Abstract
Background
The use of non-invasive brain stimulation (NIBS) combined with exercise could produce synergistic effects on chronic pain conditions. This study aims to evaluate the efficacy and safety of NIBS combined with exercise to treat chronic pain as well as to describe the parameters used to date in this combination.
Methods
The search was carried out in Medline, Central, Scopus, Embase, and Pedro until November 2019. Randomized clinical trials (RCTs) and quasi-experimental studies reporting the use of non-invasive brain stimulation and exercise on patients with chronic pain were selected and revised.
Results
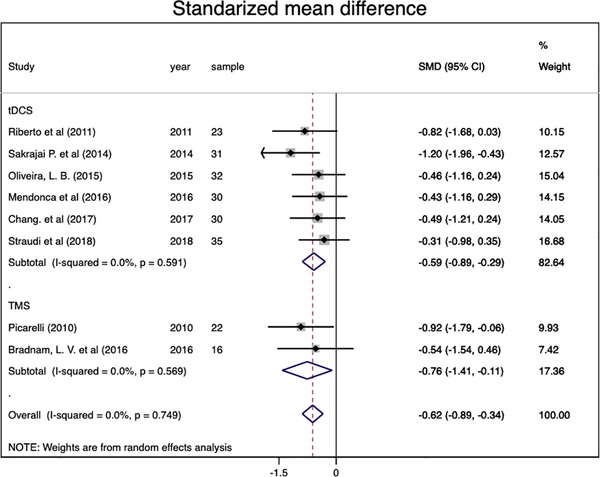
The authors included eight studies (RCTs), reporting eight comparisons (219 participants). Authors found a significant and homogeneous pain decrease (ES: −0.62, 95% CI:−0.89 to −0.34; I2= 0.0%) in favor of the combined intervention compared to sham NIBS + exercise, predominantly by excitatory (anodal tDCS/ rTMS) motor cortex stimulation. Regarding NIBS techniques, the pooled effect sizes were significant for both tDCS (ES: −0.59, 95% CI: −0.89 to −0.29, I2= 0.0%) and rTMS (ES: −0.76, 95% CI: −1.41 to −0.11, I2=0.0%).
Conclusions
This meta-analysis suggests a significant moderate to large effects of the NIBS and exercise combination in chronic pain. The authors discuss the potential theoretical framework for this synergistic effect.
Keywords: non-invasive brain stimulation, chronic pain, exercise, transcranial direct current stimulation, transcranial magnetic stimulation
1. Introduction
Pain is a significant public health problem worldwide; one out of ten people is diagnosed with chronic pain each year [1]. Pain perception is associated with psychological, neurological, and emotional components. Therefore, there are several treatment approaches involving not only drugs but other behavioral therapies targeting those other components. Regardless of the treatment’s efficacy, some are associated with adverse events as toxicity, drug abuse, or gastrointestinal events [2]. Moreover, 5% of these population doesn’t respond to conventional medications leading to a gap of new effective and safe pain treatments [3].
Over the last 20 years, the knowledge regarding non-invasive brain stimulation (NIBS) techniques such as transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS) has increased on a wide of different conditions [4,5]. Studies are supporting the efficacy of tDCS and rTMS on chronic pain by enhancing the endogenous pain modulation system [6,7]. Motor cortex stimulation effects on pain are likely a result of secondary and distant modulation on pain-related circuits such as the cingulate gyrus, insula, thalamic nuclei, subthalamic areas, and brainstem [8,9]. Indeed, studies have shown that motor cortex stimulation leads to a reduction of thalamocortical dysrhythmia [10,11], which is one important neurophysiological signature of chronic pain conditions [12]. TDCS exert its effects by changing spontaneous neuronal activity through a subthreshold current delivered via two electrodes (anode and cathode). [13]. While rTMS changes cortical excitability due to a magnetic stimulus produced by an electromagnetic pulse delivered by a coil placed over the head [14]. Both, however, depend on specific parameters such as the site of stimulation, duration, intensity, and also the neural networks state. For this reason, the state-dependent nature of the non-invasive brain stimulation needs a well-combined therapy to guide the neuroplasticity process [15,16].
Moreover, exercise increases corticothalamic excitability [17]. Exercises may enhance the inhibitory activity of thalamic areas involved with pain regulation. Furthermore, exercise is also associated with motivation and pleasure by enhancing the dopaminergic pathway and endogenous-opioid system, which could modify pain perception [18,19]. Studies are showing positive effects of exercise in healthy and pain conditions associated with a bottom-up regulation, called exercise-induced hypoalgesia, depending on the exercise type and intensity [20,21].
Combined treatment of NIBS and exercise has also been tested for psychiatric conditions. In addition, other combined protocols show effects on cognitive improvement, as well as stroke rehabilitation and reduction of chronic pain [22]. Steinberg et al. [23] describe the synergistic effect of tDCS and exercise on executive function, while Vaz et al. [24] supports the positive effects of rTMS combined with exercise-based rehabilitation therapies for gait speed improvement after a stroke [25]. Nevertheless, the efficacy of the exercise with NIBS combination on chronic pain is still unclear, and also the best parameters to produce a synergistic effect.
This systematic review aims to evaluate the efficacy and safety of NIBS combined with exercise to treat chronic pain as well as to describe the parameters used to date in this combination.
2. Methods
2.1. Protocol and registration
A systematic review of the literature and meta-analysis was conducted following the recommendation of the Cochrane group [26], including the PRISMA guidelines (online supplementary material 1)[27]. The study protocol was registered in PROSPERO with the following registration number: CRD42019126255, and it is available to review online: (http://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019126255).
2.2. Literature search and study selection
We searched in MEDLINE, Scopus, Cochrane Central, EMBASE and PEDRO databases until November 26, 2019, using a search strategy with the following non-exhaustive MeSH search terms: ”noninvasive brain stimulation” OR “transcranial magnetic stimulation” OR “transcranial direct current stimulation” AND ”Exercise” AND ”Chronic pain” OR “Neuropathic pain”. The full research strategy is shown in online supplementary Material 2. Following the initial search, new relevant studies were found on bibliographic references of the included studies.
Duplicates were eliminated before selection. Before titles and abstract selection, two reviewers (AC-R and OR-T) agreed on a standard approach. Two random samples of fifty search results were selected for training purposes. Reviewers screened these titles and abstracts, and the inter-rater agreement and kappa estimator were computed, aiming for an inter-rater agreement of at least 90% (online supplementary material 3). After this standardization process, the citations were independently screened by two reviewers (AC-R and OR-T) in terms of titles and abstracts. Discrepancies between reviewers were resolved by a third reviewer (KP-B). After the initial review, the two main reviewers independently assessed full texts of selected studies, and again, the third reviewer resolved discrepancies.
2.3. Eligibility criteria
We searched for full-text articles without language restrictions (only articles in English were found). Included articles had to: (i) enroll subjects with chronic pain, defined by pain more than three months; (ii) performed noninvasive brain stimulation including transcranial direct current stimulation or transcranial magnetic stimulation; combined with any type of exercise including aerobic, non-aerobic, resistance and dynamic strength exercise. (iii) Randomized controlled trials (RCTs) included parallel-group, crossover designs, and pilot studies, and quasi-experimental (QE) trials included non-controlled, non-randomized, and one arm studies.
