ABSTRACT
Background
Sea vegetables are rich sources of nutrients as well as bioactive components that are linked to metabolic health improvement. Algal polysaccharides improve satiety and modulate gut microbiota while proteins, peptides, and phenolic fractions exert anti-inflammatory, antioxidant, and antidiabetic effects.
Objective
We tested the hypothesis that dietary supplementation with either Pacific dulse (Palmaria mollis, red algae) or wakame (Undaria pinnatifida, brown algae) could remediate metabolic complications in high-fat diet-induced obesity.
Methods
Individually caged C57BL/6J mice (n = 8) were fed ad libitum with either a low-fat diet (LFD), 10% kcal fat; high-fat diet (HFD), 60% kcal fat; HFD + 5% (wt:wt) dulse (HFD + D); or HFD + 5% (wt:wt) wakame (HFD + W) for 8 weeks. Food intake and weight gain were monitored weekly. Glucose tolerance, hepatic lipids, fecal lipids, and plasma markers were evaluated, and the gut microbiome composition was assessed.
Results
Despite the tendency of higher food and caloric intake than the HFD (P = 0.04) group, the HFD + D group mice did not exhibit higher body weight, indicating lower food and caloric efficiency (P < 0.001). Sea vegetable supplementation reduced plasma monocyte chemotactic protein (MCP-1) (P < 0.001) and increased fecal lipid excretion (P < 0.001). Gut microbiome analysis showed that the HFD + D group had higher alpha-diversity than the HFD or LFD group, whereas beta-diversity analyses indicated that sea vegetable–supplemented HFD-fed mice (HFD + D and HFD + W groups) developed microbiome compositions more similar to those of the LFD-fed mice than those of the HFD-fed mice.
Conclusion
Sea vegetable supplementation showed protective effects against obesity-associated metabolic complications in C57BL/6J male mice by increasing lipid excretion, reducing systemic inflammatory marker, and mitigating gut microbiome alteration. While the obese phenotype development was not prevented, metabolic issues related to lipid absorption, inflammation, and gut microbial balance were improved, showing therapeutic promise and warranting eventual mechanistic elucidations.
Keywords: Palmaria mollis, Undaria pinnatifida, sea vegetable; obesity; inflammation; gut microbiome
Sea vegetable Pacific dulse and wakame supplementation exhibits protective effects against obesity-associated metabolic complications in C57BL/6J mice by increasing lipid excretion, reducing systemic inflammatory markers, and mitigating gut microbiome alteration.
Introduction
The growing obesity challenge is a globally recognized epidemic affecting more than 700 million individuals around the world, with 4 million estimated deaths mainly due to complications related to cardiovascular and renal diseases, diabetes, cancer, and musculoskeletal disorders (1). The annual economic burden of obesity in the United States is estimated to be ∼$8.65 billion for annual productivity loss and ∼$6899 annual per capita cost for direct health care (2–4). Given these significant health and economic burdens, numerous efforts are directed at addressing this challenge, from lifestyle modification and drug treatment to bariatric surgery. Since the effects of caloric restriction and increased energy expenditure are often stunted by adaptive physiological responses (5, 6), and not all patients may necessarily qualify for bariatric surgery, pharmacotherapeutic intervention remains the most exploited approach. However, options remain limited because some drugs have been withdrawn due to serious adverse effects on cardiovascular, renal, pulmonary, and psychiatric functions (3). With the high costs and risks for adverse effects associated with medical and pharmacological interventions for obesity, the bioactive potentials of compounds from natural products are continuously being explored for therapeutic applications. These compounds include lipase inhibitors, appetite repressors, energy expenditure promoters, and lipid metabolism regulators from terrestrial and aquatic biomaterial sources (7).
Numerous metabolic complications associated with diet-induced obesity are mediated by inflammation and gut microbiome reprogramming (8–10). Elucidation of the links between obesity and a multitude of pathophysiological aberrations like diabetes, atherosclerosis, and nonalcoholic fatty liver disease (NAFLD) enables increased understanding of the interplay of immunologic and metabolic functions in nutrient-sensitive cells in the body. Imbalances in homeostatic and proinflammatory immune responses trigger the activation of inflammatory pathways which eventually affect proper nutrient metabolism (11). This process shows that although the consumption of calorie-rich diets along with a sedentary lifestyle reflects the mass-balance concept behind adiposity, the influx of immune cells and increase in inflammatory markers remain crucial in the initiation and progression of obesity-related comorbidities (12–14). In fact, HFD proinflammatory effects, which are causal to the onset of numerous chronic diseases and their progression, account for associated sequelae, including increase of circulating free fatty acids, reduction of gut barrier function, and alteration of the gut microbiome, all of which may trigger a cascade of responses that sustain a systemic low-grade inflammatory state (15). Being an energy-dense diet, an HFD contributes twice as much caloric value per gram (fat 9 cal/g) than carbohydrates and protein (4 cal/g), which makes caloric excess easier to reach per gram of unit HFD consumption. HFDs also increased plasma concentrations of LPS in mouse gut, with concomitant increases in the proportions of LPS-containing microbiota (13). The decline of Bacteriodetes and increase of Firmicutes and Proteobacteria in obese humans and rodents are hallmarks of HFD consumption. This typical microbiome shift is often characterized by decreased abundance and diversity, usually reported as alpha- and beta-diversity indices/estimates.
As important dietary ingredients in many coastal regions around the world, sea vegetables have been increasingly recognized as a source of bioactive compounds with therapeutic potential in ameliorating obesity and its associated metabolic complications (16). These bioactive compounds include polysaccharides (fucoidan, carrageenan, porphyran, and alginates), proteins (phycoerythrin, phycocyanin, and lectins), pigments (carotenoids and polyphenols), and minerals (zinc and magnesium) (17). Major mechanisms involved in the antiobesogenic activities of macroalgal compounds include attenuation of nutrient absorption and adipogenic programming and inflammation, stimulation of satiety, and improvements in lipid metabolism (18). Brown algae wakame (Undaria pinnatifida) is a known source of the algal carotenoid fucoxanthin and the polysaccharide fucoidan, both reported to exert antiobesity effects by regulating weight gain, glucose tolerance, inflammation. and lipid metabolism (19–21). While the bioactivities of peptides, lipids, polysaccharides, and phenolic fractions from the red algae Atlantic dulse (Palmaria palmata) have increasingly been documented (22–26), its close but genetically isolated relative Pacific dulse (Palmaria mollis) (27) has received little attention. To our knowledge, at the time of this report only 1 reported study had investigated the bioactive potential of P. mollis in animal models. In this study the researchers showed that 2.5% (wt:wt) supplementation with P. mollis prevented hepatic steatosis and visceral adiposity in mice (NSY/HOS, Type 2 Diabetes Mellitus strain) and hyperlipidemia in zebrafish (28). While this research group showed that Pacific dulse can suppress fat deposition in an HFD-fed diabetic mouse model by downregulating lipogenic genes and upregulating the expression of beta oxidation–related proteins, the potential effects of Pacific dulse in the modulation of gut microbiome and remediation of inflammation remain unknown.
In the current study, we aimed to determine the effects of wakame (U. pinnatifida) or Pacific dulse (P. mollis) supplementation on improving lipid metabolism, inflammation, and gut microbiome variation in diet-induced obesity using a nondiabetic mouse model.
