Abstract
Endodontic pathology is a bacterial disease. It is well established that periapical disease is the result of bacteria, their product, and the host response to them. Periradicular disease will occur after microorganisms and their metabolic products affect the periradicular tissue. Aim of using antibiotics as part of a treatment regimen is to achieve, within the periodontal environment, a concentration of the drug that is sufficient either to kill (bactericidal) or arrest the growth (bacteriostatic) of pathogenic microorganisms. There are two possible approaches to improve the drug action: sustained and controlled drug release to reduce or eliminate side effects by improving the therapeutic index and site-specific drug delivery to minimize systemic effects. These two strategies have been explored by the association of drugs with different vehicles, either naturals or synthetics.
A wide variety of specialized local delivery systems (i.e. intrapocket devices) have been designed to maintain the antibiotic in the GCF (gingival crevicular fluid) at a concentration higher than the MIC (minimum inhibitory concentration). Fibres, films, strips and microparticles made of biodegradable or non-biodegradable polymers have been reported as effective methods to administer antibacterial agents for periodontal therapy. Together with these solid devices, semisolid adhesive or non-adhesive formulations have also been proposed.
Keywords: local delivery, antibiotics, delivery devices, periodontal, root canal
INTRODUCTION
Endodontic pathology is a bacterial disease. It is well established that periapical disease is the result of bacteria, their product, and the host response to them. Histological studies in general, have not been able to demonstrate viable bacteria in periapical lesions. These findings persist to the present time. Nowadays, evidence indicates that many of these lesions are indeed infected before, and after endodontic treatment. Iwu showed that 88% or 14 of 16 periapical granulomas were positive for bacteria when they were homogenized and cultured (1).
In 1992 Wayman (2) studied 58 cases of periapical lesions. He cut these lesions in half and examined one half histological and cultured the other half. In only 8 of 58 cases could he demonstrated bacteria histologicaly. However, when the other half lesion was cultured 51 of 58 cases were positive. He found 133 isolates, of which 87 were strict anaerobes, 37 were facultative anaerobes and only 9 were aerobes. The bacteria (3) were found not only in periapical abscess but also in granulomas and cystis.
Microorganisms vary in their pH tolerance ranges, and most human pathogens grow well within a range of 5 to 9 pH (4). Some strains of Escherichia coli, Proteus vulgaris, Enterobacter aerogenes and Pseudomonas aeruginosa can survive in pH 8 or 9 (5). These bacterial species have occasionally been isolated from infected root canals, usually causing secondary infections (6). Certain bacteria, such as some enterococci, tolerate very high pH values, varying from 9 to 11. Fungi generally also exhibit a wide pH range, growing within a range of 5 to 9 pH (5). It has been demonstrated that enterococci and fungi are highly resistant to calcium hydroxide (7, 8). Since these microorganisms are commonly found in cases of endodontic failure, the routine use of calcium hydroxide should be questioned.
Endodontic infections are polymicrobial, and no known medicament is effective against all the bacteria found in infected root canals. In addition, the medicament should ideally reach microorganisms located in distant areas of the root canal system in lethal concentrations.
Antibiotic therapy has been used for years as an adjunct to periodontal treatment. One of the most promising recent advances in periodontal therapy has been the development of sustained-release delivery systems to administer antibiotics directly to the periodontal pocket. Locally delivered antibiotics overcome many of the disadvantages that we see with systemic drugs. There are specific guidelines and indications for the use of locally delivered antibiotics as adjuncts to periodontal therapy in dental practice.
Periradicular disease will occur after microorganisms and their metabolic products affect the periradicular tissue. The magnitude of the host response will be directly proportional to the virulence and the number of microbial cells present. Tissue damage caused by bacteria is mediated by either direct or indirect mechanisms. Direct harmful effects caused by bacteria involve their products, such as enzymes (collagenase, hyaluronidase, condroitinase, acid phosphatase), exotoxins and metabolites (bytrate, propionate, ammonium polyamines, sulphured compounds). In addition, bacterial components such as peptidoglycan, teichoic acid, fimbriae, outer membrane proteins, capsule, and lypopolysaccharide, stimulate the development of host immune reaction capable of causing severe tissue destruction (9, 10, 11). For example, macrophages can be activated by bacterial components and can be stimulated to release chemical mediators such as cytokines (interleukin-1b, tumours necrosis factor, and interleukin-6), and prostaglandins, which are involved in the induction of bone resorbtion commonly observed in chronic periradicular diseases (10,11).