2.4. Data extraction
Data extraction from each selected study was conducted independently by two reviewers (AC-R and OR-T) and then revised by a third reviewer (KP-B). A data extraction table was used for each included study, obtaining information on the measurement of pain at baseline and after treatment of NIBS combined with exercise, to calculate the mean difference per group. We also extracted data referring to safety, parameters of the study, study population outcomes, year of publication, country, number of patients evaluated, chronic pain condition, and quality of life. The extracted data were tabulated, coded, and then imported into a datasheet for analysis.
2.5. Risk of bias assessment
The risk of bias of the selected studies was evaluated by two reviewers (OR-T and SG-L) using Jadad Scale for RCTs. This scale evaluates RCTs according to three categories: (i) randomization; (ii) blinding; and (iii) reporting data. We used the standard score system from 0 to 5 (high number means a low risk of bias) [28]. In the event of any discrepancies between the two reviewers, a consensus was solved by discussion. If a full consensus cannot be reached between the two reviewers after an exhaustive discussion, the opinion of a third reviewer was obtained, and the proceeding majority consensus was taken.
2.6. Data synthesis
We decided to present results separately according to study design (RCTs vs. QE studies), given the differences in the quality of evidence between these two types of studies. The pain outcome was categorized according to time, based on previous reviews [29], in three groups: i) short term (end of intervention or <1 week); ii) medium-term (>1 week to <6 weeks); and iv) long term (>6 weeks).
Then, with the RCTs data, we performed an exploratory meta-analysis of Hedge’s g for pain rating response. Although within the treatment categories are interventions with different parameters, we decided to do an exploratory synthesis to compare across the spectrum of the available neuromodulation techniques. When possible, we used pre and post scores of the pain analog scales, for each outcome to calculate the mean difference between groups. The difference was then converted to an effect size (ES). Given that Cohen’s d has a slight bias to overestimate in small sample sizes, we adjusted Cohen’s d to Hedge’s g by applying a correction factor [30].
We considered the likely clinical importance of the pooled effect size using the criteria proposed in the IMMPACT consensus statement[31]. Specifically, we judged a decrease in pain of less than 15% as no important change, of 15% or more as a minimally important change, of 30% or more as a moderately important change and of 50% or more as a substantially important change.
We assessed heterogeneity using an I2 statistical, and we considered low heterogeneity when I2 <40% [32]. We consider it appropriate to use random-effects models due to the overall heterogeneity evaluation (in population and intervention) [32]. The meta-regression and publication bias were not assessed since the number of studies pooled for each meta-analysis was less than ten [26]. The data was processed using Stata v15.0 software.
3. Results
3.1. Overview
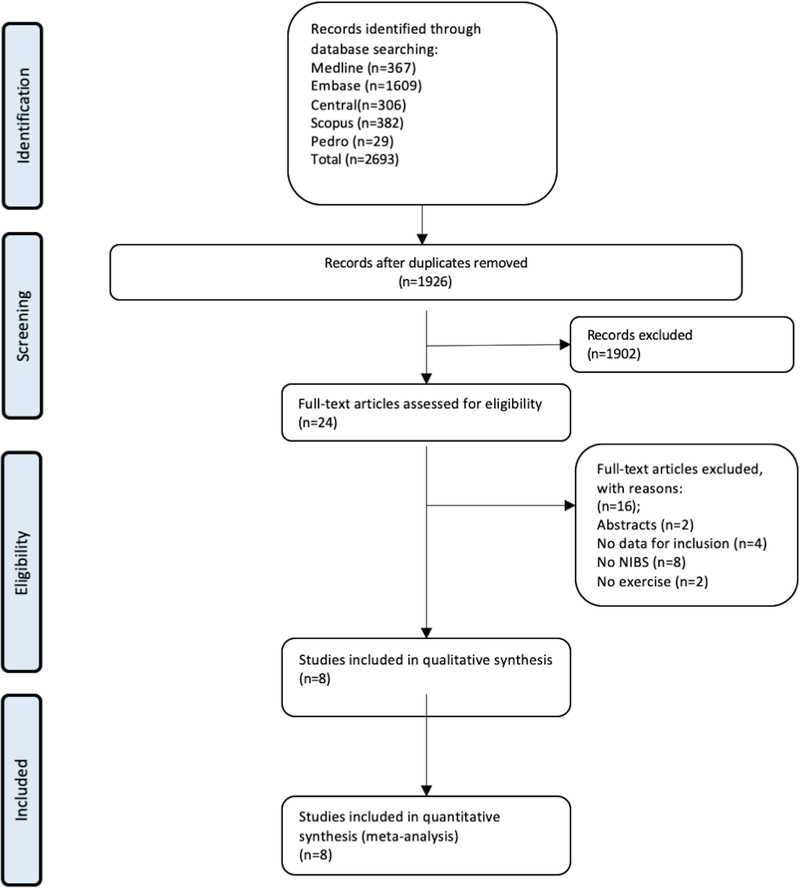
The search retrieved 2693 results; after removing duplicates, 1926 titles and abstracts were screened, and of these, 1902 were excluded. Sixteen studies were evaluated in full-text; eight studies were excluded (online supplementary material 4). And finally, eight studies (RCTs) were included [33–40], reporting eight comparisons (219 participants). A flow diagram of the searched and evaluated literature is presented in Figure 1.
Figure 1:
A flow diagram of the searched and evaluated literature
We included the following pain populations: (i) knee osteoarthritis (12.5% studies, n=1) [33], (ii) fibromyalgia (25% studies, n=2)[37,38], (iii) myofascial pain syndrome (25% studies, n=2)[34,35], (iv) low-back pain (12.5% studies, n=1)[36], (vi) cervical dystonia (12.5% studies, n=1)[40], and (vii) complex regional pain syndrome type I in upper limb (12.5% studies, n=1)[39]. Regarding the pain rating scale, six studies reported through the visual analog scale [33–36,38,39], one with the SF-36 form pain subscale [37], and one through the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) [40]. The range of included timepoints is from five days [35] to four months after baseline [37].
Among the chronic pain population, the range of chronic pain duration was variable, from 5 months in case of myofascial pain [35] to 149 months for fibromyalgia [38] (See Table 1 and Table 2). Moreover, some studies reported no drop-outs at the primary outcome [34,38,40]. On the other hand, two studies reported one drop-out [35,39], Straudi et al. [36] reported two drop-outs in the sham group and used intention to treat for the analysis, and Chang et al [33] had 16% of drop outs in the active TDCS and exercise compare to 20% in the sham tDCS and exercise. More information can be found in Table 1 and 2.
Table 1.
