Abstract
Endothelin is a recently discovered peptide composed of 21 amino acids. There are three endothelin isomers: endothelin -1 (ET-1), endothelin -2 (ET-2) and endothelin - 3 (ET-3). In humans and animals levels of ET-1, ET-2, ET-3 and big endothelin in blood range from 0,3 to 3 pg/ml. ET-1, ET-2 and ET-3 act by binding to receptors. Two main types of the receptors for endothelins exist and they are referred to as A and B type receptors. Different factors can stimulate or inhibit production of endothelin by endothelial cells. Mechanical stimulation of endothehum, thrombin, calcium ions, epinephrine, angiotensin II, vasopressin, dopamine, cytokines, growth factors stimulate the production of endothelin whereas nitric oxide, cyclic guanosine monophosphate, atrial natriuretic peptide, prostacyclin, bradykinin inhibit its production. Endothelins have different physiological roles in human body but at the same time their actions are involved in the pathogenesis of many diseases.
The aim of this review was to present some of, so far, the best studied physiological roles of endothelin and to summarize evidence supporting the potential role of ET in the pathogenesis of certain diseases.
Keywords: endothelin, physiological and pathophysio-logical effects
Introduction
Yanagisawa et al. were the first to isolate and identify endothelin from the pig arterial endothelial cell culture in 1988 (1). Endothelin (ET) is a peptide composed of 21 amino acids, with two disulfide bonds between amino acids 1 and 15, and 3 and 11. There are three endothelin isomers: endothelin 1 (ET-1), endothelin 2 (ET-2) and endothelin 3 (ET-3) coded by three independent genes. Their chemical structure and potency for smooth muscle contracting effect are different (2). ET-1, ET-2 and ET-3 act by binding to receptors. There are two main types of receptors for endothelins and they are referred to as A and B type receptors.
ET is formed by cleaving 164 amino acids from 203 amino acids of pre-pro-endothelin, which results in formation of big endothelin. Big endothelin is subsequently converted to endothelin by endothelin converting enzyme. Endothelin -1 (ET-1) can bind to ETA receptors found on adjacent vascular smooth muscle cells. The ETA receptor is coupled with a G-protein. This coupling between ET-1, ETA receptor and G-protein leads to the formation of inos-itol triphosphate (IP3). Formation of IP3 leads to calcium mobilization and smooth muscle contraction (Figure 1.). ET-1 is produced mainly by endothelial cells and vascular smooth muscle cells. Sertolli cells, mesangium, hepatocytes, neuron and astrocytes in central nervous system (CNS) also produce ET-1 but to a lesser extent. ET-2 is predominantly produced within intestine and kidney. The highest levels of ET-3 are found in brain, and it is believed that ET-3 is involved in regulation of neuronal function (3).
Figure 1.
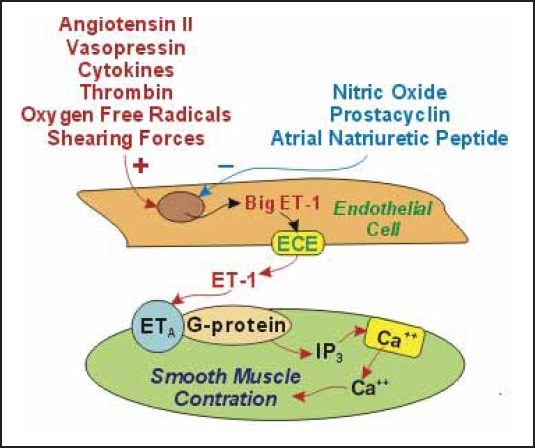
Mechanism of smooth muscle contraction induced by ET-1 (25)
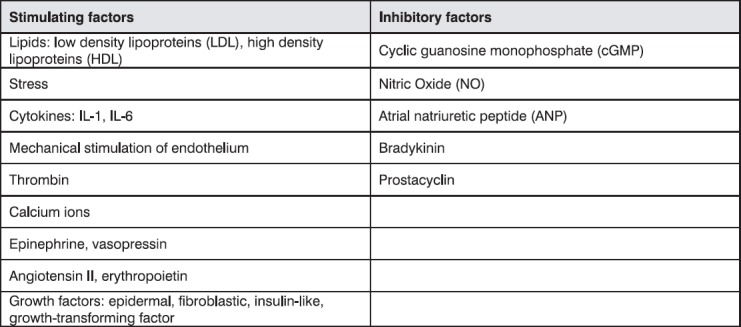
Different factors can stimulate or inhibit production of endothelin by endothelial cells. These factors are summarized in Table 1.
