Holoprosencephaly is the incomplete division of the forebrain during embryogenesis. Kruszka et al. identify 14 cases of holoprosencephaly associated with variants in cohesin complex genes. Using mouse embryo studies, they confirm that cohesin complex genes are expressed during the critical time period for forebrain division.
Keywords: holoprosencephaly, cohesin complex, X-linked inheritance, forebrain division
Abstract
Marked by incomplete division of the embryonic forebrain, holoprosencephaly is one of the most common human developmental disorders. Despite decades of phenotype-driven research, 80–90% of aneuploidy-negative holoprosencephaly individuals with a probable genetic aetiology do not have a genetic diagnosis. Here we report holoprosencephaly associated with variants in the two X-linked cohesin complex genes, STAG2 and SMC1A, with loss-of-function variants in 10 individuals and a missense variant in one. Additionally, we report four individuals with variants in the cohesin complex genes that are not X-linked, SMC3 and RAD21. Using whole mount in situ hybridization, we show that STAG2 and SMC1A are expressed in the prosencephalic neural folds during primary neurulation in the mouse, consistent with forebrain morphogenesis and holoprosencephaly pathogenesis. Finally, we found that shRNA knockdown of STAG2 and SMC1A causes aberrant expression of HPE-associated genes ZIC2, GLI2, SMAD3 and FGFR1 in human neural stem cells. These findings show the cohesin complex as an important regulator of median forebrain development and X-linked inheritance patterns in holoprosencephaly.
Introduction
Holoprosencephaly (HPE) is defined by incomplete division of the embryonic forebrain. While occurring in approximately 1 in 10 000 live births, HPE is estimated to occur in 1 in 250 embryos, making it one of the most common human developmental abnormalities (Matsunaga and Shiota, 1977). The most common cause is trisomy 13, which accounts for ∼50% of all cases (Kruszka and Muenke, 2018). Over the past two decades, four principal genes have been associated with HPE: SHH at 7q36.3, ZIC2 at 13q32.3, SIX3 at 2p21, and TGIF1 at 18p11.31. These four genes have been the mainstay for genetic testing in individuals with HPE and normal karyotypes (Pineda-Alvarez et al., 2010; Kruszka et al., 2018). At least 10 other genetic loci have been associated with HPE, but at a lower prevalence (Kruszka et al., 2018). SHH, SIX3, ZIC2 and TGIF1 account for only a fraction of the genetic aetiology in individuals with normal karyotypes. In a recent next generation sequencing study of 257 individuals with HPE, deleterious variants in SHH were most common in 5.8% of the HPE cohort, ZIC2 at 4.7%, SIX3 at 2.7% and no deleterious variants in TGIF1 (Dubourg et al., 2016); collectively, these four genes accounted for 13.2% of the aetiology in these individuals. With the introduction of whole exome sequencing (WES), driver mutations in new genes including FGFR1 and CNOT1 are being found (Simonis et al., 2013; De Franco et al., 2019; Kruszka et al., 2019). To expand the genetic aetiology of HPE and uncover novel regulators of forebrain development, we have applied WES to 277 probands with HPE and both their parents (trios), if available.
We initially identified loss-of-function (LOF) variants in cohesin complex genes in 5 of 277 (1.8%) individuals in our holoprosencephaly cohort at the National Human Genome Research Institute (NHGRI). Through our holoprosencephaly network, DECIPHER (Firth et al., 2009), and GeneMatcher (Sobreira et al., 2015), we identified 10 other individuals with holoprosencephaly and variants in cohesin complex genes. Collectively, these 15 individuals with HPE have 13 LOF variants, one in-frame deletion, and one pathogenic missense variant distributed across the four cohesin complex genes SMC1A (MIM: 300040), STAG2 (MIM: 300826), SMC3 (MIM: 606062) and RAD21 (MIM: 606462). The majority of cases (11/15) are females with variants in the X-linked genes SMC1A and STAG2. Cohesin is a highly conserved multiprotein complex with SMC1A, SMC3, RAD21 and STAG1/STAG2 as its subunits in mammals (Brooker and Berkowitz, 2014). This complex forms a ring structure that is involved in sister chromatid cohesion during DNA replications. Additional roles of this complex include transcription regulation and DNA repair (Mehta et al., 2013). Mutations in the cohesin complex and its regulators have been associated with four human genetic syndromes: Cornelia de Lange syndrome (CdLS) caused by variants in NIPBL (Krantz et al., 2004), SMC1A (Musio et al., 2006), SMC3 (Deardorff et al., 2007), RAD21 (Deardorff et al., 2012b), BRD4 (Olley et al., 2018), HDAC8 (Deardorff et al., 2012a); Roberts SC phocomelia syndrome caused by mutations in ESCO2 (Gordillo et al., 2008), CHOPS syndrome (Cognitive impairment and coarse facies, Heart defects, Obesity, Pulmonary involvement, and Short stature and skeletal dysplasia) associated with AFF4 variants (Izumi et al., 2015); and chronic atrial and intestinal dysrhythmia caused by mutations in SGOL1 (Chetaille et al., 2014). The cohesin complex genes that we associate with holoprosencephaly (STAG2, SMC1A, SMC3 and RAD21) are intolerant of variation based on the Genome Aggregation Database (gnomAD) constraint metric of observed/expected loss of function (o/e) values (Lek et al., 2016). Values <0.35 (o/e) are considered under selection against LOF (https://gnomad.broadinstitute.org) and the cohesin complex genes were well below this threshold: STAG2 0.02 [90% confidence interval (CI), 0.1–0.09], SMC1A 0.0 (90%CI, 0.0–0.06), SMC3 0.0 (90%CI, 0.0–0.04) and RAD21 0.1 (90%CI, 0.04–0.26).
Materials and methods
Subjects and clinical phenotyping
The individuals and families with HPE in this study were recruited from multiple international clinical genetics centres. Within the participating institutions, the phenotype was evaluated by clinical exam by the authors of this study and brain imaging (MRI or CT) or autopsy to confirm HPE. The study was approved by National Human Genome Research Institute Institutional Review Board (IRB) and the ethical committee of the patient’s local institutions. The subjects’ consents were obtained according to the Declaration of Helsinki.
DNA sequence analysis
Sanger sequencing
With the goal of new gene discovery, probands were prescreened for four common genes known to cause HPE: SHH (MIM 600725) on 7q36, ZIC2 (MIM 603073) on 13q32, SIX3 (MIM 603714) on 2p21, and TGIF1 (MIM 602630) on 18p11.3 using Sanger sequencing (Supplementary material). Novel variants found in this study by WES were also confirmed with Sanger sequencing.
Whole exome sequencing
WES was performed at the National Intramural Sequencing Center (NISC) on the individuals from the NHGRI HPE cohort (Supplementary material). The remaining individuals were sequenced at seven other academic and commercial laboratories (Supplementary Table 1). All WES results were verified by Sanger sequencing. Stringent variant filtering of the NHGRI cohort included: (i) de novo inheritance of variants in genes known to be intolerant of variation (Lek et al., 2016); (ii) absence in the ExAC data base (Lek et al., 2016); and (iii) combined annotation-dependent depletion (CADD) scores >20 (Kircher et al., 2014).