General information from the tDCS studies included in the meta-analysis
| Author | Country | Sample size N (M/F) | Jadad | Clinical Condition | Groups (Age±SD) | Pain duration (months±SD) | Protocol | tDCS Duration | Anode/Cathode | Intensity | Sham tDCS | Exercise | Safety | Pain outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chang. et al (2017) | Australia | 30 (10/20) | 4 | Knee osteoarthritis | (1) a-tDCS before Exercise(59.8 ± 9.1); (2) s-tDCS before Exercise() 64.1 ± 11.1) | (1) 7.2± 5.3 (2) 9 ± 7.3 | 8 weeks: tDCS and exercise twice weekly (16 sessions) and completing home exercises twice per week | 20 min | M1 contralateral /SO ipsilateral | 1mA | Electrodes over the same position and stimulation was turned on for the first 15 seconds and then turned off. | 30 min of 5 standardized quadriceps strengthening exercises with ankle cuff weights or resistance bands (3 sets of 10 repetitions with a 30 s break between sets for each exercise) provided by a physiotherapist. | (1) Active tDCS and exercise group: 1 subject with pain and swelling (gout), 1 single episode of headache. (2) Sham tDCS and exercise group: 1 subject with painful sensation under tDCS during the ramped up | VAS (0–100 mm) on walking over the past week |
| Mendonca et al (2016) | Brazil | 45 (1/44) | 4 | Fibromyalgia | (1) a-tDCS + Aerobic Exercise simultaneously(44.5±14); (2) a-tDCS+Sham Exercise simultaneously(49.9±10.6); (3) sham-tDCS + Aerobic Exercise simultaneously(48±11.8) | (1) 140.6±72.2 (2) 149.3±111.1 (3) 125.6±100.2 | 4 weeks: first week: tDCS 5 times/week and Aerobic exercise 3 times/week. The next 3 weeks: Aerobic Exercise 3 times/week | 20 min | left M1/right SO | 2mA | Electrodes over the same position and stimulation only on the initial 30s, then the power was turned off | 30 min of aerobic exercise on a treadmill (intensity of 60% - 70% of the maximum HR). Sham Exercise: the HR was maintained within 5% of the resting Heart Rate. | All were mild and did not differ between groups. Related to Aerobic Exercise: mild muscle pain; related to tDCS: headache, neck pain, tingling, skin redness, somnolence, concentration issues. | VNS (0–10 cm) at the moment |
| Oliveira, L. B. (2015) | Brazil | 32 (3/29) | 5 | Chronic temporomandibular pain -myofascial pain | (1) a-tDCS before Exercise (23.8 ± 7.3); (2) s-tDCS after Exercise(25.5 ± 6.3) | (1) 29.8 ± 17.1 (2) 33.7 ± 22.8 | 4 week: First week: exercise and tDCS for 5 days. Then, 3 weeks: exercise 2 days per week until completed 10 sessions | 20 min | M1 contralateral/ SO ipsilateral | 2mA | Electrodes over the same position and stimulation only on the initial 30s, then the power was turned off | 15 min of 6 repetitions of each exercise, progressing from lying down to the sitting position. The exercises involved cervical traction and self-stretching of the posterior muscles of the head and neck maintained for 20 s. | One subject suffered burns on the fifth day of stimulus application, due to acne in the supraorbital region. At the end of the exercise sessions, the skin was completely healed with a small scar measuring 2x2 mm | VAS (0–10 cm) at rest |
| Sakrajai P. et al (2014) | Thailand | 31 (22/9) | 4 | Myofascial Pain Syndrome | (1) a- tDCS + Exercise(49.94 ±8.25); (2) s-tDCS + Exercise(45.93±10.24) | (1) 5.91±2.55 (2) 5.69±1.69 | 5 consecutive days of tDCS and standard treatment | 20 min | M1 contralateral /SO ipsilateral | 1 mA | Electrodes over the same position and stimulation only on the initial 30s, then the power was turned off while the power indicator remained on and the control switch was covered by an opaque adhesive | Daily stretching of the group of muscles throughout the available range of motion. Treatment also included ultrasound therapy for 5–10 min followed by the application of hot packs over the affected part 20 min 3 times/week for 2 weeks. | Active tDCS: 2 patients developed a transient erythematous rash with no pruritus or pain under the reference electrode, which resolved within 1 hour. | NRS (0–10 cm) average on the last 24 hours and immediately after the treatment |
| Riberto et al (2011) | Brazil | 23 (0/23) | 4 | Fibromyalgia | (1)a-tDCS before the Rehabilitation program (58.3 ±12.1); (2) s-tDCS before the Rehabilitation program (52.4 ±11.5) | (1) 9.9 ±11.8 (2) 6.4 ±10.2 | 4 months: 10 sessions of tDCS (1 each week) and exercise 3 times a week | 20 min | M1 contralateral /SO ipsilateral | 2mA | Electrodes over the same position and stimulation only on the initial 30s, then the power was turned off | Rehabilitation program for 2 hours: 1 hour [educative interventions or cognitive behavior group therapy focused in pain. Subjects are oriented on posture and ergonomics by physical and occupational therapists and behavioral modulation by psychologists and social workers] and 1 hour [cardiovascular and strengthening training or stretching exercises] | none adverse events reported | (1) SF-36 pain subscale on the last 4 weeks. (2) VAS (0–100 mm) at the moment on each extremity (values not reported). |
| Straudi et al (2018) | Italy | 35 (9/26) | 5 | Low back pain | (1) a-tDCS before Exercise(54.3±12.4); (2) s-tDCS before Exercise(56±12.9) | (1)9.4±9.2; (2) 7.8±5.3 | 4 weeks: first week: 5 daily sessions of tDCS and 2 or 3 times per week sessions of exercise. Next 3 weeks: aerobic exercise 2 or 3 times per week (total of 11 exercise sessions) | 20 min | M1 contralateral /SO ipsilateral or M1 dominant hemisphere if pain was central / SO contralateral of anode | 2 mA | Stimulation only on the initial 30s, then the power was turned off | Group exercise (10 participants) of 1 hour including education(neurophysiology of pain and ergonomic advices), strengthening exercise (low moderate intensity of submaximal contractions on abdominal and back exercises), stretching exercise (elongation position of all structures that support the spine for 15–20 s) and relaxation techniques (correct breathing techniques, reduce stress, muscle tension and anxiety with activities) | 29 reported mild side-effects (16 in the active tDCS and 13 in sham tDCS) as skin redness, tingling, headache, sleepiness, trouble to concentrate, dizziness, mood fluctuation. | VAS (0–100 mm, doesn’t specify the time) |
tDCS: Transcranial Direct Current Stimulation; SO: Supraorbital; s-tDCS: sham-tDCS; a-tDCS: active tDCS; VAS: visual analog scale; SF-36: 36-Item Short Form Survey; NRS: Numerical Rating Scale.
Table 2.
