Abstract
Postnatal heat stress (HS) effects on pig physiology and performance are widely studied but prenatal HS studies, albeit increasing, are still limited. The objective of this study was to evaluate the chronic prenatal HS effects in growing pigs raised in postnatal thermoneutral (TN) or in HS environment. For prenatal environment (PE), mixed-parity pregnant sows were exposed to either TN (PTN; cyclic 18 to 24 °C; n = 12) or HS (PHS; cyclic 28 to 34 °C; n = 12) conditions from day 9 to 109 of gestation. Two female offspring per sow were selected at 10 wk of age and allotted to one of two postnatal growing environments (GE): GTN (cyclic 18 to 24 °C; n = 24) and GHS (cyclic 28 to 34 °C; n = 24). From 75 to 140 d of age, GTN pigs remained in GTN conditions, while GHS pigs were in GTN conditions from 75 to 81 d of age and in GHS conditions from 82 to 140 d of age. Regardless of PE, postnatal HS increased rectal and skin temperatures (+0.30 and +1.61 °C on average, respectively; P < 0.01) and decreased ADFI (−332 g/d; P < 0.01), resulting in lower ADG and final BW (−127 g/d and −7.9 kg, respectively; P < 0.01). The GHS pigs exhibited thicker backfat (P < 0.01), lower carcass loin percentage (P < 0.01), increased plasma creatinine levels (P < 0.01), and decreased plasma glucose, nonesterified fatty acids, T3, and T4 levels (P < 0.05). Prenatal HS increased feed intake in an age-dependent manner (+10 g·kg BW–0.60·d−1 for PHS pigs in the last 2 wk of the trial; P = 0.02) but did not influence BW gain (P > 0.10). Prenatal HS decreased the plasma levels of superoxide dismutase on day 3 of GHS (trend at P = 0.08) and of T4 on day 49 (P < 0.01) but did not affect T3 on day 3 nor 49 (P > 0.10). Prenatal HS increased rectal and skin temperatures and decreased temperature gradient between rectal and skin temperatures in GTN pigs (+0.10, +0.33 and −0.22 °C, respectively; P < 0.05) but not in GHS pigs (P > 0.10). There were also PE × GE interactions found with lower BW (P = 0.06) and higher backfat (P < 0.01) and perirenal adiposity (P < 0.05) for GHS–PHS pigs than the other groups. Overall, increased body temperature and altered thyroid functions and physiological stress responses suggest decreased heat tolerance and dissipation ability of pigs submitted to a whole-gestation chronic prenatal HS. Postnatal HS decreased growth performance, increased carcass adiposity, and affected metabolic traits and thyroid functions especially in pigs previously submitted to prenatal HS.
Keywords: carcass adiposity, growth, pig, postnatal heat stress, prenatal heat stress, thermoregulation
Introduction
As extreme heat events become longer, more frequent, and more intense, the impact on swine production also increases (Luber and McGeehin, 2008; IPCC, 2014). During postnatal heat stress (HS), pigs decrease their feed intake (FI) as an adaptive response to reduce heat production (HP) (Renaudeau et al., 2008, 2013). These negative effects are heightened with the selection for higher lean percentage as HP increases with higher lean tissue accretion rate (Brown-Brandl et al., 2014) resulting in less heat-tolerant pigs. In female mammals such as the sow, hyperthermia can induce physiological changes that can impair oocyte development, early embryonic development, fetal and placental growth, and nursing performance (as reviewed by Hansen, 2009) which can all affect the subsequent growth of the offspring. It is thus important to understand how prenatal HS can affect the pig’s postnatal performance.
Prenatal HS can be defined as the in utero exposure of the offspring to maternal hyperthermia (Edwards, 1969; Lary, 1986). There are many studies in mice suggesting that prenatal HS can depress brain and body growth (Shiota and Kayamura, 1989; Hinoue et al., 2001), although the effects of prenatal stress, in general, can also depend on stress duration: acute prenatal stress enhances protection of fetus from maternal corticosteroids but chronic prenatal stress weakens it (Welberg et al., 2005). In farm animals, thermal conditioning during incubation have been reported to influence adaptive HS response of poultry species (Loyau et al., 2015), while in calves, prenatal HS can alter deoxyribonucleic acid (DNA) methylation profile and reduce growth performance (Monteiro et al., 2016; Skibiel et al., 2018). Recent studies in pigs suggest that sows exposed to gestational HS produced pigs with increased core body temperature and altered metabolic processes, body composition, and thermal responses (Boddicker et al., 2014; Cruzen et al., 2015; Johnson et al., 2015a, 2015b, 2015c; Chapel et al., 2017). The objective of this study was to evaluate the effects of chronic prenatal HS on the growth performance, body composition, and physiological responses of growing pigs in postnatal thermoneutral (TN) or HS environment.
Materials and Methods
The experiment was conducted in accordance with the French legislation on animal experimentation and was approved by the French National Committee for Consideration of Ethics in Animal Experimentation (Authorization: APAFiS #11016-2017080718212019 delivered on September 26, 2017).
Experimental design and animal management
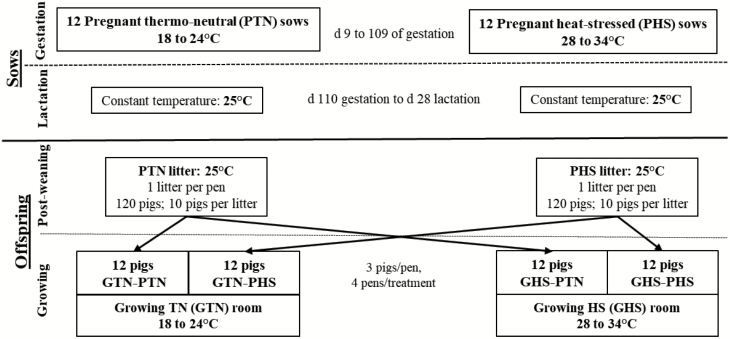
The study was conducted in the INRAE experimental facilities at the Unité Expérimentale Porcs de Rennes (UEPR) located in Saint-Gilles, France from October 2017 to June 2018. The general framework of the experimental study is presented in Figure 1.
Figure 1.
Framework of the experimental study from gestation to growing period. Pregnant sows (12 primiparous and 12 multiparous) were housed under either PTN or PHS conditions from 9 to 109 d of gestation. Their female offspring were subjected to GTN or GHS conditions from 82 to 140 d of age. Sows during lactation and piglets during post-weaning were housed under TN conditions.
Gestating and lactating sows
A total of 16 gilts and 16 multiparous sows (16 blocks of 2 sisters) were initially blocked according to parity and litter origin with additional blocking factors for the multiparous sows, that is, body weight (BW) and backfat thickness (BFT) at weaning of their respective litters. The animals were kept in one of two identical rooms during their pregnancy: one thermoneutral (PTN) room and one heat-stressed (PHS) room. Each room was equipped with two pens. In both rooms, gilts and sows were placed in separate pens (eight animals per pen of 4.5 × 4.8 m). The gilts were moved to the gestation rooms 3 wk prior to the expected date of breeding while the sows were blocked and transferred on the day of weaning immediately after weighing and BFT measurement. Sows were artificially inseminated with four different sire origins, with one sire used to inseminate four females (two multiparous and two primiparous sows) per treatment. One PHS primiparous sow was removed prior to insemination because of urogenital infection. From day 0 to 6 of gestation, the ambient temperature was kept under cyclic TN conditions (18 to 24 °C) in both experimental gestation rooms. The PTN sows were maintained at this environmental temperature regimen until day 109 of gestation. In the PHS room, the ambient temperature was gradually increased from day 6 to 9 and thereafter maintained under cyclic HS conditions (28 to 34 °C) from day 9 to 109 of gestation. Whatever the temperature treatment, the minimum and maximum temperatures were reached at 0600 and 1800 hours, respectively (Figure 2a). All animals were given a commercial gestation feed (13.6% crude protein (CP); 2,300 kcal/kg net energy [NE]) following a daily individual feed allowance calculated according to Dourmad et al. (1997). The daily ration was distributed in two meals at 0830 and at 1600 hours and water was provided ad libitum.
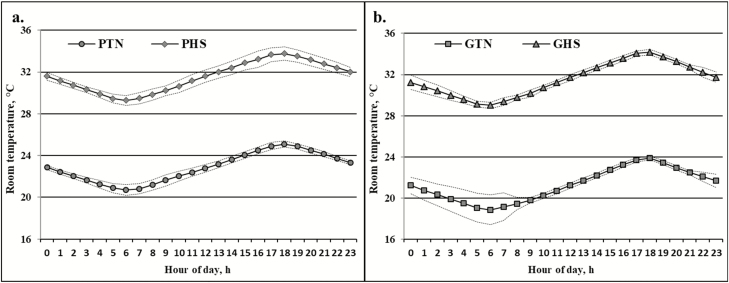
Figure 2.
Actual average hourly ambient temperature (mean ± SE) of the gestation rooms (a) from day 9 to 109 of gestation and of the growing rooms (b) from 83 to 140 d of age during the growth period.