Mouse embryo in situ hybridization
Genes that contribute to median forebrain morphogenesis and HPE pathogenesis are expressed in the prosencephalic neural folds that give rise to the forebrain during primary neurulation (Roessler et al., 2018). We therefore examined expression of Stag2 and Smc1a by in situ hybridization on mouse embryos at GD8.25 (Supplementary material), a stage representing early neurulation and within the critical period for HPE genesis (Heyne et al., 2015a). In situ hybridization was conducted as previously described and analysis was limited to the prosencephalic regions of the neural fold from which the forebrain will develop (Everson et al., 2017). This study was conducted in strict accordance with the recommendations in the ‘Guide for the Care and Use of Laboratory Animals’ of the National Institutes of Health. The protocol was approved by the University of Wisconsin-Madison School of Veterinary Medicine Institutional Animal Care and Use Committee (protocol number 13–081.0). CD-1 mice (Mus musculus) were purchased from Charles River and C57BL/6J mice from The Jackson Laboratory. Timed pregnancies were established as described previously (Heyne et al., 2015b). Embryos were dissected at gestational Day 8.25 and fixed overnight in 4% paraformaldehyde. In situ hybridization was carried out on whole C57BL/6J embryos or 50-μm sections cut from CD-1 embryos with a vibrating microtome in the transverse plane along the anterior-posterior axis. In situ hybridization was carried out as described previously (Everson et al., 2017).
Gene expression studies in human neural stem cells
To test the hypothesis that variation in cohesin genes, specifically STAG2 and SMC1A, perturb known forebrain developmental pathways, we measured selected gene expression associated with these pathways. First, knockdown of STAG2 and SMC1A with shRNA was performed on H9-derived human neural stem cells (ThermoFisher/Invitrogen, #N7800–100) (Supplementary material and Supplementary Fig. 1). Known HPE pathways were analysed at the gene expression level with RT-qPCR of SHH, SIX3, FGFR1, GLI2, ZIC2, GLI2, SMAD3 and DISP1 genes.
Data availability
The raw data that support the findings of this manuscript are available upon request to the corresponding author.
Results
Patients: phenotype and genotype
We assembled 277 individuals with HPE in our NHGRI cohort (135 trios and 142 singletons); the cohort characteristics are shown in Supplementary Table 2. In the four classic HPE genes, pathogenic variants were found in 33 (11.9%) individuals: ZIC2 was most common with 15 (5.4%) variants, followed by SHH nine (3.2%), SIX3 eight (2.9%), and TGIF1 one (0.4%). For these four genes, Supplementary Table 3 lists each variant, HPE subtype and inheritance pattern. In our HPE cohort of 277 individuals at NHGRI, four females had truncating variants (four nonsense and one splice site) in the cohesin complex genes STAG2 and SMC1A on the X chromosome, and one proband had a nonsense variant in RAD21 on chromosome 8. Another four LOF variants in STAG2 in females, two LOF variants and one missense variant in SMC1A all in females, two LOF variants in RAD21, and an in-frame deletion in SMC3 were found through our group’s HPE network, DECIPHER (Firth et al., 2009) and GeneMatcher (Sobreira et al., 2015) (genotypes: Table 1; phenotypes: Tables 2–5).
Table 1.
Individuals with HPE and variants in cohesin complex genes
| Patient ID | Gene | Variant | hg19/GRCh37 human reference genome | Inheritance | CADD score | Age | HPE type |
|---|---|---|---|---|---|---|---|
| 1 | STAG2 | c.3034C>T p.(R1012*) | chrX-123217380-C-T | De novo | 53 | Newborn | Alobar |
| 2 | STAG2 | c.205C>T p.(Arg69*) | chrX-123164892-C-T | De novo | 27 | 2 years | Semi-lobar |
| 3 | STAG2 | c.436C>T p.(R146*) | chrX-123176469-C-T | Singleton | 38 | 32-week gestation | Alobar |
| 4 | STAG2 | c.2533+1G>A | chrX-123205174-G-A | Maternal | 34 | Newborn/deceased | Semi-lobar |
| 5 | STAG2 | c.2898_2899del p.(Glu968Serfs*15) | chrX- 123215352–123215353 | De novo | 34 | 12 months | Microform |
| 6 | STAG2 | c.775C>T p.(Arg259*) | chrX-123181311-C-T | De novo | 36 | 9.5 years | Septo-optic dysplasia |
| 7 | SMC1A | c.3285+1G>C | chrX-53409426-C-G | De novo | 25.1 | 15 months | MIHV |
| 8 | SMC1A | c.1495C>T p.(Arg499*) | chrX-53436043-G-A | Singleton | 39 | 16.5 months | Microform |
| 9 | SMC1A | c.2683C>G (p. Arg895Gly) | chrX-53423417-G-C | De novo | 28.5 | 6 years | Semi-lobar/lobar |
| 10 | SMC1A | c.2394delA; p.(Lys798Asnfs*31) | chrX-53430524 | De novo | 35 | 3 years | Semi-lobar |
| 11 | SMC1A | c.2834delG; p.(Gly945Alafs*19) | chrX-53423175 | De novo | 35 | 20 months | Semi-lobar |
| 12 | RAD21 | c.1548delinsTC p.Glu518Argfs*19 | chr8–117862929 | Paternally inherited | 35 | 7 years | MIHV |
| 13 | RAD21 | c.589C>T p.(Gln197*) | chr8–117869605-G-A | Unknown | 38 | 14 years | HPE |
| 14 | RAD21 | c.1217_1224del p.(Lys406Argfs*4) | chr8–117864885 | Unknown | 35 | 2 years | Septo-optic dysplasia |
| 15 | SMC3 | c.1138_1152del p.(Gly380_Gln384del) | chr10–112343987-GG…AG (15 bp) | De novo | 21.9 | Termination after 21 weeks | Semi-lobar |
CADD = combined annotation-dependent depletion; MIHV = middle interhemispheric variant type holoprosencephaly.
Table 2.
Phenotype details of individuals with LOF variants in STAG2
| Present study | Mullegama et al., 2017 | Aoi et al., 2019 | Yuan et al., 2019 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 1 | Patient 2 | Patient 1 | Patient 2 | Patient 3 | ||
| Variant | c.3034C>T p.(R1012*) | c.205C>T p.(Arg69*) | c.436C>T p.(R146*) | c.2533+1G>A | c.2898_2899del p.(Glu968Serfs *15) | c.775C>T p.(Arg259*) | c.205C>T p.(Arg69*) | c.3097C>T p.(Arg1033*) | c.2229C>T p.(Trp743*) | c.418C>T p.Q140* | c.1605T>A p.C535* | c.1658_1660delinsT p.K533Ifs*6 |
| Inheritance | De novo | De novo | Singleton | Maternal | De novo | De novo | De novo | De novo | De novo | De novo | De novo | De novo |
| Sex | Female | Female | Female | Female | Female | Female | Female | Male | Female | Female | Female | Female |
| Age | Newborn | 2 years | 32-week gestation | Newborn/ deceased | 12 months | 9.5 years | 8 years | foetus | 7 years | 3.7 years | 4.5 years | 1.9 years |
| Brain MRI/HPE type | Alobar | Semi-lobar | Alobara | Semi-lobar | Microform | Septo-optic dysplasia | Dysgenesis of the splenium of the corpus callosum | HPE (unspecified) | White matter hypoplasia | NR | NR | Microform; agenesis of corpus callosum; colpocephaly |
| Developmental delay | NA | Global | NA | NA | + | Intellectual disability; motor delay; | Speech | NR | Intellectual disability; developmental delay | Motor and speech delay | Intellectual disability; motor and speech delay | Intellectual disability; motor and speech delay |
| Craniofacial anomalies | Midline cleft lip/palate | Cleft palate; micrognathia | Cyclopia; absent nose, microsomia, hypognathia | − | NR | − | Submucous cleft palate | Cleft lip/palate | Cleft palate | − | Micrognathia | Single central incisor; micrognathia |
| Microcephaly | + | + | + | Severe | + | − | + | NR | − | − | + | + |
| Ear anomalies and hearing | Low-set | − | Hypoplastic right ear | NR | NR | − | Bilateral microtia with hearing loss | NR | Hearing loss | Dysmorphic ears | Microtia, right; conductive hearing loss | Dysmorphic ears |
| Vertebral anomalies | Lumbar spina bifida | − | T7−T10 hemivertebrae | NR | NR | NR | Thoracic hemivertebrae and butterfly vertebrae | NR | Thoracic hemivertebrae | Vertebral clefts | NR | + |
| Congenital heart disease | NR | Patent foramen ovale and patent ductus arteriosus | Ventricular septal defect | Hypoplastic left heart; DORV | − | Ventricular septal defect | Ventricular septal defect | Hypoplastic left heart | − | Hypoplastic left heart | NR | NR |
| Growth delay | NR | + | NA | NA | + | − | NR | Short stature | − | + | + | |
| Limb anomalies | - | − | NR | − | NR | Left hip dysplasia | Bilateral fifth finger clinodactyly | NR | NR | − | Fifth finger clinodactyly | − |
| Other | Gastroesophageal reflux and has a G-tube and Nissen fundoplication | Duodenal atresia | Bilateral optic nerve hypoplasia | Seizure disorder | Seizure disorder | Seizure disorder | ||||||
DORV = double outlet right ventricle; NA = non-applicable; NR = not reported.