General information from the TMS studies included in the meta-analysis
| Author | Country | Sample size (M/F) | Jadad | Clinical Condition | Group (Age±SD) | Painduration (months±SD) | Protocol | NIBS | Number of sessions | Target area | Frequency, intensity | Pulses/sessions. No of sessions | Sham TMS | Exercise | Safety | Pain outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bradnam, L. V. et al (2010) | Australia | 16 (6/10) | 3 | Cervical Dystonia | (1) iTBS before Exercise (50.5 ± 8.5) (2) Sham iTBS before Exercise (53.4 ± 14.6) |
- | 10 sessions (5 sessions per week) of iTBS or sham iTBS before exercise | Intermittent TBS | 10 sessions | Lateral cerebellum (bilateral) | 2 s train of TBS repeated every 10 s for a total of 190 s (600 pulses) | Sham TMS coil with a similar noise to the real iTBS | Active exercise training was guided by a 10 minutes video recording at each session. The videos depicted a person sitting in a chair performing neck exercises with voice-over instructions. It included an attempt to maintain a ‘chin tuck’ neutral upper neck posture and other exercises as followed by a ‘cueing task’ (participants moved out of their dystonic posture in response to a sound cue) and motor control training | None adverse events | TWSTRS pain scale | |
| Picarelli. et al (2010) | United States | 23 (9/14) | 4 | Complex regional pain syndrome type I in upper limb | (1) rTMS + Medical treatment with exercise (43.5 ± 12.1,); (2) Sham rTMS + Medical treatment with exercise (40.6 ± 9.9) | (1) 82.33 ± 34.5, (2) 79.27 ± 32.1 | 10 sessions (5 sessions per week) of rTMS or sham rTMS. | rTMS | 10 sessions | Precentral gyrus | 10 Hz, 100% RMT | 10 sec trains with an interval of 60 sec, total of 2500 pulses delivered during 25 | Sham TMS sessions used an identical 8-shaped coil which did not generate a magnetic field but generate a similar noise. | Best medical treatment: (1) standardized pharmacological treatment (2) physical therapy program (kinesiotherapy plus low impact, aerobic, relaxation and stretching exercise) for 3 months | 1 generalized seizure after the 7th session. Others: headache, neck pain, non-painful sensation on the scalp, dizziness. | VAS (0–10 cm) (not specific time) |
rTMS: repetitive Transcranial Magnetic Stimulation; VAS: visual analog scale; TWSTRS: Toronto Western Spasmodic Torticollis Rating Scale – severity.
3.2. Combined treatment characteristics
Regarding the intervention, seven studies performed motor cortex stimulation (anodal M1 tDCS and high-frequency rTMS) [33–39] and one study theta-burst, a type of rTMS technique, over the cerebellar cortex [40]. The NIBs sessions lasted 20 min for tDCS, during a time-lapse of 1 week [35] to 4 months [37], and a frequency from 5 to 1 time per week, two of the studies used 1 mA [33,35] and four of them 2 mA as the intensity[34,36–38]. For the control group, seven of the studies used sham NIBS combined with active exercise, while only Mendoca et al. [38] had 3 arms ( active tDCS and exercise, sham tDCS and exercise, active tDCS and sham exercise). The sham tDCS protocol was homogeneous as they used the electrodes on the same location, most of them 30 seconds of stimulation at the beginning and the end of the stimulation and in-between had an off-period. The rTMS sham protocol included a sham coil with a similar noise. Moreover, only one study considered sham exercise, described as the 5% of the baseline heart rate [38]. Exercise interventions were heterogeneous and could include several activities such as walking, running, body stretching, and muscle contraction. The exercise session lasted from 10 to 60 min and the exercise period was around 5 days to 4 months, and the frequency vary from daily to 2 or 3 times a week, only two studies [36,38] performed moderate protocols, and six don’t specify the intensity. Regarding the exercise type, 62.5% (five studies) performed stretching including myofascial pain [34,35], low back pain[36], complex regional pain syndrome [39] and fibromyalgia [37], 37.5% (three studies) performed strength including chronic pain due to knee osteoarthritis [33], fibromyalgia [37] and low back pain[36]; and 25% (two studies) performed aerobic exercise, one in fibromyalgia considering the range of 60%−70% of the maximum heart rate [38] and the other study in complex regional pain syndrome [39]. Moreover, 37.5% (three) of the studies was restricted to only one type of exercise[33,34,38], while the other 62.5% (six) had a combination of different types of exercises and/or therapies (postural, educational sessions, occupational therapy, behavior, ultrasound therapy)[35–37,39,40]. Also, group sessions were described in 37.5% (three) of the studies [35–37]. Regarding the combination modality, 50% (four)[33,36,37,40], 12.5% (one)[38], and 12.5%(one)[34] of the studies performed the exercise prior, during, and after NIBS, respectively, and 25% (two)[35,39] do not specify the time.. A qualitative summary of included articles is provided in Tables 1 and 2.
3.3. Effect on outcomes
We analyzed eight RCTs (eight comparisons) with chronic pain conditions (n=219) to evaluate the combined effect of NIBs and exercise in chronic pain. We found a significant and homogeneous pain decrease (ES: −0.62, 95% CI:−0.89 to −0.34; I2= 0.0%) in favor of the combined intervention compared to sham NIBS and active exercise. When analyzing NIBs techniques separately, results were significant for both tDCS (ES: −0.59, 95% CI: −0.89 to −0.29, I2= 0.0%) and rTMS (ES: −0.76, 95% CI: −1.41 to −0.11, I2=0.0%) (Figure 2). Regarding the stimulation location, the motor cortex stimulation (seven studies) shown a pooled effect size of −0.62 (95% CI: −0.91 to −0.34, I2= 0.0%), while the cerebellar cortex stimulation (one study) was no significant (ES= −0.54, 95% CI: −1.54 to 0.46). Besides, the pooled estimates by conditions only were significant for fibromyalgia (ES: −0.59, 95% CI: −1.15 to −0.04) and myofascial pain (ES: −0.81, 95% CI: −1.53 to −0.09) (online supplementary material 5).
Figure 2.
Forest plot of pain score effects by Non-Invasive Brain Stimulation technique.
Also, two studies [38,40] reported single- and paired-pulse transcranial magnetic stimulation (TMS) results, we could not performed a meta-analysis since they performed different type of stimulations (anodal M1 tDCS and cerebellar theta burst). Both studies did not find statistically significant differences on the combined group versus sham, however Mendoca et al.[38] reported a trend of increasing the motor evoked potential (MEP) and intracortical inhibition (ICI) after the combine intervention (anodal M1 tDCS + exercise).
Following the criteria proposed in the IMMPACT consensus statement [31] and previous meta-analysis [29], we back transformed the SMD to a mean difference using the mean standard deviation of the post-treatment sham group scores of the studies included in this analysis. We then used this to estimate the real percentage change on a 0 to 10 pain intensity scale of active stimulation compared with the mean poststimulation score from the sham groups of the included studies. We found that the combined intervention compared with sham + exercise produce a 28.5% reduction in pain (tDCS=28% and rTMS=31.7%) considered as a clinical important change for chronic pain conditions.
3.4. Safety
Six out of the eight articles reported side effects[33–36,38,39]. The 64% and 56% of the active and sham NIBS groups respectively, had common side effects as tingling, itching, skin redness, headache, sleepiness, trouble concentrating, dizziness and mood fluctuations. One article reported burn on the fifth day of stimulation due to acne lesions on the side of the stimulation [34], another article reported a transient erythematous rash. One article with rTMS reported mild side effects (headache, neck pain, dizziness, non-painful sensation) and one episode of generalized seizure[39].
3.5. Risk of bias assessment
The overall quality of the selected articles was greater than 3. The third reviewer participation was not needed to solved discrepancies. All of them [33,35,37–40] except two [34,36] obtained one point in the first question regarding randomization. Even though all achieved to specify their designs as randomized, they lack to specify how the sequence was generated. Besides, only one study didn’t describe the withdrawals and dropouts adequately. All the studies achieved to report and describe in full detail how the double-blinding was performed. See Table 1 and 2.