On day 110 of gestation, 12 sows (6 primiparous and 6 multiparous) from each temperature treatment were selected based on litter origin, BW, and BFT and were distributed equally to one of two identical farrowing rooms equipped with 12 pens and maintained in constant TN conditions of 25 °C. Cross-fostering was done within each pregnancy treatment (PTN or PHS) and within the parity, for example, piglet of a primiparous PTN sow can only be fostered to another primiparous PTN sow. Sows were placed in individual pens (1.79 × 2.38 m) until weaning (day 28 of lactation). From day 1 to 5 of lactation, sows were given individual rations of 0.5, 1.5, 3.0, 5.0, and 6.0 kg/d, respectively. From day 6 to 28 of lactation, feed and water were provided ad libitum. The commercial lactation feed (16.5% CP; 2,343 kcal/kg NE) was distributed three times per day (0800, 1300, and 1600 hours).
Weanlings and growing pigs
At weaning, 10 littermate piglets (3 entire males, 4 castrated males, and 3 females) were selected from each of the 24 sows (total of 240 piglets). The pigs selected were those closest to average weaning BW of pigs from PTN and PHS groups (i.e., 9.7 and 9.5 kg, respectively). During post-weaning, the 10 selected littermates were housed in one pen (1.45 × 2.72 m), hence one litter per pen. The 24 litters were allocated to two rooms, with 6 PTN and 6 PHS litters in each room. The piglets were given a standard pre-starter diet (18.9% CP; 2,498 kcal/kg NE) for 1 wk and a standard starter diet (18.0% CP; 2,262 kcal/kg NE) until the end of post-weaning. Water and feed were provided ad libitum, and the rooms were maintained in constant TN conditions (25 °C) throughout the 6 wk of post-weaning.
At 10 wk of age, 48 females were randomly selected from these 240 piglets (choice of 2 out of the 3 females per litter). Considering the prenatal environment (PE: PTN vs. PHS), the two selected females per litter were allotted to one of two similar growing (G) rooms with two different thermal environments (GE: GTN or GHS). Each room was equipped with eight pens (2 × 2 m) designed for housing three pigs each. To balance out the experimental design, the pigs were blocked according to litter origin so that in one pen, all pigs were half-sisters (same sire). Moreover, each pig of one pen in the GTN room has a full sister in the corresponding pen of the GHS room.
The experiment, which started after 5 d of adaptation, was divided into two main periods. The first main period was from 75 to 81 d of age where all pigs were kept under cyclic TN conditions (18 to 24 °C). The second main period started at 82 d of age (day 0; transition day for the GHS pigs from TN to HS conditions) when the temperature in the GHS room was gradually changed at a rate of 1 °C/h from 0600 to 1800 hours and was thereafter maintained in cyclic HS conditions of 28 to 34 °C until 140 d of age (Figure 2b). The GTN room was maintained in cyclic TN conditions from 82 to 140 d of age. Ad libitum access to a standard growing-finishing feed (16.3% CP; 2,495 kcal/kg NE) and to water was provided throughout the growing period. Meals were distributed three times daily (0900, 1300, and 1600 hours). The pigs were slaughtered at 140 d of age.
Measurements
Growth and slaughter performance
For the overall growth performance, the two main periods previously described (i.e., 75 to 81 and 82 to 140 d of age) were considered. The pigs were individually weighed at the beginning of each main period, every 1 or 2 wk during the experimental period, and on the day before slaughter. The daily FI was measured for each pen as the difference between offered and refused feed. Refusals and spillages were collected daily at 0800 hours before the first feed distribution of the day; their dry matter (DM) (103 °C for 24 h) were also determined daily.
Pigs were slaughtered in the experimental slaughterhouse of INRAE-UEPR after 24 h fasting. Pigs were slaughtered by electrical stunning and exsanguination in compliance with the current national regulations applied in slaughterhouses. Hot carcass, perirenal fat, and head were weighed just after slaughter. Weights of the hypothalamus and pituitary gland were also recorded. Backfat (G2) and muscle (M2) depths were measured on one dorsal spot between the third and fourth last ribs at 6 cm of the spinal canal axis, using a Capteur Gras Maigre (Lean Fat Sensor) (DGM) device (Fives Syleps, Lorient, France). BFT was measured on carcass split at three different locations: on the first and last ribs and on the Gluteus muscle (minimum fat). The length of the left side of the carcass was also measured. On the day after slaughter, cold carcass and wholesale cuts from the right carcass side (ham, loin, shoulder, belly, and backfat) were weighed.
Physiological parameters
Rectal temperature was measured using a digital thermometer (Microlife Corporation, Paris, France; accuracy ±0.1 °C) and skin temperature by a Type K thermocouple probe (HH-21 model, Omega, Stamford, CT, USA; accuracy ±0.1 °C). These measurements were done on the sows at days 4, 9, 12, 29, 60, 106, and 110 of gestation and days 2, 6, 13, 20, and 26 of lactation. In the growing pigs, rectal and skin temperatures were measured at 1300 hours on days −5, 0, 2, 3, 7, 29, 43, and 53 of the second main experimental period. Skin temperature was not measured on day 3. On all pigs, at 1330 hours on days −4, 3, and 49, blood was collected at the jugular vein in heparin tubes, centrifuged (3,000 × g; 10 min; 4 °C), and plasma was stored at −20 °C until analysis. Commercially available kits were used to measure the plasma levels of creatinine (Creatinine [Jaffe], Thermo Fisher Scientific Oy, Vantaa, Finland), glucose (Glucose [HK], Thermo Fisher Scientific Oy, Vantaa, Finland), nonesterified fatty acids or NEFA (FUJIFILM Wako Chemicals Europe GmbH, Neuss, Germany), and biological antioxidant potential or BAP (Diacron Labs srl, Grosseto, Italy). Intra-assay coefficient of variation (CV) was 4.9%, 1.7%, 0.4%, and 3.5%, respectively. Inter-assay CV was 7.0%, 8.4%, 2.4%, and 5.1%, respectively. For the enzymatic activities, creatine kinase or CK (CK [IFCC], Thermo Fisher Scientific Oy, Vantaa, Finland), lactate dehydrogenase or LDH (LDH [IFCC], Thermo Fisher Scientific Oy, Vantaa, Finland), and superoxide dismutase or SOD (Sigma-Aldrich, St Louis, MO) were measured. Intra-assay CV was 11.1%, 1.1%, and 4.1%, respectively. Inter-assay CV were 16.8% and 17.2% for CK and LDH, respectively. The plasma levels of thyroid hormones of T3 and T4 (ST AIA-PACK TT3 and ST AIA-PACK T4, Tosoh Corporation, Tokyo, Japan) were also determined. Intra-assay CV was 3.8% and 3.9%, respectively.
Calculations
Live BW measured on 75, 82, and 140 d of age were considered. Growth performance was calculated in two ways. First, for the overall growth performance, the average performance of the two main periods was considered (75 to 82 and 82 to 140 d of age). Since pen was the experimental unit, the average daily gain (ADG) for a given period corresponded to the mean of the three individual ADG. The average daily feed intake (ADFI) (measured per pen and divided by 3) was expressed in two ways: as the classical ADFI (g/d) and as the ADFI per metabolic BW (g·kg–0.60·d–1). The feed conversion ratio (FCR) was calculated as the FI divided by the BW gain for a given pen and for a given period. For data collected only from 82 to 140 d of age, this second main period was split into five subperiods: subperiod 1 (day 0 to 6; with day 0 as the transition of the GHS room to cyclic HS conditions) and subperiods 2, 3, 4, and 5 (day 7 to 15, 16 to 29, 30 to 43, and 44 to 58, respectively). Growth performance (ADFI per metabolic BW and ADG) was calculated for each subperiod.
For the carcass traits, carcass dressing was calculated as the percentage of hot carcass to slaughter BW (sBW). Wholesale cut weights were expressed as a percentage of the cold right carcass side. Carcass lean meat content was calculated using the CGM measurements (G2 and M2) according to the equation by Daumas et al. (2010): Lean meat content (%) = 62.19 − 0.729 G2 + 0.144 M2. Average BFT was calculated as the mean of the measurements from the three different locations previously described. For the thermoregulation parameters, temperature gradient was calculated as the difference between rectal and skin temperatures. Data of enzymes CK and LDH were log-transformed to follow normal distribution.
Statistical analyses
According to the factorial design based on 2 postnatal growing environments (GE; GTN and GHS) and 2 prenatal environments (PE; PTN and PHS), there were 4 treatments (i.e., GTN–PTN, GTN–PHS, GHS–PTN, and GHS–PHS) with 4 pens per treatment, for a total of 12 pigs per treatment.