aAutopsy finding.
Table 5.
Phenotype details of individuals with LOF variants in SMC3
| Present study | Gil-Rodríguez et al., 2015 | |
|---|---|---|
| Patient 15 | n = 16 | |
| Variant | c.1138_1152del p.(Gly380_Gln384del) | Missense (9/16); in-frame deletions/duplications (6/16); nonsense (1/16) |
| Inheritance | De novo | De novo (10/10) |
| Sex | Male | Female 7/16 |
| Age | Foetus | NR |
| Brain imaging | Semilobar HPE | Corpus callosum dysgenesis (2/11); porencephalic cyst (1/11) |
| Developmental delay | NA | Intellectual disability (13/13) |
| Craniofacial anomalies | Median cleft lip | Cleft palate 1/14; synophrys (11/15); thick eyebrows (9/13); anteverted nostrils (8/14); thin upper lip vermilion (13/16) |
| Microcephaly | NR | 6/12 |
| Ear anomalies and hearing | NR | Low-set ears (6/11); hearing loss 7/13 |
| Vertebral anomalies | NR | Butterfly vertebrae (1/12); scoliosis (1/12) |
| Congenital heart disease | Tetralogy of Fallot | 9/16 |
| Growth delay | NA | Height Z-score < −3.0 (6/16); weight Z-score < −3.0 (5/16) |
| Limb anomalies | Hand/feet cutaneous syndactyly; ulnar deviation of second digit of hands bilaterally; proximally set thumbs | Small hands (11/14); small feet (11/13); proximally set thumbs (12/16) |
| Other | Hypospadias; anal atresia | Seizures (3/12) |
NA = non-applicable; NR = not reported.
STAG2
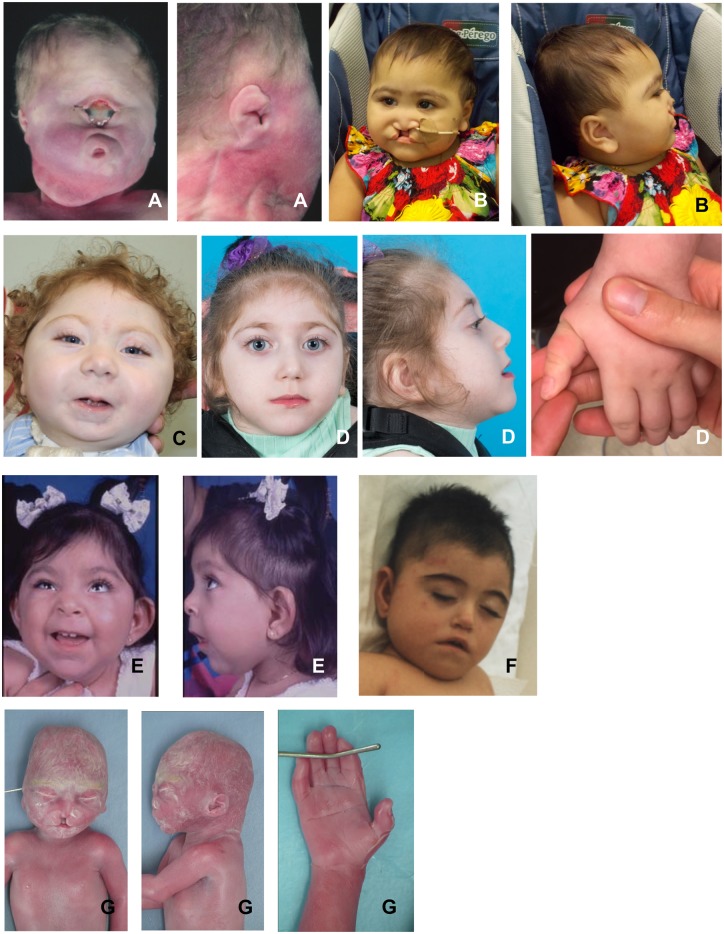
The phenotypes of four of six patients with STAG2 pathogenic variants in the present study included the most severe forms of HPE: alobar HPE with cyclopia, alobar without cyclopia, and semilobar HPE (Patients 1–4; Tables 1 and 2). The other two patients with STAG2 variants had milder forms of HPE (Patients 5–6; Table 1): Patient 5 had microform HPE, which is characterized by midline clefting, hypotelorism and depressed nasal bridge without brain anomalies (Fig. 1B), and Patient 6 is classified with septo-optic dysplasia type of HPE (Hahn et al., 2010) based on ophthalmology exam showing optic nerve hypoplasia and MRI findings of a mildly dysmorphic neurohypophysis. In Table 2, we compare the genotypes and phenotypes of the six cases in the present study with six cases with LOF variants from the medical literature (Mullegama et al., 2017; Aoi et al., 2019; Yuan et al., 2019). Overlapping clinical features of the six individuals in the present study and the six individuals in the medical literature include two of the six cases from the medical literature with HPE: Patient 1 from Aoi et al. (2019) has a structural brain malformation consistent with HPE, and Patient 3 from Yuan et al. (2019) has the microform HPE subtype. Additionally, three of the four cases in the medical literature have midline brain malformations including HPE as noted above, agenesis of the corpus callosum, and dysgenesis of the corpus callosum. Most of the present study and the cases in the medical literature have vertebral anomalies: six of seven that reported spine anomalies. Vertebral anomalies are not part of the clinical features associated with classic CdLS, but are commonly found in individuals with variants in SMC3 and RAD21 (Kline et al., 2018). Also, seven of nine total had congenital heart disease. All LOF STAG2 variants in the medical literature are de novo; interestingly, in the present study, Patient 4, (1/5) is inherited maternally which may be explained by skewed X-inactivation (not tested) or incomplete penetrance. Additionally, there is a LOF variant in the gnomAD database of presumptively healthy individuals (allele count 1/178 804), which raises the possibility of the rare case of incomplete penetrance (https://gnomad.broadinstitute.org accessed 1 May 2019). Collectively from the 12 cases in the present study and medical literature with LOF variants in STAG2, only one individual was male and he was reported to have HPE (Aoi et al., 2019); the most likely conclusion is that LOF variants in STAG2 are lethal or result in the most severe phenotype (HPE). Coincidentally, Mullegama et al. (2017) reported a patient with a STAG2 with an identical variant as in Patient 2 (Fig. 1), c.205C>T; p.Arg69*. The patient in the Mullegama et al. (2017) report had dysgenesis of the splenium of the corpus callosum and the patient in this study had semilobar HPE, showing that STAG2 LOF variants are responsible for a spectrum of midline brain anomalies.