3.6. Subgroup and sensitivity analysis
Sensitivity analysis showed no difference when the study with the largest effect was removed from the analysis, and neither if we excluded one study at a time.
We classified the interventions by the number of NIBS sessions (more or less than ten sessions). We found significant results for both subgroups: (i) ten or more sessions (ES= −0.68, 95% CI: −1.11 to −0.26) and (ii) less than ten session (ES=−0.57, 95% CI: −0.95 to −0.20). Additionally, by classifying the exercise intervention in less than eight weeks (ES= −0.61, 95% CI: −0.93 to −0.30) and more than eight weeks (ES=−0.63, 95% CI: −1.18, −0.07), the effect size was still significant for both. We performed a subgroup analysis with the tDCS + exercise studies and the intensity of 1 mA and 2 mA, the result was significant in both cases (ES= −0.83, 95% CI: −1.52 to −0.14, I2=41.7%; ES=−0.48, 95% CI: −0.84 to –0.11, I2=0.0%; respectively), however, heterogeneity was high with 1 mA of intensity. Moreover, the risk of bias level, the number of NIBS sessions, and the exercise duration were not important sources of heterogeneity in our analysis (online supplementary material 5).
4. Discussion
4.1. Summary of results
In this systematic review and meta-analysis, we included eight RCTs that have evaluated the effects of the combination of non-invasive brain stimulation (tDCS and rTMS) with exercise to treat chronic pain. We found a significant and homogenous decrease in pain scores in favor of the combined treatment compared to the combination of sham NIBS and exercise. The excitatory motor cortex stimulation (both tDCS and rTMS) seems to be an effective NIBS intervention, however, the absence of comparative studies except for a cerebellar comparator [40] does not allow us to evaluate the comparative efficacy of the montages. The exercise protocols were heterogeneous, predominantly stretching, and strength protocols before brain stimulation. The number of NIBS sessions and the exercise duration were not significant sources of heterogeneity in our analysis.
4.2. Exploration of synergistic effects
These results are consistent with the previous meta-analysis of NIBS in chronic pain [29], where a significant decrease in pain rating with both rTMS and tDCS was found. Moreover, they reported an effect size of −0.22 and −0.43 for rTMS and tDCS alone, respectively. Our estimates are slightly higher (ES=−0.62), suggesting a potential synergistic effect of the combination with exercise (though other factors may explain this higher effect size than the treatment). To confirm this superior effect, it is essential to include studies that contemplate the interventions alone and combined. In our meta-analysis, only Mendoca et al.[38] compared three intervention arms (combined, sham tDCS and aerobic exercise alone) reporting significant results towards the combination, despite it showed a non-significant moderate effect size in our meta-analysis probably due to the small sample size. Consequently, Castelo-Branco et al. [41] proposed a 4-arms factorial RCT design to confirm potential synergistic effects and to address possible placebo effects of the interventions. Comparing interventions alone and combined either by factorial or parallel studies allow for detangling the individual effects of each intervention and in the case of factorial studies to assess placebo effects and conclude interaction between independent variables [42]. Therefore, it is important for future meta-analysis to compare NIBS, exercise-alone, and combined protocols to evaluate a real synergistic effect.
Another issue to address with potential influences on synergic effects is the moment to combine the interventions. In our meta-analysis, we included two studies on myofascial pain that showed large significant results; however, the study of Oliveira et al.[34] did not achieve significance. This might be explained by the applied order of the interventions, as they performed the tDCS after the exercise protocol. Although Oliveira et al. [34] reported positive results towards the combined treatment, the results may not have been optimized. It has been shown in previous physiological studies that tDCS after-effects begin after 5–7 minutes of stimulation and may last for approximately 1 hour [43]. Ideally, combined therapies with tDCS should be performed either simultaneously or right after for better effects [15,43]. Thus, future studies should contemplate the application moment of the interventions to assess better any potential synergistic effect.
In a meta-analysis on Osteoarthritis (OA), daily or three days per week exercise protocols decrease pain with a moderate effect size at eight weeks after the treatment [44]. This result contrasts with the non-significant effect of our single OA included study. This difference might be explained by the number of sessions performed (bi-weekly vs daily or three days per week from the previous meta-analysis) suggesting a possible role of the number of sessions on the effects of tDCS on pain. Among our analysis, we assess the amount of NIBS sessions with a splitting threshold of ten. Even though, we obtained significant moderate effects in both groups, the studies with ten or more sessions showed slightly higher estimates (ES=−0.68) than those with less than ten sessions (ES=−0.57) supporting the influence of the number of sessions from the interventions on pain relieve [45]. However, the ideal dose and the possible number of boost sessions to keep the obtained effects are still unknown and should be addressed in future studies.
4.3. Main sources of heterogeneity
4.3.1. Stimulation parameters
Six of our studies used tDCS, and the stimulation parameters were almost consistent in all the studies with 20 minutes of motor cortex stimulation with an intensity of 2 mA; only two of them used an intensity of 1 mA. However, the main heterogeneity was the number and the frequency of tDCS sessions. The number of sessions varied between five sessions daily to 16 sessions in eight weeks. Moreover, some of the studies performed five consecutive days weekly; Chang et al. reported biweekly tDCS sessions for eight weeks. Furthermore, in fibromyalgia, different protocols were performed. Whereas Mendonca et al. [38] tested five consecutive sessions of M1 anodal tDCS (in one week), Riberto et al. [37] implemented ten sessions of anodal M1 tDCS (one per week) for four months. Despite these sources of heterogeneity, the results were consistent after the subgroup analysis, suggesting a robust effect of the combined treatment even though in small sample sizes studies.
Additionally, despite the significant results of rTMS, the protocols were more heterogeneous in terms of the stimulation location, one significant protocol in complex regional pain with rTMS was described, whereas other use with cerebellar intermittent theta-burst stimulation for cervical dystonia. To identify the adequate location, further comparative studies are needed.
4.3.2. Exercise protocol
The different exercise protocols as aerobic, strengthening, flexibility, and movement therapies might have a significant impact on pain perception [46]. Busch et al. [47] support the use of aerobic and strengthening exercises for fibromyalgia but not for stretching exercise [47,48], but the lack of comparison between types of exercises in RCTs hamper these interpretations. Moreover, there is a lack of description of the exercise protocol combined with NIBS, only three of the studies described clearly the type of exercise and only two the intensity. Mendonca et al. [38] considered aerobic exercise according to the monitor of the heart rate, Chang et al. [33] describe an extensive protocol of strengthening exercise on OA patients, and Straudi et al.[36] describe a combination of different low to moderate exercise in low back pain patients. It is recommended to describe the frequency, the type of exercise and the intensity in order to make the exercise protocol more reproductible for future studies.
The mechanisms of exercise for pain control are still unclear. Previous studies suggested top-down mechanisms [49,50], related to the cortical activation in motor- and attentional-related areas which can generate an influence on descending pain modulatory circuits feeding to midbrain and further to the medulla [51], producing a opioidergic tone[52]. Also, these effects could be due to a bottom-up mechanism (an enhancement of the sensory system activity), in that case then strengthening exercises or moderate aerobic exercise may have similar effects. In a previous study, we have shown the impact of sensory stimulation on pain perception and motor cortex excitability [53]. Thus, we hypothesized that this may be the main effect. If the effects are mainly due to secondary cardiovascular effects such as the elevation in the resting blood pressure [54], then aerobic exercises may have a greater effect. However, comparative studies with different types of exercises are needed to elucidate their mechanisms.