Recently, it has been demonstrated that bacterial DNA may activate macrophages and dendritic cells triggering release of pro-inflammatory cytokines (12). Another example refers to the tissue damage associated with the acute periradicular abscess. Host defense mechanisms against bacteria aggressing from the root canal appear to be the most important factor involved in the pus formation associated with acute periradicular abscess. Formation of oxygen derived free radicals, such as superoxyde and hydrogen peroxyde, together with the release of lysosomal enzymes by polymorphonuclear neutrophils, such as elastase, collagenase, and gelatinase, induce the destruction of the extracellular matrix, leading to the pus formation (13). Therefore, bacteria can exert indirect destructive effects, which seems to be more significant in the tissue damage associated with acute and chronic periradicular lesions.
DRUG DELIVERY DEVICES
There are two possible approaches to improve the drug action:
- sustained and controlled drug release to reduce or eliminate side effects by improving the therapeutic index;
- site-specific drug delivery to minimize systemic effects.
These two strategies have been explored by the association of drugs with different vehicles, either naturals or synthetics.
Drug delivery systems can be classified according to the mechanism controlling drug release. We distinguish three categories:
- “solvent controlled” matrix systems based on macromolecular matrix permeability to small molecules after matrix swelling into hydrated medium;
- “reservoir systems” controlled by drug diffusion across a polymeric membrane;
- “chemically controlled systems” where the rate of drug release is controlled by the rate and extent of degradation of chemical bonds and the erosion of the polymeric matrix.
For all these systems, the basic polymer can be of natural origin such as proteins (14) or collagen (15), semi-synthetic such as cellulose derivatives (16,17) or synthetic, all of which must preferably degrade during use. Natural polymers have been considered as biodegradable carriers (18). However, most of them have disadvantages inherent to their structure, including limited half-life, complexity of composition and immunogenicity due to the polymer itself or to its degradation by-products. Many polymer-based systems for antibiotic delivery in the treatment of periodontal diseases have been studied and evaluated in vitro and/or in vivo.
PERIODONTAL LOCAL DELIVERY DEVICES
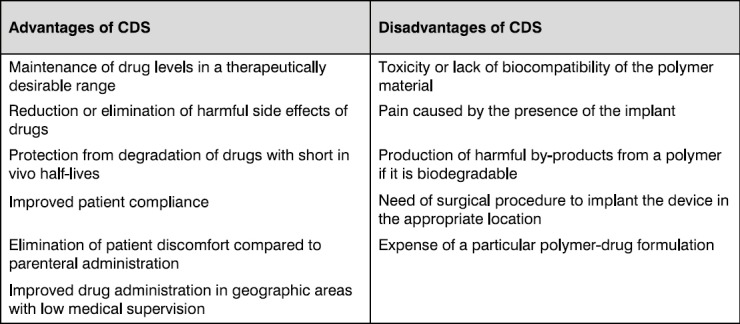
Local delivery devices were widely studied for various applications. Table 1 includes a list of advantages and potential disadvantages of controlled release devices. Regardless of the carrier system used, a candidate polymer for the design of a controlled delivery system must comply with a range of characteristics valid for most biomaterials:
Table 1.
Main advantages and potential disadvantages of controlled delivery systems (CDS) for the treatment of periodontitis
- it must be free of elutable impurities, additives, stabilizers, catalyst residues, and emulsifers;
- with the exception of bioerodable systems, the physical, chemical, and mechanical properties of the polymer should not be altered by the biological environment;
- it must have sufficient mechanical and thermal stability;
- it must be able to be readily processed, cast, or moulded in films, rods, tubing systems, and so forth;
- the material should not be carcinogenic, toxic, or inflammatory;
- the system must be able to be sterilized or prepared under aseptic conditions.
A wide variety of specialized local delivery systems (i.e. intrapocket devices) have been designed to maintain the antibiotic in the GCF (gingival crevicular fluid) at a concentration higher than the MIC (minimum inhibitory concentration). Fibres, films, strips and micro particles made of biodegradable or non-biodegradable polymers have been reported as effective methods to administer antibacterial agents for periodontal therapy. Together with these solid devices, semisolid adhesive or non-adhesive formulations have also been proposed.