Figure 1.
Patient images. (A) Patient 3 with alobar HPE and a c.436C>T p.(Arg146*) variant in STAG2; (B) Patient 5 with microform HPE and a c.2898_2899del p.(Glu968Serfs*15) in STAG2; (C) Patient 2 with semi-lobar HPE and a c.205C>T p.(Arg69*) variant in STAG2; (D) Patient 9 with semi-lobar HPE and a c.2683C>G p.( Arg895Gly) variant in SMC1A; (E) Patient 7 with middle interhemispheric variant HPE and a c.3285+1G>C variant in SMC1A; (F) Patient 8 with microform HPE and a c.1495C>T p.(Arg499*) variant in SMC1A; (G) Patient 15 with semi-lobar HPE and an SMC3 variant c.1138_1152del p.(Gly380_Gln384del). See Table 1 for further details.
SMC1A
The other five individuals with X-linked HPE were all females (Patients 7–11) (Table 3 and Fig. 1D–F) with four truncating variants and one with a missense variant in SMC1A, a cohesin complex gene known to be associated with CdLS (Deardorff et al., 2007). Variants in SMC1A account for 4–6% of individuals with CdLS (Patients 12–14) (Ansari et al., 2014; Boyle et al., 2015; Yuan et al., 2015) and are most commonly missense and in-frame deletions (Huisman et al., 2013). Four of five individuals in the present study had LOF variants; therefore, we used 16 cases with LOF variants in SMC1A from the medical literature with phenotype information for comparison in Table 3 (Hoppman-Chaney et al., 2012; Goldstein et al., 2015; Lebrun et al., 2015; Jansen et al., 2016; Symonds et al., 2017). The most severe phenotype in the LOF variants in the medical literature was HPE found in 2 of 16 individuals (Hoppman-Chaney et al., 2012; Symonds et al., 2017). In both the present study and in the medical literature, when parents were available, all LOF variants were de novo and all individuals were females. In addition to midline brain defects, the most striking phenotype is seizure disorders. In the present study, four of five individuals had seizures and 15 of 16 in the medical literature. In the largest study of 10 individuals with truncating variants in SMC1A, nine of nine reporting seizures had severe drug-resistant epilepsy (Symonds et al., 2017). All 16 cases in the present study and medical literature had developmental delay. Two individuals in the present study have facial characteristics consistent with mild CdLS, Patients 9 and 10 both had synophrys and small hands. In the largest study of LOF variants in SMC1A (Table 3), the authors report few phenotype characteristics consistent with CdLS (Symonds et al., 2017).
Table 3.
Phenotype details of individuals with variants in SMC1A
| Present study | Symonds et al., 2017 | Jansen et al., 2016 | Goldstein et al., 2015 | Lebrun et al., 2015 | Hoppman-Chaney et al., 2012 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient 11 | n = 10 | n = 2 | n = 2 | n = 1 | n = 1 | |
| Variant | c.3285+1G>C | c.1495C>T p.(Arg499*) | c.2683C>G (p. Arg895Gly) | c.2394delA p.(Lys798Asnfs*31) | c.2834delG p.(Gly945Alafs*19) | Truncating variants (n = 10) | Truncating variants (n = 2) | Truncating variants (n = 2) | c.1911+1G > T | 8.2-kb deletion in SMC1A; 45,X[7]/46,XX[23] |
| Inheritance | De novo | Singleton | De novo | De novo | De novo | De novo 10/10 | De novo 2/2 | De novo 2/2 | De novo | De novo |
| Sex | Female | Female | Female | Female | Female | Female 10/10 | Female 2/2 | Female 2/2 | Female | Female |
| Age | 15 months | 16.5 months | 6 years | 3 years | 20 months | 11 months–14 years | 14–46 years | 3–4 years | 7 years | 10 years |
| Brain MRI | MIHV HPE | Triventricular ectasia | Semi-lobar/lobar HPE | Semi-lobar HPE | Semi-lobar HPE | Semi-lobar HPE 1/10; thin corpus callosum 1/10 | Enlarged ventricles and cerebellar vermis hypotrophy (1/2) | Thinning of corpus callosum 1/2 | Small frontal lobes, thin corpus callosum | Semi-lobar HPE |
| Developmental delay | + | + | + | + | + | 10/10 | 2/2 | 2/2 | + | + |
| Craniofacial anomalies | NR | Single central incisor; depressed nasal bridge | Brachycephaly, synophrys, arched eyebrows, long eyelashes | Synophrys; long eyelashes; upturned nose | Sloping forehead, metopic ridging, upslanting palpebral fissures, midface flattening, bitemporal narrowing | Cleft palate 2/10 | Cleft palate 1/2 | 0/2 | Retrognathia | Skull asymmetry with right-sided flattening, Prominent metopic suture, and bitemporal narrowing |
| Microcephaly | + | + | + | + | + | Average Z-score −3.0 (9/9) | 1/2 | 0/2 | + | + |
| Ear anomalies and hearing | NR | NR | NR | Prominent ears | NR | Posteriorly rotated ears (3/10) | Small ears and prominent anti-helix 1/2 | 0/2 | − | − |
| Vertebral anomalies | Spina bifida (L-spine) | NR | NR | NR | NR | Bifid thoracic vertebrae 2/10 | Scoliosis 2/2 | 0 | − | T7−T12 butterfly vertebrae and partial hemivertebrae |
| Congenital heart disease | − | − | NR | − | Patent foramen ovale | 4/10 | 0/2 | 0/2 | − | Tetralogy of Fallot |
| Growth delay | + | + | − | + | NR | Average Z-score −3.0 (9/9) | 2/2 | 1/2 | + | + |
| Limb anomalies | NR | Small hands | Small hands; proximal implant of thumbs | Small hands/feet | NR | Minor limb anomalies 7/10 | Small hands 2/2 | Small hands/feet 1/2 | Small hands/feet | Multiple minor limb anomalies |
| Other | NR | Seizure disorder; periodic fevers | Seizure disorder; swallowing problems; congenital hip dysplasia; visual impairment | Seizure disorder; feeding problems | Seizure disorder | Seizure disorder 9/9 | Seizure disorder 2/2 | Seizure disorder 2/2 | Seizure disorder, gastroesophageal reflux | |
MIHV = middle interhemispheric variant; NR = not reported.
