Abstract
Cardiovascular diseases are the major cause of mortality in uraemic patients treated by hemodialysis. Left ventricular hypertrophy (LVH) is considered to be a major cardiac risk factor.
AIM:
To investigate the presence of some potential adverse risk factors in hemodialysis patients with developed LVH echocardiography verified and determine their relative contribution to the LVH in comparison with patients with normal LV.
METHOD:
The study included 50 patients with end-stage renal disease in the first 2 years of hemodialysis treatment, who were followed up during one year. All participants have the echocardiography performed as well as serial measurements of potential modifiable cardiovascular risk factors.
RESULTS:
This investigation showed that LVH is present in high percentage (72%) in uraemic patients, even at the beginning of hemodialysis treatment. This LV morphological abnormality is statistically significantly related to anaemia (p<0,001), systolic (p<0,001) and diastolic hypertension (p<0,001)), elevated mean arterial pressure (p<0,001) and hyperparathyroidism (p=0,002).
CONCLUSION:
Modification of existing risk factors in uraemic patients could contribute to prevention and treatment of LV hypertophy and thus reduce cardiovascular morbidity and mortality.
Keywords: left ventricular hypertrophy, uraemia, cardiovascular factors
INTRODUCTION
Left ventricular hypertrophy (LVH) is commonly present in uraemic patients and it has been recognized as adaptive mechanism of LV on volume and pressure overload (1). It is especially associated with high mortality of uraemic patients (2,3) and presents an independent risk factor of adversely impact on outcome in praedialysis, as an in haemodialysis patients.
Left ventricular hypertrophy is resulting from hypertrophy of myocytes and hyperplasia of non-myocytes elements, especially cardiac fibroblasts (4). Myocytes are unable to replicate and consequently enlarge in hypertrophic condition, which can be a basis for distinguishing the type of LVH. In concentric hypertrophy of LV myocytes increase in thickness. The increased LV mass is associated with increased thickness of both the interventricular septum and left ventricular posterior wall, with preserved normal ventricular volume. In concentric LV hypertrophy, the relative wall thickness is higher than 45%. In eccentric LV hypertrophy myocytes grow longitudinally, and the increased LV mass is associated with increased LV volume.
End-stage renal disease includes many factors which have possible role in development of LVH. Among them, the most important are considered to be anaemia, hypertension, hypervolemia and arterio-vein fistula, hyperparathyreoidism, hyperlipoproteinemia and malnutrition. A relative contribution of all of this factors in development of LVH in chronic uraemia has not yet been determined. Risk factors should be clearly idenfified and assessed, as their reduction might diminish overall cardiovascular morbidity and mortality.
Aim
To assess the presence of some predisponing risk factors in haemodialysis patients with echocardiographically verified LVH and in haemodialysis patients without LVH
To compare echocardiografic features of haemodialysis patients after a 12 months period with special relations to presence of observed risk factors and their individual contribution in LVH development.
SUBJECTS AND METHODS
The study included 50 patients with end-stage renal disease, who had started haemodialysis treatment within the period of 2 years before the beginning of the investigation. All patients were dialysed under the same conditions, which consisted of three sessions of 4 hours haemodialysis treatment per week, using machines with controlled ultrafiltration, bicarbonate puffer for dialysis, as well as biocompatible polysulphone membranes, with the same vascular access (A-V fistula) and with adequate doses of delivered dialysis (Kt/V 1,31±0,13, urea reduction rate 63,45±4,54%). This prospective and comparative study was carried out at the Institute of Nephrology of Clinical Centre University of Sarajevo.
The study did not include patients with diabetes mellitus and those with organic heart valves defects.
All participants had the echocardiography performed at the beginning of the study (baseline) and after the period of 12 months. It was done when so called «dry body weight» of a patient was achieved, always in a period within the 24 hours after the last haemodialysis. In that way the interdialysis increment of internal LV diameter, caused by increased blood volume due to fluid ingestion and loss of excretory renal functon, was avoided. Namely, calculated LV mass index before dialysis is usually higher up to 25 g/m2 as compared to postdialysis values, although the actual LV mass remains unchanged (5). Echocardiography was performed by a cardiologist, using ATL Ullramark-9 echocardiograph equiped with 2.5 MHz transducer, enabled for M-mod, two-dimensional and pulse Doppler measurements.