For the overall growth performance, the pen (n = 16) was considered as the experimental unit and data were analyzed using a repeated measure of the PROC MIXED procedure (SAS Inst. Inc., Cary, NC) considering PE (n = 2), GE (n = 2), the two main periods (n = 2; n = 3 for live BW), their interactions, and sire (n = 4) as fixed effects. The average growth performance during the second main period was also analyzed using PROC MIXED model with the PE (n = 2), GE (n = 2), their interaction, and sire (n = 4) as fixed effects, and including the growth performance during the first main period as covariates. For growth performance calculated per subperiod (ADG and ADFI per metabolic BW), data were analyzed using a repeated measure of the PROC MIXED procedure considering the PE (n = 2), GE (n = 2), subperiod (n = 5), their interactions, and sire (n = 4) as fixed effects and with performance measured during the first main period as covariates.
For carcass and physiological parameters, the pig (n = 48) was used as the experimental unit. Individual pig data were analyzed using the PROC MIXED procedure with the PE (n = 2), GE (n = 2), their interaction, pen (n = 16), and sire (n = 4) as fixed effects. Slaughter BW was included in the model as a covariate for data analysis of carcass traits. Thermoregulation and blood parameters were subjected to a repeated measurement PROC MIXED procedure based on the days of measurement (n = 8 for rectal temperature, n = 7 for skin temperature and temperature gradient, and n = 3 for blood parameters) and the interactions with PE and with GE. Thermoregulation responses were also subjected to another repeated measurement analysis but only considering measurements during the second main period (days 2, 3, 7, 29, 43, and 53 measurements).
Results
The average hourly temperature of the rooms (Figure 2) indicates that the actual average minimal temperatures (20 to 25 °C for PTN; 29 to 34 °C for PHS; 19 to 24 °C for GTN; 29 to 34 °C for GHS) were slightly higher than the targeted temperatures.
Thermoregulatory responses
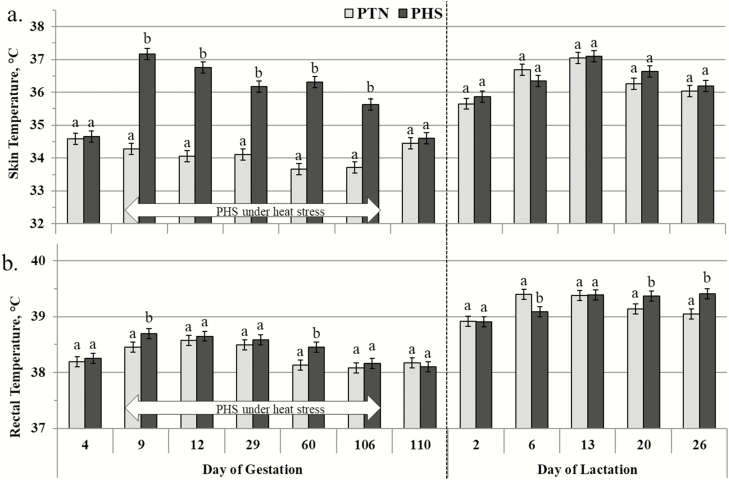
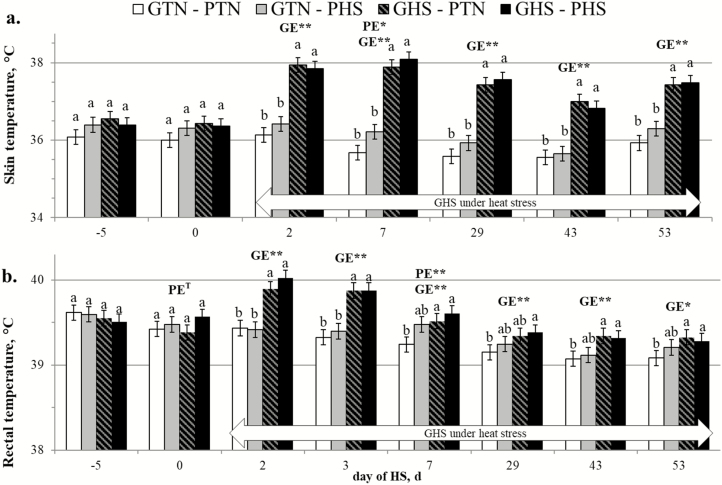
The results for the skin and rectal temperatures of the sows during gestation and lactation are presented in Figure 3. For the overall duration of HS exposure, gestating PHS sows had higher skin temperature (36.40 vs. 33.96 °C on average; P < 0.001) and rectal temperature (38.51 vs. 38.35 °C on average; P = 0.020) than PTN sows. For growing pigs, thermoregulation data of two pigs from GTN-PHS group were removed because they were sick from day 0 to 2 of the second main period. The skin and rectal of the growing pigs are presented in Figure 4. Considering only the second main period, regardless of the PE, GHS pigs had higher skin temperature (37.55 vs. 35.94 °C on average; P < 0.001) and rectal temperature (39.57 vs. 39.27 °C on average; P < 0.001) compared with their GTN counterparts. On day 2 of the second main period, GHS pigs had higher skin temperature (37.89 vs. 36.27 °C on average; P < 0.001) and rectal temperature (39.96 vs. 39.43 °C on average; P < 0.001) compared with GTN pigs. Thereafter, skin temperature remained significantly and constantly higher in GHS than in GTN pigs until day 53 (37.45 vs. 36.11 °C on average; P < 0.001), whereas rectal temperature gradually decreased and by day 53, the difference was less pronounced although still significant (39.30 vs. 39.15 °C on average; P = 0.011).
Figure 3.
Effect of the climatic environment on the skin (a) and rectal (b) temperatures of mixed parity sows from gestation to lactation (LSmeans ± SEM). From day 9 to 109 of pregnancy, sows (n = 24) were subjected to one of two environments: thermoneutral (PTN; 18 to 24 °C) and heat-stressed (PHS; 28 to 34 °C). During the whole lactation period, sows were kept at 25 °C. a,bWithin each day, LSmeans with different superscript letters differ according to the experimental group (P < 0.05).
Figure 4.
Effect of climatic environment (TN or HS) during prenatal development (PE; PTN vs. PHS) and during growing (GE; GTN vs. GHS) on the skin (a) and rectal (b) temperatures of growing pigs (LSMeans ± SEM). a,bWithin each day, LSmeans with different superscript letters differ according to the experimental group (P < 0.05). PE = effect of the prenatal environment, regardless of the growing environment. GE = effect of the growing environment regardless of the prenatal environment. *P < 0.05, **P < 0.01.
Regarding the interaction between PE × GE, the rectal temperature of the two GHS groups was not different from those of GTN–PHS pigs (P > 0.100) but was higher than those of GTN–PTN pigs on days 7, 43, and 53 of HS (P < 0.050). Considering all repeated measures during the second main period, an overall significant PE × GE interaction was also observed for skin temperature (P = 0.005) and for temperature gradient (P = 0.018; data not shown). In GTN conditions, PHS pigs had higher skin temperature (36.10 vs. 35.77 °C on average; P < 0.001) and narrower temperature gradient (3.20 vs. 3.42 °C on average; P = 0.015) than PTN pigs, but not in GHS conditions (37.55 °C on average, P = 0.979 for skin temperature; and 1.96 °C on average P = 0.997 for temperature gradient). In GTN conditions, the rectal temperature of PHS pigs was also higher than those of PTN pigs (39.32 vs. 39.22 °C on average; P = 0.038) but not in GHS conditions (39.57 °C on average; P = 0.735).
Growth performance
Table 1 shows the summary of the growth performance of the growing pigs starting at 35.9 ± 0.8 kg BW. The interaction between PE and GE treatments was not significant except a trend (P = 0.059) for the live BW, with PHS pigs tending to be lighter at final BW than PTN pigs when raised in GHS conditions but not in GTN conditions. Regardless of PE treatment, GHS pigs had lower final BW than GTN pigs (97.2 vs. 105.1 kg; P < 0.001). Neither GE nor PE had significant effect on the FCR (P = 0.221 and P = 0.549, respectively). In the second main period (82 to 140 d of age), the GHS pigs had overall lower performance than GTN pigs in terms of ADG (1,003 vs. 1,130 g/d on average; P = 0.017), ADFI (2,327 vs. 2,659 g/d on average; P = 0.008), and ADFI per metabolic BW (199 vs. 181 g·kg–0.60·d-1 on average; P = 0.045). When corrected for the same performance during the first main period, PHS tended to have higher ADFI per metabolic BW than PTN pigs in the second main period (190 vs. 185 g·kg–0.60·d-1 on average; P = 0.097).
Table 1.
