Abstract
There is no clear evidence about the influence of programmed physical activity (training) on growth hormone (GH) response to acute physical exercise.
The aim of this study was to estimate the relationship between the level of physical activity and the serum growth hormone concentration in response to acute physical exercise. The study was performed on 20, healthy male subjects. Based on the level of their physical activities they were divided in two groups of equal size: group 1, trained, and group 2, untrained subjects. All subjects performed one boot of exercise on cycle ergometer, lasting 30 minutes. Work intensity was approx. 65% of VO2 max, and the rate of cycling was 60/min. Serum GH concentrations were measured by IRMA (immunoradiometric assays) method in blood samples obtained in the period of rest, during exercise and in the recovery period. There were marked differences in the dynamics of changes in the seram GH concentrations during exercise period between the groups of various level of physical activity despite the lack of the significant differences in basal level and maximal level of serum GH concentration at the end of exercise. Untrained subjects showed faster increase in serum GH concentration than trained subjects, but in trained subjects the restoration of the basal values in the recovery period was faster. These results indicate that the level of physical activities in young, healthy male subjects has no influence on GH response to acute physical exercise.
Keywords: growth hormone, acute physical exercise, trained, untrained
Introduction
Growth hormone (GH) is a peptide hormone synthesized and secreted by the anterior segment of the pituitary gland. There are many factors that may influence GH secretion. Physical activity is the one of the most potent stimuli (1). The mechanism underlying exercise stimulated rise in GH is still unclear. It was observed that peripheral metabolic signals influence GH response to exercise. GH response to physical exercise is attenuated by fat and carbohydrate ingestion (2). It is enhanced by the opiate antagonist naloxone in untrained subjects, but blunted in trained subjects (3). Central cholinergic pathways are also thought to be involved in GH response augmenting it bv niridostiemine. but attenuatine bv atronine (4).
Serum GH concentration, as previously reported, rise in response to acute physical exercise with a threshold level of approximately 30% of VO2 max. It begins to rise within 10 minutes from the exercise onset. The level is dependent on the type and the intensity of exercise and could rise up to 100-fold (5). The serum GH concentration during acute exercise is also influenced by sex, body composition, nutrition status and training status. The ef-feet of training status on GH response to acute exercise is unclear with different results being reported. Some studies reported attenuated GH response after training period and greater GH response in untrained subjects (6). Other studies showed no influence of training stams on GH response to acute exercise (7). Soriguer et al. (8) found significantly higher GH response in subjects with higher level of VO2 max. Results of Manneta et al. (9) supported this finding showing threefold GH response to acute exercise in trained vs. untrained subjects.
Due to these inconsistencies we aimed, in this study, to estimate the influence of the level of physical activity on GH serum response to acute physical exercise in young healthy male.
Methods
Subjects
The study involved a group of 20 healthy males, 21 years of age on average, who, based on the level of their physical activity, were divided into two groups of equal size: trained and untrained subjects. Enrollment criteria for the trained group included regular participation in active training programs for at least two years before the study. Untrained, sedentary subjects were students of School of Medicine with no active or recreative sport activities for the same period. Each subject underwent a detailed medical history and physical examination, and no history of pituitary, renal, hepatic, metabolic, or other system disease was found. Subjects refrained from exercise for 24 h before the study. Informed consent in written was obtained from all subjects before the study.
Exercise Protocol
Each subject completed a single boot of exercise on bicycle-ergometer. The testing was performed between 8-10 a.m. to avoid daily variation in growth hormone secretion. Subjects were requested to consume their evening meal at, or before 17.00 p.m. the previous day in order to avoid possible confounding effects of meals on GH secretion. Subjects fasted until the end of the test.
Table 1.
Physical characteristics of the subjects
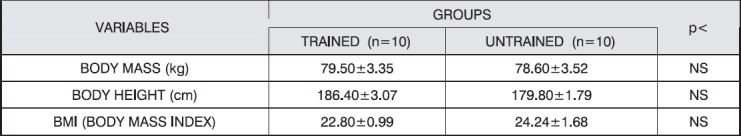
Acute exercise of 30 minutes duration was carried out on bicycle-ergometer (LODE-Corival 400) follows the protocol of individually adjusted constant workload. Workload was approximately 65 % of the workload achieved at VO2 max. Cycling cadence was 60 rpm.