RAD21
Four variants were found in the two cohesin complex genes that are not X-linked, three were in the gene RAD21. The three RAD21 variants (Patients 12 and 13) (Table 4) were LOF; interestingly, Patient 12 with the c.1548delinsTC p.(Glu518Argfs*19) variant in RAD21 is a paternally inherited variant with the father having synophrys and a submucous cleft palate. In Table 4, the three LOF variants in the present study are compared to LOF and deletions involving RAD21 in the medical literature (Wuyts et al., 2002; McBrien et al., 2008; Deardorff et al., 2012b; Minor et al., 2014; Boyle et al., 2017). A much higher fraction of LOF variants are inherited compared to STAG2 and SMC1A: present study one (1/1) and in the medical literature, 2 of 5 (when parents where available). Both the present study and the medical literature presented individuals with cardinal features of CdLS (Kline et al., 2018), including: synophrys or thick eyebrows in 8/10, short or upturned nose in 5/10, long philtrum 5/10, and microcephaly in 6/10.
Table 4.
Phenotype details of individuals with LOF variants in RAD21
| Present study | Boyle et al., 2017 | Minor et al., 2014 | Deardorff et al., 2012b | McBrien et al., 2008 | Wuyts et al., 2002 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 12 | Patient 13 | Patient 14 | Patient 1 | Patient 2 | Patient 1 | Patient 4 | ||||
| Variant | c.1548delinsTC p.Glu518Argfs*19 | c.589C>T p.(Gln197*) | c.1217_1224del p.(Lys406Argfs*4) | c.704delG p.(Ser235Ilefs*19)b | Heterozygous 665-bp deletion including exon 13 | c.592_593dup p.Ser198Argfs*6 | Heterozygous chr8:117, 708,713–121,024,193 (hg18) deletionc | Heterozygous chr8: 116,950,003–118,944,486 (hg18) deletionc | Heterozygous chr8:117, 640,909–119,330,085 (hg18) deletionc | Heterozygous chr8:117237890–122631628 (hg18) deletionc |
| Inheritance | Paternala | Unknown | Unknown | Maternal | Maternal | Not found in mother; paternal sample unavailable | De novo | NR | De novo | De novo |
| Sex | Female | Male | Male | Female | Male | Male | Male | NR | Male | Male |
| Age | 7 years | 14 years | 2 years | 26 years | 3 years | 12 years | 7 years | NR | 26 months | 18 years |
| Brain MRI | MIHV | HPE non-specified | Septo-optic dysplasia | Normal | NR | NR | NR | NR | Focal hypersignal in T2 weight images at the level of tuber cinereum | |
| Developmental delay | + | + | + | + | + | + | − | Cognitive delay | Borderline | + |
| Craniofacial anomalies | Submucous cleft palate; synophrys, hypertelorism | Hypotelorism, upturned nose, long philtrum | Cleft palate, synophrys, brachycephaly, short nose, long philtrum, thin vermilion border; prominent eyebrows | Long philtrum, thin upper lip vermillion, short nose with up turned nasal tip (from images) | Scaphocephaly, coarse facial features, frontal bossing, mild synophrys, right ptosis, depressed nasal bridge, short nose, micrognathia | Brachycephaly; synophrys; anteverted nose; long philtrum; hirsutism | Full arched eyebrows; synophrys, cleft palate | Thick eyebrows | Prominent metopic ridge; thick eyebrows | Synophrys; long philtrum, and thin vermilion border; hirsutism |
| Microcephaly | NR | + | + | + | − | + | + | − | + | − |
| Ear anomalies and hearing | Low−set ears | − | Posteriorly rotated ears | Low-set and posteriorly rotated ears | − | − | NR | − | ||
| Vertebral anomalies | NR | NR | − | NR | NR | NR | Thoracic vertebral cleft | NR | Hemivertebrae at T10 and T11 | Kyphosis |
| Congenital heart disease | − | NR | − | − | NR | NR | − | − | Patent foramen ovale | NR |
| Growth delay | − | − | − | − | + | − | − | + | − | − |
| Limb anomalies | − | − | Small hands, fifth finger clinodactyly | Fifth finger clinodactyly | Minor hand and feet anomalies | Minor hand and feet anomalies | Minor hand and feet anomalies | Proximal thumb | Minor hand and feet anomalies | Clinodactyly first finger |
| Other | − | Seizure disorder | Gastroesophageal reflux | Hypospadias; bifid scrotum; undescended testes; inguinal hernia | Exostoses | Exostoses | Bifid scrotum; exostoses | Seizure disorder; exostoses | ||
aFather of proband is affected with synophrys, and a submucous cleft palate.
bMother affected with microcephaly and facial features consistent with CdLS.
cRAD21 is only cohesin complex gene in minimal overlapping interval (RAD21, EIF3H, UTP23, SLC30A8, MED30, EXT1, RAD21-AS1, AARD).
NR = not reported.
SMC3
The fourth non-X-linked gene is SMC3 and the SMC3 variant (Patient 15; Table 5) was a de novo in-frame deletion that is likely pathogenic (Richards et al., 2015). In Table 5, we compare to the largest and most comprehensive series of individuals with variants in SMC3 (n = 16) (Gil-Rodríguez et al., 2015). The present study found an in-frame deletion in SMC3 in a foetus with semilobar HPE, median cleft lip, tetralogy of Fallot, hypospadias, anal atresia and limb anomalies. Gil-Rodríguez et al. (2015) found two of their study participants to have midline brain malformations: corpus callosum dysgenesis (2/11) and no cases of holoprosencephaly. Based on reviewing the present study’s case and the cohort presented by Gil-Rodríguez et al., intellectual disability (13/13) and congenital heart disease (10/17) were prevalent. The facial features are difficult to characterize because of the early gestation in the present study (Fig. 1G); however, Gil-Rodríguez et al. found a majority of cases to have facial features consistent with CdLS (Table 5).
Mouse in situ hybridization
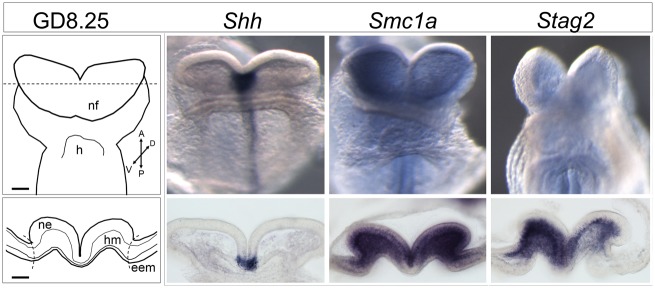
As a control, we first examined the expression of Shh (Fig. 2), a gene with a well-characterized expression domain and role in forebrain patterning and HPE (Chiang et al., 1996; Solomon et al., 2012; Hong et al., 2016). Expression of Shh is restricted to the ventromedial neuro-ectoderm (Fig. 2) as described previously (Echelard et al., 1993). Both Smc1a and Stag2 are also strongly detected in the anterior neural folds with expression observed in both the neuro-ectoderm and adjacent mesenchyme (Fig. 2). The specificity of the observed expression domains for these genes is supported by the absence of staining in extra-embryonic membrane tissue lateral to the neural folds.
Figure 2.
Gestational day (GD) 8.25 mouse embryos were stained by in situ hybridization to determine gene expression patterns. A ventral view is shown for whole mounts. Transverse sections through the prosencephalic neural folds (at the level of the dashed line in schematic) were stained to visualize gene expression in specific cellular compartments. eem = extra-embryonic membranes; h = heart; hm = head mesenchyme; ne = neuroectoderm; nf = neural folds. Scale bar = 100 µm.