The following parameters were followed up in a monthly intervals: blood pressure and body weight, as well as the laboratory tests including: haemoglobin, BUN, creatinine, albumin, total cholesterol, triglycerides, calcium, phosphorus, parathormone (PTH). For each participant clinical and laboratory values at the beginning were considered as basal, while the average mean monthly levels in a period of 12 months, until the second echocardiogaphy, were considered as comparative ones.
LV mass was calculated according the modified Devereux’s cubic formula accepted by American Association of Cardiologists. LV mass index was calculated as LV mass divided by body surfice area:
LV mass index ={0,00083 [(LVEDD+IVS+PW)3-(LVEDD)3]+0,6}/BSA (g/m2)
LV volume was calculated according to Pombo’s et als. formula:
LVV=[(LVEDD)3x0,001047]/BSA (ml/m2).
The following criteria were used for distinguishing the comparative patients groups:
LV hypertrophy: LV mass index in males >131 g/m2, in females >100 g/m2 (6)
LV dilatation: LV volume >90 ml/m2 (7)
concentric LVH: LV hypertrophy with normal LV volume eccentric LVH: LV hypertrophy with LV volume>90 ml/m2
Statistical Analysis
Data were expressed as mean values with standard deviation. The significance of the differences between the mean values of the comparable groups were tested by Student’s-t test with accepted statistical significance at the level of p<0.05. Logistic regression (Odds ratio) was used to identify independent association of various risk factors related to LV hypertrophy. Multilinear regression (beta coefficient) was used to identify LV mass index predictors.
Statistical analysis was facilitated using the statistical programme SigmaStat, version 2.
RESULTS
Echocardiography at thebeginning of the study
There were 14 (28%) patients with normal finding of the left ventricle (LV), 19 (38%) were found to have concentric LVH, while17 (34%) showed echocardiographic signs of eccentric LVH (Figure 1).
Figure 1.
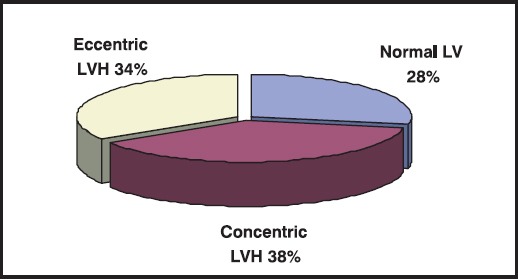
Prevalence of the LV morphologic changes on the first echocardiography
Regarding functional status of LV, 9 (16,7%) patients showed impaired systolic function, 21 (38,9%) had diastolic disfunction, while the remainder of 20 (44,4%) showed normal function of LV (Figure 2).
Figure 2.
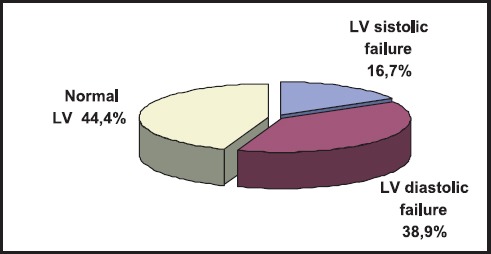
The prevalence of the LV function status on the first echocardiography
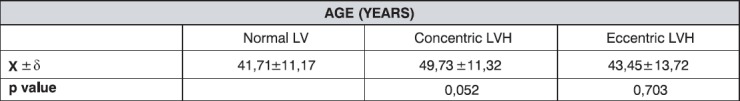
The age structure of participants is shown at Table 1. They included 26 males and 24 females. It is shown that the average age was over 40 years in all groups, with lowest values in the group with normal LV function, and without statistically significant difference regarding age between the groups with concentric and eccentric LVH.
Table 1.
Age structure by LV functional status
Biochemical Indicators
Haemoglobin
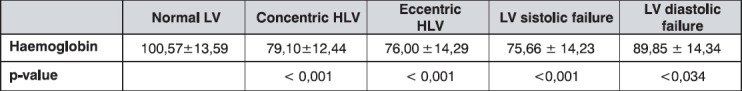
As a main indicator of anaemia, haemoglobin (Hb) showed highly significant difference between the group with normal LV and those with concentric and eccentric LVH (Tabela 2). Patients with normal LV echocardiographic mass, were found to have significantly higher mean Hb values as compared to a group with systolic impairment of LV (p<0,001) and diastolic disfunction of LV (p<0,034). The average Hb values of the total sample were below the lower cut-off point of normal range for the general population (normal range Hg 120-175 g/L).
Table 2.