Effect of the prenatal and postnatal (growing) climatic environment on the growth performance of growing pigs1
| Items | GTN–PTN | GTN–PHS | GHS–PTN | GHS–PHS | RSD2 | Statistics3 |
|---|---|---|---|---|---|---|
| Live BW, kg | ||||||
| 75 d | 35.8 | 36.1 | 36.6 | 35.2 | 1.7 | GE**, P**, S* PE × GET, GE × P** |
| 82 d | 42.3 | 42.6 | 43.7 | 41.6 | 1.69 | |
| 140 d | 104.4a | 105.8a | 98.8b | 95.6b | ||
| ADG, g · d−1 | ||||||
| 75 to 81 d | 859 | 888 | 997 | 943 | 70 | P**, GE × P** |
| 82 to 140 d | 1,147 | 1,114 | 1,017 | 989 | ||
| 82 to 140 d4 | 1,110a | 1,129a | 983b | 967b | 52 | GE** |
| ADFI, g · d−1 | ||||||
| 75 to 81 d | 1,729 | 1,884 | 1,996 | 1,901 | 168 | P**, GE × P** |
| 82 to 140 d | 2,635 | 2,683 | 2,347 | 2,307 | ||
| 82 to 140 d4 | 2,602a | 2,680a | 2,215b | 2,255b | 84 | GE**, S* |
| ADFI per metabolic BW, g · kg–0.60·d–1 | ||||||
| 75 to 81 d | 198 | 208 | 218 | 210 | 11 | P**, GE × P* |
| 82 to 140 d | 196 | 202 | 180 | 181 | ||
| 82 to 140 d4 | 198a | 202a | 172b | 178b | 6 | PET, GE**, S* |
| FCR | ||||||
| 75 to 81 d | 2.02 | 2.12 | 2.01 | 2.02 | 0.09 | P** |
| 82 to 140 d | 2.30 | 2.41 | 2.31 | 2.33 | ||
| 82 to 140 d4 | 2.34 | 2.37 | 2.29 | 2.31 | 0.08 | ns |
1A total of 48 female pigs (housed three pigs per pen) were distributed to a 2 x 2 factorial design based on their prenatal environment (PE) and their postnatal growing environment (GE): TN – thermoneutral (18 to 24˚C), HS = heat-stressed (28 to 34˚C). First main period: Pigs were maintained in TN conditions from 75 to 81 d of age. Second main period: The GTN room was maintained in TN conditions until 140 d of age; in GHS room, temperature transition started at 82 d of age and full-blown thermal challenge was from 84 to 140 d of age.
2Residual standard deviation.
3The pen was considered as the experimental unit. Data were analyzed using PROC MIXED model with PE, GE, period (P), their interactions, and sire (S) as fixed effects. LS means with different superscript letters differ according to the experimental group TP < 0.10, *P < 0.05, **P < 0.01.
4Adjusted performance based on the average value measured during first main period (944 g/d, 1,915g/d, 212 g·kg BW−0.60·d−1, and 2.03 for ADG, ADFI, ADFI per metabolic BW, and FCR, respectively).
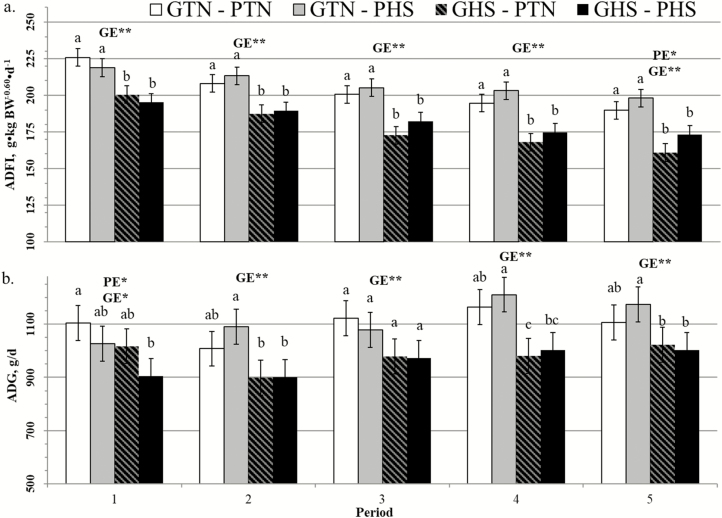
Looking at the subperiods during the second main period (Figure 5), there were no significant interactions between GE and subperiod for ADFI per metabolic BW (P = 0.819) and ADG (P = 0.467), because GHS pigs performed consistently lower than GTN pigs (P < 0.050). Meanwhile, there was a significant interaction between PE and subperiod for ADFI per metabolic BW (P = 0.012). Regardless of the GE group, PHS pigs ate more than PTN pigs starting only from the subperiod 3 with this difference being significant on subperiod 5 (186 vs. 175 g·kg–0.60·d-1 on average for PTN and PHS pigs, respectively; P = 0.022). There was also a tendency for a PE and subperiod interaction (P = 0.061) for ADG but no significant difference was seen between PHS and PTN pigs in any of the subperiods.
Figure 5.
Effect of climatic environment (TN or HS) during prenatal development (PE; PTN vs. PHS) and during growing (GE; GTN vs. GHS) on ADFI per metabolic BW (a) and ADG (b) in growing pigs using TN performance as covariate (LSMeans ± SEM). Subperiod 1: day 0 to 6 of GHS; subperiod 2: day 7 to 15; subperiod 3: day 16 to 29; subperiod 4: day 30 to 43; and subperiod 5: day 44 to 58. a,b,cWithin each subperiod, LSmeans with different superscript letters differ according to the experimental group (P < 0.05). PE = effect of the prenatal environment, regardless of the growing environment. GE = effect of the growing environment regardless of the prenatal environment. *P < 0.05, **P < 0.01.
Carcass and organ traits
The slaughter performance of the pigs is presented in Table 2. The sBW of GHS pigs was lower than those of GTN pigs (P < 0.001), while the hot carcass weight of GHS–PHS pigs was lower than those of the two GTN groups (P < 0.006) but not different from those of the GHS–PTN pigs (P = 0.431). Postnatal HS decreased carcass length (P < 0.001) and tended to increase carcass dressing (P = 0.068) but was not affected by the prenatal treatment. Expressed as a percentage of the sBW, PHS pigs tended to have lower head weight (P = 0.066) than PTN pigs, regardless of the GE.
Table 2.
Effect of the prenatal and postnatal (growing) climatic environments on the carcass and organ traits of growing pigs1
| Items | GTN–PTN | GTN–PHS | GHS–PTN | GHS–PHS | RSD2 | Statistics3 |
|---|---|---|---|---|---|---|
| No. of pigs | 12 | 12 | 12 | 12 | ||
| Slaughter BW (sBW), kg | 102.3ab | 103.6a | 96.8bc | 93.7c | 5.6 | GE** |
| Hot carcass weight, kg | 80.8a | 81.7a | 77.0ab | 74.2b | 4.5 | GE** |
| Carcass dressing, % | 79.1 | 78.8 | 79.6 | 79.2 | 0.9 | GET |
| Carcass length, cm | 95.7ab | 97.4a | 94.9bc | 93.4c | 1.9 | GE**, PE × GE** |
| Head, % sBW | 4.47ab | 4.31b | 4.59a | 4.49ab | 0.24 | PET, GE* |
| Carcass composition4 | ||||||
| Lean meat, % | 63.5a | 63.4a | 63.2a | 61.8b | 1.2 | PE*, GE*, PE × GET, sBW** |
| Average BFT, mm | 18.7bc | 17.0c | 19.3ab | 20.9a | 1.7 | GE**, PE × GE**, sBW** |
| Perirenal fat, g/kg sBW | 7.6ab | 7.0b | 7.6ab | 9.2a | 1.5 | PE × GE* |
| Carcass cuts4, % cold carcass wt | ||||||
| Loin | 29.0a | 29.2a | 27.8b | 27.9b | 0.9 | GE** |
| Ham | 26.8bc | 26.3c | 27.5a | 27.1ab | 0.6 | PE*, GE** |
| Belly | 12.1 | 12.0 | 12.2 | 12.5 | 0.6 | sBW** |
| Backfat | 4.9ab | 4.6b | 4.9ab | 5.4a | 0.6 | GET, PE × GE*, sBW** |
| Organ wt., mg/kg BW | ||||||
| Hypothalamus | 11.2a | 9.8b | 10.1ab | 10.8ab | 1.5 | PE × GE* |
| Pituitary gland | 2.8ab | 2.7b | 2.7b | 3.0a | 0.3 | PE × GE* |
1A total of 48 female pigs (housed three pigs per pen) were distributed to a 2 x 2 factorial design based on their prenatal environment (PE) and their postnatal growing environment (GE): TN – thermoneutral (18 to 24˚C), HS = heat-stressed (28 to 34˚C).
2 Residual standard deviation.
3The pig was considered as the experimental unit. Data were analyzed using PROC MIXED model with PE, GE, their interaction, pen, and sire as fixed effects. TP < 0.10, *P < 0.05, **P < 0.01. LS means with different superscript letters differ according to the experimental group.
4Slaughter BW was used as covariate (adjusted sBW = 99.1 kg).
Looking at carcass traits adjusted for the same sBW, lean meat content was decreased both by postnatal HS (P = 0.028) and prenatal HS (P = 0.029), with GHS–PHS pigs having the lowest lean meat content compared with the three other groups (P < 0.040). A PE × GE interaction was also observed for average BFT (P = 0.003) and for cold carcass proportions of backfat (P = 0.016) and of perirenal fat (P = 0.017), with PHS pigs being fatter than their PTN counterparts when they were submitted to postnatal HS. Irrespective of the prenatal treatment, postnatal HS also increased average BFT (P < 0.001) and ham percentage (P = 0.001) and decreased loin percentage (P < 0.001). Meanwhile, prenatal HS decreased ham percentage (P = 0.007) regardless of the GE treatment. Neither postnatal nor prenatal HS affected belly percentage (P = 0.171 and P = 0.514, respectively).