Gene expression studies in human neural stem cells
As noted in the ‘Materials and methods’ section, we analysed the expression level of genes known to be involved in HPE pathways with RT-qPCR, which include SHH, SIX3, FGFR1, GLI2, ZIC2, GLI2, SMAD3 and DISP1. SHH, SIX3, ZIC2 and FGFR1 were chosen as variants in these genes known to cause HPE (Kruszka et al., 2018; Kruszka and Muenke, 2018). DISP1 is part of the sonic hedgehog pathway and has been associated with HPE (Roessler et al., 2009; Dubourg et al., 2016); also part of the sonic hedgehog pathway, GLI2 is an often HPE tested gene that is associated with HPE spectrum anomalies including pituitary insufficiency, midface hypoplasia, hypotelorism, and cleft lip/palate (Kruszka et al., 2018). Although not known to contain driver mutations associated with HPE, SMAD3 physically interacts with ZIC2 and controls transcription in a NODAL-dependent manner and variant forms of ZIC2 associated with HPE in humans and the mouse have difficulty with SMAD-dependent transcription, making SMAD3 of interest (Houtmeyers et al., 2016). Compared to controls, SMC1A knockdown in human neural stems cells resulted in significantly increased expression in GLI2 (P < 0.01), ZIC2 (P < 0.05), and SMAD3 (P < 0.05) (Supplementary Fig. 2). For STAG2 knockdown (Supplementary Fig. 3), significantly increased expression was seen in ZIC2 (P < 0.0001) and FGFR1 (P < 0.01). Thus, there was overexpression in ZIC2 from knockdown of both SMC1A and STAG2. Similar to a previous experiment (Cotney et al., 2015), SHH and SIX3 expression was undetectable in human neural stem cells.
Discussion
HPE research and clinical care has focused on sonic hedgehog pathway and the genes SHH, ZIC2,and SIX3 for the last two decades (Roessler and Muenke, 2010; Roessler et al., 2018). This study introduces new genes in the cohesin complex as important components of early forebrain division and the holoprosencephaly spectrum. Evaluating the holoprosencephaly study at NHGRI with WES, five of 277 probands were identified with variants in cohesin complex genes. Ten additional individuals with HPE were identified from other institutions. Eleven of the 15 individuals had variants in the X-linked genes STAG2 and SMC1A. STAG2 has only recently been associated with human disease (Mullegama et al., 2017, 2019; Soardi et al., 2017; Aoi et al., 2019; Yuan et al., 2019). A small number of cases with cohesin complex HPE have been reported in the medical literature in the past: two HPE cases with LOF variants in STAG2 (Aoi et al., 2019; Yuan et al., 2019), two cases associated with SMC1A (Hoppman-Chaney et al., 2012; Symonds et al., 2017), and no HPE cases have been reported that we are aware of in RAD21 and SMC3. Knowing that all individuals with CdLS have not had brain imaging, the incidence of HPE associated with cohesinopathy genes may be more common than previously reported.
Interestingly, the 11 individuals in this study with STAG2 and SMC1A variants were females, thus we propose that LOF variants in the X-linked cohesin genes are usually lethal in males; certainly, there are possible exceptions in males including mosaicism, 47,XXY, and gene duplications. Notably, STAG2 undergoes complete X-inactivation and SMC1A undergoes partial X-inactivation (Cotton et al., 2013). For Patient 2 with a STAG2 nonsense variant (c.205C>T p.(Arg69*), X-inactivation studies were consistent with random X-inactivation, implying that haploinsufficiency is required for the HPE phenotype in STAG2. The one exception to LOF in STAG2 and SMC1A is Patient 9, who had a missense variant (Table 1) located in the conserved second coiled-coil domain and is likely pathogenic (Richards et al., 2015). Based on the LOF variants in SMC1A in the other four individuals in this report, we hypothesize that the SMC1A variant (c.2683C>G (p. Arg895Gly)) has a LOF variant or a dominant negative effect. To evaluate X-linked inheritance from our HPE registry, we performed a binomial distribution on 700 individuals with HPE. Of these 700 individuals, 409 were female (P = 0.000005). If we subtract individuals with known pathogenic variants in SHH, SIX, and ZIC2, there are 645 individuals, of whom, 378 were female (P = 0.000015). Although STAG2 and SMC1A variation most likely does not explain this significant trend towards female sex in our registry, X-linked dominant inheritance likely plays an important role.
A previous study has shown that antagonizing the hedgehog signalling pathway between gestational days 7.0 and 8.25 of mouse development (approximately corresponding to the 15th to 22nd days of human gestation) results in HPE (Heyne et al., 2015a). As forebrain patterning genes are expected to be expressed in the prosencephalic neural folds during primary neurulation (Geng and Oliver, 2009), we assessed expression of cohesin complex genes during this critical period for HPE in the mouse. The finding that both Smc1a and Stag2 are expressed in the prosencephalic neural folds complements the human genetic evidence in this study and supports the role of cohesion complex genes in forebrain morphogenesis. Being expressed in both the neuro-ectoderm and adjacent mesenchyme suggests that the cohesion complex may interact with other critical regulators of forebrain patterning and HPE pathogenesis.
To elucidate the relationship between forebrain division in early embryogenesis and the cohesin complex further, we knocked down cohesin complex gene expression in human progenitor cells and measured canonical HPE gene expression. Upregulation in gene expression was seen in GLI2, ZIC2 and SMAD3 for SMC1A knockdown (Supplementary Fig. 2), and ZIC2 and FGFR1 for STAG2 knockdown (Supplementary Fig. 3). LOF in ZIC2 has been associated with HPE in the past and the mechanism of increased ZIC2 expression in SMC1A and STAG2 knockdown human neural stem cells is not completely clear. In the mouse model, LOF of Zic2 results in the failure to activate specific genes in the mid-gastrula node including Foxa2, which is required to activate Shh in the prechordal plate (Warr et al., 2008). There is evidence in the Xenopus that overexpression of zic2 may contribute depletion of foxa2 in the Spemann organizer (Houtmeyers et al., 2016). Overexpression by injection of zic2 mRNA into Xenopus embryos at the four- to eight-cell stage resulted in reduced foxa2 expression (Houtmeyers et al., 2016). SMAD3 is upregulated in SMC1A knockdown, which is of interest as SMAD3 and ZIC2 physically interact with each other in cell culture (A549 cells) to occupy a binding site in the promoter region of FOXA2 (Houtmeyers et al., 2016). FGFR1 expression increased in STAG2 knockdown neural progenitor cells. FGFR1 variants are associated with Hartsfield syndrome, which has HPE and split hands and feet as phenotype elements. It is unclear how overexpression of FGFR1 is related to HPE as the mechanism of FGFR1 in HPE is a dominant negative effect (Hong et al., 2016). GLI2 is overexpressed in the SMC1A knockdown neural progenitor cells. GLI2 is both a transcriptional activator and repressor in the sonic hedgehog pathway (Sasaki et al., 1999) and although it does not cause HPE, LOF variants in GLI2 are associated with Culler-Jones syndrome, which presents with hypopituitarism, polydactyly and facial features often found in HPE (Kruszka et al., 2018).
In conclusion, we present 15 patients with HPE spectrum malformations who have variants in cohesin complex genes STAG2, SMC1A, SMC3 and RAD21. Although the precise mechanism of abnormal forebrain development is unknown in LOF variants in cohesin complex genes, Stag2 and Smc1a are expressed in neural fold at the critical time of forebrain division in the mouse model. Additionally, we show that knockdown of STAG2 and SMC1A in human neural stem cells perturbs known HPE genes. Currently, there are no cohesin complex or X-linked genes that are commonly tested for in individuals with HPE (Kruszka et al., 2018). This report of X-linked and cohesin complex HPE has broad implications for future genetic testing, genetic counselling and HPE research.