Methods
Experimental animal and diets
Thirty-two 8-wk-old male C57BL/6J mice were obtained from Jackson Laboratory. Upon arrival, mice were distributed into individual cages for acclimation for 1 wk. This was done to monitor individual food intake and prevent microbiota sharing among littermates. Eight weight-matched mice were assigned to each diet group, namely the LFD (10% kcal fat), HFD (60% kcal fat), HFD + wakame (HFD + W), and HFD + dulse (HFD + D) groups, for 8 wk. Individually caged mice were housed at constant temperature (22 ± 2°C) with a 12-h light/dark cycle and free access to both food and water in the Linus Pauling Science Center Vivarium at Oregon State University. Food intake and spillage and weight gain were monitored weekly, and food efficiency was calculated based on the gram weight gain per gram of food consumed times 100. Caloric efficiency was calculated based on the gram weight gain per calorie intake times 100. Fresh fecal samples were obtained from each animal for gut microbiome analysis. Mice (n = 31) were killed by carbon dioxide inhalation followed by cervical dislocation at the end of a feeding period without fasting. One mouse was lost during a glucose tolerance test at the fourth week. Blood samples were obtained by cardiac puncture using heparinized syringes. Liver, epididymal white adipose tissue (WAT), and interscapular brown adipose tissue (BAT) samples were obtained postmortem and stored at −80°C until further analysis. The experimental protocol concerning the ethical treatment of animals was approved by the Oregon State University Animal Care and Use Committee (IACUC 5030.02/18/2018) and the experiments were conducted according to the protocol.
Diet preparation
Fresh Pacific dulse (P. mollis) was provided by Dr. Christopher Langdon at the Hatfield Marine Science Center, Oregon State University, and fresh wakame (U. pinnatifida) was purchased from a local farm in South Korea and freeze-dried by Sunmarine Biotech Co. Ltd. Fresh sea vegetables were washed serially to remove debris, epiphytes, and salts, frozen overnight, and freeze-dried. Fresh sea vegetable powders were prepared by grinding dried material in a food-grade pulverizer (commercial Electric Grain Mill, 25,000 rpm with 2800-W motor). Test diets were formulated and supplied by DYETS, Inc. These included an HFD with 60% of calories from fat with and without sea vegetable powder supplementation (5% wt:wt) and an LFD with 10% of calories from fat. Diet composition is provided in Supplemental Table 1.
Glucose tolerance test
To determine the state of glucose intolerance among test animals, a standard intraperitoneal glucose tolerance test (IPGTT) was performed during week 4 of the HFD-feeding period. This was done to ensure that the diet-driven obesity induction was working in the rodent model. Mice fasted for 6 h were administered a d-glucose solution (1 g/kg body weight). With a sterile lancet, a small puncture was made on the dorsoposterior tail region for periodic monitoring of blood glucose concentrations for time points 0, 15, 30, 60, 90, and 120 min after intraperitoneal glucose administration. Readings were obtained using a commercial blood glucose monitoring system (LifeScan, Inc.). Blood glucose values over time were analyzed using the AUC, which is reflective of the rate at which exogenous glucose was cleared from the systems of test animals.
Plasma, liver, and fecal markers
Plasma was recovered from heparinized blood samples after refrigerated centrifugation for 10 minutes at 2000 × g. Frozen liver tissues and vacuum-dried fecal samples were homogenized with ceramic beads (Bertin Technologies) prior to lipid content quantification. Plasma, liver, and fecal lipids were quantified through colorimetric enzymatic assays using Infinity triglycerides (Thermo Fisher Scientific) and Infinity cholesterol (Thermo Fisher Scientific) liquid-stable reagents (29) with signal detection using a microplate reader (Molecular Devices). Plasma glucose was quantified using a commercial kit (Wako Autokit Glucose 997-03001). Plasma monocyte chemotactic protein 1 (MCP-1) concentrations were determined using an ELISA kit (eBioscience, Inc.).
Microbiome analysis
Using fecal samples that were freshly collected at the end of the feeding period, whole-genomic DNA was extracted and subjected to 16S rRNA gene amplification and sequencing as previously described (30). Briefly, fecal DNA was extracted using the Qiagen Powersoil kit and subsequently subjected to PCR amplification of the V4 hypervariable region of the 16S rRNA gene. Amplicons were sequenced on an Illumina MiSeq at the Center for Genome Research and Biocomputing at Oregon State University. The resulting V4 16S rRNA forward-end sequence reads were quality controlled and subjected to amplicon sequence variance (ASV) clustering, taxonomic annotation, and phylogenetic reconstruction using the DADA2 workflow (31, 32). The Phyloseq R package (33) was used to quantify community alpha- and beta-diversity as well as conduct data visualizations and the statistical tests described alongside the results.
Statistical analysis
Data are presented as means ± SDs. One-way ANOVA was used to compare data sets with Tukey's multiple comparison test for post hoc analysis. Significant difference was set at P ≤ 0.05. All statistical analyses were carried out using GraphPad Prism 8 software (GraphPad Software).
Results
Improvement of caloric management via lower food efficiency under the sea vegetable–supplemented diets
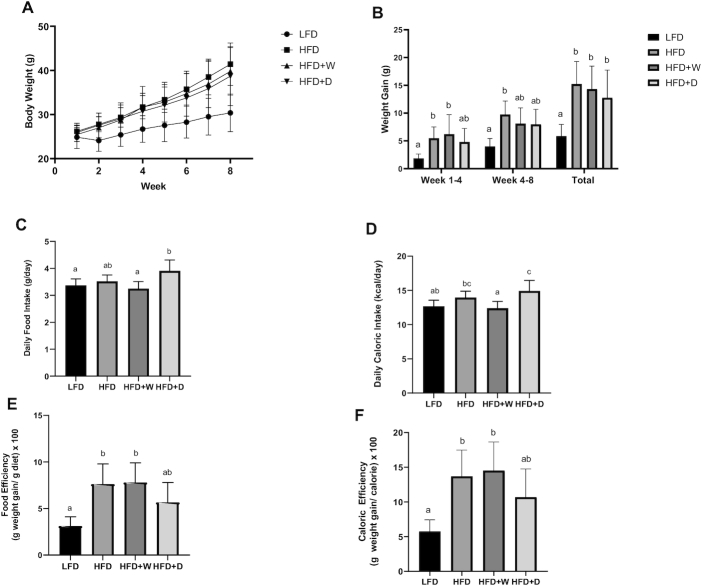
The final body weight of HFD-fed mice (41.4 ± 1.7 g) was significantly higher than that of the LFD- fed mice (30.4 ± 1.5 g; P ≤ 0.01), but comparable to that of the sea vegetable–supplemented groups (HFD + W, 39.8 ± 1.9 g; HFD + D, 38.7 ± 2.4 g; P > 0.05) at week 8 (Figure 1A). The weekly body weight of the sea vegetable–supplemented groups was similar to that of the LFD group until week 3 for the HFD + W group (P = 0.1402) and week 5 for the HFD + D group (P = 0.0623), results showing short-term weight gain suppression by the supplementation. These mice had weight gain that was almost 3-fold (HFD, 15.1 ± 1.5 g) and 2-fold (HFD + W, 13.2 ± 0.9 g; HFD + D, 11.4 ± 1.1 g) higher than that of the LFD group (5.9 ± 0.7 g) (Figure 1B). The average daily food intake of the HFD + D group (3.91 ± 0.14 g) was significantly higher than that of the HFD + W (3.25 ± 0.09 g) and LFD (3.37 ± 0.08 g) groups (P ≤ 0.01), but comparable to that of the HFD group (3.52 ± 0.08 g) (Figure 1C). The HFD + W group had lower daily caloric intake (12.4 ± 0.35 kcal/d) than the HFD group (13.95 ± 0.33 kcal/d) and the HFD + D group (14.93 ± 0.09 kcal/day) (P ≤ 0.05), and the HFD + D group had higher daily caloric intake than the LFD and HFD + W groups (Figure 1D). While statistically nonsignificant, the HFD + D group showed a tendency of lower food efficiency (5.08 ± 0.54) than the HFD (7.64 ± 0.83) and HFD + W (7.33 ± 0.64) groups. Additionally, the food efficiency of the HFD + D group was statistically comparable to that of the LFD group (3.11 ± 0.35) (P = 0.08; Figure 1E). The same trend was observed in caloric efficiency. The HFD + D group had a tendency of lower caloric efficiency (10.68 ± 1.44) than the HFD (13.69 ± 1.34) and HFD + W (14.53 ± 1.45) groups, as well as caloric efficiency statically comparable to that of the LFD group (5.75 ± 0.59) (P = 0.06; Figure 1F). These findings may indicate better caloric management in the HFD + D group.