For the brain parts, a PE and GE interaction was observed for the weight of the hypothalamus (P = 0.022) and of the pituitary gland (P = 0.038) expressed per kg BW. Hypothalamus of GTN–PTN pigs were heavier (P = 0.027) than that of GTN–PHS pigs but were not different from the two GHS groups (P > 0.050). Meanwhile, pituitary gland of GHS–PHS pigs was heavier (P < 0.050) than those of GTN–PHS and GHS–PTN pigs but not different (P = 0.223) to that of GTN–PTN pigs.
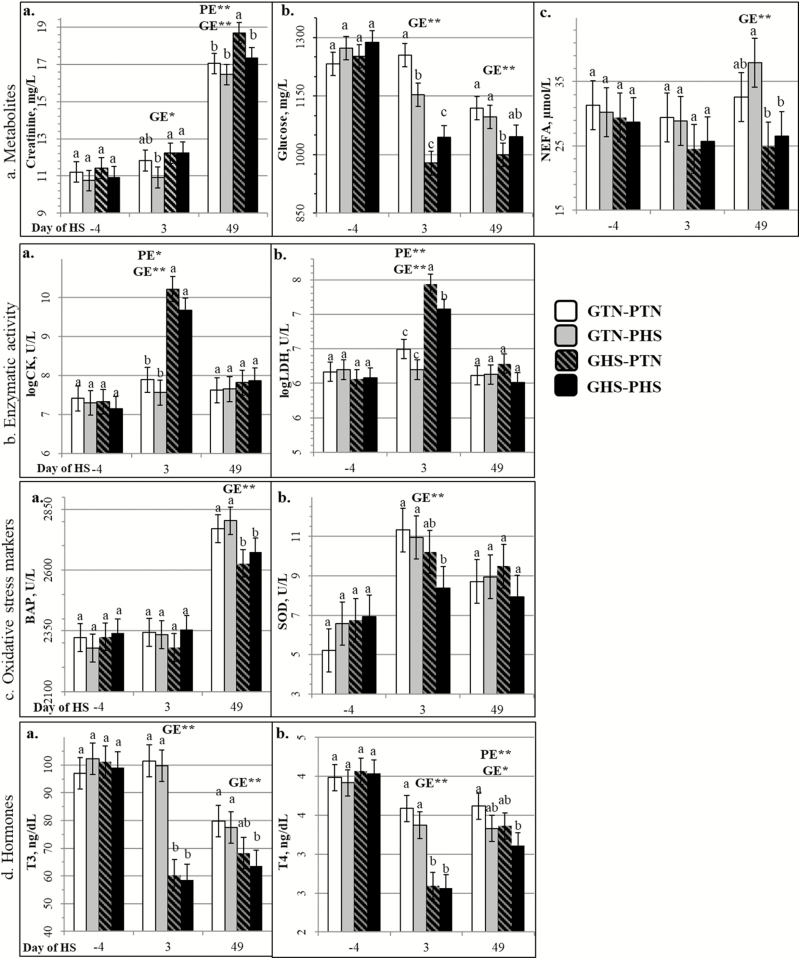
Plasma parameters
Whatever the parameter, plasma concentration was similar among all four treatments on day −4 (Figure 6). The PE × GE interaction was significant for some blood parameters at some specific days. Plasma creatinine concentration was higher in GHS pigs (GHS–PTN and GHS–PHS) compared with GTN–PHS pigs on day 3 (P = 0.049 and P = 0.026, respectively) and was highest in GHS–PTN pigs among all groups on day 49 (P < 0.030). Plasma glucose of the GTN pigs (GTN–PTN and GTN–PHS) was higher than the GHS–PTN pigs on day 49 (P = 0.008 and P = 0.036, respectively). Plasma NEFA of the GHS pigs (GHS–PTN and GHS–PHS) were lower only when compared with GTN–PHS pigs (P = 0.001 and P = 0.008, respectively) and only on day 49. The GTN groups (GTN–PTN and GTN–PHS) had higher SOD plasma levels on day 3 (P = 0.004 and P = 0.031, respectively) and higher plasma T3 levels on day 49 (P = 0.006 and P = 0.025, respectively) compared with GHS–PHS pigs. And on day 49, plasma T4 was higher in GTN–PTN pigs than in GHS–PHS pigs (P = 0.003).
Figure 6.
Effect of climatic environment (TN or HS) during prenatal development (PE; PTN vs. PHS) and during growing (GE; GTN vs. GHS) on plasma parameters of growing pigs (LSMeans ± SEM). a,bWithin each day, LSmeans with different superscript letters differ according to the experimental group (P < 0.05). PE = effect of the prenatal environment, regardless of the growing environment. GE = effect of the growing environment regardless of the prenatal environment. *P < 0.05, **P < 0.01.
Regardless of PE treatment, acute and chronic postnatal HS (on days 3 and 49, respectively) increased creatinine (P = 0. 012 on day 3; P < 0. 001 on day 49) and decreased glucose (P < 0. 001 on day 3; P = 0. 002 on day 49), T3 (P < 0. 001 on days 3 and 49), and T4 (P < 0. 001 on day 3; P = 0. 020 on day 49) plasma concentrations. Compared with GTN pigs, plasma NEFA levels of GHS pigs were lower on day 3 (trend at P = 0.074) and on day 49 (P < 0.001). Higher activities of CK and LDH (P < 0.001) were observed in GHS pigs but only on day 3. Postnatal HS also decreased plasma SOD levels on day 3 (P = 0.004) and BAP levels on day 49 (P < 0.001). Regardless of the postnatal treatment, plasma SOD level tended to be lower (P = 0.082) in PHS pigs than in PTN pigs on day 3. Prenatal HS also decreased plasma T4 on day 49 (P = 0.007) without a significant decrease in plasma T3 (P = 0.305).
Discussion
In this study, we investigated the effects of both prenatal and postnatal thermal environments on performance and physiological responses in growing pigs. The cyclic HS treatments applied in the experiment were enough to elicit responses from both the PHS sows and GHS pigs as they exhibited biphasic acclimation HS responses similar to previous studies (Black et al., 1993; Renaudeau et al., 2010). This response, as described by Renaudeau et al. (2010), is characterized by a short-term heat acclimation where pigs experience rapid physiological changes as shown by the spike in rectal temperature and followed by a long-term heat acclimation where animals show improved heat tolerance by increasing the ability to dissipate heat and to decrease HP. The higher skin temperature of PHS sows and GHS pigs throughout the thermal challenge can be related to the shift of the blood flow more toward the peripheral tissues and away from internal tissues (Collin et al., 2001a). The GHS pigs in our study were able to adapt to chronic HS as reflected in their decreasing rectal temperatures. Exposure to HS has been shown to decrease FI and to decrease metabolic HP due to a reduction in maintenance requirements as adaptation responses (Quiniou et al., 2000; Renaudeau et al., 2013). The FI reduction was not observed in gestating sows submitted to HS (data not shown) because a large part of the ration was given at 0800 hours when the ambient temperature was low and because they had a strict daily feed allowance, thus leaving no room for refusals.
Effects of postnatal HS on growth performance and metabolism
In the present study, postnatal HS decreased ADFI per metabolic BW of growing pigs by 39 g·kg–0.60·d–1 on average, which is slightly lower than the values reported by Renaudeau et al. (2008) in younger pigs (−40, −65, and −104 g·kg–0.60·d–1 at constant temperatures of 28, 32, and 36 °C, respectively, compared with controls). It is possible that implementing cyclic rather than constant HS conditions allowed the pigs in the present study to compensate by eating more feed during the colder parts of the day (decrease in ADFI is lower at 28 °C than at 32 °C). Nevertheless, the reduction in ADFI had negative consequences on the growth rate due to a reduction in nutrient intake similar to a previous study (Collin et al., 2001b).
Carcass traits were adjusted for the same sBW in our study to evaluate the strict effect of HS on carcass composition and to take into account the study limitations of not having the pigs slaughtered at the same live BW. Based on the fact that maximum protein deposition can be reached at 80 kg and maximum fat deposition is not reached even in the range of 110 to 130 kg BW (Van Milgen and Noblet, 2003), it can be assumed that GHS pigs (average of 95.3 kg at slaughter) would deposit more fat than lean if they had been slaughtered at the same BW (but older age) as GTN pigs (average of 103.0 kg at slaughter). In our study, the GHS pigs were fatter than GTN pigs which is in contrast to other studies with ad libitum-fed pigs subjected to constant high ambient temperature (32 to 33 °C) being leaner and having less lipid deposition than pigs in TN conditions (Collin et al., 2001b; Cruzen et al., 2015). Differences in results could be related to the previously discussed higher level of ADFI reduction in pigs under constant HS conditions compared with the cyclic HS conditions implemented in our study. Kouba et al. (2001) reported increased triglyceride uptake and storage in HS pigs compared with their pair-fed TN counterparts and the “additional” FI in our study could have been deposited as fat since pigs decrease their metabolic HP during chronic postnatal HS and it is more energy efficient to deposit fat (Van Milgen and Noblet, 2003). The fatter carcasses of GHS pigs are logically associated to lower loin (lean cut) percentage, whereas the increased ham percentage of GHS pigs may be explained by the changes in conformation as shown by their shorter carcass.