Table 1.
Stimulating or inhibitory factors of endothelin production
In humans and animals, levels of ET-1, ET-2, ET-3 and big endothelin in blood range from 0,3 to 3 pg/ml. Concentrations of endothelin in body fluids, especially in urine, saliva, milk, cerebrospinal fluid are several times higher then the concentration in plasma (4).
The half-life time of endothelin in plasma is very short. It is estimated that about 90 % of circulating endothelin is eliminated by pulmonary uptake, and the remaining portion mainly through kidneys and very small quantity through liver (3,4).
Physiological effects of endothelin on:
Blood vessels
One of the main effects of endothelin is a marked and sustained vasoconstriction. This vasoconstriction is caused by the modulation of dihydropyridine channel function by endothelin as well as by an increase of intracellular calcium level. This increase in calcium level in sarcoplasma leads to the contraction of smooth muscle cells (4). Vasoconstricting actions of endothelin are dependent not only on its concentration, but also on the condition of the vascular endothelium. If the endothelium is intact, stimulation of the sympathic system increases smooth muscle contractility caused by endothelin. If the endothelium is damaged, vasoconstricting effect of endothelin is even more marked because it overlaps the effects of serotonin. Endothelin also affects blood flow in various regions of circulation. In mesenteric and cerebral circulation it reduces blood flow but in pulmonary vessels it causes an increase in blood flow (5).
Numerous studies have shown that endothelin causes an increase of arterial blood pressure in a dose-dependent manner.
Heart
Studies have shown that endothelin has positive inotropic and chronotropic effect on isolated left atrium of guinea pig’s heart (1,4). It is believed that this effect is mediated by protein G and phospholipase C. ET-1 is also a potent growth factor for cadiomyocites.
Kidney
Enothelin has a strong influence on kidney function. ET-1 decreases renal blood flow and glomerular filtration and increases vascular resistance (6). Studies in vitro have shown that ET-1 reduces the production of renin in isolated juxtaglomerular cells (7,8). It also reduces excretion of sodium and potassium in urine.
Lungs
ET-1 has also effects on lungs. When stimulated by ET-1 pulmonary fibroblasts produce collagen, and at the same time stimulation by ET-1 results in increased mucous secretion and contraction of bronchial smooth muscle (9).
Other tissues and functions. Numerous studies carried out with purpose to elucidate effects of endothelin in the human body have shown that this compound affects conductivity of parasympathetic ganglia and nerves, regulates the function of myofibroblasts in the process of wound healing, increases the expression of protooncogens, exerts a mitogenic effect and increases human renal interstitial fibroblasts proliferation (3,4).
Endothelin and diseases
During the past decade research on endothelin has become very extensive as the increase or decrease of blood endothelin was observed in patients with certain diseases.
Blood vessels
In hypertensive patients the levels of ET-1 were significantly higher than in normotensive controls. Also, the level of ET-1 in plasma of hypertensives significantly correlated with mean arterial pressure (10). The involvement of endothelin in etiopathogenesis of hypertension led to a research on possible use of endothelin receptor blockers in the treatment of hypertension. Currently, few endothelin receptor blockers are being tested for the treatment of hypertension. So far, the use of Bosentan (non-specific ET receptor antagonist) leads to a decrease in mean blood pressure in aorta, peripheral vascular blood pressure and reduction of elevated left ventricular end diastolic pressure (11). This drug also reduces pulmonary arterial pressure and is used in the treatment of pulmonary hypertension (12).