The mean values of heamoglobin (g/L) by groups
Parathormon
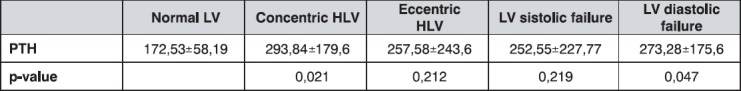
The average mean values of serum parathormone (PTH) in the group with normal LV function was significantly lower than in patients with concentric LVH (p=0,021), as well as in the group with diastolic disfunction (p=0,047), as is shown in Table 3. It is also obvious that all the groups have higher PTH values than the referent values for the general population (range 10-65 pg/ml).
Table 3.
The mean PTH levels (pg/ml) by groups
In regard to other laboratory serum parameters (lipids, albumin, BUN, creatinine, calcium, phosphorus) we did not show significant differences between the groups.
Clinical indicators
Systolic blood pressure
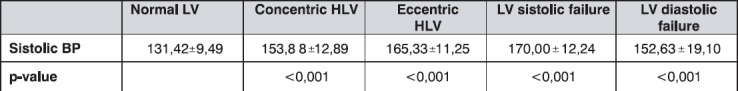
Patients with normal LV function showed normal average systolic blood pressure (SBP) values (131 mmHg), while the patients with concentric and eccentric LVH had significantly elevated SBP (p<0,001), with the highest average values in the group with systolic impairment (170 mmHg). The results are presented in Table 4.
Table 4.
The mean sistolic blood pressure values (mmHg) by groups
Diastolic blood pressure
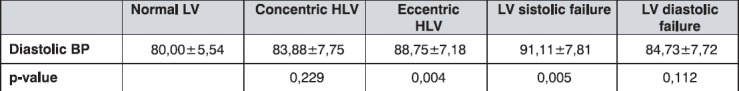
Patients with normal LV function and structure, and patients with concentric LVH and diastolic disfunction had normal values of diastolic blood pressure (DBP), while the others had hypertensive diastolic values as shown in Table 5.
Table 5.
The mean diastolic blood pressure values (mmHg) by groups
The mean arterial pressure
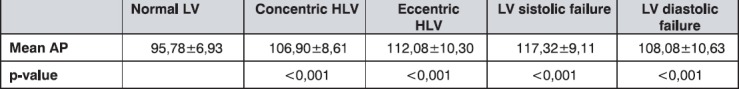
The lowest average values of mean arterial pressure were shown in patients with normal LV. As presented in Table 6, these are significantly different in comparison with all the other groups.
Table 6.
The mean arterial pressure (MAP) values (mmHg) by groups
Echocardiography outcome
The changes in echocardiography findings in 12 months period by groups are presented in Table 7.
Table 7.
Echocardiography outcome after one year follow-up by groups
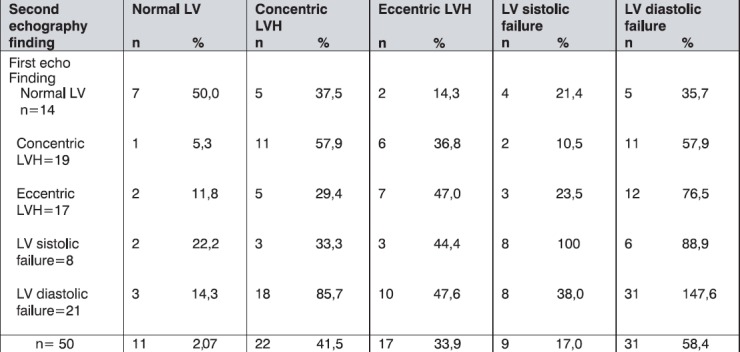
We found that after 12 months period, in regard to echocardiographic morphological changes, overall 21% of patients showed normal LV, 45% had signs of concentric LVH, and 34% of eccentric LVH.
Also, we noted that, after 12 months period, almost 58% of participants showed the signs of LV diastolic disfunction, 17% of LV systolic impairment, while only 25% patients were found with normal LV function.
Significant predictors of concentric LVH appearance are presented in Table 8.
Table 8.