FIGURE 1.
Sea vegetable supplementation affects weight gain, caloric intake, and food and caloric efficiency in HFD-fed C57BL/6J male mice. (A) Weekly body weight, (B) average weight gain, (C) daily food intake, (D) daily caloric intake, (E) food efficiency, and (F) caloric efficiency. HFD, high-fat diet; HFD + D, 5% dulse–supplemented HFD; HFD + W, 5% wakame-supplemented HFD; LFD, control low-fat diet.
Modulation of lipid metabolic markers by the sea vegetable–supplemented diets
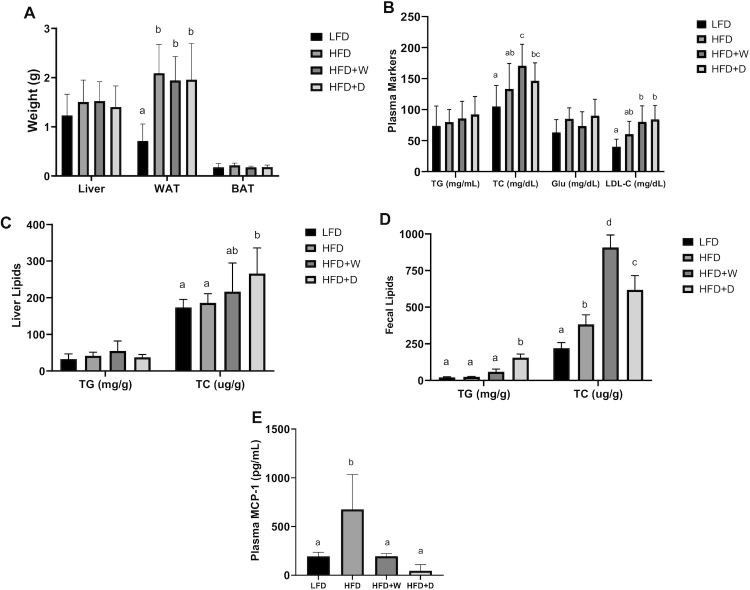
Liver and BAT weights were comparable among all test groups, with ranges of 1.2–1.5 and 0.17–0.2 g, respectively. The HFD + D (1.78 ± 0.21 g), HFD + W (1.88 ± 0.18 g), and HFD (2.2 ± 0.2 g) groups had significantly higher WAT weights than the LFD group (0.7 ± 0.1 g) (P ≤ 0.01), showing that 8-wk HFD feeding was effective in inducing obesity (Figure 2A). Plasma triglyceride and glucose concentrations were comparable among all test groups (P > 0.1). The HFD + D (146.41 ± 10.17 mg/dL) and HFD + W (170.70 ± 12.23 mg/dL) groups had higher plasma total cholesterol concentrations than the LFD group (104.88 ± 12.85 mg/dL) (P ≤ 0.01). While the total cholesterol concentrations in the HFD + D group were comparable to those in the HFD group (133.19 ± 14.52 mg/dL), the values observed for the HFD + W group were significantly higher (P = 0.03). Plasma LDL cholesterol concentrations were higher in sea vegetable–supplemented HFD groups than in the LFD group (40.18 ± 4.53 mg/dL) (P ≤ 0.05) (Figure 2B). Hepatic triglyceride concentrations were comparable among all of the test groups (P > 0.1), a pattern similar to that of the plasma lipid markers. The HFD + D group showed higher amounts of liver total cholesterol (267.57 ± 28.54 μg/g) than the HFD group (190.43 ± 8.74 μg/g) (P ≤ 0.01). Comparably, the high amounts of cholesterol observed in the HFD + W group (214.43 ± 31.93 μg/g) were statistically indistinguishable those in both the HFD and LFD groups (173.57 ± 8.10 μg/g) (P > 0.1). Both the HFD + D (606.43 ± 37.51 μg/g) and HFD + W (923.86 ± 29.54 μg/g) groups had higher fecal total cholesterol than the HFD (375.14 ± 25.54 μg/g) and LFD (220.00 ± 14.44 μg/g) groups, (P ≤ 0.01). This finding is parallel to the observed elevated plasma total cholesterol, suggesting that cholesterol synthesis and excretion may have been affected by sea vegetable supplementation. Fecal triglyceride concentrations were significantly higher in the HFD + D (155.18 ± 10.25 mg/g) than in the HFD (25.06 ± 1.38 mg/g) and LFD (21.29 ± 1.49 mg/g) groups (P ≤ 0.01), suggesting improved triglyceride disposal/excretion (Figure 2C and D).
FIGURE 2.
Sea vegetable supplementation increases plasma and liver total cholesterol while improving lipid excretion and inflammation in HFD-fed C57BL/6J male mice. (A) Weight of liver, WAT (epididymal fat), and BAT, (B) concentrations of TG, TC, Glu, and LDL cholesterol in the plasma, (C) concentrations of TG and TC in the liver, (D) concentrations of TG and TC in the fecal matter, and (E) plasma MCP-1 concentrations. BAT, brown adipose tissue; Glu, glucose; HFD, high-fat diet; HFD + D, 5% dulse–supplemented HFD; HFD + W, 5% wakame-supplemented HFD; LFD, control low-fat diet; TC, total cholesterol; TG, triglyceride; WAT, white adipose tissue.
Alleviation of systemic inflammation by the sea vegetable–supplemented diets
The plasma inflammatory marker MCP-1 was significantly lower in the HFD + D (45.99 ± 23.14 pg/mL) and HFD + W (195.03 ± 10.77 pg/mL) groups than in the HFD (675.97 ± 134.98 pg/mL) group, (P ≤ 0.01). Sea vegetable supplementation ameliorated an increase in the systemic inflammatory marker MCP-1 to a concentration comparable to that of the LFD group (192.99 ± 16.26 pg/mL) (P > 0.5), (Figure 2E). Fasting glucose concentrations were comparable in all of the groups, suggesting that the mice were not hyperglycemic at week 4 of the feeding period. Significantly higher glucose concentrations were observed across all of the HFD groups than in the LFD control for an entire 120-min monitoring period after glucose administration (P ≤ 0.01). This finding shows that the rate of exogenous blood glucose clearance is dysregulated, affirming the state of glucose intolerance in HFD-fed mice. At the 4-wk intervention, no significant glycemic improvement was observed with sea vegetable supplementation (Figure 3).