Root canal therapy
The most important elements of root canal preparation are effective access and aseptic biomechanical preparation. Medicaments such as Ledermix paste and calcium hydroxide pastes have been recommended as routine intracanal medicaments.
Early investigations evaluated two antibiotic-containing preparations: Grossman’s polyantibiotic paste, which contains penicillin, bacitracin or chloramphenicol and streptomycin (19) and the other a mixture of neomycin, polymixin and nystatin (20). Both of these had some efficacy as intracanal medicaments. A more recent study has shown that clindamycin gave no advantage as a root canal dressing when compared with conventional root canal dressings (21). Further in vitro investigations have produced more favourable results with antibiotic mixtures such as ciprofloxacin, metronidazole and minocycline that were used as topical root canal agents (22, 23). However, the consensus of clinical opinion is that calcium hydroxide is the most appropriate agent for the purpose of controlling bacterial activity (24). Even though, it has been demonstrated that enterococci and fungi are highly resistant to calcium hydroxide (7, 8). Since these microorganisms are commonly found in cases of endodontic failure, the routine use of calcium hydroxide should be questioned.
Medication used inside root canals:
Kenacomb: corticosteroid, antibiotic cream (Bristol-Myers Squibb Company, Cairo, Egypt) purchased on the open market. Each gram of the cream contained the following:
- Nystatin (mycostatin), 100 000 units
- Neomycin (as neomycin sulphate), 2.5 mg
- Gramicidin, 0.25 mg
- Triamcinolone acetonide 1.0 mg
These ingredients were combined in an aqeous cream base.
ADVANTAGES AND DISADVANTAGES OF LOCAL DELIVERY
I Advantages of local delivery when compared to systemic delivery of antimicrobial agents:
- higher concentration
- fewer side effects
- sustained / controlled delivery
- patient compliance
II Disadvantages of local delivery when compared to systemic delivery of antimicrobial agents:
- more expensive
- more time consuming
- no effect on bacterial reservoirs
TYPES OF LOCAL ANTIBIOTIC THERAPY WITH SLOW RELEASE DEVICES
A. Tetracycline impregnated fibres
- FDA approved as Actisite (Alza Corp., USA) in the United States (25)
- controlled release-10 days
- demonstrated to decrease pocket depth, increase attachment gain and reduce periodontal disease recurrence
- non-resorbable
- comparison-tetracycline
Local delivery
12.2 mg / fibre
Ten days
Total: 12.5 mg
Concentration > 1,300 μg / ml
B. Doxycycline gel for subgingival delivery.
- FDA approved as Atridox (Atrix, The Block Drug Co.) for use in the United States
- Sustained release: 27 days resorbable
- 8.5% doxycycline; 420 μg/ml in GCF
C. Metronidazole gel for subgingival delivery
- Sustained release: one day resorbable
- Elyzol (Durnex, Denmark)
- 25% metronidazole; 24 hrs > 1 μg/ml in GCF
D. Minocycline gel or powder (2%) for subgingival delivery
- Dentomycin- Great Britain, Periocline - Japan (Cyanamid International)
- Sustained Release: resorbable
- 2% Minocycline
E. Indications for Local Delivery of Antibiotic Agents
- Adult periodontitis - localized pockets 5 mm with bleeding
- Pocket in anterior area of mouth, where if peri-odontal surgery done, it may pose all aesthetic problem
- Recurrent/Refractory periodontitis
- Medically compromised patients where periodontal surgery is not indicated
Tetracyclines
The tetracyclines are a group of broad-spectrum antibiotic agents that were introduced into clinical practice in the late 1940s. There are now numerous compounds on the market, all based on the congeneric derivatives of the polycyclic naphthacene carboxamide (26). Tetracycline, doxycycline and minocycline are used extensively in the management of periodontal diseases.
They are bacteriostatic antibiotics, which interfere with bacterial protein synthesis and also inhibit tissue collagenase activity (27). They have a broad spectrum of activity inhibiting both Gram-negative and Gram-positive organisms, including the beta-lactamase producing strains which occur in approximately 50% of 6-7 mm deep periodontal pockets and against which penicillins are ineffective.