Laboratory Measurements
Before the exercise a plastic cannula was placed in subject’s cubital vein on the left arm for blood sampling. Growth hormone concentration in blood was measured in the resting period, during exercise (in 6th, 12th, 18th, 24th. and 30th. minutes) and during recovery (15th, 30th and 45th minutes) period. The standard immunoradiometric (IRMA) method was used to determine serum growth hormone level.
Results And Discussion
Serum growth hormone concentrations in the resting period and the dynamics of changes during exercise and in the recovery period are presented in the Figure 1. Physical exercise is the most potent physiological stimulus of GH secretion (1,3,4). All previous studies reported a rise of serum GH concentration during acute exercise, but the results about influence of subjects’ training status on this response are contradictory. Our results show that serum growth hormone concentration in the rest period, is 2.6 times higher in the trained subjects (3.26 mU/l) compared to the untrained subjects (1.22 mU/l), but the difference is not statistically significant. Serum growth hormone concentration was increasing successively in both groups during acute physical exercise. Statistically significant increase of serum GH concentration untrained group achieved in 12th minute and untrained subjects in 18th minute of exercise. Our results are in accordance with those previously reported. Bunt et al. (10) also found faster incipient GH response in untrained subjects and suggested that this is due to higher sympathic sensitivity in subjects with lower level of physical activity. Mazzeo et al. (11) showed that sympathic activity is one of the most important mediators of GH response during acute exercise. We found no statistically significant differences between mean values of growth hormone concentrations in the groups, neither during workload nor during recovery period. Reports on the influence of the training on GH response during exercise are inconsistent. Both augmented (8) and attenuated (6) GH response in trained vs. untrained subjects was reported. Our results are in accordance with the results by Mc. Gall et al (12). They found that 12 weeks of intensive aerobic exercise training in sedentary subjects had no influence on the magnititude of GH response to acute exercise. Although maximal increase in growth hormone concentration was measured at the end of exercise (30th min.) in both groups, there were marked differences between groups in the dynamics of the increase of serum GH concentration during exercise. The increase in serum growth hormone concentration during exercise in the relation to the concentration in the resting period was smaller in trained vs. untrained subjects. Decrease in serum growth hormone concentration during recovery period, in relation to the concentration at the end of exercise, was faster in the trained subjects. In recent study, Jenkins (5) also reported higher magnitude of GH response to acute exercise and longer maintenance of elevated GH serum concentration after acute exercise in sedentary subjects. This difference in the dynamics of GH response to acute exercise between the trained and untrained subjects is probably the result of celular adaptation in the form of enhanced celular sensitivity after the training.
Figure 1.
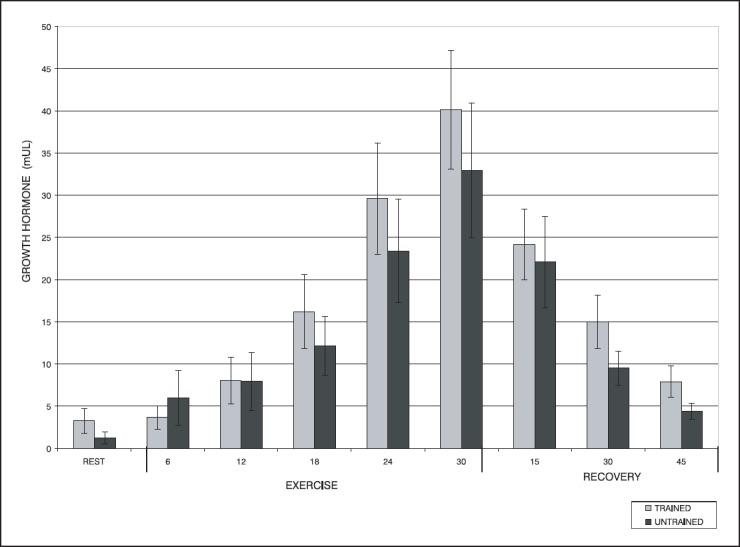
Dynamics of changes in serum GH concentrations during exercise and in the recovery period in trained and untrained subjects
Conclusions
It can be concluded that the level of physical activity, training status, has no influence on absolute values of serum GH concentration in response to acute physical exercise. There were marked differences in the dynamics of changes in the serum GH concentrations during the exercise period between the groups with various level of physical activity, despite the lack of the significant differences in basal level and maximal level of serum GH concentration at the end of exercise. Untrained subjects showed faster increase in serum GH concentration than the trained subjects, but trained subjects had faster restoration of the basal values in the recovery period.