FIGURE 3.
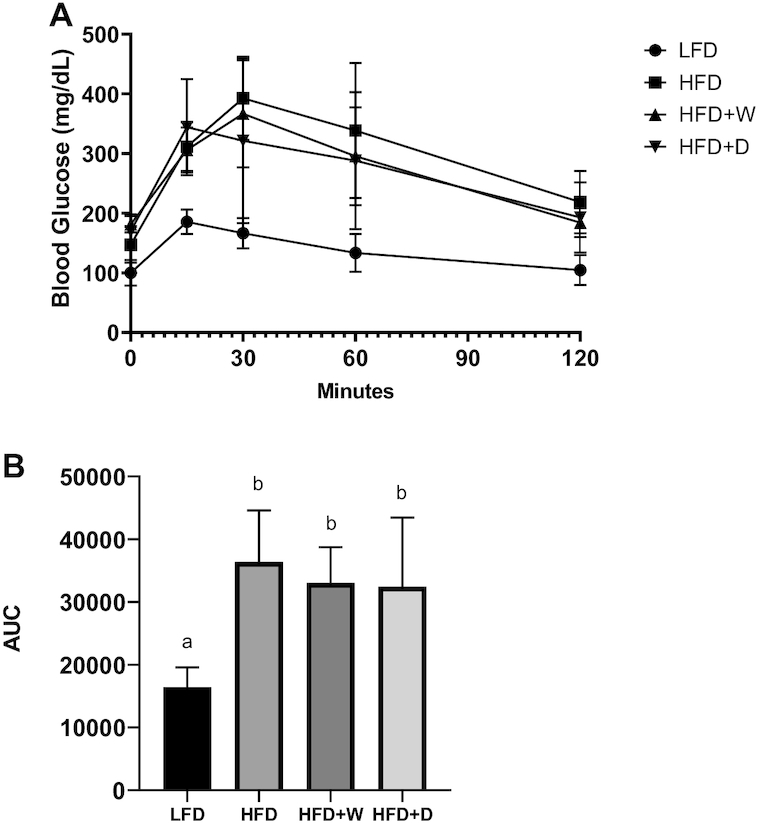
Sea vegetable supplementation shows no glycemic improvements in HFD-fed C57BL/6J male mice. (A) Blood glucose concentration analyzed from IPGTT. (B) AUC calculated from IPGTT. AUC, area under the curve; IPGTT, intraperitoneal glucose tolerance test; HFD, high-fat diet; HFD + D, 5% dulse–supplemented HFD; HFD + W, 5% wakame-supplemented HFD; LFD, control low-fat diet.
Impact of the sea vegetable–supplemented diets on the gut microbiome
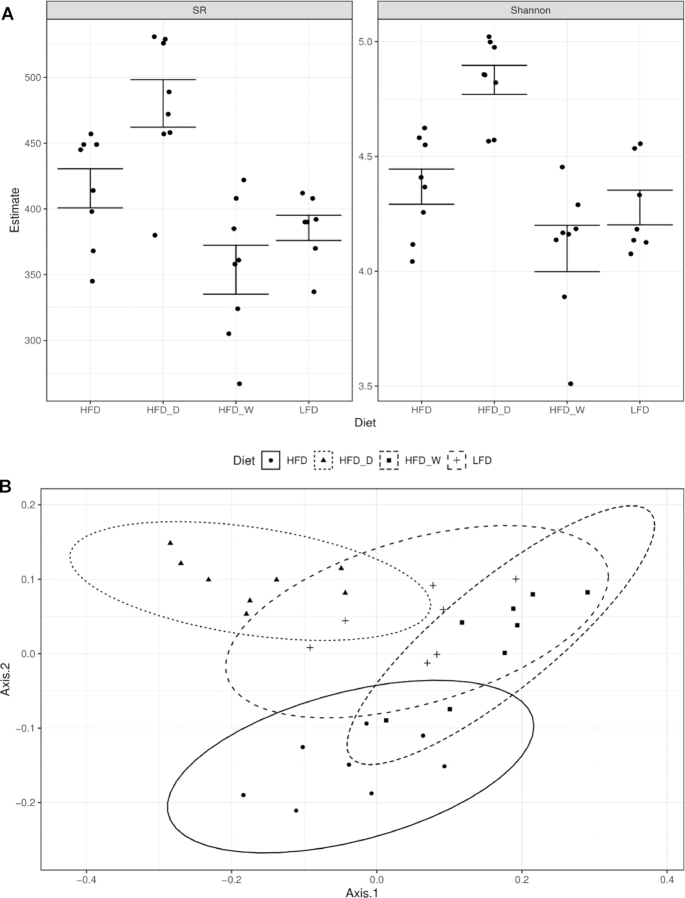
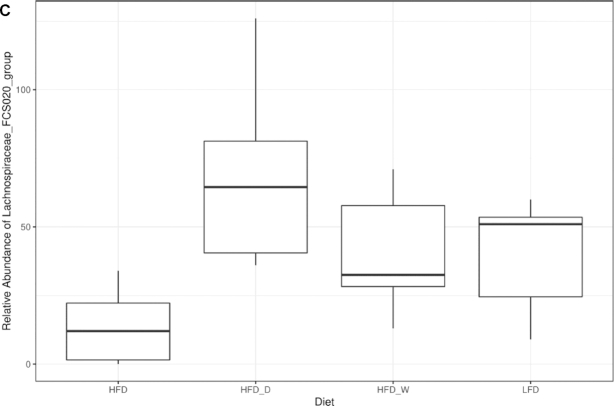
Microbiome analysis of fresh fecal samples revealed diet-driven changes in both alpha- and beta-diversity indices (Figure 4). Samples were rarified to 141,138 sequence reads to reveal a total of 3142 unique ASVs identified in the study. Both species richness (SR) and Shannon entropy were influenced by diet (ANOVA; P < 0.001), with the HFD + D group showing higher diversity estimates (Figure 4A). Microbiome species richness showed a strong correlation with fecal fat content (ANOVA; P = 0.006). Using the Bray-Curtis dissimilarity metric, we determined that the microbiomes of the sea vegetable–supplemented HFD groups were comparable to the microbiome of the LFD group, suggesting that the commonly observed HFD-driven gut microbiome alteration has been averted (Figure 4B). This diet-driven estimate (Adonis; P < 0.001) was closely associated with the weight gain of the mice (P = 0.037). Sea vegetable supplementation was associated with differential relative abundance of 34 genera (FDR-corrected Kruskal-Wallis, P < 0.05), including the Lachnospiraceae FCS020 group, whose relative abundance increased to levels more similar to those in the LFD treatment group (Figure 4C).
FIGURE 4.
Sea vegetable supplementation averts gut dysbiosis in HFD-fed C57BL/6J male mice. (A) Microbiome alpha-diversity analyzed by SR (left) and Shannon entropy (Shannon, right), (B) microbiome ordination analyzed by Bray-Curtis dissimilarity beta-diversity metrics where ellipses represent 95% CIs, and (C) rarefied abundance of the Lachnospiraceae FCS020 group. HFD, high-fat diet; HFD + D, 5% dulse–supplemented HFD; HFD + W, 5% wakame-supplemented HFD; LFD, control low-fat diet; SR, species richness.