Tetracycline analogues such as doxycycline and minocycline, although more expensive, have a number of theoretical advantages over tetracycline. They exhibit greater oral absorption, they have more prolonged half-lives, and they show enhanced lipid solubility, which is important for their antibacterial action (26). The inhibitory effect of tetracycline on oxygen radicals may also prevent a wider spectrum of tissue destruction. Thus, tetracyclines may have general antiproteolytic properties.
Anticollagenase inhibition
In addition to the antibiotic effects of tetracyclines, a further mechanism has been proposed to explain their efficacy in the treatment of periodontal disease, notably their anticollagenase action (28). This action appears to be related to the source of the enzyme and the tetracycline used. Doxycycline is the most potent tetracycline for collagenase inhibition. Collagenases derived from neutrophils (mature metalloprotcinases-8) are more susceptible to a tetracycline-induced inhibition while collagenases derived from human fibroblasts or gingival cervicular fluid collagenase harvested from deep perio-dontal pockets appear to be more resistant to the drug.
Tetracycline inhibition of collagenase may relate to the drug’s ability to bind with calcium and zinc ions (28). Zn2+ are located at the active site of the enzyme, whilst Ca2+ are on an exogenous co-factor. A further mechanism may be associated with the ability of the tetracyclines to scavenge reactive oxygen radicals (e.g. hydroxyl groups or hypochlorous acid) produced by PMN5, These oxygen radicals activate latent collagenases. Inhibition of collagenase may result in further antiproteolytic effects such as inactivation of a-1 proteinase inhibitor and neutrophil elastase.
Metronidazole
Among the antibiotics that have been considered for peri-odontal treatment, metronidazole has often been chosen because of its selective efficacy against obligate anaerobes (29). Metronidazole acts by inhibiting DNA synthesis. It is known to convert into a reactive reduced form and affects specifically anaerobic rods and spirochetes in the subgingival microflora.
Metronidazole has also been successful in refractory and advanced cases when used for a 1-week period (16). Other studies reported that adjunctive metronidazole therapy was more effective in adults with deep pockets than with less advanced periodontitis (30).
Clindamycin
Clindamycin has been investigated for treatment of peri-odontal disease in a limited number of studies (31, 32). Systemic clindamycin therapy, as an adjunct to scaling, decreased the incidence of active disease from an annual rate of 8.0 to 0.5% of sites per patient (33, 34).
Following gel insertion of clindamycin in conjunction with subgingival scaling, motile rods and spirochetes were not detected after 1 month. Prevotella intermedia and Porphyromonas gingivalis were eliminated or below detectable levels after 1 week.
CONCLUSION
Antibiotic therapy has been used for years as an adjunct to periodontal treatment. One of the most promising recent advances in periodontal therapy has been the development of sustained-release delivery systems to administer antibiotics directly to the periodontal pocket. Locally delivered antibiotics overcome many of the disadvantages which wee see with systemic drugs. There are specific guidelines and indications for the use of locally delivered antibiotics as adjuncts to periodontal therapy in dental practice.
Endodontic infections are polymicrobial, and no known medicament is effective against all the bacteria found in infected root canals. In addition, the medicament should ideally reach microorganisms located in distant areas of the root canal system in lethal concentrations.
It has been demonstrated that enterococci and fungi are highly resistant to calcium hydroxide (7,8). Since these microorganisms are commonly found in cases of endodontic failure, the routine use of calcium hydroxide should be questioned.
