Abstract
In order to achieve the multi-claim products required for the dental care category, it is necessary for the formulator to use a variety of different ingredients. This places a number of demands on the development process. Innovations in the areas of pharmaceutical technology have contributed to the formulation of the products having superior efficacy as well as other attributes that may contribute to clinical response and patient acceptability. Improved clinical efficacy and tolerability, along with conditioning signals, should encourage patient compliance with oral hygiene further complementing professional efforts directed at disease prevention. The most effective way of preventing the development of dental disease is in controlling the production of dental plaque. It is formed by microbial action. The removal of plaque from the teeth and related areas is essential for the maintenance of a healthy mouth.
In this paper we have presented the main components of toothpastes and mouthwashes. For the active ingredients, their supposed effect as therapeutic agents is also explained.
Keywords: formulation, ingredients, toothpastes, mouth-washes
Introduction
The most effective way of preventing the development of dental disease is in controlling the production of dental plaque. Plaque is a soft thin layer which deposits on teeth gums and all appliances fitted in the mouth. It is formed by microbial action. Dietary sugars, in particular sucrose, contribute to the formation of plaque and their presence increases the rate of formation and thickness of plaque. The removal of plaque from the teeth and related areas is essential for the maintenance of a healthy mouth (1). In this paper we have presented the main components of toothpastes and mouthwashes. For the active ingredients, their supposed effect as therapeutic agents is also explained.
Ingredients of toothpastes and mouthwashes
A toothpaste in defined as a semi-solid material for removing naturally occurring deposits from teeth and is supposed to be used simultaneous with a toothbrush. A mouthwash is defined as a non-sterile aqueous solution used mostly for its deodorant, refreshing or antiseptic effect. Mouthwashes or rinses are designed to reduce oral bacteria, remove food particles, temporary reduce bad breath and provide a pleasant taste.
Mouthwashes (mouthrinses) are generally classified as either cosmetic or therapeutic or a combination of the two. Cosmetic rinses are commercial products that remove oral debris before or after brushing, temporary suppress bad breath, diminish bacteria in the mouth and refresh the mouth with a pleasant taste. Therapeutic rinses often have the benefits of their cosmetic counterparts, but also contain an added active ingredient, (for example fluoride or chlorhexidine), that help protect against some oral diseases. The amount of the different ingredients in mouthwashes varies from product to product. Some practically have the same composition as toothpastes, although they do not contain abrasives. Distinct from toothpastes most mouth-washes contain alcohol, as a preservative and a semi-active ingredient. The amount of alcohol is usually ranging from 18 - 26 %.
Abrasives
Abrasives are the substances that are used for abrading, grinding or polishing. They remove substances adhering to the surface of the teeth without scratching it and bring out their natural luster.
One of the mayor properties of the abrasive is hardness. The degree of abrasivity depends on the hardness of the abrasive, the morphology of the particles, and on the concentration of abrasive in the paste. As the hardness of the enamel on the tooth surface is 6-7 on the Moh’s scale, the hardness of an abrasive should be 3 or less. For practical purposes, the particle size should be 20μm or less; if it is more than this they may damage the tooth surface and gums. The abrasives found in toothpastes are often not as hard as the enamel, but as hard or harder than the dentine. Abrasives are most often found as crystals, small and smooth particles are preferred to avoid tooth wear. Needie and rod-shaped particles must be avoided (2).
Although many methods have been suggested for measuring the abrasive effect powders incorporated in toothpastes, the RDA method (Radioactive Dentine Abrasion) is the most widely accepted in the world today. In this method, an extracted human tooth is irradiated to convert the 31P in its dentine to 32p. The tooth is then put into an abrasion testing machine together with an abrasive and the abrasion of 32p is measured using a radioactivity counter. The pH of abrasives should range from weakly acidic to weakly alkaline and they should be white powders which are insoluble in water, flavourless and odourless. The following substances are widely used abrasives, which satisfy these conditions:
Calcium carbonate (CaCO3)
A fine, white, odourless, microcrystalline powder, practically insoluble in water (3). This abrasive has been used for a very long time. Its abrasiveness is generally higher than that of calcium phosphate. There are two types-a heavy and precipitated type. The raw material for the former is limestone and for the latter calcium hydroxide.