Discussion
Our results demonstrated that sea vegetable supplementation exerts short-term weight gain suppression without affecting the liver, visceral, or brown adipose tissues. The progressive weight gain in the HFD group was modestly suppressed by Pacific dulse and wakame supplementation, with the mean weight gain values intermediate between those for the LFD and HFD groups. Significant weight gain was observed after week 4 and week 6 for the wakame- and dulse-supplemented groups, respectively. In the present study we observed an effect similar to that reported in HFD-fed NSY/HOS (T2DM strain) mice with 2.5%, wt:wt P. mollis supplementation (28). The suppression of visceral adiposity in mice was reportedly related to the downregulation of peroxisome proliferator-activated receptor γ (PPAR-γ) in the adipose tissue and upregulation of beta oxidation–related genes (PPAR-α and Acox1) in the liver (28). While we used a higher supplementation dose (5%) for P. mollis, we were unable to observe similar effects in terms of visceral adiposity suppression. This result can be attributed to the fact that earlier researchers used CT scans for visceral adipose tissue volume quantification, which is a much more sensitive method. In addition, the use of different mice strains with distinctive metabolic phenotypes and significantly older age is a potential source of different metabolic outcomes in feeding trials.
While both carotenoid fucoxanthin and polysaccharide fucoidan from wakame (U. pinnatifida) are known to facilitate weight loss by modulating fat mass accumulation in mice (19–21), this was not observed in this study. The weight gain of wakame-supplemented groups was only briefly suppressed. It is of value to point out that the short-term weight gain suppression observed in sea vegetable–supplemented HFD groups were associated with reduced food efficiency for Pacific dulse and reduced food intake for wakame. The possibility of reduced fat absorption and satiety stimulation can be considered since algal phenolics and polysaccharides are known to modulate digestive enzyme activities, satiety-associated signals, and digestive tract motility (34).
Lack of significant difference between treatments in terms of liver and BAT weight indicates that the 8-wk HFD feeding was not enough to induce hepatic steatosis, which is an associated obesity risk factor for NAFLD. Most studies have found that HFD induces discernable lipid deposition in the liver after at least 12 wk. Furthermore, since BAT is associated with thermogenesis, it is likely that 5% (wt:wt) sea vegetable supplementation did not exert discernable thermogenic effect in our model.
While the elevated cholesterol concentrations observed in both plasma and liver under sea vegetable supplementation were not expected, considering the well-documented hypolipidemic properties of sea vegetables, a similar effect was reported in ICR and KKAy mice with fucoxanthin supplementation. Specifically, repeated oral administration of fucoxanthin (500–1000 mg/kg) for 30 days resulted in an almost 2-fold increase in plasma total cholesterol in both male and female ICR mice (35). The same was observed in a 4-wk study in diabetic/obese (KKAy) mice with 0.2% fucoxanthin supplementation (36). Both total and non-HDL cholesterol concentrations in the serum were elevated in fucoxanthin-supplemented groups (36), suggesting that carotenoid fucoxanthin exerts hypercholesterolemic effects. Researchers then showed that these effects were mediated by the induction of SREBP (sterol regulatory element binding proteins) expression in the liver, which is associated with the cholesterol biosynthetic pathway. It is worth noting, however, that hepatic cholesterol content was reduced in their study, which was attributed to the reduction of hepatic LDL receptor (LDL-R) and SR-B1 proteins, and the increased mRNA expression of PCSK9, with the former 2 proteins responsible for cholesterol uptake in the liver, and the latter was associated with intracellular degradation of LDL-R in the lysosomes (36). However, this was not the case in our results. On the contrary, both Pacific dulse and wakame supplementation increased hepatic total cholesterol, with a parallel increase in fecal cholesterol. This finding is comparable to the result of different statins on cholesterol metabolism in C57BL/6J mice. While it is generally known that statins lower cholesterol concentrations through HMG-CoA reductase competitive inhibition, researchers demonstrated that this is accomplished by a paradoxical increase in hepatic cholesterol synthesis coupled by the potent stimulation of cholesterol secretion (37). Specifically, rosuvastatin and lovastatin caused increased cholesterol excretion via the biliary route while atorvastatin treatment stimulated the transintestinal disposal pathway, eventually resulting in reduced plasma cholesterol concentrations in their rodent model (37). The higher total cholesterol concentrations in the liver observed in this study may suggest increased biosynthesis or that LDL-R–driven uptake was enhanced. Since the former is more plausible, it can be considered that the increased cholesterol excretion in the feces may have been stimulated as a buffering mechanism to ensure tightly regulated cholesterol homeostasis in the body.
While the increased amounts of cholesterol can generally be disconcerting considering that LDL particles are closely linked to cardiovascular diseases, specifically atherosclerosis, there is a growing understanding now as to the atherogenic risk associated with this cholesterol particle and its apolipoprotein (apoB). The variability of cholesterol loading in apolipoproteins accounts for the growing advocacy to use apoB as a more reliable marker for atherogenicity than LDL cholesterol. Since lipid deposition in the vascular tissues is mainly driven by particle size and mass, and the latter can vary greatly for LDL particles, the protein marker attached to it can be more receptive to getting trapped and eventually becoming a foam cell (38, 39).
Pacific dulse supplementation also increased triglyceride excretion in the feces, which is similar to the effects observed in red algae Gelidium amansii supplementation in Sprague-Dawley rats fed with a high-fructose diet (40) and in streptozotocin-nicotinamide–induced diabetic rats (41). Beneficial effects of algal polysaccharides, particularly soluble fiber, in increasing lipid excretion have been well reported. While major polysaccharide families constituting the dietary fiber profile in red and brown algae may be distinct (42), both Pacific dulse (P. mollis) and wakame (U. pinnatifida) have favorably high fiber content (>25%) (43, 44). Hypolipidemic effects linked to high-fiber diets are mainly associated with soluble fibers, as these may decrease pancreatic lipase activity, reduce lipid emulsification and lipolysis, and lower lipid absorption, thereby increasing fecal lipid excretion (45).
Obesity-induced inflammation is considered the major link to numerous health perturbations in metabolic syndrome. In particular, chronic low-grade inflammation in the adipose tissue, marked by the recruitment and accumulation of macrophages, is crucial to insulin resistance development (46). Being a chemoattractant protein specific to monocytes and macrophages (47, 48), MCP-1 plays a critical role in the progression of the adiposity-related inflammatory cascade. As early as 7 d of HFD feeding in mice, MCP-1 expression in the adipose tissue is increased, with significant elevation of plasma concentrations observed during the fourth week (49). In humans, circulating concentrations of MCP-1 are significantly higher in obese subjects than nonobese controls, showing positive relations to other systemic inflammatory markers, such as C-reactive protein (CRP) and IL-6 (50). The same observations have been reported in patients with type 2 diabetes and cardiovascular diseases (51). Considering this integral role in disease progression, numerous efforts have been directed at lowering MCP-1 concentrations. Treatments involving MCP-1 reduction for health improvement in humans include statins, rosiglitazone, colestimide (hypolipidemic drug), vitamin E, and physical interventions like bypass surgery and exercise (51).