Supplementary Material
Acknowledgements
We thank Dr Seychelle M. Vos of the Max Planck Institute for Biophysical Chemistry for critical review of this manuscript. This study makes use of data generated by the DECIPHER community. A full list of centres who contributed to the generation of the data is available from http://decipher.sanger.ac.uk and via email from decipher@sanger.ac.uk. Funding for the project was provided by the Wellcome Trust.
Glossary
Abbreviations
- CdLS
Cornelia de Lange syndrome
- HPE
holoprosencephaly
- LOF
loss-of-function
Funding
This work was supported by the National Human Genome Research Institute Intramural Research Program. Work in the lab of K.S.W. was funded by the Dutch Cancer Society (KWF) grant EMCR 2015–7857 and by the by the Netherlands Organisation of Scientific Research (NWO) grant 737.016.014. Work in the R.J.L. lab was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under award numbers R01ES026819 and T32ES007015.
Competing interests
The authors report no competing interests.
References
- Ansari M, Poke G, Ferry Q, Williamson K, Aldridge R, Meynert AM et al. Genetic heterogeneity in Cornelia de Lange syndrome (CdLS) and CdLS-like phenotypes with observed and predicted levels of mosaicism. J Med Genet 2014; 51: 659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoi H, Lei M, Mizuguchi T, Nishioka N, Goto T, Miyama S et al. Nonsense variants in STAG2 result in distinct sex-dependent phenotypes. J Hum Genet 2019; 64: 487–92. [DOI] [PubMed] [Google Scholar]
- Boyle MI, Jespersgaard C, Brondum-Nielsen K, Bisgaard AM, Tumer Z. Cornelia de Lange syndrome. Clin Genet 2015; 88: 1–12. [DOI] [PubMed] [Google Scholar]
- Boyle MI, Jespersgaard C, Nazaryan L, Bisgaard AM, Tumer Z. A novel RAD21 variant associated with intrafamilial phenotypic variation in Cornelia de Lange syndrome: review of the literature. Clin Genet 2017; 91: 647–9. [DOI] [PubMed] [Google Scholar]
- Brooker AS, Berkowitz KM. The roles of cohesins in mitosis, meiosis, and human health and disease. Methods Mol Biol 2014; 1170: 229–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetaille P, Preuss C, Burkhard S, Cote JM, Houde C, Castilloux J et al. Mutations in SGOL1 cause a novel cohesinopathy affecting heart and gut rhythm. Nat Genet 2014; 46: 1245–9. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 1996; 383: 407–13. [DOI] [PubMed] [Google Scholar]
- Cotney J, Muhle RA, Sanders SJ, Liu L, Willsey AJ, Niu W et al. The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment. Nat Commun 2015; 6: 6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton AM, Ge B, Light N, Adoue V, Pastinen T, Brown CJ. Analysis of expressed SNPs identifies variable extents of expression from the human inactive X chromosome. Genome Biol 2013; 14: R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Franco E, Watson RA, Weninger WJ, Wong CC, Flanagan SE, Caswell R et al. A Specific CNOT1 mutation results in a novel syndrome of pancreatic agenesis and holoprosencephaly through impaired pancreatic and neurological development. Am J Hum Genet 2019; 104: 985–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deardorff MA, Bando M, Nakato R, Watrin E, Itoh T, Minamino M et al. HDAC8 mutations in Cornelia de Lange syndrome affect the cohesin acetylation cycle. Nature 2012a; 489: 313–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deardorff MA, Kaur M, Yaeger D, Rampuria A, Korolev S, Pie J et al. Mutations in cohesin complex members SMC3 and SMC1A cause a mild variant of cornelia de Lange syndrome with predominant mental retardation. Am J Hum Genet 2007; 80: 485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deardorff MA, Wilde JJ, Albrecht M, Dickinson E, Tennstedt S, Braunholz D et al. RAD21 mutations cause a human cohesinopathy. Am J Hum Genet 2012b; 90: 1014–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubourg C, Carre W, Hamdi-Roze H, Mouden C, Roume J, Abdelmajid B et al. Mutational spectrum in holoprosencephaly shows that FGF is a new major signaling pathway. Hum Mutat 2016; 37: 1329–39. [DOI] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 1993; 75: 1417–30. [DOI] [PubMed] [Google Scholar]
- Everson JL, Fink DM, Yoon JW, Leslie EJ, Kietzman HW, Ansen-Wilson LJ et al. Sonic Hedgehog regulation of Foxf2 promotes cranial neural crest mesenchyme proliferation and is disrupted in cleft lip morphogenesis. Development 2017; 144: 2082–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth HV, Richards SM, Bevan AP, Clayton S, Corpas M, Rajan D et al. DECIPHER: database of chromosomal imbalance and phenotype in humans using ensembl resources. Am J Hum Genet 2009; 84: 524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Oliver G. Pathogenesis of holoprosencephaly. J Clin Invest 2009; 119: 1403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Rodríguez MC, Deardorff MA, Ansari M, Tan CA, Parenti I, Baquero-Montoya C et al. De novo heterozygous mutations in SMC3 cause a range of Cornelia de Lange syndrome-overlapping phenotypes. Hum Mutat 2015; 36: 454–62. [DOI] [PubMed] [Google Scholar]
- Goldstein JH, Tim-Aroon T, Shieh J, Merrill M, Deeb KK, Zhang S et al. Novel SMC1A frameshift mutations in children with developmental delay and epilepsy. Eur J Med Genet 2015; 58: 562–8. [DOI] [PubMed] [Google Scholar]
- Gordillo M, Vega H, Trainer AH, Hou F, Sakai N, Luque R et al. The molecular mechanism underlying Roberts syndrome involves loss of ESCO2 acetyltransferase activity. Hum Mol Genet 2008; 17: 2172–80. [DOI] [PubMed] [Google Scholar]
- Hahn JS, Barnes PD, Clegg NJ, Stashinko EE. Septopreoptic holoprosencephaly: a mild subtype associated with midline craniofacial anomalies. AJNR Am J Neuroradiol 2010; 31: 1596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyne GW, Melberg CG, Doroodchi P, Parins KF, Kietzman HW, Everson JL et al. Definition of critical periods for Hedgehog pathway antagonist-induced holoprosencephaly, cleft lip, and cleft palate. PLoS One 2015a; 10: e0120517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyne GW, Plisch EH, Melberg CG, Sandgren EP, Peter JA, Lipinski RJ. A simple and reliable method for early pregnancy detection in inbred mice. J Am Assoc Lab Anim Sci 2015b; 54: 368–71. [PMC free article] [PubMed] [Google Scholar]
- Hong S, Hu P, Marino J, Hufnagel SB, Hopkin RJ, Toromanovic A et al. Dominant-negative kinase domain mutations in FGFR1 can explain the clinical severity of Hartsfield syndrome. Hum Mol Genet 2016; 25: 1912–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppman-Chaney N, Jang JS, Jen J, Babovic-Vuksanovic D, Hodge JC. In-frame multi-exon deletion of SMC1A in a severely affected female with Cornelia de Lange Syndrome. Am J Med Genet A 2012; 158A: 193–8. [DOI] [PubMed] [Google Scholar]
- Houtmeyers R, Tchouate Gainkam O, Glanville-Jones HA, Van den Bosch B, Chappell A, Barratt KS et al. Zic2 mutation causes holoprosencephaly via disruption of NODAL signalling. Hum Mol Genet 2016; 25: 3946–59. [DOI] [PubMed] [Google Scholar]
- Huisman SA, Redeker EJ, Maas SM, Mannens MM, Hennekam RC. High rate of mosaicism in individuals with Cornelia de Lange syndrome. Journal of medical genetics 2013; 50: 339–44. [DOI] [PubMed] [Google Scholar]