Vasoconstricting effects of endothelin have been intensively studied for possible pathogenic roles in diabetic vascular complications. Results of Kakizawa et al. have shown that plasma ET-1 was higher in poorly controlled diabetic patients than in healthy controls. Improved glycemic control did not affect plasma ET-1 concentration (13). Migdalis et al. have also demonstrated involvement of ET-1 in diabetes and atheromatosis (14).
Key aspect of atherosclerosis is the imbalance of vasoac-tive factors. Bousette et al. have demonstrated correlation between the expression of endothelin and the underlying atherosclerotic lesion. Endothelin’s role in atherosclerosis must stem from its varying physiological activities, including vasoconstriction, mitogenesis, neutrophil adhesion, and platelet aggregation, and hypertrophy, as well as its propensity to induce the formation of reactive oxygen species (15).
Endothelin is also one of causative substances in cerebral vasospasam after subarachnoid hemorrhage (5,16,17).
Heart
Prolonged exposure to ET-1 can lead to myocardial hypertrophy and cellular damage of cardiac myocytes. The cellular effects of ET, including constriction of vascular smooth muscle, cell proliferation, hypertrophy of cardiac myocytes, and activation of cardiac fibroblasts, are those that are associated with both the clinical manifestations of heart failure and the pathological remodeling of the heart that results in progressive cardiac dysfunction. ET has also been shown to exert effects that may be involved in the pathogenesis of heart failure (18). These include effects on the central and autonomic nervous system, the baroreflex response, and sodium excretion in kidneys. In patients with heart failure, levels of ET in blood are elevated and a rough correlation between circulating ET levels and the severity of heart failure has been observed (18). The cause of this increase remains uncertain. According to Parker et al. the cause of elevated ET-1 concentrations in patients with chronic CHF is increased ET-1 production rather than decreased clearance of ET-1 (19).
Increased blood levels of endothelin were also observed in patients with acute myocardial infarction, in congenital and acquired heart defects, ishemic heart disease and following heart transplantation treated with cyclosporin (20). Results of Ficai et al. have demonstrated that ET-1 has an important role in atrial hypertension (21).
Endothelins are associated with cardiac remodeling. This remodeling can be a direct effect of this hormone or indirect response to a relative ischemia promoted by vasoconstrictor effect. Ramires et al. have demonstrated that endothelin may have a pivotal role in myocardial fibrosis by direct stimulation of collagen accumulation (22).
Kidney
Elevated blood concentrations of endothelin were noted in patients with acute and chronic renal failure (6). This increase was even higher in advanced uremia and in patients on dialysis.
Mesangial cells (MCs) play a central role in the physiology and pathophysiology of endothelin-1 (ET-1) in kidneys. MCs release ET-1 in response to a variety of factors. Release of ET-1 can lead to MC hypertrophy, proliferation, contraction, and extracellular matrix accumulation. Excessive stimulation of ET-1 production by MC is likely to be of pathogenic importance in glomerular damage in the setting of diabetes, hypertension, and glomera-lonephritis (23).
Lungs
It is considered that endothelin has effects on ethiopatho-genesis of pulmonary hypertension, asthma and fibrinous alvelolitis (9,12).
The production of endothelin is increased in pulmonary arterial hypertension. In addition to its potent pulmonary vasoconstricting effects, endothelin in tlungs causes hypertrophy, fibrosis and inflammation. Endothelin expression correlates significantly with disease severity and outcome in patients with pulmonary arterial hypertension. These findings suggest that endothelin, through binding to both ETA and ETB receptor subtypes, is a key causative agent in the pathophysiology of pulmonary arterial hypertension (12).
Endothelin-1 is a potent bronchoconstrictor both in vitro and by inhalation in animal models in vivo. The bronchial epithelial cells of asthmatic patients show increased expression of ET-1 and increased sensitivity to ET-1. Plasma levels of ET-1 were reported to be elevated in acute exacerbation of asthma and to correlate with clinical severity of asthma (24). The mechanism of ET-1 activity in human airways still needs to be clarified.