Our study shows that sea vegetable supplementation significantly reduces plasma MCP-1 in HFD-fed groups of mice. Both sea vegetables exerted anti-inflammatory effects, ably remediating the commonly observed obesity-induced systemic inflammation. This finding is in agreement with the effects observed in U. pinnatifida lipid (fucoxanthin-rich) supplementation in C57BL/6J mice (20) and polysaccharide fucoidan treatment in 3T3L1 (52). As for the use of Pacific dulse (P. mollis), this is to our knowledge the first report on its anti-inflammatory effect in a murine model. While the anti-inflammatory effects of phycobiliprotein and chlorophyll from P. palmata have been documented in vitro and in vivo (26), dietary intervention using the same sea vegetable material showed contrary results in humans. Specifically, researchers reported that consumption of P. palmata–supplemented bread (5 g/d) increased serum CRP (53). As an acute phase protein, CRP can be drastically increased to 1000-fold and quickly return to normal concentrations in the blood within 24–48 h. Since the main biological function of CRP is for host defense against bacterial pathogens and clearance of apoptotic and necrotic cells (54), CRP concentrations can greatly and rapidly fluctuate. Since obesity-associated inflammation is chronic in nature and the stability of CRP in the system is rather brief, using it as a marker for chronic inflammation can be misleading.
Given that most obesity-associated proinflammatory cytokines can block the insulin signaling pathway (55) and that averting inflammation may improve glycemic condition, as in the case of the anti-inflammatory drug salsalate in obese nondiabetic human subjects (56), the use of inflammation-targeted antidiabetic treatments hold promise (57). While antidiabetic activities have been reported in both red and brown algae extracts that exerted anti-inflammatory effects (58, 59), this was not observed in this current study. The observed potent anti-inflammatory effects in sea vegetable–supplemented HFD groups did not cause significant glycemic improvements among test animals at 4 wk of feeding of the experimental diets. Although the dulse-supplemented group showed relatively faster clearance of exogenous blood glucose during IPGTT (Figure 3A), this was not enough to account for a significant difference in terms of the AUC. It is also important to point out that the inflammatory marker MCP-1 was determined at a much later period (week 8) than IPGTT, which was determined during week 4 of diet supplementation.
The gut microbiome plays an important role in nutrient absorption, energy harvest and storage, immune function, and endocrine response. Alterations to the gut microbiome can impair these critical functions to adversely impact host physiology. These impairments include decline in gut barrier function, increased susceptibility to metabolic endotoxemia (elevated lipopolysaccharide concentrations), augmented energy harvest and storage, and subsequent progression of inflammation, insulin resistance, and other metabolic complications (60). Considering these links to metabolic health and disease progression, the gut microbiome is considered a promising therapeutic target (61). Therefore, interventions that prevent functional disruption of the gut microbiome or that restore the microbiome to a state that contributes to homeostasis are being explored.
HFDs are known to cause significant alterations to the gut microbiome, in both obese and nonobese states (62, 63). HFD consumption is generally accompanied by significant phylum-level microbiota shifts, characterized by the decrease of Bacteroidetes and increase of Firmicutes and Proteobacteria (60). A decrease in bacterial density and an increase in the relative proportion of Bacteroidales, Clostridiales, and Enterobacteriales in HFD-fed Sprague-Dawley rats for both obesity-prone and nonprone strains has been reported (64). While both strains showed microbiome alteration mediated by HFD, inflammatory progression was crucial for the onset of hyperphagia and obese phenotype development (64).
Our study showed that the gut microbiome of HFD + D–fed mice, as assessed from fresh stool samples, was more diverse as indicated by higher SR and Shannon Entropy estimates. As a measure of distinguishable taxa in each sample, the evenness of species abundances are effective indicators of how Pacific dulse supplementation favored a more diverse gut community. The gut microbiome of the HFD + W–fed mice had lower alpha-diversity, which was comparable to the observed reduction of gut microbiome SR in polysaccharide fucoidan–supplemented HFD feeding in BALB/c mice (65). Researchers, however, noted the favorable increase in abundance of the phylum Bacteroidetes, which is often reduced during HFD exposure.
Bray-Curtis dissimilarity analysis revealed that the gut microbiome profile of mice fed with a sea vegetable–supplemented HFD was comparable to that of mice fed with an LFD. The same clustering was observed in terms of the relative abundance of Lachnospiraceae FCS020, a member of a common butyrate-producing gut bacterial taxa associated with diets high in fiber and complex carbohydrates (66). While there seems to be no unified view on the diversity, abundance, and major taxonomic shifts in diet-induced obesity animal models, it is considered that an “inflammation-associated microbiome” predominates in subjects with obesity-associated metabolic disorders. Specifically, it is thought to be attributable to the reduction of bacterial diversity and/or gene richness, as well as the lower potential for butyrate production. Lower bacterial gene count, which can arise from decreased abundance and diversity, has been associated with altered gut microbial functions and linked to increased fat accumulation, LPS-induced inflammation, insulin resistance, obesity, metabolic syndrome, and higher risks for diabetes, cardiovascular disorders, and inflammatory bowel disease (10). The decline of gut microbiota diversity observed in Westernized diets was recreated in human microbiota–carrying mice (Swiss Webster) fed with low microbiota-accessible carbohydrate (MAC). Researchers have reported early reversibility of this decline at a single generation, but subsequent generations required microbiota reintroduction and dietary MAC intervention for the restoration of healthy gut conditions (67). Algal polysaccharides are rich sources of both soluble and insoluble fiber, which can serve as a carbohydrate source for gut microbiota. In human trials, it has been reported that increased dietary fiber intake can increase microbiota richness and stability (68). The prebiotic effects reported in sea vegetable polysaccharides include selective enrichment of probiotic taxa, improved short-chain fatty acid production, colonic morphology and immune function enhancement, and downregulation of proinflammatory cytokine and carcinogenic protein expression (44).
While wakame (U. pinnatifida) has been an established source of bioactive compounds such as carotenoid fucoxanthin and polysaccharide fucoidan, Pacific dulse (P. mollis) has been barely investigated. A previous study has shown that Pacific dulse supplementation can ameliorate diet-induced obesity in both mice (NSY/HOS) and zebra fish models (28). In this present study, short-term weight gain suppression was observed using a different mouse model (C57BL/6J) and a higher supplementation dose (5%, wt:wt). While improvements in adiposity and glycemic condition were not observed, this study reports for the first time, to our knowledge, the beneficial effects of dulse supplementation in HFD-fed mice, which involved the reduction of food efficiency and the modulation of both inflammation and the gut microbiome.
Protein-rich rhodophytes (red algae) are a known source of bioactive proteins (lectins and phycobiliproteins), peptides, and amino acids (69), and sizeable amounts of soluble (sulfated galactans) and insoluble (xylan, mannan, and cellulose) fibers (42). Prebiotic xylooligosaccharides, which have been shown to bring about dramatic shifts in gut microbiota in heathy and prediabetic subjects (70), have been reported to be produced from Palmaria sp. in Japan (71).
In conclusion, we demonstrated that 5% (wt:wt) sea vegetable powder supplementation can exert protective effects in HFD-fed mice by increasing lipid excretion, suppressing inflammation, and averting gut dysbiosis. To our knowledge, this is the first work to show the therapeutic potential of P. mollis for remediating gut microbiome shift and inflammation in a high-fat fed mouse model. Considering that dulse is an extensively grown sea vegetable in the Pacific Northwest, with a high specific growth rate (4.9–5.1%) (72) and considerable amounts of protein and fiber (23.5–25.7% and 26.9–43.0%, dry weight) (43), identification of active fractions and elucidation of therapeutic mechanisms are deemed necessary for optimum resource utilization.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Dr. Christopher Langdon for supplying Pacific dulse for the experiments and Alexandra Becraft and Marlena Sturm for their assistance during tissue collection.