The main predictors of concentric LVH appearance
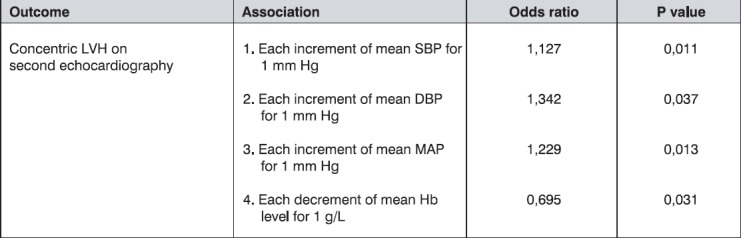
Multiple logistic regression, using age, sex, Hb, SBP, DBP, MAP and PTH as covariables, showed an independent association of concentric LVH with each increment of mean SBP, mean DBP, mean MAP for 1 mmHg (p=0,011; p=0,037; p=0,013, respectively) and each decrement of mean Hb values for 1 g/L (p=0,031).
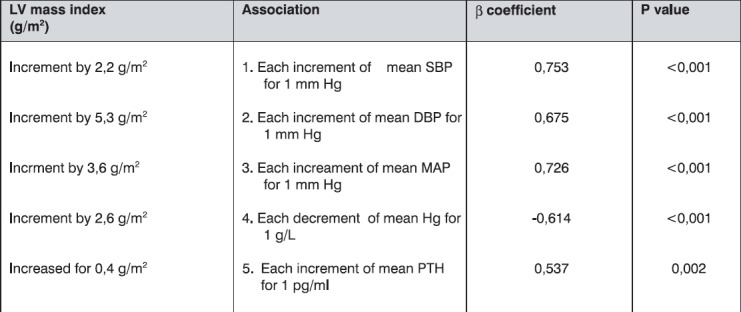
Linear regression, using the same covariables, indicated significant predictors and their association with the echocardiographic changes in LV mass index, as is shown in Table 9.
Table 9.
The main predictors of LV mass index changes
DISCUSSION
Cardiovascular diseases are the leading cause of mortality and accounts for about 50% of overall mortality in haemodialysis patients (8,9). Pathogenesis of cardiovascular disease in these patients includes risk factors already known for general population, but as well factors related to renal failure. Relative contribution of these factors in various disorders of left cardiac ventricle is possible to derermine by serial measurements of risk factors. Echocardiography presents one of the most important means among non-invasive methods for evaluation of morphological and functional heart characteristics in uraemic patients. In our study, assessment by echocardiography in haemodialysis patients showed that 72% of patients, without clinical signs of heart failure, had abnormal LV echocardiographical finding. Among them, we found concentric LVH in 38% patients, and eccentric one in 34% patients. Systolic impairment was found in 16,7%, and diastolic disfunction in 38,9% patients. Only 28% participants did not show any echocardiographic abnormality. LV mass index was especially high in the group with diastolic LV disfunction and in patients with systolic LV impairment (higher for about 50% as compared to those with normal LV finding).
Some other studies, using echocardiography as an evaluation method, showed prevalence of LV hypertrophy of 57% to 93% in the population of patients with end stage renal failure (10,11).
Similar finding was observed in uraemic patients at the beginning of haemodialysis treatment in prospective Canadian multicentric study, which included 433 patients followed in a period of 41 months (12). Systolic disfunction was observed in 16%, LV dilatation in 28%, LVH in 40,7% patients, while only 16 % patients had normal echocardiographyc finding.
Parfrey et al. in 1996 confirmed that average survival of haemodialysis patients with LV abnormalities at the beginning of dialysis, is significantly poorer in comparison to those with normal echocardiographical finding (13), especially to those with systolic impairment of LV. High proportion of patients with LVH at the beginning of haemodialysis treatmen indicates that predisposing factors for development of LVH are existing even in preterminal phasis of chronic renal failure.
After a year of follow up, we found echocardiogram abnormalities in almost 79,3% of our patients, while only 20,7% han normal echocardiogram of LV. Proportion of patients with concentric LVH increased for 6.0% in comparison to baseline findings (38% to 44%), and proportion on patients with normal finding decreased for 6% (28% to 22%). The number of patients with systolic failure is the same as at the beginning of the study, while the proportion of patients with diastolic LV disfunction increased for 47,6%. Diastolic disfunction mostly accompanies concentric LVH (in 58% of cases).
Evolution of normal to hyperthrophic LV may be under relative influence of various risk factors present at the time of echocardiography performance.