Conclusion
In all the above-mentioned diseases, the role of endothelin is still not fully understood and therefore, this important compound is still subject to extensive research in biomedical world.
References
- (1).Yanagisawa M, Kurihara H, Kimura S, Tombe Y, Kobayashi M. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- (2).Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc. Natl. Acad. Sci. USA. 1989;86:2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Yanagisawa M, Masaki T. Endothelin a novel endothelium-derived peptide. Biochem. Pharmacol. 1989;38:1877–1886. doi: 10.1016/0006-2952(89)90484-x. [DOI] [PubMed] [Google Scholar]
- (4).Donckier J.E, Hanet C, Berbinschi A, Galannti L, Robert A, Van Mechelin H. Cardiovascular and endocrine effects of ET-1 at pathophysiological and pharmacological plasma concentration in conscious dogs. Circulation. 1991;84:2476–2484. doi: 10.1161/01.cir.84.6.2476. [DOI] [PubMed] [Google Scholar]
- (5).Josko J, Gwozdz B, Hendryk S, Jedrzejewska-Szypulka H, Słowiński J, Jochem J. Expression of vascular endothelial growth factor (VEGF) in rat brain after subarachnoid haemorrhage and endothelin receptor blockade with BQ-123. Folia Neuropathol. 2001;39:243–251. [PubMed] [Google Scholar]
- (6).Laghmani K, Preisig P.A, Alpern R.J. The role of endothelin in proximal tubule proton secretion and the adaptation to a chronic metabolic acidosis. J. Nephrol. 2002;15(suppl. 5):75–87. [PubMed] [Google Scholar]
- (7).Lin H, Mariem S, Smith M.J, Young D.B. Effect of endothelin-1 on glomerular hydraulicpressure and renin release in dogs. Hypertension. 1993;21:845–851. doi: 10.1161/01.hyp.21.6.845. [DOI] [PubMed] [Google Scholar]
- (8).Tian X, Tang G, Chen Y. The effects of endothelin-1 selective endothelin receptor-type. A antagonist on human renal interstitial fibroblasts in vitro. Zhoghua Yi Xue Za Zhi. 2002;82:5–9. [PubMed] [Google Scholar]
- (9).Bauer M, Wilkens H, Langer F, Schneider S.O, Lausberg H, Schafers H.J. Selective upregulation of endothelin Breceptor gene expression in serve pulmonary hypertension. Circulation. 2002;105:1034–1036. doi: 10.1161/hc0902.105719. [DOI] [PubMed] [Google Scholar]
- (10).Parissos J.T, Venetsanou K.F, Mentzikof D.G, Kalantzi M.V, Georgopoulou M.V, Christopoulos N, Karas S.M. Plasma levels of soluble cellular adhesion molecules in patients with arterial hypertension. Correlations with plasma endothelin-1. Eur. Intern. Med. 2001;12:350–356. doi: 10.1016/s0953-6205(01)00125-x. [DOI] [PubMed] [Google Scholar]
- (11).Ding S.S, Qiu C, Hess P, Xi J.F, Clozel J.P, Clozel M. Chronic endothelin receptor blockade prevents renal vasoconstriction and sodium retention in rats with chronic heart failure. Cardiovasc. Res. 2002;53:963–970. doi: 10.1016/s0008-6363(01)00558-2. [DOI] [PubMed] [Google Scholar]
- (12).Clozel M. Effects of bosentan on cellular processes involved in pulmonary arterial hypertension: do they explain the longterm benefit? Ann. Med. 2003;35:605–613. doi: 10.1080/07853890310017477. [DOI] [PubMed] [Google Scholar]