Calcium phosphate, dibasic; Calcium phosphate, dibasic, dihydrate (CaHPO4, CaHPO4x 2H20)
There is a dihydrate form and an anhydride form. As the anhydride form is harder than the dihydrate form, it is not often used by itself. The dihydrate form has a mild abrasive effect and feels good on use. It is neutral in pH and has good compatibility with other ingredients. However, when it is in toothpaste for a long period of time, it loses its water of crystallisation, changes to the anhydride form and makes the toothpaste go hard. For this reason a magnesium salt or other stabiliser is added (2).
Silica, silica hydrate (SiO2, SiO2 x nH2O)
The main ingredient of the silica used in abrasives is high purity amorphous silicon dioxide and there are varieties of different types whose properties vary with the method of production. Silica is very suitable for use in toothpastes containing fluoride because no insoluble salt is formed when it reacts with fluoride. As its refractive index is lower than that of other abrasives, silica can be used to make clear gel tootpastes.
Other abrasives
Aluminium hydroxide is also used as an alternative to calcium phosphate, dibasic, because it is cheaper. Other abrasives such as calcium pyrophosphate, insoluble sodium metaphosphate, magnesium carbonate and alumna may also be used for special types.
Binders
Binders are used to prevent the separation of powder and liquid ingredients and give an appropriate degree of viscoelasticity and form to the toothpaste. They can prevent the toothpaste from drying out by binding water. Also, they have an influence on the dispersion, foaming, rinsing and other qualities of the toothpaste in the oral cavity. The most widely used binder at present is sodium carboxym-ethylcellulose (CMC).
Carboxymethylcellulose is physiologically inactive, it dissolves in water, it is very compatible with other ingredients, highly stable and relatively low in price. There are many types of CMC having a variety of different characteristics stemming from different degrees of hydroxy group substitution and polymerisation, so it is necessary to select the most appropriate one for the purpose in mind. Other known cellulose derivatives include methylcellulose, hydroxyethylcellulose and hydroxypropylcellulose. Examples of other binders used are polysaccharides such as sodium alginate, carrageenan and xanthan gum; synthetic polymers like sodium polyacrylate and inorganic clay minerals as bentonite and laponite.
Sodium alginate
It is obtained from algae belonging to the Phaeophyceae, mainly species of Laminaria (4). It consists chiefly of the sodium salt of alginic acid. A white or pale yellowish-white powder which is odourless or almost odourless and tasteless. Slowly soluble in water, forming a viscous, colloidal solution; practically insoluble in alcohol and in ether.
Sodium alginate has little surface activity and its emulsifying power is achieved by increasing the viscosity of the aqueous phase. It is used as a suspending and thickening agent and in the preparation of water-miscible pastes, creams and gels. According to the viscosity required, from 1 to 10 % is used in the preparation of pastes and creams.
Carrageenan
A dried aqueous extract from species of Chondras, Gigartina, Eucheuma or other members of the families Gigarti-naceae, Solieriaceae, Hypneaceae and Furcellariaceae. A white to yellowish coarse or fine, almost odourless powder with a mucilaginous taste. Soluble 1 in 100 of water at 85°c. It disperses more readily if first mixed with alcohol. It is used as an emulsifying, suspending and thickening agent in formulations of toothpastes, creams and emulsions. Carrageenans are galactans or polymers of D-galactose, are heavily sulfated, and are anions with multiple electrolytes of molecular weight ranging from 105 to 106. All carrageenans have a linear structure of (AB)n type, with alternating 1,3 and 1,4 bonds. Classically, seven types of carrageenans are distinguished as a function of the nature of the sequence. These are t, κ, λ, μ, ν, θ, ξ carrageenans (5).
Carbomers
These are synthetic high molecular weight polymers of acrylic acid cross-linked with polyalkenyl ethers of sugars or polyalcohols. They are produced in several grades characterised by the viscosity of a defined solution. White, hygroscopic powders with a slight characteristic odour. They swell in water and in other polar solvents after dispersion and neutralisation with sodium hydroxide solution (6). It also soluble in water, alcohol and glycerol. Carbomer is used in toothpastes as a binder (thickener).
Xanthan gum
Xanthomonas campestris is a bacterium which commonly develops on certain species of Brassicaceae where, by using the vegetable substrate, it produces a gummy exudate: xanthan “gum”, a high-molecular-mass anionic polysac-charide. It exists as the sodium, potassium or calcium salt (6).
Industrially, this “gum” is produced by a bacterial culture on correctly buffered and aerated media containing carbohydrates with Xanthomonas campestris. Upon completion of fermentation, the polymer is recovered by precipitation with isopropanol, filtered, dried, and crushed (7).