REFERENCES
- 1.Byers M.R, Taylor P.E, Khayat B.G, Kimberly C.L. Effect of injury and inflammation on pulpal and periapical nerves. J Endod. 1990;16:78–84. doi: 10.1016/S0099-2399(06)81568-2. [DOI] [PubMed] [Google Scholar]
- 2.Jontell M, Bergenholtz G, Scheynius A, Ambrose W. Dendritic cells and macrophages expressing class II antigens in the normal cat incisor pulp. J Dent. Res. 1988;67:1263–1266. doi: 10.1177/00220345880670100301. [DOI] [PubMed] [Google Scholar]
- 3.Barkhodar R.A, Desouza Y.G. Human T- lymphocyte subpopulation in periapical lesions. Oral Surg. 1988;65:763. doi: 10.1016/0030-4220(88)90026-6. [DOI] [PubMed] [Google Scholar]
- 4.Padan E, Zilberstein D, Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981;650:151166. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- 5.Atlas R.M. Principles of microbiology. 2nd ed. Dubuque, Iowa: WCB Publishers; 1997. [Google Scholar]
- 6.Haapasalo M, Ranta H, Ranta K.T. Facultative gram-negative enteric rods in persistent periapical infections. Acta Odont Sc. 1983;41:19–22. doi: 10.3109/00016358309162299. [DOI] [PubMed] [Google Scholar]
- 7.Byström A, Claesson R, Sundqvist G. The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod Dent Traumatol. 1985;1:170–175. doi: 10.1111/j.1600-9657.1985.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 8.Waltimo T.M.T, Sirén E.K, Orstavik D, Haapasalo M.P. Susceptibility of oral Candida species to calcium hydroxide in vitro. Int. Endod. J. 1999;32:94–98. doi: 10.1046/j.1365-2591.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- 9.Salyers A.A, Whitt D.D. Bacterial pathogenesis: A molecular approach. Washington ASM Press; 1994. p. 418. [Google Scholar]
- 10.Henderson B, Poole S, Wilson M. Bacterial modules: A novel class of virulence factors which cause host tissue pathology bay inducing cytokine synthesis. Microbiol. Rev. 1996;60:316–341. doi: 10.1128/mr.60.2.316-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair S.P, Meghji S, Wilson M, Reddi K, White P, Henderson B. Bacterially induced bone destruction: mechanisms and misconceptions. Infect. Immun. 1996;64:2371–2380. doi: 10.1128/iai.64.7.2371-2380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heeg K, Sparwasser T, Lipford G.B, Hacker H, Zimmermann S, Wagner H. Bacterial DNA as an evolutionary conserved legend signalling danger of infection to immune cells. Eur J Clin. Microbiol. Infect. Dis. 1998;17:464–469. doi: 10.1007/BF01691128. [DOI] [PubMed] [Google Scholar]
- 13.Siqueira J.F., Jr . Tratamento das infecço es endodônticas. Rio de Janeiro: MEDSI; 1997. [Google Scholar]
- 14.Steinberg D, Friedman M, Soskolne A, Sela M.N. A new degradable controlled release device for treatment of periodontal disease. In vitro release study. J Periodontol. 1990;61:393–398. doi: 10.1902/jop.1990.61.7.393. [DOI] [PubMed] [Google Scholar]
- 15.Minabe M, Uematsu A, Nishijima K, Tomomatsu E, Tamura T, Hori T, Umemoto T, Hino T. Application of a local drug delivery system to periodontal therapy I Development of collagen preparations with immobilized tetracycline. J Periodontol. 1989;60:113–117. doi: 10.1902/jop.1989.60.2.113. [DOI] [PubMed] [Google Scholar]
- 16.Loesche W.J, Giordano J, Soehren S, Hutchinson R, Rau C.F, Walsh L, Schork A, Arbor A, Mich D. Non-surgical treatment of patients with periodontal disease. Oral Surg Oral Med Oral Pathol Endod. 1996;81:533–543. doi: 10.1016/s1079-2104(96)80042-4. [DOI] [PubMed] [Google Scholar]
- 17.Paquette D.W, Waters G.S, Stefanidou V.L, Lawrence H.P, Friden P.M, O’Connor S.M, Sperati J.D, Oppenheim F.G, Hutchens L.H, Williams R.C. Inhibition of experimental gingivitis in beagle dogs with topical salivary histatins. J Clin. Periodontol. 1997;24:216–222. doi: 10.1111/j.1600-051x.1997.tb01834.x. [DOI] [PubMed] [Google Scholar]