4.3.3. Pain outcomes
The main outcome in the included studies is a self-reported subjective pain perception of a noxious stimulus. The Visual Analog scale (VAS), numerical rating scales (NRS), and verbal rating scales (VRS) are the most common and validated scales to measure pain used in the included studies. Over time, the measurement of pain has been a controversial topic. Hence, the report of this variable needs to be deeply described. According the IMMPACT Consensus Group recommendations [55], these three measurements are valid and reliable and no one scale demonstrated greater responsiveness to detect improvements associated with pain treatments, however, inside the core outcome measures for clinical trials on chronic pain, the numerical rating scale (11-points, from 0 to 10) is the preferred. This recommendation should be adopted in future RCTs on the combination of NIBS and exercise. Also, we also suggested to add the outcome of usage of rescue pain-treatments, since the combine intervention could produce a reduction of analgesic pills usage and not only a pain scale reduction. The variability of chronic pain perception might change due to different factors, as psychological factors, medication, activities, time of the day reported, among others [56]. For that reason, the inclusion of physical and emotional functioning outcomes are needed. For instance, Riberto et al. [37] reported the pain improvement using the SF-36 scale over the last 4 weeks [34]. Therefore, the standardization of the way to collect pain information and the included outcomes are important for future studies to elucidate the real effects of the NIBS and exercise combination on the pain construct.
4.3.4. Neurophysiological outcomes
Two studies reported no difference in TMS results before and after combine intervention compared with control groups. Clinically, chronic pain conditions are often associated with motor disturbances, potentially due to physiological impairment, limb immobilization, or “kinesiophobia”, some studies reported alterations on cortical networks regulating motor functions. For instance, in patients with chronic low back pain, decreased excitability in the primary motor cortex (M1) [57] and diminished intracortical motor inhibition in M1 circuits[58] have been reported. It is therefore plausible that exercise intervention combine with NIBS may generate a normalization of these parameters leading to higher excitability and higher inhibition on M1, however, the scarce number of studies, small sample sizes, and the lack of healthy controls hamper the interpretation of these results.
4.4. The theoretical framework of NIBS and exercise effects on chronic pain
Chronic pain is a complex syndrome comprising of emotional, psychological, and mechanical components. Although it is initially a protective mechanism, long-term noxious stimuli can modify the pain pathway leading to chronic pain, by a maladaptive response in the descending pain modulatory system.
This system consists of a complex process that starts with pain facilitation between the primary and secondary sensorimotor neurons on the dorsal horn of the medulla. This activates cortical and subcortical pain related areas – such as subnucleus reticularis dorsalis (SRD) and periaqueductal grey matter (PAG) [59]. Consequently, the descending pain modulation system is activated, and through serotonergic, noradrenergic, and dopaminergic pathways, it exerts an inhibitory effect over the dorsal horn by the release of enkephalins [59,60]. In chronic pain, this endogenous pain modulation is disrupted, leading to decrease capacity to inhibit pain [61].
The hypoalgesic response of the exercise could be explained by central mechanisms that may include increased secretion of β-endorphins, attention mechanisms, interaction of the cardiovascular and pain regulatory systems, and an activation of the descending pain modulatory system due to the peripheral afferents originating in the muscle which can undergo thalamic modulation and could diminish the intensity perceived pain [57,62]. On the other hand, excitatory motor cortex (both tDCS and rTMS) can induce changes in thalamic and subthalamic nuclei [8] which can produce changes on pain-related areas such as the anterior cingulate and the periaqueductal grey and it could enhance the endogenous pain modulation system [9,60].
Thus, the combination of tDCS and exercise might restore the endogenous pain modulation system and decrease pain perception. We hypothesized that exercise would generate a bottom-up modulation, affecting large neural circuits due to the increases of afferent input in the sensorimotor area and generating neuroendocrine responses (increases activity of dopaminergic pathways and opioid-endogenous system), while tDCS would generate a top-down modulation enhancing the endogenous pain modulation system by increasing the sensorimotor-thalamic connectivity and periaqueductal grey matter activation. Also, it is plausible a synergistic top-down mechanism, previous literature reported that exercise may modulate attentional and pre-motor networks involved in executive and spatial cognitive functions [58,63,64], for that this central activation could lead to modulation on interference control processes [49] and reduce the attention allocation on the pain stimulus (ergo reducing the pain perception). Finally, another explanation, could be a synergistic effect on the endogenous pain modulation system for both interventions. Recent literature supports the hypothesis that exercise generates a motor cortex inhibition during an acute pain stimulus [50], this effect in addition to the NIBS modulation of similar cortical areas (sensorimotor cortices) could lead to an enhancement of the endogenous pain modulation system and produce an opioidergic tone.
This combination could be a potential innovative and feasible alternative approach to treat chronic pain due to its safety profile. Moreover, studies suggest a cost-effective component of non-invasive brain stimulation techniques and exercise [65–67], however, future studies should explore the cost-effectiveness of combining NIBS and exercise for chronic pain conditions.
4.5. Limitations
This exploratory analysis has some limitations. First, the control group used was sham NIBS and active exercise, only one study compared the combination of exercise and tDCS against exercise combine with sham tDCS and tDCS combined with sham exercise [38], hence in order to conclude that the combined intervention is more efficient than the use of only one of them, more studies with a factorial design are needed. Second, regardless of the constant parameters of the tDCS protocols, as the duration of the session, the intensity, and stimulation location (motor cortex), the number and the frequency of the sessions was variable. On the other hand, rTMS protocols were more heterogenous on the stimulation location (motor cortex and cerebellar stimulation). Moreover, despite an extensive database search, only English papers were found. However, this systematic review has important strengths: it followed the PRISMA statement and was registered in the PROSPERO database. In addition, we performed a comprehensive search strategy across multiple databases.
4.6. Research recommendations
In this exploratory analysis, despite the heterogeneity of the studies and the lack of information about exercise protocols, a positive mean difference was found, favoring the combination of NIBS and exercise against sham NIBS and active exercise. For future studies, more factorials designs including the effect of each intervention alone would be necessary in order to support the theory of the synergistic effect of NIBS combined with exercise against exercise or NIBS alone. Furthermore, a clear description of the exercise protocol should be reported including the type, intensity, the number of sessions and the attrition rate. Moreover, they should also follow the exercise guidelines for safety and homogeneity of the results [68].
5. Conclusion
This meta-analysis provides evidence of significant moderate to large effects of the NIBS and exercise combination compare to sham NIBS and exercise on chronic pain patients. This effect was predominantly due to excitatory motor cortex stimulation. More RCTs are needed to evaluate the benefits and risks of this combined intervention in patients with chronic pain, especially using a factorial design to elucidate the comparative effectiveness of the intervention components. These studies need to be well-powered, adequately reported, and be more detailed in the description of the interventions.
Supplementary Material
Article highlights.
The combination of NIBS and exercise has a moderate to large effect in chronic pain conditions.
Common side effects were reported in both groups (active NIBS + exercise and sham NIBS + exercise). Two moderate-severe side effects were reported in the active NIBS + exercise.