In our study we found significant differences in mean systolic, diastolic and mean arterial pressure in the group with normal LV finding as compared with the group with LV hypertrophy, both of concentric and eccentric type. We found significant independent association of systolic, diastolic and mean arterial pressure with concentric LVH. This findings clearly demonstrate the impact of hypertension on LV hypertrophy progression and the need for maintaining the target blood pressure values up to 130/80 mmHg in these population of patients. Moreover, some studies described LV hypertrophy regression by tight control of blood pressure values with combination of beta blockers, calcium antagonists and ACE inhibitors (14), with ACE inhibitor monotherapy (15) or angiotensin receptor blockers, losartan (16).
Anaemia appears to have an independent impact to echocardiographycal outcome in haemodialysis patients. We noted significant independent association between the reduction of average haemoglobin by 1 g/L with appearance of concentric LVH after a period of 1 year, as well as with the increment of LV mass index for 2,6 g/m2 (p<0,001). This finding strongly indicates significance of connection between anaemia and LV hypertrophy, which was also shown in the study carried out by Foley et al. (17). Correction of anaemia by erythropoietin raises possibility for LV hypertrophy regression in this population (18, 19).
We noted significantly higher PTH levels in the group with LVH (p=0,043) as compared with the group with normal LV mass in haemodialysis patients. Pathways which can explain the role of hyperparathyreoidism in development of LV hypertrophy may include direct or indirect effects. Direct trophic effects can be mediated by increment of protein synthesis in myocardial myocytes and induction of creatin kinase BB through protein kinase C activation, via functional domain of 28-34 aminoacids (20) and by direct trophic effect to interstitial fibroblasts (21). Indirect trophic effects include increment of blood pressure values via hypercalcaemia, as well as anaemia and changes in small and intermediate blood vessels.
As LVH presents major cardiovascular risk factor in these patients, prevention of hypertrophy, its early detection and LV mass index reduction by modifying risk factors is promising process by which we may expect reduction of overall cardiovascular morbidity and mortality in patients on chronic haemodialysis treatment.
CONCLUSIONS
Echocardiographic abnormalities are commonly found in patients with end-stage renal disease at the beginning of haemodialysis treatment. Even higher proportion of the changes is found during the follow up of the treatment. Anaemia is an independent risk factor for development of concentric LV hypertrophy.
Increased blood pressure values is significantly associated with LV hypertrophy.
Parathormone appears to be significant LV mass index changes predictor, which gives this “uraemic toxine” the character of cardiovascular risk factor.
Pharmacological impact to potentially reversible risk factors in uraemic patients, gives possibility for LV hypertrophy regression.
Presence of cardiac abnormalities and its risk factors at early stage of haemodialysis treatment suggests the importance of their earlier detection and correction, even in the predialysis period of chronic renal failure.
REFERENCES
- 1.London G.M, Fabian F, Marchais S.j, et al. Uremic cardiomyopathy: an inaddequate left ventricular hypertrophy. Kidney Int. 1987;31:973–980. doi: 10.1038/ki.1987.94. [DOI] [PubMed] [Google Scholar]
- 2.Silberberg J.S, Barre P.E, Prichard S.S, Sniderman A.D. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int. 1989;36:286–290. doi: 10.1038/ki.1989.192. [DOI] [PubMed] [Google Scholar]
- 3.Cannella G. Left ventricular hypertrophy in dialysed patients. What can be done about it? Nephrol Dial Transplant. 1996;11:418–420. [PubMed] [Google Scholar]
- 4.Hutchins G.M. Cardiac pathology in chronic renal failure. In: Parfrey P.S, Harnett J.D, editors. Cardiac Dysfunction in Chronic Uremia. Boston: Kluwer Academic Publishers; 1992. p. 85. [Google Scholar]