The authors’ responsibilities were as follows—JYK, JFS, and CM: designed the research; RLM, CM, CRA, and TJS: conducted the research and analyzed the data; RLM and JYK: wrote the manuscript; JYK: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
Supported by the Agricultural Research Foundation, Oregon State University (ARF8855A), Oregon Agricultural Experiment Station.
Author disclosures: The authors report no conflicts of interest.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: ASV, amplicon sequence variance; BAT, brown adipose tissue; CRP, C-reactive protein; HFD, high-fat diet; IPGTT, intraperitoneal glucose tolerance test; LFD, low-fat diet; LDL-R, LDL receptor; MAC, microbiota-accessible carbohydrate; MCP-1, monocyte chemotactic protein 1; NAFLD, nonalcoholic fatty liver disease; PPAR, peroxisome proliferator-activated receptor; SR, species richness; WAT, white adipose tissue.
References
- 1. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak LB, Mokdad AH, Moradi-Lakeh M, Naghavi M et al.. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tremmel M, Gerdtham UG, Nilsson PM, Saha S. Economic burden of obesity: a systematic literature review. Int J Environ Res Public Health. 2017;14(4):E435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kakkar AK, Dahiya N. Drug treatment of obesity: current status and future prospects. Eur J Intern Med. 2015;26(2):89–94. [DOI] [PubMed] [Google Scholar]
- 4. An R. Health care expenses in relation to obesity and smoking among U.S. adults by gender, race/ethnicity, and age group: 1998–2011. Public Health. 2015;129(1):29–36. [DOI] [PubMed] [Google Scholar]
- 5. Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–604. [DOI] [PubMed] [Google Scholar]
- 6. Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond). 2015;39(8):1188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yun JW. Possible anti-obesity therapeutics from nature—a review. Phytochemistry. 2010;71(14–15):1625–41. [DOI] [PubMed] [Google Scholar]
- 8. Ellulu MS, Patimah I, Khaza'ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13(4):851–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121(6):2111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. Diabetes. 2006;582(1):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72. [DOI] [PubMed] [Google Scholar]
- 14. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duan Y, Zeng L, Zheng C, Song B, Li F, Kong X, Xu K. Inflammatory links between high fat diets and diseases. Front Immunol. 2018;9:2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lange KW, Hauser J, Nakamura Y, Kanaya S. Dietary seaweeds and obesity. Food Sci Hum Wellness. 2015;4:87–96. [Google Scholar]
- 17. Kumar SA, Brown L. Seaweeds as potential therapeutic interventions for the metabolic syndrome. Rev Endocr Metab Disord. 2013;14;(3):299–308. [DOI] [PubMed] [Google Scholar]
- 18. Wan-Loy C, Siew-Moi P. Marine algae as a potential source for anti-obesity agents. Mar Drugs. 2016;14(12):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maeda H, Hosokawa M, Sashima T, Funayama K, Miyashita K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem Biophys Res Commun. 2005;332(2):392–7. [DOI] [PubMed] [Google Scholar]
- 20. Maeda H, Hosokawa M, Sashima T, Murakami-Funayama K, Miyashita K. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol Med Rep. 2009;2(6):897–902. [DOI] [PubMed] [Google Scholar]
- 21. Kim MJ, Jeon J, Lee JS. Fucoidan prevents high-fat diet-induced obesity in animals by suppression of fat accumulation. Phyther Res. 2014;28(1):137–43. [DOI] [PubMed] [Google Scholar]
- 22. Harnedy PA, O'Keeffe MB, Fitzgerald RJ. Purification and identification of dipeptidyl peptidase (DPP) IV inhibitory peptides from the macroalga Palmaria palmata. Food Chem. 2015;172:400–6. [DOI] [PubMed] [Google Scholar]
- 23. Fitzgerald C, Mora-Soler L, Gallagher E, O'Connor P, Prieto J, Soler-Vila A, Hayes M. Isolation and characterization of bioactive pro-peptides with in vitro renin inhibitory activities from the macroalga Palmaria palmata. J Agric Food Chem. 2012;60(30):7421–7. [DOI] [PubMed] [Google Scholar]
- 24. Wang T, Jónsdóttir R, Kristinsson HG, Hreggvidsson GO, JÓ Jónsson, Thorkelsson G, Olafsdottir GE. Enzyme-enhanced extraction of antioxidant ingredients from red algae Palmaria palmata. LWT-Food Sci Technol. 2010;43:1387–93. [Google Scholar]
- 25. Banskota AH, Stefanova R, Sperker S, Lall SP, Craigie JS, Hafting JT, Critchley AT. Polar lipids from the marine macroalga Palmaria palmata inhibit lipopolysaccharide-induced nitric oxide production in RAW264.7 macrophage cells. Phytochemistry. 2014;101:101–8. [DOI] [PubMed] [Google Scholar]
- 26. Lee D, Nishizawa M, Shimizu Y, Saeki H. Anti-inflammatory effects of dulse (Palmaria palmata) resulting from the simultaneous water-extraction of phycobiliproteins and chlorophyll a. Food Res Int. 2017;100(Pt 1):514–21. [DOI] [PubMed] [Google Scholar]
- 27. Meer JP van der, Bird CJ. Palmaria mollis stat. nov.: a newly recognized species of Palmaria (Rhodophyceae) from the northeast Pacific Ocean. Can J Bot. 1985;63(3):398–403. [Google Scholar]
- 28. Nakayama H, Shimada Y, Zang L, Terasawa M, Nishiura K, Matsuda K, Toombs C, Langdon CS, Nishimura N. Novel anti-obesity properties of palmaria mollis in zebrafish and mouse models. Nutrients. 2018;10(10):E1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rulifson IC, Collins P, Miao L, Nojima D, Lee KJ, Hardy M, Gupte J, Hensley K, Samayoa K, Cam C et al.. In vitro and in vivo analyses reveal profound effects of fibroblast growth factor 16 as a metabolic regulator. J Biol Chem. 2017;292(5):1951–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gaulke CA, Barton CL, Proffitt S, Tanguay RL, Sharpton TJ. Triclosan exposure is associated with rapid restructuring of the microbiome in adult zebrafish. PLoS One. 2016;11(5):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Torres ERS, Akinyeke T, Stagaman K, Duvoisin RM, Meshul CK, Sharpton TJ, Raber J. Effects of sub-chronic MPTP exposure on behavioral and cognitive performance and the microbiome of wild-type and mGlu8 knockout female and male mice. Front Behav Neurosci. 2018;12(July):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mcmurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8(4):561–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chater PI, Wilcox MD, Houghton D, Pearson JP. The role of seaweed bioactives in the control of digestion: implications for obesity treatments. Food Funct. 2015;6(11):3420–7. [DOI] [PubMed] [Google Scholar]
- 35. Beppu F, Niwano Y, Tsukui T, Hosokawa M, Miyashita K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J Toxicol Sci. 2009;34(5):501–10. [DOI] [PubMed] [Google Scholar]
- 36. Beppu F, Hosokawa M, Niwano Y, Miyashita K. Effects of dietary fucoxanthin on cholesterol metabolism in diabetic/obese KK-A(y) mice. Lipids Health Dis. 2012;11:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schonewille M, De Boer JF, Mele L, Wolters H, Bloks VW, Wolters JC, Kuivenhoven JA, Tietge UJ, Brufau G, Groen AK. Statins increase hepatic cholesterol synthesis and stimulate fecal cholesterol elimination in mice. J Lipid Res. 2016;57(8):1455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Contois JH, Mcconnell JP, Sethi AA, Csako G, Devaraj S, Hoefner DM, Warnick GR. Apolipoprotein B and cardiovascular disease risk: position statement from the AACC Lipoproteins and Vascular Diseases Division Working Group on Best Practices. Clin Chem. 2009;55(3):407–19. [DOI] [PubMed] [Google Scholar]