- (13).Kakizawa H, Itoh M, Itoh Y, Imamura S, Ishiwata Y, Matsumoto T, Yamamoto K, Kato T, Ono Y, Nagata M, Hayakawa N, Suzuki A, Goto Y, Oda N. The relationship between glycémie control and plasma vascular endothelial growth factor and endothelin-1 concentration in diabetic patients. Metabolism. 2004;53:550–555. doi: 10.1016/j.metabol.2003.12.002. [DOI] [PubMed] [Google Scholar]
- (14).Mgdalis I.N, Kalogeropoulou K, Karmaniolas K.D, Varvarigos N, Mortzos G, Cordopatis P. Plasma levels of endothe-lin and early carotid atherosclerosis in diabetic patients. Res. Commun. Mol. Pathol. Pharmacol. 2000;108:15–25. [PubMed] [Google Scholar]
- (15).Bousette N, Giaid A. Endothelin-1 in atherosclerosis and other vasculopathies. Can. J. Physiol. Pharmacol. 2003;81:578–587. doi: 10.1139/y03-010. [DOI] [PubMed] [Google Scholar]
- (16).Giaid A, Gibson S.J, Herrero M.T, Gentelmaon S, Legon S, Yanagisawa M, Masaki T, Ibrahim B.N, Roberts G.W, Rossi M.L, Polak J.M. Topographical localization of endothelin mRNA and peptide immunoreactivity in neurons of the human brain. Histochemistry. 1991;95:303–314. doi: 10.1007/BF00266781. [DOI] [PubMed] [Google Scholar]
- (17).Mostafa M.G, Mima T, Taniguchi T, Mori K. Doxorubicin, an RNA synthesis inhibitor, prevents vasoconstriction and inhibits aberrant expression of endothelin-1 the cerebral vasospam model of the rat. Neurosci. Lett. 2000;283:197–200. doi: 10.1016/s0304-3940(00)00940-x. [DOI] [PubMed] [Google Scholar]
- (18).Greenberg B.H. Endothelin and endothelin receptor antagonists in heart Failure. CHF. 2002;8:257–261. doi: 10.1111/j.1527-5299.2002.01153.x. [DOI] [PubMed] [Google Scholar]
- (19).Parker J.D, Thiessen J.J. Increased endothelin-1 production in patients with chronic heart failure. Am. J. Physiol. Heart. Circ. Physiol. 2004;286:H1141–H1145. doi: 10.1152/ajpheart.00239.2001. [DOI] [PubMed] [Google Scholar]
- (20).Letizia C, De Biase L, Caliumi C, Verrelli C, Semeraro R, Subioli S, Cerci S, D’ Erasmo E. Endothelin-1 circulating levels increase in patients with orthotopic heart transplantation and in chronic therapy with cyclosporine. Minerva Cardioangiol. 2001;49:15–22. [PubMed] [Google Scholar]
- (21).Ficai S, Herizi A, Mimran A, Jover B. Endothelin blockade in angiotensin. II. Hypertension: prevention and treatment studies in the rat. Clin. Exp. Pharmacol. Physiol. 2001;28:1100–1103. doi: 10.1046/j.1440-1681.2001.03568.x. [DOI] [PubMed] [Google Scholar]
- (22).Ramires F.J, Nunes V.L, Fernandes F, Mady C, Ramires J.A. Endothelins and myocardial fibrosis. J. Card. Fail. 2003;9:232–237. doi: 10.1054/jcaf.2003.26. [DOI] [PubMed] [Google Scholar]
- (23).Sorokin A, Kohan D.E. Physiology and pathology of endothelin-1 in renal mesangium. Am. J. Physiol. Renal Physiol. 2003;285:F579–F589. doi: 10.1152/ajprenal.00019.2003. [DOI] [PubMed] [Google Scholar]
- (24).George W, Chalmers D, Stuart A, Kantilal R, Neil C.T. Endothelin-1 induced bronchoconstriction in asthma. Am. J. Respir. Crit. Care Med. 1997;156:382–388. doi: 10.1164/ajrccm.156.2.9702066. [DOI] [PubMed] [Google Scholar]
- (25).Picture taken from web page. http://www.cvphysiology.com/Blood%20Flow/BF012.htm .