The drop in plasma thyroid hormone levels of GHS pigs can be linked to the reduction of their ADFI and to the lower growth and HP (Collin et al., 2002). Since thyroid hormones stimulate dietary and endogenous fat breakdown (Sinha et al., 2018), the lower T3 and T4 levels found in postnatal HS pigs in our study and in agreement with Sanz Fernandez et al. (2015) could contribute to the increased body fat content of GHS compared with GTN pigs, as also shown in rats (Iossa et al., 2001). Plasma NEFA of GHS pigs were also lower which can be due to lower amounts of lipid ingested because of the reduced FI or to a limited ability of HS pigs to mobilize fat as demonstrated by Pearce et al. (2013). Indeed, it has been previously suggested that pigs subjected to postnatal HS had lower metabolic flexibility with a lower fatty acid oxidation in their skeletal muscles than pair-fed TN pigs (Baumgard and Rhoads, 2013; Zhao et al., 2018). The elevated plasma creatinine level and CK activity in GHS pigs can also suggest increased muscle protein catabolism instead of fat catabolism for energy production (Clarkson et al., 2006). Lower plasma SOD (in acute HS) and plasma BAP (in chronic HS) levels measured in GHS pigs could be indications of reduced antioxidant capacities similar to previous reports in pigs (Yang et al., 2014; Liu et al., 2018) which, in humans, could be markers of metabolic syndrome such as lower adiponectin and increased insulin (Kim et al., 2014). Altogether, the results of our study thus validate the effects of postnatal HS as reported in existing literature, such as reduction in growth performance, increased carcass adiposity, and altered physiology and metabolism.
Effects of prenatal HS on growth performance and metabolism in growing pigs raised in postnatal TN conditions
The effects of prenatal HS on growth performance, metabolism, and physiology in swine have been studied in the past few years as reviewed by Johnson and Baumgard (2018). According to Johnson et al. (2015c), prenatal HS increased the core temperature of growing pigs, which is comparable to our results. Prenatal stress and increased cytokines in the fetal environment have both been reported to alter set point in the hypothalamic–pituitary–adrenal (HPA) axis (Glover et al., 2010; Dreier et al., 2014), which plays a role in the body temperature control of mammals including pigs (Bligh, 1966; Baldwin and Ingram, 1968). In our study, there were indeed differences in the relative weight of hypothalamus and pituitary gland among the treatments. The anterior and posterior parts of these glands have different functions which could explain why the glands did not have the same pattern of increase or decrease in weight. However, the gland parts were not weighed separately in our study, so this remains to be theoretical. Nevertheless, pituitary gland size itself does not signify a lower or higher hormone activity; it can only be suggested that these observed changes could be related to morphological changes and/or abnormalities that, in humans, are related to altered pituitary function (Maghnie et al., 1991; Cooper et al., 2017). These changes could also be related to smaller head size of GHS pigs observed in our and in other studies (Cruzen et al., 2015; Johnson et al., 2015b). It can thus be hypothesized that the higher rectal temperature of prenatal heat-stressed pigs observed at many stages during the growing phase could be a consequence of an altered HPA set point due to their fetal environment.
Chapel et al. (2017) suggested that the elevated core temperature of pigs submitted to in utero HS pigs during mid-gestation could be associated to higher fasting HP and circulating T3 levels. Meanwhile, Johnson et al. (2015c) found that although in utero HS pigs in their study also exhibited increased body temperature, they found no effect in plasma levels of T4 (a pro-hormone) nor of T3 (active form of T4) regardless of the timing (first or second half of gestation) and the length of gestational HS. In our study, however, PHS pigs subjected to whole-gestation HS had lower levels of plasma T4 with no significant change in plasma T3 levels. Nevertheless, these results suggest that prenatal HS, depending on the timing and duration, can alter thyroid functions which to some extent could also be associated to the previously discussed hypothesis of an altered HPA set point. Between the two GTN groups in our study, those subjected to prenatal HS also had higher skin temperature denoting increased heat dissipation activity (possibly from the increased rectal temperature) and had a narrower temperature gradient implying a lower capacity to further dissipate heat (Cuddy et al., 2014).
The increased core temperature could also be partly attributed to a higher thermic effect of feeding linked to the age-dependent ADFI increase of PHS pigs in our study. This higher ADFI of prenatally heat-stressed pigs, however, failed to translate to a significant ADG increase in agreement to previous studies (Cruzen et al., 2015; Safranski et al., 2015; Wilmoth et al., 2015). There is also an age-dependent decrease in feed efficiency in in utero HS pigs as it was observed during the finishing phase (Johnson et al., 2015b) but not in the growing phase (Johnson et al., 2015a). These results suggest the limited ability of PHS pigs to convert increased intake to gain which can be an effect of intrauterine growth restriction due to fetal undernutrition (Ji et al., 2017). This can be related to reports of prenatal HS causing placental insufficiency or inefficiency (Galan et al., 1999; Zhao et al., 2019) perhaps due to blood redirection away from the placenta in heat-stressed sows. According to Boddicker et al. (2014), the positive effect of prenatal HS on subsequent pig FI is dependent on the HS timing, and pigs from sows heat-stressed in the first half of gestation ate more than pigs from sows heat-stressed in the second half of gestation. Altogether, this suggests that prenatal HS can indeed affect postnatal pig performance. Whether this effect is neutral, positive, or negative seems to depend, among other things, on the age of the offspring and the timing and duration of the prenatal HS.
In utero heat-stressed pigs reared in TN conditions have been described to have increased fat deposition in some studies (Boddicker et al., 2014; Johnson et al., 2015b); however, other studies found no effect (Cruzen et al., 2015; Johnson et al., 2015a) comparable to our results. Differences between studies could be due to several factors, such as pig physiological stage considered or sex: fatter carcasses of prenatal HS in TN-reared pigs have been observed in mixed-sex (barrows and females) finishing pigs (Johnson et al., 2015b), but not in mixed-sex young and growing pigs (Boddicker et al., 2014; Johnson et al., 2015a) nor in finishing barrows (Cruzen et al., 2015). These discrepancies may be at least partly explained by differential effects of prenatal HS on pig FI between studies, as the increased FI generally observed with increasing pig age and BW is associated to increased fat and decreased protein deposition (Van Milgen and Noblet, 2003). In our study, the increased FI of PHS pigs was significant only at the end of the experimental growth period, which was probably insufficient to significantly influence body fatness of PHS pigs in postnatal TN conditions. Finally, as mentioned previously, the prenatal HS effect can depend on the timing and duration. For example, pigs exposed to prenatal HS only in the first half of gestation had thicker subcutaneous fat than control pigs, but pigs exposed during the entire gestation exhibited no significant response (Boddicker et al., 2014), in agreement with present results. Overall, the results from our study and from existing literature show that in pigs in postnatal TN conditions, exposure to prenatal HS can increase body temperature attributed to a possible alteration in the HPA axis and in thyroid functions. Depending on the timing and duration, prenatal HS can also increase carcass adiposity but this is not proven for TN-raised pigs exposed to a whole-gestation prenatal HS.
Effects of prenatal HS on growth performance and metabolism in growing pigs subjected to postnatal HS
Despite the increasing literature in prenatal HS in pigs, there is still little known on the effect of prenatal HS on the ability of pigs to cope with postnatal HS. In our study, prenatal HS tended to reduce plasma SOD during acute postnatal HS. This result is in agreement with previous studies (Lista et al., 2010; Yin et al., 2018), which suggests a decreased anti-oxidative capacity previously reported in the offspring of stressed mammals. This decreased oxidative stress tolerance and other prenatal HS effects discussed so far can influence the pig’s postnatal life and production efficiency. Indeed, prenatal HS seemed to aggravate the production performance of GHS pigs in our study as GHS–PHS pigs had slightly lower ADG and final BW than GHS–PTN pigs even with similar overall ADFI. Under postnatal HS, both GHS groups still had higher rectal temperature than GTN–PTN pigs even after 53 d of chronic HS suggesting they were unable to dissipate all heat necessary to lower body temperature to a similar level with pigs kept in prenatal and postnatal thermoneutrality. In the previous section, the decreased ability of pigs to dissipate heat as a consequence of prenatal HS was discussed. At 28 to 34 °C, pigs in our study may have already reached the maximum heat dissipation level through the skin as GHS–PHS and GHS–PTN had similar skin temperature and temperature gradient. With a reduced anti-oxidative capacity, one could hypothesize a decreased heat tolerance in PHS pigs as they were subjected to postnatal HS wherein heat dissipation has already been maximized.