- 18.McLeod A.D, Tolentino L, Tozer T.N. Glucocorticoid-dextran conjugates as potential prodrugs for colon-specific delivery: steady-state pharmacokinetics in the rat. Biopharm. Drug Disposition. 1994;15:151–161. doi: 10.1002/bdd.2510150207. [DOI] [PubMed] [Google Scholar]
- 19.Curson I. Endodontic techniques - the antibacterial treatment of root canals. British Dental Journal. 1966;120:381–383. [PubMed] [Google Scholar]
- 20.Grieve A.R, Friend L.A, Plant C.G. A clinical trial of three root canal medicaments. British Dental Journal. 1973;134:188–93. doi: 10.1038/sj.bdj.4802977. [DOI] [PubMed] [Google Scholar]
- 21.Molander A, Reit C, Dahlen G. Microbiological evaluation of clindamycin as a root canal dressing in teeth with apical periodontitis. Int. Endod. J. 1990;23:113–118. doi: 10.1111/j.1365-2591.1990.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 22.Hoshino E, Kurihara-Ando N, Sato I, Uematsu H, Sato M, Kota K, Iwaku M. In vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. Int. Endod. J. 1996;29:125–130. doi: 10.1111/j.1365-2591.1996.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 23.Sato T, Hoshino E, Uematsu H, Noda T. In vitro antibacterial sensitivity to combinations of drugs on bacteria from carious and endodontic lesions of human deciduous teeth. Oral Microbiol. Immunol. 1993;8:172176. doi: 10.1111/j.1399-302x.1993.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 24.Fava L.R.G, Saunders W.P. Calcium hydroxide pastes: classification and clinical indications. International Endodon J. 1999;32:257–282. doi: 10.1046/j.1365-2591.1999.00232.x. [DOI] [PubMed] [Google Scholar]
- 25.Niderman R, Abdelshehid G, Goodson J.M. Periodontal therapy using local delivery of antimicrobial agents. Dent. Clin N Am. 2002;46:665–677. doi: 10.1016/s0011-8532(02)00030-7. [DOI] [PubMed] [Google Scholar]
- 26.Seymour R.A, Heasman P.A. Tetracyclines in the management of periodontal diseases: a review. J Clin Periodontol. 1995;22:22–35. doi: 10.1111/j.1600-051x.1995.tb01767.x. [DOI] [PubMed] [Google Scholar]
- 27.Yu Z, Ramamurthy N.S, Leung M, Chang K.M, McNamara T.F, Golub L.M. Chemically-modified tetra-cycline normalizes collagen metabolism in diabetic rats. A dose-response study. J Periodontol Res. 1993;28:420–428. [PubMed] [Google Scholar]
- 28.Seymour R.A, Heasman P.A. Pharmacological control of periodontal disease. II Antimicrobial agents. J. Dent. 1995;23:5–14. doi: 10.1016/0300-5712(95)90654-z. [DOI] [PubMed] [Google Scholar]
- 29.Noyan U, Yilmaz S, Kuru B, Kadir T, Acar O, Buget E. A. clinical and microbiological evaluation of systemic and local metronidazole delivery in adult periodontitis patients. J Clin Periodontol. 1997;24:158165. doi: 10.1111/j.1600-051x.1997.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 30.Fiorellini J.P, Paquette D.W. The potential role of controlled release delivery systems for chemotherapeutic agents in periodontics. J Periodontol. 1992;66:870–877. [PubMed] [Google Scholar]
- 31.Higashi K, Matsushita M, Morisaki K, Hayashi S.I, Mayumi T. Local drug delivery systems for the treatment of periodontal disease. J Pharmacobio Dyn. 1991;14:72–81. doi: 10.1248/bpb1978.14.72. [DOI] [PubMed] [Google Scholar]
- 32.Sauvetre E, Glupczynsky Y, Yourassowsky E, Pourtois M. The effect of clindamycin gel insert in peri-odontal pockets, as observed on smears and cultures. Infection. 1993;21:245–247. doi: 10.1007/BF01728900. [DOI] [PubMed] [Google Scholar]
- 33.Gordon J, Walker C, Hovliaras C, Socransky S. Efficacy of clindamycin hydrochloride in refractory periodontitis:24-month results. J Periodontol. 1990;61:686–691. doi: 10.1902/jop.1990.61.11.686. [DOI] [PubMed] [Google Scholar]
- 34.Magnusson I, Low S.B, McArthur W.P, Marks R.G, Walker C.B, Maruniak J, Taylor M, Padgett P, Jung J, Clark W.B. Treatment of subjects with refractory periodontal disease. J Clin Periodontol. 1994;21:628–637. doi: 10.1111/j.1600-051x.1994.tb00755.x. [DOI] [PubMed] [Google Scholar]