It is a cream-coloured powder. Soluble in hot and cold water, xanthan gum forms aqueous solutions of which the viscosity remains practically unchanged by temperature changes, as well as pH changes.
The behaviour of these solutions is of the pseudoplastic-type: decrease in viscosity proportional to shearing and instant recovery of the initial viscosity upon discontinuation of shearing. Incompatibilities are rare (borates, hypo-chlorites, peroxydes, free radical generators). The gum is compatible with most salts, with moderate surfactant concentrations, and with most preservatives; it tolerates alcohol concentrations up to 50% percents. Compatible with most vegetable hydrocolloids, it does not form gels by itself, but it forms thermally reversible gels. It is devoid of toxicity. Xanthan gum is used as a stabiliser, binder (thickener), and emulsifier.
Humectants
They prevent loss of water, and subsequent hardening of the paste in the tube or when it is exposed to air. They also provide creamy texture. These are short-chained polyalcohols such as glycerol, sorbitol (highly concentrated aqueous solution), propylene glycol and polyethylene glycol.
Solvents
Water is the most common solvent used in toothpaste. It dissolves the ingredients and allows them to be mixed. Alcohol is used in mouth rinses (mouthwashes) as a solvent and taste enhancer.
Foaming agents
The functions of foaming agents are to disperse the toothpaste throughout the oral cavity in order to enhance the cleaning effect and, acting as a surfactant, clean away the dirt inside it. Also, by means of their volume of foam, they give a feeling of thickness, and satisfaction. Surfactants having excellent foaming, dispersion, suspension, permeation, cleansing and hard water resistance qualities as well as no toxicity or irritation, are selected for foaming agents.
Surfactants lower the surface tension of the liquid environment in the oral cavity so that the substances in the toothpaste/mouthwash can contact the teeth more easily. They penetrate and dissolve plaque. This makes it easier to clean the teeth. The foaming effect produced by the surfactants is also beneficial in cleaning the teeth, and contributes to remove debris and gives a feeling of cleanness. Another function of the surfactant is in dispersing the flavours in the toothpaste/mouthwash. Because, they go into the mouth, attention is also paid to taste and smell. The one most frequently used at present is sodium lauryl sulfate; other examples are sodium lauryl sarcosinate, sodium alkylsulfo succinate, sodium cocomonoglyceride sulfonate and sucrose fatty acid esters.
Sodium lauryl sulphate (SLS)
A mixture of sodium alkyl sulphates, consisting mainly of sodium dodecyl sulphate. It is a white or pale yellow powder or crystals with a slight characteristic odour. Freely soluble in water; partly soluble in alcohol (6). It exhibits high affinity for proteins and is a strong denaturing agent. Incompatible with cationic materials and with acids below pH 2.5. Sodium lauryl sulphate may be irritant to the skin and mucosa. It may also damage the mucosal mucin layer by denaturing its glycoproteins (8). The epithelium will then be more exposed for irritants and this can result in aphtous ulcerations in some patients. It has also been claimed that there is a connection between the use of toothpaste or mouthwash containing SLS and an increased frequency of recurrent aphthous ulcers (RAU) in some patients. A product without SLS may thus be recommended for patients with RAU (8). The adverse effects of SLS have resulted in the development of toothpaste and mouthwashes with alternative surfactants such as sodium lauryl sarcosinate, socamidopopybetaine. Common for these surfactants are that they are less irritating to the oral mucosa. It is effective in both acid and alkaline solution and in hard water. Also, it has antimicrobial activity due to its ability to interfere with membranes and a variety of biologic processes in microorganisms.
Flavouring agents
They get rid of the unpleasant smell and taste of the other raw materials and give a cold, refreshing taste. Combinations of water-insoluble essential oils, such as spearmint, peppermint, eucalyptus and menthol are often used as flavouring agents in toothpastes and mouthwashes. The flavouring agents are solubilised and dispersed through the paste or liquid via the surfactant.
Sweeteners
Sweeteners also improve the taste of toothpastes and mouthwashes and give them a mild and sweet taste. The most common used sweeteners are sodium saccharin, sorbitol and glycerol. Xylitol is a sweetener that is also claimed to provide anti-caries activity.
Colouring agents
Most toothpastes and mouthwashes contain colour-substances which give them an attractive appearance. The colour-substances are classified by the Colour Index (CI), published by the Society of Dyers and Colourists and the American Association of Textile Chemists and Colourists, or by a system called the FD&C Colours. Titanium dioxide is often added to toothpastes to give them a white colour.