- 39. Sniderman AD, Thanassoulis G, Glavinovic T, Navar AM, Pencina M, Catapano A, Ference BA. Apolipoprotein B particles and cardiovascular disease: a narrative review. JAMA Cardiol. 2019;4(12):1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu HC, Chang CJ, Yang TH, Chiang MT. Long-term feeding of red algae (Gelidium amansii) ameliorates glucose and lipid metabolism in a high fructose diet-impaired glucose tolerance rat model. J Food Drug Anal. 2017;25(3):543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang TH, Yao HT, Chiang MT. Red algae (Gelidium amansii) reduces adiposity via activation of lipolysis in rats with diabetes induced by streptozotocin-nicotinamide. J Food Drug Anal. 2015;23(4):758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jiménez-Escrig A, Sánchez-Muniz FJ. Dietary fibre from edible seaweeds: chemical structure, physicochemical properties and effects on cholesterol metabolism. Nutr Res. 2000;20:585–98. [Google Scholar]
- 43. Rosen G, Langdon CJ, Evans F. The nutritional value of Palmaria mollis cultured under different light intensities and water exchange rates for juvenile red abalone Haliotis rufescens. Aquaculture. 2000;185:121–36. [Google Scholar]
- 44. de Jesus Raposo MF, de Morais AM, de Morais RM. Emergent sources of prebiotics: seaweeds and microalgae. Mar Drugs. 2016;14(2):E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lairon D. Soluble fibers and dietary lipids. In: Kritchevsky D, Bonfield C. Dietary fiber in health and disease. Advances in experimental medicine and biology.1997;vol 427:pp. 99–108. [DOI] [PubMed] [Google Scholar]
- 46. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Solé J, Nichols AP, Ross JS, Tartaglia LA. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Boring L, Gosling J, Chensue SW, Kunkel SL, Farese R V, Broxmeyer HE, Charo IF. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100(10):2552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North RJ, Gerard C, Rollins BJ. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187(4):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen A, Mumick S, Zhang C, Lamb J, Dai H, Weingarth D, Mudgett J, Chen H, Macneil DJ, Reitman ML. Diet induction of monocyte chemoattractant protein-1 and its impact on obesity. Obes Res. 2005;13(8):1311–20. [DOI] [PubMed] [Google Scholar]
- 50. Kim CS, Park HS, Kawada T, Kim JH, Lim D, Hubbard NE, Kwon B, Erickson KL, Yu R. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes. 2006;30(9):1347–55. [DOI] [PubMed] [Google Scholar]
- 51. Panee J. Monocyte chemoattractant protein 1 (MCP-1) in obesity and diabetes. Cytokine. 2012;60(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim KJ, Lee BY. Fucoidan from the sporophyll of Undaria pinnatifida suppresses adipocyte differentiation by inhibition of inflammation-related cytokines in 3T3-L1 cells. Nutr Res. 2012;32(6):439–47. [DOI] [PubMed] [Google Scholar]
- 53. Allsopp P, Crowe W, Bahar B, Harnedy PA, Brown ES, Taylor SS, Smyth TJ, Soler-vila A, Magee PJ, Gill CI. The effect of consuming Palmaria palmata-enriched bread on inflammatory markers, antioxidant status, lipid profile and thyroid function in a randomised placebo-controlled intervention trial in healthy adults. Eur J Nutr. 2016;55(5):1951–62. [DOI] [PubMed] [Google Scholar]
- 54. Volanakis JE. Human C-reactive protein: Expression, structure, and function. Mol Immunol. 2001;38(2–3):189–97. [DOI] [PubMed] [Google Scholar]
- 55. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. [DOI] [PubMed] [Google Scholar]
- 56. Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31(2):289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pollack RM, Donath MY, LeRoith D, Leibowitz G. Anti-inflammatory agents in the treatment of diabetes and its vascular complications. Diabetes Care. 2016;39(Suppl 2):S244–52. [DOI] [PubMed] [Google Scholar]
- 58. Oh JH, Kim J, Lee Y. Anti-inflammatory and anti-diabetic effects of brown seaweeds in high-fat diet-induced obese mice. Nutr Res Pract. 2016;10(1):42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Choi J, Kim KJ, Koh EJ, Lee BY. Gelidium elegans extract ameliorates type 2 diabetes via regulation of MAPK and PI3K/Akt signaling. Nutrients. 2018;10(1):E51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Murphy EA, Velazquez KT, Herbert KM. Influence of high-fat diet on gut microbiota: a driving force for chronic disease risk. Curr Opin Clin Nutr Metab Care. 2015;18(5):515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cani PD, Delzenne NM. The gut microbiome as therapeutic target. Pharmacol Ther. 2011;130(2):202–12. [DOI] [PubMed] [Google Scholar]
- 62. Mullin GE. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Nutr Clin Pract. 2010;25(3):310–11. [DOI] [PubMed] [Google Scholar]
- 63. Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight ROB, Ahima RS, Bushman FD, Wu GD. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137(5):1716–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. De La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299(2):G440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu M, Ma L, Chen Q, Zhang P, Chen C, Jia L, Li H. Fucoidan alleviates dyslipidemia and modulates gut microbiota in high-fat diet-induced mice. J Funct Foods. 2018;48:220–7. [Google Scholar]
- 66. O'Keefe SJD, Li JV, Lahti L, Ou J, Carbonero F, Mohammed K, Posma JM, Kinross J, Wahl E, Ruder EH et al.. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. 2015;6:6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529(7585):212–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tap J, Furet JP, Bensaada M, Philippe C, Roth H, Rabot S, Lakhdari O, Lombard V, Henrissat B, Corthier G et al.. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environ Microbiol. 2015;17(12):4954–64. [DOI] [PubMed] [Google Scholar]
- 69. Harnedy PA, Fitzgerald RJ. Bioactive proteins, peptides, and amino acids from macroalgae. J Phycol. 2011;47(2):218–32. [DOI] [PubMed] [Google Scholar]
- 70. Yang J, Summanen PH, Henning SM, Hsu M, Lam H, Huang J, Tseng C, Dowd SE, Finegold SM, Heber D et al.. Xylooligosaccharide supplementation alters gut bacteria in both healthy and prediabetic adults: a pilot study. Front Physiol. 2015;6:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yamamoto Y, Kishimura H, Kinoshita Y, Saburi W, Kumagai Y, Yasui H, Ojima T. Enzymatic production of xylooligosaccharides from red alga dulse (Palmaria sp.) wasted in Japan. Process Biochem. 2019;82:117–22. [Google Scholar]
- 72. Gadberry BA, Colt J, Maynard D, Boratyn DC, Webb K, Johnson RB, Saunders GW, Boyer RH. Intensive land-based production of red and green macroalgae for human consumption in the Pacific Northwest: an evaluation of seasonal growth, yield, nutritional composition, and contaminant levels. Algae. 2018;33(1):109–25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.