Exercise protocols included aerobic, strength, flexibility exercises. The description of the protocols were heterogenous, hence, future studies should considerer a description including, type, intensity, duration and frequency of the exercise protocol.
The NIBS protocol for tDCS was almost homogeneous including the location of the electrodes and duration. For rTMS, two different protocols were described including rTMS and theta burst stimulation
More factorial design studies are needed in order to confirm the efficacy of NIBS + exercise from exercise or NIBS alone.
Footnotes
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Goldberg DS, McGee SJ. Pain as a global public health priority. BMC public health. 2011. October 6;11:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SJaO C. Chronic Pain: How to Approach These 3 Common Conditions. J Fam Pract. 2017;66(3):145–157. [PubMed] [Google Scholar]
- 3.Torrance N, Ja Fau Ferguson - Afolabi E, Fau Afolabi E - Bennett MI, et al. Neuropathic pain in the community: more under-treated than refractory? (1872–6623 (Electronic)). [DOI] [PMC free article] [PubMed]
- 4.Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2017. January;128(1):56–92. [DOI] [PubMed] [Google Scholar]
- 5.Lefaucheur J-P, André-Obadia N, Antal A, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clinical Neurophysiology. 2014. 2014/11/01/;125(11):2150–2206. [DOI] [PubMed] [Google Scholar]
- 6.DosSantos MF, Martikainen IK, Nascimento TD, et al. Building up Analgesia in Humans via the Endogenous μ-Opioid System by Combining Placebo and Active tDCS: A Preliminary Report. PLOS ONE. 2014;9(7):e102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DosSantos MF, Oliveira AT, Ferreira N, et al. The Contribution of Endogenous Modulatory Systems to TMS- and tDCS-Induced Analgesia: Evidence from PET Studies. Pain Research and Management. 2018;2018:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Larrea L, Peyron R, Mertens P, et al. Positron emission tomography during motor cortex stimulation for pain control. Stereotactic and functional neurosurgery. 1997;68(1–4 Pt 1):141–8. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Larrea L, Peyron R, Mertens P, et al. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain. 1999. November;83(2):259–73. [DOI] [PubMed] [Google Scholar]
- 10.Gutmann B, Mierau A, Hulsdunker T, et al. Effects of physical exercise on individual resting state EEG alpha peak frequency. (1687–5443 (Electronic)). [DOI] [PMC free article] [PubMed]
- 11.Sato G, Osumi M, Morioka S. Effects of wheelchair propulsion on neuropathic pain and resting electroencephalography after spinal cord injury. J Rehabil Med. 2017. 2017/01//;49(2):136–143. [DOI] [PubMed] [Google Scholar]
- 12.Walton KD LR. Central Pain as a Thalamocortical Dysrhythmia: A Thalamic Efference Disconnection? Vol. Chapter 13. Boca Raton, FL: CRC Press/Taylor & Francis; 2010. (Kruger LLA, editors, editor. Translational Pain Research: From Mouse to Man). [PubMed] [Google Scholar]
- 13.Stagg CJ, Antal A, Nitsche MA. Physiology of Transcranial Direct Current Stimulation. The journal of ECT. 2018. September;34(3):144–152.** This review provide insightful information regarding physiological principles of tDCS (Summary of the tDCS effects, neurophysiological information and network effects).
- 14.Bestmann S The physiological basis of transcranial magnetic stimulation. Trends in cognitive sciences. 2008. March;12(3):81–3. [DOI] [PubMed] [Google Scholar]
- 15.Woods AJ, Antal A, Bikson M, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2016. February;127(2):1031–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valero-Cabre A, Amengual JL, Stengel C, et al. Transcranial magnetic stimulation in basic and clinical neuroscience: A comprehensive review of fundamental principles and novel insights. Neuroscience and biobehavioral reviews. 2017. December;83:381–404.**This review provide elemental information about physiological principles of TMS.
- 17.Neva JA-O, Brown KE, Mang CS, et al. An acute bout of exercise modulates both intracortical and interhemispheric excitability. (1460–9568 (Electronic)). [DOI] [PubMed]
- 18.Tajerian M, Clark JD. Nonpharmacological Interventions in Targeting Pain-Related Brain Plasticity. Neural Plast. 2017;2017:2038573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakaizumi K, Kondo T, Hamada Y, et al. Involvement of mesolimbic dopaminergic network in neuropathic pain relief by treadmill exercise: A study for specific neural control with Gi-DREADD in mice. Molecular pain. 2016;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geneen LJ, Moore RA, Clarke C, et al. Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. The Cochrane database of systematic reviews. 2017. January 14;1:Cd011279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brosseau L, Taki J, Desjardins B, et al. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part two: strengthening exercise programs. Clinical rehabilitation. 2017. May;31(5):596–611.* The part II of the Ottawa panel clinical practice guideline based on a systematical search recommend strenghening exercises for knee osteoarthritis
- 22.Rice D, Nijs J, Kosek E, et al. Exercise-Induced Hypoalgesia in Pain-Free and Chronic Pain Populations: State of the Art and Future Directions. J Pain. 2019. November;20(11):1249–1266. [DOI] [PubMed] [Google Scholar]
- 23.Steinberg F, Pixa NH, Fregni F. A Review of Acute Aerobic Exercise and Transcranial Direct Current Stimulation Effects on Cognitive Functions and Their Potential Synergies [Review]. Frontiers in Human Neuroscience. 2019. 2019-January-11;12(534). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaz PG, Salazar A, Stein C, et al. Noninvasive brain stimulation combined with other therapies improves gait speed after stroke: a systematic review and meta-analysis. Top Stroke Rehabil. 2019. April;26(3):201–213. [DOI] [PubMed] [Google Scholar]
- 25.Rice D, Nijs J, Kosek E, et al. Exercise-Induced Hypoalgesia in Pain-Free and Chronic Pain Populations: State of the Art and Future Directions. The Journal of Pain. 2019;20(11):1249–1266. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Medicine. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jadad AR, Ra Fau Moore - Carroll D, Fau Carroll D - Jenkinson C, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? (0197–2456 (Print)). [DOI] [PubMed]
- 29.O’Connell NE, Marston L, Spencer S, et al. Non-invasive brain stimulation techniques for chronic pain. The Cochrane database of systematic reviews. 2018. April 13;4:Cd008208.** This meta-analysis gives a scope of the effects of different Non-invasive stimulation techniques on chronic pain conditions.
- 30.Calculating Lakens D. and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013. November 26;4:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008. February;9(2):105–21.**Provides information regarding the minimun clinical important difference in chronic pain.
- 32.Fau DerSimonian R - Laird N, Laird N. Meta-analysis in clinical trials. (0197–2456 (Print)).