In the present study, the GHS–PHS pigs had lower carcass lean meat content than GHS–PTN pigs and tented to exhibit higher fatness at both subcutaneous and internal levels. Reasons for this decrease in leanness are still unclear but one theory could be related to the hypothesized fetal growth restriction that, in other mammals, is linked to impaired glucose and insulin metabolism (Thureen et al., 1992; Lesage et al., 2004). Indeed, Johnson et al. (2015b) also reported that insulin per unit of glucose or per kg of FI was higher in in utero HS pigs but only if raised in the postnatal HS environment. Boddicker et al. (2014) reported that it is the exposure to prenatal HS during the first half of the gestation that increases circulation insulin levels regardless of the environment during the second half of gestation. A whole-gestation prenatal HS has also been reported to increase postnatal stress response of pigs (Merlot et al., 2018) possibly linked to the hypothesized altered HPA set point. Hypersecretion of chronic stress hormones in humans is attributed to increased visceral fat accumulation and muscle loss (Pervanidou and Chrousos, 2012) and could be related to the increased visceral adiposity of pigs subjected to both prenatal and postnatal HS in our study.
With effects such as heightened postnatal stress responses, altered thyroid functions, decreased heat dissipation ability, and reduced anti-oxidative capacity, chronic prenatal HS seems to impair long-term heat tolerance of pigs in our study, as indicated by their reduced growth, increased body adiposity, and overall decreased productive efficiency. However, it must be noted that the present study considered only a chronic whole-gestation HS effect. Existing literature in pigs and other mammals suggests that impacts on pig performance and carcass composition could differ depending on the timing, duration, and intensity of the prenatal HS applied, and this still remains to be further investigated.
Conclusion
Studies about prenatal HS on growing pigs are still limited. Chronic prenatal HS in our study decreased heat tolerance of growing pigs due to increased body temperature and altered thyroid functions and physiological stress responses. With the increasing impact of climate change, this implies a decreased global swine production efficiency. Existing literature and present data suggest that multiple factors can influence prenatal HS effects, such as duration and timing during gestation, postnatal thermal environment, and physiological stage of pigs. More studies are needed to further elucidate its effects and biological mechanism in pigs.
Acknowledgments
We want to thank F. Le-Gouevec, A. Chauvin, M. Genissel, J. Georges, J. Delamarre, H. Demay, D. Boutin, F. Guerin, Y. Surel, and P. Touanel from the UEPR, INRAE, 35590 Saint-Gilles, France for animal care and sample collection, A. Constentin, P. Ganier, A. Marchais, C. Mustière, C. Perrier, G. Robin, and C. Trefeu for lab analyses. We also want to thank Lallemand SAS for the PhD grant of A. M. Serviento. This study was funded by INRAE, France AgriMer and to the French Ministry of Agriculture (CASDAR financial support; SCANALI project).
Glossary
Abbreviations
- ADFI
average daily feed intake
- ADG
average daily gain
- BAP
biological antioxidant potential
- BFT
backfat thickness
- BW
body weight
- CGM
Capteur Gras Maigre (Lean Fat Sensor)
- CK
creatine kinase
- CP
crude protein
- DM
dry matter
- FCR
feed conversion ratio
- FI
feed intake
- GE
growing environment
- GHS
growing heat stress
- GTN
growing thermoneutral
- HP
heat production
- HPA
hypothalamic–pituitary–adrenal
- HS
heat stress
- LDH
lactate dehydrogenase
- NE
net energy
- NEFA
nonesterified fatty acids
- PE
prenatal environment
- PHS
prenatal heat stress
- PTN
prenatal thermoneutral
- sBW
slaughter BW
- SOD
superoxide dismutase
- TN
thermoneutral
Conflict of interest statement
The authors declare that there is no conflict of interest.
Literature Cited
- Baldwin B. A., and Ingram D. L.. . 1968. The influence of hypothalamic temperature and ambient temperature on thermoregulatory mechanisms in the pig. J. Physiol. 198:517–529. doi: 10.1113/jphysiol.1968.sp008622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgard L. H., and Rhoads R. P. Jr. 2013. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 1:311–337. doi: 10.1146/annurev-animal-031412-103644 [DOI] [PubMed] [Google Scholar]
- Black J. L., Mullan B. P., Lorschy M. L., and Giles L. R.. . 1993. Lactation in the sow during heat stress. Livest. Prod. Sci. 35(1–2):153–170. doi: 10.1016/0301-6226(93)90188-N [DOI] [Google Scholar]
- Bligh J. 1966. The thermosensitivity of the hypothalamus and thermoregulation in mammals. Biol. Rev. Camb. Philos. Soc. 41:317–368. doi: 10.1111/j.1469-185x.1966.tb01496.x [DOI] [PubMed] [Google Scholar]
- Boddicker R. L., Seibert J. T., Johnson J. S., Pearce S. C., Selsby J. T., Gabler N. K., Lucy M. C., Safranski T. J., Rhoads R. P., Baumgard L. H., . et al. 2014. Gestational heat stress alters postnatal offspring body composition indices and metabolic parameters in pigs. PLoS One. 9:e110859. doi: 10.1371/journal.pone.0110859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Brandl T. M., Hayes M. D., Xin H., Nienaber J. A., Li H., Eigenberg R. A., Stinn J. P., and Shepherd T.. . 2014. Heat and moisture production of modern swine. In: Advances in Measurement and Modeling of Indoor Environmental Quality of Animal Buildings, ASHRAE 2014 Winter Conference; July 18 to 22, 2014; New York, NY. p. 534.
- Chapel N. M., Byrd C. J., Lugar D. W., Morello G. M., Baumgard L. H., Ross J. W., Safranski T. J., Lucy M. C., and Johnson J. S.. . 2017. Determining the effects of early gestation in utero heat stress on postnatal fasting heat production and circulating biomarkers associated with metabolism in growing pigs. J. Anim. Sci. 95:3914–3921. doi: 10.2527/jas2017.1730 [DOI] [PubMed] [Google Scholar]
- Clarkson P. M., Kearns A. K., Rouzier P., Rubin R., and Thompson P. D.. . 2006. Serum creatine kinase levels and renal function measures in exertional muscle damage. Med. Sci. Sports Exerc. 38:623–627. doi: 10.1249/01.mss.0000210192.49210.fc [DOI] [PubMed] [Google Scholar]
- Collin A., Lebreton Y., Fillaut M., Vincent A., Thomas F., and Herpin P.. . 2001a. Effects of exposure to high temperature and feeding level on regional blood flow and oxidative capacity of tissues in piglets. Exp. Physiol. 86:83–91. doi: 10.1113/eph8602102 [DOI] [PubMed] [Google Scholar]
- Collin A., van Milgen J., Dubois S., and Noblet J.. . 2001b. Effect of high temperature and feeding level on energy utilization in piglets. J. Anim. Sci. 79:1849–1857. doi: 10.2527/2001.7971849x [DOI] [PubMed] [Google Scholar]
- Collin A., Vaz M. J., and Le Dividich J.. . 2002. Effects of high temperature on body temperature and hormonal adjustments in piglets. Reprod. Nutr. Dev. 42:45–53. doi: 10.1051/rnd:2002005 [DOI] [PubMed] [Google Scholar]
- Cooper O., Bonert V., Moser F., Mirocha J., and Melmed S.. . 2017. Altered pituitary gland structure and function in posttraumatic stress disorder. J. Endocr. Soc. 1:577–587. doi: 10.1210/js.2017-00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzen S. M., Boddicker R. L., Graves K. L., Johnson T. P., Arkfeld E. K., Baumgard L. H., Ross J. W., Safranski T. J., Lucy M. C., and Lonergan S. M.. . 2015. Carcass composition of market weight pigs subjected to heat stress in utero and during finishing. J. Anim. Sci. 93:2587–2596. doi: 10.2527/jas.2014-8347 [DOI] [PubMed] [Google Scholar]
- Cuddy J. S., Hailes W. S., and Ruby B. C.. . 2014. A reduced core to skin temperature gradient, not a critical core temperature, affects aerobic capacity in the heat. J. Therm. Biol. 43:7–12. doi: 10.1016/j.jtherbio.2014.04.002 [DOI] [PubMed] [Google Scholar]
- Daumas G., Causeur D., and Predin J.. . 2010. Validation de l’équation française de prédiction du taux de muscle des pièces (TMP) des carcasses de porc par la méthode CGM. J. Rech. Porcine 43:229–230. [Google Scholar]
