on data outside of pregnancy) (5). It is worth noting that although significant differences were observed, these are still small sample sizes and that larger numbers would be beneficial in future work. Other limitations of this study were that we did not have detailed dietary information on the timing of meal, snack, or drink ingestion, which means that although it is likely, we cannot definitively say that the raised daytime glucose was due to this.
In summary, FDA of CGM glucose profiles gives important information about differences in glucose control, which is largely undetectable by standard CGM metrics, including detail on the timing and duration of these differences. While FDA is best suited to explore population-level differences in glucose profiles, the equivalent on an individual basis clinically would be the ambulatory glucose profile. Regular review of this throughout pregnancy would enable a focus on meal choices, together with a more aggressive approach to bringing forward insulin bolus timing and increasing insulin doses especially mid-pregnancy, aiming for small, but sustained, improvements in daytime glucose levels.
Supplementary Material
Article Information
Acknowledgments. The authors thank all of the women with type 1 diabetes who participated. The authors also thank the 31 clinical care teams and the CONCEPTT Steering Committee for invaluable support: Denice Feig (Mt. Sinai Hospital, Toronto, Ontario, Canada), Helen Murphy (Institute of Metabolic Science, Cambridge University Hospitals NHS Foundation Trust, Cambridge, U.K.), Elisabeth Asztalos (Sunnybrook Research Institute, Toronto, Ontario, Canada), John F.R. Barrett (Sunnybrook Research Institute, Toronto, Ontario, Canada), Rosa Corcoy (Hospital de la Santa Creu i Sant Pau, Barcelona, Spain), Alberto De Leiva (Hospital de la Santa Creu i Sant Pau, Barcelona, Spain), Lois Donovan (University of Calgary, Calgary, Alberta, Canada), Moshe Hod (Helen Schneider Hospital for Women, Rabin Medical Center, Petah Tikva, Israel), Lois Jovanovic (Santa Barbara, CA), Erin Keely (The Ottawa Hospital, Riverside Campus, Ottawa, Ontario, Canada), Craig Kollman (Jaeb Center For Health Research, Tampa, FL), Ruth McManus (St. Joseph Health Care London, London, Ontario, Canada), Kellie Murphy (Mt. Sinai Hospital, Toronto, Ontario, Canada), Katrina Ruedy (Jaeb Center For Health Research, Tampa, FL), and George Tomlinson (University Health Network, Toronto General Hospital, Toronto, Ontario, Canada).
Funding. The CONCEPTT trial was funded by a JDRF grant (17-2011‐533) and grants under JDRF Canadian Clinical Trial Network, a public‐private partnership including JDRF and FedDev Ontario and supported by JDRF (80-2010‐585). Medtronic supplied the CGM sensors and CGM systems at reduced cost. G.R.L. and E.M.S. were funded by the Higher Education Funding Council for England. H.R.M. was funded by the National Institute for Health Research (CDF-2013-06-035).
The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the U.K. Department of Health.
Duality of Interest. E.M.S. has received honoraria for speaking from Abbott Diabetes Care and Eli Lilly and Company. H.R.M. serves on the Medtronic European Scientific Advisory Board. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. E.M.S. and G.R.L. wrote the manuscript. All authors critically reviewed the manuscript. G.R.L. and E.M.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. These data were presented in abstract form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019, and the 51st Annual Meeting of the Diabetes Pregnancy Study Group of the European Association for the Study of Diabetes, Graz, Austria, 5–8 September 2019.
Footnotes
A complete list of the CONCEPTT Collaborative Group can be found in the Supplementary Data online.
This article contains Supplementary Data online at https://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-2527/-/DC1.