- 33.Chang WJ, Bennell KL, Hodges PW, et al. Addition of transcranial direct current stimulation to quadriceps strengthening exercise in knee osteoarthritis: A pilot randomised controlled trial. PLoS One. 2017;12(6):e0180328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliveira LB, Lopes TS, Soares C, et al. Transcranial direct current stimulation and exercises for treatment of chronic temporomandibular disorders: a blind randomised-controlled trial. J Oral Rehabil. 2015. October;42(10):723–32. [DOI] [PubMed] [Google Scholar]
- 35.Sakrajai P, Janyacharoen T, Jensen MP, et al. Pain reduction in myofascial pain syndrome by anodal transcranial direct current stimulation combined with standard treatment: a randomized controlled study. Clin J Pain. 2014. December;30(12):1076–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Straudi S, Buja S, Baroni A, et al. The effects of transcranial direct current stimulation (tDCS) combined with group exercise treatment in subjects with chronic low back pain: a pilot randomized control trial. Clinical rehabilitation. 2018. October;32(10):1348–1356. [DOI] [PubMed] [Google Scholar]
- 37.Riberto M, Marcon Alfieri F, Monteiro de Benedetto Pacheco K, et al. Efficacy of transcranial direct current stimulation coupled with a multidisciplinary rehabilitation program for the treatment of fibromyalgia. The open rheumatology journal. 2011;5:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendonca ME, Simis M, Grecco LC, et al. Transcranial Direct Current Stimulation Combined with Aerobic Exercise to Optimize Analgesic Responses in Fibromyalgia: A Randomized Placebo-Controlled Clinical Trial. Frontiers in human neuroscience. 2016;10:68–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Picarelli H, Teixeira MJ, de Andrade DC, et al. Repetitive transcranial magnetic stimulation is efficacious as an add-on to pharmacological therapy in complex regional pain syndrome (CRPS) type I. J Pain. 2010. November;11(11):1203–10. [DOI] [PubMed] [Google Scholar]
- 40.Bradnam LV, McDonnell MN, Ridding MC. Cerebellar Intermittent Theta-Burst Stimulation and Motor Control Training in Individuals with Cervical Dystonia. Brain sciences. 2016. November 23;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castelo-Branco L, Uygur Kucukseymen E, Duarte D, et al. Optimised transcranial direct current stimulation (tDCS) for fibromyalgia—targeting the endogenous pain control system: a randomised, double-blind, factorial clinical trial protocol. BMJ Open. 2019;9(10):e032710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. Pearson/Prentice Hall; 2009. [Google Scholar]
- 43.Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2011. February;17(1):37–53. [DOI] [PubMed] [Google Scholar]
- 44.Goh SL, Persson MSM, Stocks J, et al. Efficacy and potential determinants of exercise therapy in knee and hip osteoarthritis: A systematic review and meta-analysis. Annals of physical and rehabilitation medicine. 2019. September;62(5):356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brunoni AR, Nitsche MA, Bolognini N, et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain stimulation. 2012. July;5(3):175–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ambrose KR, Golightly YM. Physical exercise as non-pharmacological treatment of chronic pain: Why and when. Best practice & research Clinical rheumatology. 2015. February;29(1):120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Busch AJ, Webber SC, Richards RS, et al. Resistance exercise training for fibromyalgia. The Cochrane database of systematic reviews. 2013. December 20(12):Cd010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hooten WM, Qu W, Townsend CO, et al. Effects of strength vs aerobic exercise on pain severity in adults with fibromyalgia: a randomized equivalence trial. Pain. 2012. April;153(4):915–23. [DOI] [PubMed] [Google Scholar]
- 49.Wang C-H, Moreau D, Yang C-T, et al. The influence of aerobic fitness on top-down and bottom-up mechanisms of interference control. Neuropsychology. 2019;33(2):245. [DOI] [PubMed] [Google Scholar]
- 50.Hautasaari P, McLellan S, Koskio M, et al. Acute exercise modulates pain-induced response on sensorimotor cortex∼ 20 Hz oscillation. Neuroscience. 2020. [DOI] [PubMed] [Google Scholar]
- 51.De Felice M, Ossipov MH. Cortical and subcortical modulation of pain. Pain management. 2016;6(2):111–120. [DOI] [PubMed] [Google Scholar]
- 52.Scheef L, Jankowski J, Daamen M, et al. An fMRI study on the acute effects of exercise on pain processing in trained athletes. PAIN®. 2012;153(8):1702–1714. [DOI] [PubMed] [Google Scholar]
- 53.Volz MS, Suarez-Contreras V, Mendonca ME, et al. Effects of sensory behavioral tasks on pain threshold and cortical excitability. PLoS One. 2013;8(1):e52968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bruehl S, Chung OY. Interactions between the cardiovascular and pain regulatory systems: an updated review of mechanisms and possible alterations in chronic pain. Neuroscience and biobehavioral reviews. 2004. July;28(4):395–414. [DOI] [PubMed] [Google Scholar]
- 55.Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1):9–19. [DOI] [PubMed] [Google Scholar]
- 56.Menendez ME, Ring D. Factors Associated with Greater Pain Intensity. Hand Clinics. 2016. 2016/02/01/;32(1):27–31. [DOI] [PubMed] [Google Scholar]
- 57.O’Connor PJ, Cook DB. Exercise and pain: the neurobiology, measurement, and laboratory study of pain in relation to exercise in humans. Exercise and sport sciences reviews. 1999;27:119–66. [PubMed] [Google Scholar]
- 58.Schmitt A, Upadhyay N, Martin JA, et al. Modulation of Distinct Intrinsic Resting State Brain Networks by Acute Exercise Bouts of Differing Intensity. Brain Plasticity. 2019. (Preprint):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care. 2014. June;8(2):143–51.*Describe possible physiopatological causes of chronic pain conditions.
- 60.Benarroch EE. Descending monoaminergic pain modulation: bidirectional control and clinical relevance. Neurology. 2008. July 15;71(3):217–21.*Explain the relevance of the main descending inhibitory pathways of the endogeneous control system.
- 61.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14(7):502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kosek E, Lundberg L. Segmental and plurisegmental modulation of pressure pain thresholds during static muscle contractions in healthy individuals. European journal of pain (London, England). 2003;7(3):251–8. [DOI] [PubMed] [Google Scholar]
- 63.Tsai C-L, Chen F-C, Pan C-Y, et al. Impact of acute aerobic exercise and cardiorespiratory fitness on visuospatial attention performance and serum BDNF levels. Psychoneuroendocrinology. 2014;41:121–131. [DOI] [PubMed] [Google Scholar]
- 64.Du Rietz E, Barker AR, Michelini G, et al. Beneficial effects of acute high-intensity exercise on electrophysiological indices of attention processes in young adult men. Behavioural brain research. 2019;359:474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Voigt J, Carpenter L, Leuchter A. Cost effectiveness analysis comparing repetitive transcranial magnetic stimulation to antidepressant medications after a first treatment failure for major depressive disorder in newly diagnosed patients - A lifetime analysis. PloS one. 2017;12(10):e0186950–e0186950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miyamoto GC, Lin C-WC, Cabral CMN, et al. Cost-effectiveness of exercise therapy in the treatment of non-specific neck pain and low back pain: a systematic review with meta-analysis. Br J Sports Med. 2019;53(3):172–181. [DOI] [PubMed] [Google Scholar]
- 67.Rancic N, Mladenovic K, Ilic NV, et al. Patient-Controlled Intravenous Morphine Analgesia Combined with Transcranial Direct Current Stimulation for Post-Thoracotomy Pain: A Cost-Effectiveness Study and A Feasibility For Its Future Implementation. Int J Environ Res Public Health. 2020;17(3):E816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Services. USDoHaH. Physical Activity and Health: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.