- Izumi K, Nakato R, Zhang Z, Edmondson AC, Noon S, Dulik MC et al. Germline gain-of-function mutations in AFF4 cause a developmental syndrome functionally linking the super elongation complex and cohesin. Nat Genet 2015; 47: 338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen S, Kleefstra T, Willemsen MH, de Vries P, Pfundt R, Hehir-Kwa JY et al. De novo loss-of-function mutations in X-linked SMC1A cause severe ID and therapy-resistant epilepsy in females: expanding the phenotypic spectrum. Clin Genet 2016; 90: 413–9. [DOI] [PubMed] [Google Scholar]
- Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet 2014; 46: 310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline AD, Moss JF, Selicorni A, Bisgaard AM, Deardorff MA, Gillett PM et al. Diagnosis and management of Cornelia de Lange syndrome: first international consensus statement. Nat Rev Genet 2018; 19: 649–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz ID, McCallum J, DeScipio C, Kaur M, Gillis LA, Yaeger D et al. Cornelia de Lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophila melanogaster Nipped-B. Nat Genet 2004; 36: 631–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszka P, Berger SI, Weiss K, Everson JL, Martinez AF, Hong S et al. CCR4-NOT transcription complex, subunit 1 (CNOT1) variant associated with holoprosencephaly. Am J Hum Genet 2019; 104: 990–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszka P, Martinez AF, Muenke M. Molecular testing in holoprosencephaly. Am J Med Genet C 2018; 178: 187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszka P, Muenke M. Syndromes associated with holoprosencephaly. Am J Med Genet C 2018; 178: 229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun N, Lebon S, Jeannet PY, Jacquemont S, Billuart P, Bienvenu T. Early-onset encephalopathy with epilepsy associated with a novel splice site mutation in SMC1A. Am J Med Genet A 2015; 167A: 3076–81. [DOI] [PubMed] [Google Scholar]
- Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016; 536: 285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga E, Shiota K. Holoprosencephaly in human embryos: epidemiologic studies of 150 cases. Teratology 1977; 16: 261–72. [DOI] [PubMed] [Google Scholar]
- McBrien J, Crolla JA, Huang S, Kelleher J, Gleeson J, Lynch SA. Further case of microdeletion of 8q24 with phenotype overlapping Langer-Giedion without TRPS1 deletion. Am J Med Genet A 2008; 146A: 1587–92. [DOI] [PubMed] [Google Scholar]
- Mehta GD, Kumar R, Srivastava S, Ghosh SK. Cohesin: functions beyond sister chromatid cohesion. FEBS Lett 2013; 587: 2299–312. [DOI] [PubMed] [Google Scholar]
- Minor A, Shinawi M, Hogue JS, Vineyard M, Hamlin DR, Tan C et al. Two novel RAD21 mutations in patients with mild Cornelia de Lange syndrome-like presentation and report of the first familial case. Gene 2014; 537: 279–84. [DOI] [PubMed] [Google Scholar]
- Mullegama SV, Klein SD, Mulatinho MV, Senaratne TN, Singh K, Center UCG et al. De novo loss-of-function variants in STAG2 are associated with developmental delay, microcephaly, and congenital anomalies. Am J Med Genet A 2017; 173: 1319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullegama SV, Klein SD, Signer RH, Center UCG, Vilain E, Martinez-Agosto JA. Mutations in STAG2 cause an X-linked cohesinopathy associated with undergrowth, developmental delay, and dysmorphia: expanding the phenotype in males. Mol Genet Genomic Med 2019; 7: e00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musio A, Selicorni A, Focarelli ML, Gervasini C, Milani D, Russo S et al. X-linked Cornelia de Lange syndrome owing to SMC1L1 mutations. Nat Genet 2006; 38: 528–30. [DOI] [PubMed] [Google Scholar]
- Olley G, Ansari M, Bengani H, Grimes GR, Rhodes J, von Kriegsheim A et al. BRD4 interacts with NIPBL and BRD4 is mutated in a Cornelia de Lange-like syndrome. Nat Genet 2018; 50: 329–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda-Alvarez DE, Dubourg C, David V, Roessler E, Muenke M. Current recommendations for the molecular evaluation of newly diagnosed holoprosencephaly patients. Am J Med Genet C 2010; 154C: 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17: 405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler E, Hu P, Muenke M. Holoprosencephaly in the genomics era. Am J Med Genet C 2018; 178: 165–74. [DOI] [PubMed] [Google Scholar]
- Roessler E, Ma Y, Ouspenskaia MV, Lacbawan F, Bendavid C, Dubourg C et al. Truncating loss-of-function mutations of DISP1 contribute to holoprosencephaly-like microform features in humans. Hum Genet 2009; 125: 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler E, Muenke M. The molecular genetics of holoprosencephaly. Am J Med Genet C 2010; 154C: 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development 1999; 126: 3915–24. [DOI] [PubMed] [Google Scholar]
- Simonis N, Migeotte I, Lambert N, Perazzolo C, de Silva DC, Dimitrov B et al. FGFR1 mutations cause Hartsfield syndrome, the unique association of holoprosencephaly and ectrodactyly. J Med Genet 2013; 50: 585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soardi FC, Machado-Silva A, Linhares ND, Zheng G, Qu Q, Pena HB et al. Familial STAG2 germline mutation defines a new human cohesinopathy. NPJ Genom Med 2017; 2: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobreira N, Schiettecatte F, Valle D, Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum Mutat 2015; 36: 928–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon BD, Bear KA, Wyllie A, Keaton AA, Dubourg C, David V et al. Genotypic and phenotypic analysis of 396 individuals with mutations in Sonic Hedgehog. J Med Genet 2012; 49: 473–9. [DOI] [PubMed] [Google Scholar]
- Symonds JD, Joss S, Metcalfe KA, Somarathi S, Cruden J, Devlin AM et al. Heterozygous truncation mutations of the SMC1A gene cause a severe early onset epilepsy with cluster seizures in females: detailed phenotyping of 10 new cases. Epilepsia 2017; 58: 565–75. [DOI] [PubMed] [Google Scholar]
- Warr N, Powles-Glover N, Chappell A, Robson J, Norris D, Arkell RM. Zic2-associated holoprosencephaly is caused by a transient defect in the organizer region during gastrulation. Hum Mol Genet 2008; 17: 2986–96. [DOI] [PubMed] [Google Scholar]
- Wuyts W, Roland D, Ludecke HJ, Wauters J, Foulon M, Van Hul W et al. Multiple exostoses, mental retardation, hypertrichosis, and brain abnormalities in a boy with a de novo 8q24 submicroscopic interstitial deletion. Am J Med Genet 2002; 113: 326–32. [DOI] [PubMed] [Google Scholar]
- Yuan B, Neira J, Pehlivan D, Santiago-Sim T, Song X, Rosenfeld J et al. Clinical exome sequencing reveals locus heterogeneity and phenotypic variability of cohesinopathies. Genet Med 2019; 21: 663–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B, Pehlivan D, Karaca E, Patel N, Charng WL, Gambin T et al. Global transcriptional disturbances underlie Cornelia de Lange syndrome and related phenotypes. J Clin Invest 2015; 125: 636–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data that support the findings of this manuscript are available upon request to the corresponding author.