Preservatives
Preservatives prevent the growth of micro-organisms in toothpastes and mouthwashes. Mostly, they include sodium benzoate, methylparaben and ethylparaben.
Pharmaceutical agents
One or more therapeutic agents are usually added to toothpastes and mouthwashes. Most toothpastes today contain fluorides to prevent caries. Recently there has been a development of different toothpastes with additional purposes, such as stain and calculus removal, and prevention of gingivitis, sensitive teeth and gum problems. In the following text the different pharmaceutical therapeutic agents are categorised according to their claimed ef-feet.
Anticaries agents
Fluoride
Fluoride is considered to be the most effective caries-inhibiting agent, and almost all toothpastes today contain fluoride in one form or the other. The most common form is sodium fluoride (NaF), but mono-fluoro-phosphate (MFP) and stannous fluoride (SnF) are also used. The fluoride amount in toothpaste is usually between 0.10-0.15 %. Fluoride is most beneficial when the mouth is not rinsed with water after tooth brushing. In this way a bigger amount of fluoride is retained in the oral cavity. Toothpastes are the main vehicle for fluoride. The combined therapeutic and cosmetic mouthwashes usually also contain fluoride, but in a non-therapeutic dose. However, there are fluoride-rinses with higher fluoride concentrations.
The mechanism by which fluoride prevents caries is not clearly understood. It is known that the fluoride ion (F-) can replace the hydroxyl ion (OH-) in hydroxyapatite, the major crystalline structure of enamel. The substituted crystal, called fluorapatite, is more resistant to acids, such as those produced by plaque bacteria, than the original hydroxyapatite (9).
As the tooth develops and enamel is formed, ingested fluoride is incorporated into the enamel. Therefore, because enamel develops its outer layer first, more fluoride can be expected to be deposited on the outer layers as compared to the inner layers. It is this surface enamel layer containing fluoride that imports caries resistance to a tooth. The incorporation of fluoride into enamel can be represented as a chemical reaction:
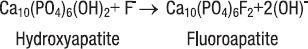
It is also suggested that fluoride has anti-bacterial actions. In an acidic environment, if fluoride is present, hydrogen fluoride (HF) is formed. HF is an undissociated, week acid that can penetrate the bacterial cell membrane. The entry of HF into the alkaline cytoplasmic compartments resuits in dissociation of HF to H+ and F. This has two separate, major effects on the physiology of the cell. The first is that the released F- interacts with cellular constituents, including various F-sensitive enzymes. The second effect is an acidification of the cytoplasmic compartment caused by the released protons. Normally protons are pumped out of the cell, but fluoride inhibits these processes. The decreased intracellular pH will make the environment less favourable for many of the essential enzymes required for cell growth (10).
As the most important anti-caries effect is claimed to be due to the formation of calcium fluoride (CaF2) in plaque and on the enamel surface during and after rinsing or brushing with fluoride. CaF2 serves as a fluoride reservoir. When the pH drops, fluoride and calcium are released into the plaque fluid. Fluoride diffuses with the acid from plaque into the enamel pores and forms fluoroapatite (FAP). FAP incorporated in the enamel surface is more resistant to a subsequent acid attack since the critical pH of FAP (pH=4.5) is lower than that of hydroxyapatite (HA) (pH=5.5). Fluoride decreases the demineralisation and increases the remineralisation of the enamel between pH 4.5-5.5, and hence the demineralisation period is shortened (10).
Xylitol
A polyhydrie alcohol (polyol) related to the pentose sugar, xylose. White crystals or crystalline powder. Very soluble in water; sparingly soluble in alcohol (6). It has a sweet taste and produces a cooling sensation in the mouth. Xylitol cannot be fermentated by oral microorganisms. It is considered to be a cariostatic agent since it can inhibit the carbohydrate metabolism in different oral micro-organisms. Xylitol seems to be unique among the sugar alcohols in its inhibitory effect on glycolysis. The inhibitory effect on glycolysis has been related to the uptake of xylitol via a constitutive fructose specific PTS (phospho-transferase) system and subsequent intracellular accumulation of xylitol-5-phosphate. Such a mechanism leads to reduced acid formation from glucose, and a reduction in the Streptococcus mutans content in both plaque and saliva (11).
Calcium / Phosphate
Calcium and phosphate supplementation in a toothpaste or mouth rinse will increase the concentration of these ions in the oral cavity. In this way they improve remineralisation and increase fluoride uptake (12).