- 5.Harnett J.D, Murphy B, Collingwood P, Purchase L, Kent G, Parfrey P.S. The reliability and validity of echocardiographic measurement of left ventricular mass index in hemodialysis patients. Nephron. 1993;65:212. doi: 10.1159/000187476. [DOI] [PubMed] [Google Scholar]
- 6.Levy D, Savage D.D, Garrison R.J, Anderson K.M, Kannel W.B, Castelli W.P. Echocardiographic criteria for left ventricular hypertrophy: the Framingham Study. Am J Cardiol. 1987;59:956–960. doi: 10.1016/0002-9149(87)91133-7. [DOI] [PubMed] [Google Scholar]
- 7.Pombo J.F, Troz B.L, Russell R.O., Jr Left ventricular low volumes and ejection fraction bz echocardiography. Circulation. 1971;43:480–490. doi: 10.1161/01.cir.43.4.480. [DOI] [PubMed] [Google Scholar]
- 8.Raine AEG, Margreiter R, Brunner F.P, et al. Report on management of renal failure in Europe, XXII 1991. Nephrol Dial Transplant. 1992;2:7–35. [PubMed] [Google Scholar]
- 9.US Renal Data System: USRDS 1994 Annual Data Report. VII Causes of death. AJKD. 1994;24(Suppl.2):88–95. [Google Scholar]
- 10.Parferey P.S, Harnett J.D, Griffiths S.M, et al. The clinical course of left ventricular hypertrophy in dialisys patinets. Nephron. 1990;55:114–120. doi: 10.1159/000185937. [DOI] [PubMed] [Google Scholar]
- 11.London G.M, Pannier B, Guerin A.P, et al. Alterations of left ventricular hypertrophy and survival of patients receiving haemodialysis: follow up of an interventional study. J Am Soc Nephrol. 2001;12:2759–2767. doi: 10.1681/ASN.V12122759. [DOI] [PubMed] [Google Scholar]
- 12.Parfrey P.S, Foley R.N, Harnett J.D. Outcome and risk factors of left ventricular disorders in chronic uremia. Nephrol Dial Transplant. 1996;11:1277–1285. [PubMed] [Google Scholar]
- 13.Parfrey P.S, Collingwood P, Foley R.N, Bahole A. Left ventricular disorders detected by M-mode echocardiography in chronic uraemia. Nephrol Dial Transplant. 1996;11:1328–1331. [PubMed] [Google Scholar]
- 14.Cannella G, Paoletti E, Delfino R, Pelaso G, Molinari S, Battista-Traverso G. Regression of left ventricular hypertrophy in hypertensive dialysed uremic patients on long-term antihypertensive therapy. Kidney Int. 1993;44:881–886. doi: 10.1038/ki.1993.326. [DOI] [PubMed] [Google Scholar]
- 15.Ecder T, Edelstein C.L, Chapman A.B, Jonson A.M, Tison L, Gill E.A, Brosnahan G.M, Schrier R.W. Reversal of left ventricular hypertrophy with angiotensin converting enzyme inhibition in hypertensive patients with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 1999;14:1113–1116. doi: 10.1093/ndt/14.5.1113. [DOI] [PubMed] [Google Scholar]
- 16.Dickstein K, Timmermans S, Segal R. Losartan: a selective angiotensin II type 1 (AT1) receptor antagonist for the treatment of heart failure. Exp Opin Invest Drugs. 1998;7(11):1897–1914. doi: 10.1517/13543784.7.11.1897. [DOI] [PubMed] [Google Scholar]
- 17.Foley R.N, Parfrey P.S, Harnett J.D, et al. The impact of anemia on cardiomyopathy, morbidity and mortality in end-stage renal disease. Am J Kid Dis. 1996;28:53–61. doi: 10.1016/s0272-6386(96)90130-4. [DOI] [PubMed] [Google Scholar]
- 18.Eckardt K.U. Cardiovascular consequences of renal anaemia and erythropoietin therapy. Nephrol Dial Transplant. 1999;14(5):1317–1323. doi: 10.1093/ndt/14.5.1317. [DOI] [PubMed] [Google Scholar]
- 19.Rasic S, Kulenovic I, Zulic I, Haracic A, Cengic M, Uncanin S, Dzemidzic J. The effect of erythropoi-etin treatment on left ventricular hypertrophy in haemodialysis patients. Bos J Bas Med Sciences. 2003;3(4):11–15. doi: 10.17305/bjbms.2003.3485. [DOI] [PubMed] [Google Scholar]
- 20.Schluter K.D, Piper H.M. Cardiovascular action of parathyroid hormone and parathyroid hormone-related peptide. Cardiovascular Research. 1998;37:34–41. doi: 10.1016/s0008-6363(97)00194-6. Rostand SG, Drueke TB. Parathyroid hormone, vitamin Dand cardiovascular disease in chronic renal failure. Kidney Int 1999; 56:383-392. [DOI] [PubMed] [Google Scholar]
- 21.Y K, Ritz E, Wiest G, Klaus G, Mall G. A role of parathyroid hormone for the activation of cardiac fibroblasts in uremia. J Am Soc Nephrol. 1994;4:1814–1819. doi: 10.1681/ASN.V4101814. [DOI] [PubMed] [Google Scholar]