Contributor Information
Collaborators: The CONCEPTT Collaborative Group, Helen Murphy, Jeannie Grisoni, Carolyn Byrne, Sandra Neoh, Katy Davenport, Lois Donovan, Claire Gougeon, Carolyn Oldford, Catherine Young, Stephanie Amiel, Katharine Hunt, Louisa Green, Helen Rogers, Benedetta Rossi, Denice Feig, Barbara Cleave, Michelle Strom, Rosa Corcoy, Alberto de Leiva, Juan María Adelantado, Ana Isabel Chico, Diana Tundidor, Erin Keely, Janine Malcolm, Kathy Henry, Damian Morris, Gerry Rayman, Duncan Fowler, Susan Mitchell, Josephine Rosier, Rosemary Temple, Jeremy Turner, Gioia Canciani, Niranjala Hewapathirana, Leanne Piper, Ruth McManus, Anne Kudirka, Margaret Watson, Matteo Bonomo, Basilio Pintaudi, Federico Bertuzzi, Giuseppina Daniela Corica, Elena Mion, Julia Lowe, Ilana Halperin, Anna Rogowsky, Sapida Adib, Robert Lindsay, David Carty, Isobel Crawford, Fiona Mackenzie, Therese McSorley, John Booth, Natalia McInnes, Ada Smith, Irene Stanton, Tracy Tazzeo, John Weisnagel, Peter Mansell, Nia Jones, Gayna Babington, Dawn Spick, Malcolm MacDougall, Sharon Chilton, Terri Cutts, Michelle Perkins, Eleanor Scott, Del Endersby, Anna Dover, Frances Dougherty, Susan Johnston, Simon Heller, Peter Novodorsky, Sue Hudson, Chloe Nisbet, Thomas Ransom, Jill Coolen, Darlene Baxendale, Richard Holt, Jane Forbes, Nicki Martin, Fiona Walbridge, Fidelma Dunne, Sharon Conway, Aoife Egan, Collette Kirwin, Michael Maresh, Gretta Kearney, Juliet Morris, Susan Quinn, Rudy Bilous, Rasha Mukhtar, Ariane Godbout, Sylvie Daigle, Alexandra Lubina Solomon, Margaret Jackson, Emma Paul, Julie Taylor, Robyn Houlden, Adriana Breen, Anita Banerjee, Anna Brackenridge, Annette Briley, Anna Reid, Claire Singh, Jill Newstead-Angel, Janet Baxter, Sam Philip, Martyna Chlost, Lynne Murray, Kristin Castorino, Lois Jovanovic, Donna Frase, Sonya Mergler, Kathryn Mangoff, Johanna Sanchez, Gail Klein, Katrina Ruedy, Craig Kollman, Olivia Lou, and Marlon Pragnell
References
- 1.Metzger BE, Lowe LP, Dyer AR, et al.; HAPO Study Cooperative Research Group . Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 2.Tan E, Scott EM. Circadian rhythms, insulin action, and glucose homeostasis. Curr Opin Clin Nutr Metab Care 2014;17:343–348 [DOI] [PubMed] [Google Scholar]
- 3.Prasai MJ, Mughal RS, Wheatcroft SB, Kearney MT, Grant PJ, Scott EM. Diurnal variation in vascular and metabolic function in diet-induced obesity: divergence of insulin resistance and loss of clock rhythm. Diabetes 2013;62:1981–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol 1999;180:903–916 [DOI] [PubMed] [Google Scholar]
- 5.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Law GR, Ellison GTH, Secher AL, et al. Analysis of continuous glucose monitoring in pregnant women with diabetes: distinct temporal patterns of glucose associated with large-for-gestational-age infants. Diabetes Care 2015;38:1319–1325 [DOI] [PubMed] [Google Scholar]
- 8.Law GR, Alnaji A, Alrefaii L, et al. Suboptimal nocturnal glucose control is associated with large for gestational age in treated gestational diabetes mellitus. Diabetes Care 2019;42:810–815 [DOI] [PubMed] [Google Scholar]
- 9.Feig DS, Donovan LE, Corcoy R, et al.; CONCEPTT Collaborative Group . Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet 2017;390:2347–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feig DS, Corcoy R, Donovan LE, et al.; CONCEPTT Collaborative Group . Pumps or multiple daily injections in pregnancy involving type 1 diabetes: a prespecified analysis of the CONCEPTT randomized trial. Diabetes Care 2018;41:2471–2479 [DOI] [PubMed] [Google Scholar]
- 11.Feig DS, Asztalos E, Corcoy R, et al.; CONCEPTT Collaborative Group . CONCEPTT: Continuous Glucose Monitoring in Women with Type 1 Diabetes in Pregnancy Trial: a multi-center, multi-national, randomized controlled trial - study protocol [published correction appears in BMC Pregnancy Childbirth 2016;16:249]. BMC Pregnancy Childbirth 2016;16:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardosi J, Francis A, Turner S, Williams M. Customized growth charts: rationale, validation and clinical benefits. Am J Obstet Gynecol 2018;218:S609–S618 [DOI] [PubMed] [Google Scholar]
- 13.Ramsay JO, Hooker G, Graves S. Functional Data Analysis with R and MATLAB. Dordrecht, New York, Springer, 2009 [Google Scholar]
- 14.StataCorp Stata Statistical Software: Release 12. College Station, TX, StataCorp LP, 2011 [Google Scholar]
- 15.R Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: 2008 [Google Scholar]
- 16.Neoh SL, Yamamoto JM, Feig DS, et al. Dietary patterns of insulin pump and multiple daily injection users during type 1 diabetes pregnancy. Diabetes Care 2020;43:e5–e7 [DOI] [PubMed] [Google Scholar]
- 17.Kristensen K, Ögge LE, Sengpiel V, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes: an observational cohort study of 186 pregnancies. Diabetologia 2019;62:1143–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neoh SL, Grisoni JA, Feig DS, Murphy HR; CONCEPTT Collaborative Group . Dietary intakes of women with Type 1 diabetes before and during pregnancy: a pre-specified secondary subgroup analysis among CONCEPTT participants. Diabet Med. 20 February 2019 [Epub ahead of print]. DOI: 10.1111/dme.13937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy HR, Elleri D, Allen JM, et al. Pathophysiology of postprandial hyperglycaemia in women with type 1 diabetes during pregnancy. Diabetologia 2012;55:282–293 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.