Sodium bicarbonate
Several studies have shown that bicarbonate is one of the salivary components that potentially modifies the formation of caries. It increases the pH in saliva, and in this way creates a hostile environment for the growth of aciduric bacteria. Sodium bicarbonate can also change the virulence of the bacteria that cause tooth decay.
Animal studies have shown that toothpastes containing sodium bicarbonate reduce the amount of both Streptococcus sobrinus and Streptococcus mutans, and this may reduce caries. Studies on human show a statistically reduction in number of mutans streptococci. Sodium bicarbonate can also prevent caries by reducing enamel solubility and increase remineralisation of enamel (13).
Anti-plaque agents
Sodium lauryl sulphate
It has been shown that the enzymes glucosyltransferase and fractosyltransferase are incorporated in an active form into the pellicle; and by Synthesising glucan in situ from sucrose, can provide a surface for colonisation by Streptococcus mutans. These enzymes can be inhibited by SLS. Such inhibition can clearly retard the regrowth of plaque (14).
Triclosan
Triclosan is a non-ionic chlorinated phenolic agent with antiseptic qualities. Triclosan has a broad-spectrum efficacy on Gram-positive and most Gram-negative bacteria. It is also effective against mycobacterium and strictly anaerobic bacteria, and against the spores and fungi of the Candida species. The mechanism of its antiseptic action is by acting on the microbial cytoplasmic membrane, inducing leakage of cellular constituents and thereby causing lysis of the micro-organisms. In spite of its activity in vitro, clinical plaque studies have revealed only moderate levels of antiplaque activity.
Evidence has accumulated to suggest that triclosan itself does not produce optimal plaque inhibitory effects without the addition of other chemicals which increase its antibacterial effect. Most commonly used are copolymer PVM/MA [Poly(Methylvinylether/Maleic anhydride)] and zinc citrate. They enhance surface retention of tríelosan (15). An antiseptic has to be retained in the oral cavity for a certain amount of time in order to have antiplaque activity. The retention sites for triclosan are not yet established, but the teeth and the micelles in saliva are suggested. Triclosan also has antiinflammatory effect by acting on the eicosanoid-cascade. Triclosan inhibits both cyclooxygenase (COX) and lipoxygenase (LOX), and thereby inhibits the production of prostaglandins and leukotri-enes. Clinical studies also indicate that triclosan reduces oral mucosal irritation caused by sodium lauryl sulphate (16,17).
Metal-ions
The most widely used metal-ions in dental preparations are zinc (Zn2+) and stannous (Sn2+). These metals have the ability to limit bacterial growth, inhibit plaque formation, inhibit the glycolytic sequence in oral anaerobic bacteria, and to restrict the ability of plaque bacteria to convert urea to ammonia (14). They can also inhibit some bacterial enzymes. It is also possible that they can reduce the bacteria’s ability to colonise the tooth surfaces.
a) Stannous-ions
Stannous-ions are added to toothpastes and mouthwashes in the form of stannous fluoride or stannous pyrophosphate. Stannous fluoride was frequently used as a vehicle for fluoride in dental preparations. At present time it is rarely used, although extensive research during the last two decades has established that stannous fluoride possesses several interesting properties.
It has been claimed that stannous fluoride is more effective in caries inhibition than sodium fluoride and mono-flourophoshate. This is probably because stannous fluoride has additional properties compared with other fluoride vehicles. However such differences are not always statically significant in small-scale studies (18). Mouth rinses containing stannous fluoride have been found to reduce the relative amounts of Streptococcus mutans and Streptococcus sanguis in plaque, to reduce the population of Streptococcus mutans in saliva and to increase the salivary levels of Lactobacilli (14).
The stannous fluoride treated enamel becomes hydropho-bic, a property which may contribute to the antiplaque effect of stannous fluoride, since hydrophobic surfaces are less easily colonised by bacteria (18). The cariostatic protection provided by stannous fluoride is dependent on a deposition of CaF reservoir on the tooth surface. Both the antiplaque effect and the inhibition of acid formation by stannous fluoride are most likely caused by the oxidation of thiol groups which stannous fluoride is known to perform. Stannous ions may inhibit bacterial glycolysis because the enzymes depend on the thiol group for their biological activity (18). The antiplaque effect of SnF can clearly also contribute to the cariostatic activity.
b) Zinc-ions
Zinc is added to toothpastes and mouthwashes as zinc chloride or zinc citrate. Zinc is a relatively non-toxic, noncumulative essential trace element Zinc inhibits the PTS pathway of glucose uptake by Streptococcus mutans, Streptococcus sanguis and Actinomyses naeslundii, and the metabolism of glucose to lactic acid. The effects of zinc are believed to be intracellular, resulting from the inhibition of sulphydryl enzymes, specifically enzyme I in the phosphotransferase transport system and aldolase and glyceraldehyde dehydrogenase in the glycolytic pathway. Zinc also inhibits the trypsin-like protease activity of Porphyromonas gingivalis and of Capnocytophaga gingivalis (14). The role of zinc in plaque inhibition or as a calculus inhibitory agent when used in toothpastes has been established by a number of workers (14). It is shown that surfactants enhance the plaque-inhibitory role of zinc (19).