References
- (1).Nindl B.C, Hymer W.C, Deaver D.R, Kraemer W.J. Growth hormone pulsatility profile characteristics following acute heavy resistance exercise. J Apply Physiol. 2001;91(1):163–175. doi: 10.1152/jappl.2001.91.1.163. [DOI] [PubMed] [Google Scholar]
- (2).Hartman M.L. Juul A, Jorgensen J.O.L. Growth hormonein adults. 2nd.Ed. UK: Cambridge University Press; 2000. Physiological regulators of growth hormone secreti on; pp. 3–53. [Google Scholar]
- (3).Grossman A, Bouloux P, Price P, Drury P.L, Lam K.S, et al. The role of the opioide peptides in the hormonal response to acute exercise in men. Clin Scien. 1984;67:483–91. doi: 10.1042/cs0670483. [DOI] [PubMed] [Google Scholar]
- (4).Cappa M, Grossi A, Benedetti S, Drago F, Loches, et al. Effect of the enhancement of the cholinergic tone by pyridostigmine on the exercise-induced growth hormone release in man. J Endocrinol Invest. 1993;16:421–424. doi: 10.1007/BF03348871. [DOI] [PubMed] [Google Scholar]
- (5).Jenkins P.J. Growth hormone and exercise. Clin Endocrinol. 1999;50:683–689. doi: 10.1046/j.1365-2265.1999.00784.x. [DOI] [PubMed] [Google Scholar]
- (6).Weltman A, Weltman J.Y, Womack C.J, et al. Exercise training decreases the growth hormone (GH) response to acute constant-load exercise. Med Sci Sports Exerc. 1997;29(5):669–676. doi: 10.1097/00005768-199705000-00013. [DOI] [PubMed] [Google Scholar]
- (7).Vigas M, Celko J, Koska J. Role of body temperature in exercise-induced growth hormone and prolactin releasein non-trained and physically fit subjects. Endocr regulat. 2000;34:175–180. [PubMed] [Google Scholar]
- (8).Soriguer Ecfofet F.J, Sebastian G.D, Campos A.V, et al. The response of the growth hormone to acute effort is a function of training. Med Clin (Bare) 1992;98(16):601–606. [PubMed] [Google Scholar]
- (9).Manetta J, Brun J.F, Maimoun L, Callis A, et al. Effect of training on the GH/IGF-l axis during exercise in middle-aged men: relationship to glucose homeostasis. Am J Physiol Endocrinol Metab. 2002;283:929–936. doi: 10.1152/ajpendo.00539.2001. [DOI] [PubMed] [Google Scholar]
- (10).Bunt J.C, Boi I, Eau R.A, Bahr J.M, Nelson R.A. Sex and training differences i ’ human growth hormone Ievās during prolonged exercise. JApply Physiol. 1986;61(5):1796–1801. doi: 10.1152/jappl.1986.61.5.1796. [DOI] [PubMed] [Google Scholar]
- (11).Mazzeo R.S, Marshall P. Influence of plasma catecholamines on the I aetate threshold during graded exercise. J. Appi. Physiol. 1989;67:1319–1322. doi: 10.1152/jappl.1989.67.4.1319. [DOI] [PubMed] [Google Scholar]
- (12).McGall G.E, Byrnes W.C, Fleck S.J, Dickinson A, et al. A cute and chronic hormonal responses to resistance training designed to promote muscle hypertrophy. Can. J. Appi. Physiol. 1999;24:96–107. doi: 10.1139/h99-009. [DOI] [PubMed] [Google Scholar]