Essential oils
Essentials oils of thymol, menthol, eucalyptol and methyl salicylate are thought to have anti-bacterial activity by altering the bacterial cell wall. Mouth rinses containing these active ingredients have been reported to reduce plaque and gingivitis significantly.
Chlorhexidine
Chlorhexidine formulations are considered to be the “gold standard” antiplaque mouthwashes due to their prolonged broad spectrum antimicrobial activity and plaque inhibitory potential (20). The mechanism of action of chlorhex-idine is related to a reduction in pellicle formation, alteration of bacterial absorption and/or attachment to teeth, and an alternation of the bacterial cell wall so that lysis occurs (9). Chlorhexidine is effective against both Gram-positive and Gram-negative bacteria, but has most effect against Gram-positive bacteria.
Chlorhexidine is bacteriostatic at very low concentrations, especially against Streptococcus mutans. It also has effect against fungi, but non or little effect against spores. It also has effect against some viruses.
Chlorhexidine is retained in the oral cavity for 24 hours by binding to phosphate, sulphate and carboxyl groups in bacteria, plaque, saliva and on the enamel surface. The anti-bacterial action is due to a disturbance of the transport through the cell membrane and of the bacterial metabolism, and by causing leakage through the cell membrane. Its antiviral effect is caused by interaction with the viral protein cap. Local side effects of Chlorhexidine including disturbance of taste and staining of teeth, tongue and restorative materials have tended to restrict its use to only a short term (20).
Anti-calculus agents
These agents act by delaying dental plaque calcification, thereby promoting plaque removal with normal tooth brushing (21). Of the anti-calculus agents, the crystal growth inhibitors have been most extensively tested clinically.
Pyrophosphate
Pyrophosphate has introduced in toothpastes to inhibit the formation of supragingival dental calculus (21). Pyrophosphate is added as tetrasodium pyrophosphate, tetra-potassium pyrophosphate or disodium pyrophosphate. It has been shown that pyrophosphate has high affinity to hydroxyapatite (HA) surfaces, probably by an interaction with Ca2+ in the hydration layer. By interacting with HA and the enamel surface, pyrophosphate reduces their protein-binding capacity. It also has the ability to inhibit calcium phosphate formation. It is therefore conceivable that pyrophosphate introduced in the oral cavity through toothpastes may affect pellicle formation. However, the P-O-P bond of pyrophosphate is known to be susceptible to enzymatic hydrolysis by plaque and salivary phosphatases, and the effect may thus be of limited duration in the oral cavity (14). Consequently, the tartar control toothpastes that contain pyrophosphate as a calculus inhibitor also incorporate phosphates inhibitors that prolong the activity of pyrophosphate in the mouth.
Studies have indicated that fluoride in combination with PVM/MA Copolymer gives a significant protection of pyrophosphate against phosphatases (22). The clinical consequences of a poorly formed or partly missing pellicle are not known. Suggested consequences are abrasion of teeth, increased demineralisation, and hypersensitivity of teeth (14).
Zinc-ions
Zinc has anti-calculus effect due to its anti-plaque properties, but in addition it is thought to influence calculus formation by inhibiting crystal growth.
Anti-dentine hypersensitivity agents
Although the condition is referred to as “dentine hyper-sensitivity” it isn’t really the dentine that is sensitive. The sensitivity of dentine is caused by fluid-filled tubules in communication with the pulp (14).
Potassium salts
Potassium ions are thought to act by blocking action potential generation in intradental nerves (23). It is claimed that potassium salts in dental preparations increase the concentration of potassium ions around the pulpal nerves, and thereby depolarises the nerve. This can inhibit a nerve response from different stimuli.
Anti-aphtous agents
Aminoglucosidase and glucose oxidase
Enzymatic toothpastes and mouthwashes do not contain surfactants like SLS because the surfactant can denaturate the enzymes. SLS may induce adverse effects in oral soft tissues and increases the frequency of ulcers in patients suffering from recurrent aphthous ulcers (RAU). The ulcers were generally reported to be smaller and less painful, to have a shorter healing time and the frequencies of aphthous ulcers episodes were decreased (24).
Whitening agents
Whitening toothpastes do not lighten the colour of the tooth structure; they simply remove surface stains with abrasives or special chemical or polishing agents, or prevent stain formation.
Abrasives
An abrasive is required for the effective removal of a discoloured pellicle. Abrasives provide a significant whitening benefit, particularly on smooth surfaces, but are of limited use for areas along the gum line and interproximally. Some whitening toothpastes contain coarse abrasives that can damage the dental tissue.
Dimethicones
Dimethicones are versatile substances that ranges from low molecular weight polydimethylsioxane fluids to high molecular weight polymers that are gum-like in nature. They cause a smooth surface on the tooth that prevents stain formation.
Papain
Papain is a sulfhydryl protease consisting of a single polypeptide chain, extracted from the Carica papaya plant (4). It is able to hydrolyse peptid bonds, and can also catalyse the transfer of an acyl group. It is used in toothpastes as an non-abrasive whitening agent.
Sodium bicarbonate
It is known that toothpastes containing high concentrations of sodium bicarbonate are more effective in removing intrinsic tooth stain than those not containing sodium bicarbonate (25).
Anti-halistosis agents
Zinc-ions
Bad breath or halitosis originates mainly from the oral cavity. The unpleasant smell is due to the retention of anaerobic, Gram-negative bacteria. These bacteria use sul-phurcontaining amino acids as substrates in their production of volatile sulphur-containing compounds (VSC). VSC have a distinctly unpleasant odour even in low concentrations. Zinc inhibits the production of VSC in the oral cavity by interacting with sulphur in the amino acids or their metabolism. Zinc can be retained in the oral cavity for approximately 2-3 hours after tooth brushing by binding to acidic substances on the oral mucosa, in the saliva or on bacterial surfaces.
Conclusion
In order to achieve the multi-claim products required for the dental care category, it is necessary for the formulator to use a variety of different ingredients. This places a number of demands on the development process. Innovations in the areas of pharmaceutical technology have contributed to the formulation of the products having superior efficacy as well as other attributes that may contribute to clinical response and patient acceptability. Improved clinical efficacy and tolerability, along with conditioning signals, should encourage patient compliance with oral hygiene further complementing professional efforts directed at disease prevention.
References
- (1).Winfield A.J, Richards R.M.E. Pharmaceutical practice. Churchil Livingstone, Edunburgh, London, New York, Philadelphia, San Francisco, Sydney, Toronto: 1998. pp. 421–423. [Google Scholar]
- (2).Mitsui T. New cosmetic science. Elsevier, Amsterdam, Lausanne, New York, Oxford, Shannon, Tokyio: 1997. pp. 480–490. [Google Scholar]
- (3).Sweetman S.C, editor. Martindale: The complete drug reference. 33rd Edition. London: Pharmaceutical Press; 2002. [Google Scholar]
- (4).Bruneton J. Pharmacognosy, phytochemistry, medicinal plants. Paris: Intercept Ltd; 1999. pp. 45–52. 53-57. [Google Scholar]
- (5).Matshuhiro B, Urzua C.C. Heterogeneity of carrageenans from Chondrus crispus. Phytochemistry. 1992;31:531–534. doi: 10.1016/0031-9422(92)90032-l. [DOI] [PubMed] [Google Scholar]
- (6).European Pharmacopoeia. 4th Edition. Strasbourg: Council of Europe; 2002. [Google Scholar]
- (7).Becker A, Katzen F, Püshler A, Ielpi L. Xanthan gum biosynthesis and application: A biochemical/genetic perspective. Appi. Microbiol. Biotechnol. 1998;50:145–152. doi: 10.1007/s002530051269. [DOI] [PubMed] [Google Scholar]
- (8).Herlofson B.B, Barkvoll P. Sodium lauryl sulfate and recurrent aphthous ulcers. A preliminary study. Acta Odont. Scand. 1994;52:257–259. doi: 10.3109/00016359409029036. [DOI] [PubMed] [Google Scholar]
- (9).Ciancio S.G. Dental products. In: Swarbrick J, Boylan J.C, editors. Encyclopedia of Pharmaceutical Technology. Vol. 3. New York and Basel: Marcel Dekker, Inc; 1990. pp. 425–456. [Google Scholar]
- (10).Fejerskov O, Ekstrand J, Burt B.A. Fluoride in dentistry. 2nd edition. Copenhagen: Munksgard; 1996. [Google Scholar]
- (11).Scheie A.A, Fejerskov O.B. Xylitol in caries prevention: what is the evidence for clinical efficacy? Oral Dis. 1998;4:268–278. doi: 10.1111/j.1601-0825.1998.tb00291.x. [DOI] [PubMed] [Google Scholar]
- (12).Schemehorn B.R, Orban J.C, Wood G.D, Fisher G.M, Winston A.E. Remineralization by fluoride enhanced with calcium and phosphate ingredients. J. Clin. Dent. 1999;10:13–16. [PubMed] [Google Scholar]
- (13).Legier-Vargas K, Mundorff-strestha S.A, Featherstone J.B.D, Gwinner L.M. Effects on sodium bicarbonate dentifrice on the levels of cariogenic bacteria in human saliva. Caries Res. 1995;29:143–147. doi: 10.1159/000262056. [DOI] [PubMed] [Google Scholar]
- (14).Embery G, R?lla G. Clinical and biological aspects of dentifrices. Oxford: Oxford University Press; 1992. [Google Scholar]
- (15).Moran J, Addy M, Newcombe R.G, Marlow I. A study to assess the plaque inhibitory actions of a newly formulated tri-closan toothpaste. J. Clin. Periodontol. 2001;28:86–89. doi: 10.1034/j.1600-051x.2001.280113.x. [DOI] [PubMed] [Google Scholar]
- (16).Wåler S.M, Rølla G, Skjørland K.K, Øgaard B. Effects of oral rinsing with triclosan and sodium lauryl sulfate on dental plaque formation: apilot study. Scand. J. Dent. Res. 1993;101:192–195. doi: 10.1111/j.1600-0722.1993.tb01103.x. [DOI] [PubMed] [Google Scholar]
- (17).Skåare A, Eide G, Herlofson B.B, Barkvoll P. The effect of toothpaste containing triclosan on oral mucosal desquamation. J. Clin. Periodontol. 1996;23:1100–1103. doi: 10.1111/j.1600-051x.1996.tb01810.x. [DOI] [PubMed] [Google Scholar]
- (18).Rølla G, Ellingsen J.E. Clinical effects and possible mechanism of action of stannous fluoride. Int. Dent. J. 1994;44:99105. [PubMed] [Google Scholar]
- (19).Giertsen E, Scheie A.A, R?lla G. Plaque inhibitition by a combination of zinc citrate and sodium lauryl sulphate. Caries. Res. 1989;23:278–283. doi: 10.1159/000261192. [DOI] [PubMed] [Google Scholar]
- (20).Sheen S, Owens J, Addy M. The effect of toothpaste on the propensity of Chlorhexidine and cetyl pyridinium chloride to produce staining in vitro: a possible predictor of inactivation. J. Clin. Periodontol. 2001;28:46–51. doi: 10.1034/j.1600-051x.2001.280107.x. [DOI] [PubMed] [Google Scholar]
- (21).Segreto V.A, Stevens D.P, Schulte M.C, Fortna R.H, Gerlach R.W. Safety and efficacy of novel tartar control dentifrice containing 3.3 % phyrophosphate: a controlled six-month clinical trial. J. Clin. Dent. 1998;9:26–29. [PubMed] [Google Scholar]
- (22).Gaffar A, Poletka T, Afflitto J, Esposito A, Smith S. In vitro evaluations of pyrophosphate/ copolymer/NaF as an anti-calculus agent. Compend. Contin. Educ. Dent. 1987;8(Suppl):242–250. [Google Scholar]
- (23).Sowinsky J, Ayad E, Petrone M, De Vizio W, Ellwood R, Davies R. Comparative investigation of the desensitising efficacy of a new dentifrice. J. Clin. Periodontol. 2001;28:1032–1036. doi: 10.1034/j.1600-051x.2001.281107.x. [DOI] [PubMed] [Google Scholar]
- (24).Fridh G, Koch G. Effect of a mouthrinse containing amyloglucosidase and glucose oxidase on recurrent aphthous ulcers in children and adolescents. Swed. Dent. J. 1999;23:49–57. [PubMed] [Google Scholar]
- (25).Kleber C.J, Moore M.H, Nelson B.J. Laboratory assessments of tooth whitening by sodium bicarbonate dentifrices. J. Clin. Dent. 1998;9:72–75. [PubMed] [Google Scholar]