- Dourmad J. Y., Etienne M., Noblet J., and Causeur D.. . 1997. Prediction de la composition chimique des truies reproductrices a partir du poids vif et de l’epaisseur de lard dorsal: application à la définition des besoins énergétiques. J. Rech. Porcine 29:255–262. [Google Scholar]
- Dreier J. W., Andersen A. M., and Berg-Beckhoff G.. . 2014. Systematic review and meta-analyses: fever in pregnancy and health impacts in the offspring. Pediatrics 133:e674–e688. doi: 10.1542/peds.2013-3205 [DOI] [PubMed] [Google Scholar]
- Edwards M. J. 1969. Congenital defects in guinea pigs: prenatal retardation of brain growth of guinea pigs following hyperthermia during gestation. Teratology 2:329–336. doi: 10.1002/tera.1420020407 [DOI] [PubMed] [Google Scholar]
- Galan H. L., Hussey M. J., Barbera A., Ferrazzi E., Chung M., Hobbins J. C., and Battaglia F. C.. . 1999. Relationship of fetal growth to duration of heat stress in an ovine model of placental insufficiency. Am. J. Obstet. Gynecol. 180:1278–1282. doi: 10.1016/s0002-9378(99)70629-0 [DOI] [PubMed] [Google Scholar]
- Glover V., O’Connor T. G., and O’Donnell K.. . 2010. Prenatal stress and the programming of the HPA axis. Neurosci. Biobehav. Rev. 35:17–22. doi: 10.1016/j.neubiorev.2009.11.008 [DOI] [PubMed] [Google Scholar]
- Hansen P. J. 2009. Effects of heat stress on mammalian reproduction. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 364:3341–3350. doi: 10.1098/rstb.2009.0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinoue A., Fushiki S., Nishimura Y., and Shiota K.. . 2001. In utero exposure to brief hyperthermia interferes with the production and migration of neocortical neurons and induces apoptotic neuronal death in the fetal mouse brain. Brain Res. Dev. Brain Res. 132:59–67. doi: 10.1016/s0165-3806(01)00295-4 [DOI] [PubMed] [Google Scholar]
- Iossa S., Lionetti L., Mollica M. P., Crescenzo R., Barletta A., and Liverini G.. . 2001. Fat balance and serum leptin concentrations in normal, hypothyroid, and hyperthyroid rats. Int. J. Obes. Relat. Metab. Disord. 25:417–425. doi: 10.1038/sj.ijo.0801516 [DOI] [PubMed] [Google Scholar]
- IPCC. 2014. Climate change 2014: synthesis report. In: Core Writing Team, Pachauri R.K., and Meyer L.A., editors. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva, Switzerland: IPCC; p. 151. [Google Scholar]
- Ji Y., Wu Z., Dai Z., Wang X., Li J., Wang B., and Wu G.. . 2017. Fetal and neonatal programming of postnatal growth and feed efficiency in swine. J. Anim. Sci. Biotechnol. 8(1):42. doi: 10.1186/s40104-017-0173-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. S., and Baumgard L. H.. . 2018. PHYSIOLOGY SYMPOSIUM: postnatal consequences of in utero heat stress in pigs. J. Anim. Sci. 97(2):962–971. doi: 10.1093/jas/sky472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. S., Sanz Fernandez M. V., Gutierrez N. A., Patience J. F., Ross J. W., Gabler N. K., Lucy M. C., Safranski T. J., Rhoads R. P., and Baumgard L. H.. . 2015a. Effects of in utero heat stress on postnatal body composition in pigs: I. Growing phase. J. Anim. Sci. 93(1):71–81. doi: 10.2527/jas.2014-8354 [DOI] [PubMed] [Google Scholar]
- Johnson J. S., Sanz Fernandez M. V., Patience J. F., Ross J. W., Gabler N. K., Lucy M. C., Safranski T. J., Rhoads R. P., and Baumgard L. H.. . 2015b. Effects of in utero heat stress on postnatal body composition in pigs: II. Finishing phase. J. Anim. Sci. 93(1):82–92. doi: 10.2527/jas.2014-8355 [DOI] [PubMed] [Google Scholar]
- Johnson J. S., Sanz Fernandez M. V., Seibert J. T., Ross J. W., Lucy M. C., Safranski T. J., Elsasser T. H., Kahl S., Rhoads R. P., and Baumgard L. H.. . 2015c. In utero heat stress increases postnatal core body temperature in pigs. J. Anim. Sci. 93:4312–4322. doi: 10.2527/jas.2015-9112 [DOI] [PubMed] [Google Scholar]
- Kim J. H., Baik H. W., Yoon Y. S., Joung H. J., Park J. S., Park S. J., Jang E. J., Park S. W., Kim S. J., Kim M. J., . et al. 2014. Measurement of antioxidant capacity using the biological antioxidant potential test and its role as a predictive marker of metabolic syndrome. Korean J. Intern. Med. 29:31–39. doi: 10.3904/kjim.2014.29.1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouba M., Hermier D., and Le Dividich J.. . 2001. Influence of a high ambient temperature on lipid metabolism in the growing pig. J. Anim. Sci. 79:81–87. doi: 10.2527/2001.79181x [DOI] [PubMed] [Google Scholar]
- Lary J. M. 1986. Chapter 6. Hyperthermia and teratogenicity. In: Anghileri L. J., and Robert J., editors. Hyperthermia in cancer treatment. Vol. 1. Boca Raton (FL): CRC Press. [Google Scholar]
- Lesage J., Del-Favero F., Leonhardt M., Louvart H., Maccari S., Vieau D., and Darnaudery M.. . 2004. Prenatal stress induces intrauterine growth restriction and programmes glucose intolerance and feeding behaviour disturbances in the aged rat. J. Endocrinol. 181:291–296. doi: 10.1677/joe.0.1810291 [DOI] [PubMed] [Google Scholar]
- Lista G., Castoldi F., Compagnoni G., Maggioni C., Cornélissen G., and Halberg F.. . 2010. Neonatal and maternal concentrations of hydroxil radical and total antioxidant system: protective role of placenta against fetal oxidative stress. Neuro Endocrinol. Lett. 31:319–324. [PubMed] [Google Scholar]
- Liu F., Celi P., Chauhan S. S., Cottrell J. J., Leury B. J., and Dunshea F. R.. . 2018. A short-term supranutritional vitamin E supplementation alleviated respiratory alkalosis but did not reduce oxidative stress in heat stressed pigs. Asian-Australas. J. Anim. Sci. 31:263–269. doi: 10.5713/ajas.17.0256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyau T., Bedrani L., Berri C., Métayer-Coustard S., Praud C., Coustham V., Mignon-Grasteau S., Duclos M. J., Tesseraud S., Rideau N., . et al. 2015. Cyclic variations in incubation conditions induce adaptive responses to later heat exposure in chickens: a review. Animal 9:76–85. doi: 10.1017/S1751731114001931 [DOI] [PubMed] [Google Scholar]
- Luber G., and McGeehin M.. . 2008. Climate change and extreme heat events. Am. J. Prev. Med. 35:429–435. doi: 10.1016/j.amepre.2008.08.021 [DOI] [PubMed] [Google Scholar]
- Maghnie M., Triulzi F., Larizza D., Preti P., Priora C., Scotti G., and Severi F.. . 1991. Hypothalamic-pituitary dysfunction in growth hormone-deficient patients with pituitary abnormalities. J. Clin. Endocrinol. Metab. 73:79–83. doi: 10.1210/jcem-73-1-79 [DOI] [PubMed] [Google Scholar]
- Merlot E., Constancis C., Resmond R., Serviento A. M., Prunier A., Renaudeau D., and Quesnel H.. . 2018. Exposition à la chaleur pendant la gestation: adaptation de la femelle gestante et conséquences sur la composition du lait, la santé néonatale et la réactivité de l’axe corticotrope de la descendance.Presented at 4. Congrès de la SF-Dohad; Grenoble, France; November 8 to 9, 2018. [Google Scholar]
- Monteiro A. P. A., Guo J. R., Weng X. S., Ahmed B. M., Hayen M. J., Dahl G. E., Bernard J. K., and Tao S.. . 2016. Effect of maternal heat stress during the dry period on growth and metabolism of calves. J. Dairy Sci. 99(5):3896–3907. doi: 10.3168/jds.2015-10699 [DOI] [PubMed] [Google Scholar]
- Pearce S. C., Gabler N. K., Ross J. W., Escobar J., Patience J. F., Rhoads R. P., and Baumgard L. H.. . 2013. The effects of heat stress and plane of nutrition on metabolism in growing pigs. J. Anim. Sci. 91(5):2108–2118. doi: 10.2527/jas.2012-5738 [DOI] [PubMed] [Google Scholar]
- Pervanidou P., and Chrousos G. P.. . 2012. Metabolic consequences of stress during childhood and adolescence. Metabolism 61:611–619. doi: 10.1016/j.metabol.2011.10.005 [DOI] [PubMed] [Google Scholar]
- Quiniou N., Dubois S., and Noblet J.. . 2000. Voluntary feed intake and feeding behaviour of group-housed growing pigs are affected by ambient temperature and body weight. Livest. Prod. Sci. 63(3):245–253. doi: 10.1016/S0301-6226(99)00135-9 [DOI] [Google Scholar]
- Renaudeau D., Anais C., Tel L., and Gourdine J. L.. . 2010. Effect of temperature on thermal acclimation in growing pigs estimated using a nonlinear function. J. Anim. Sci. 88:3715–3724. doi: 10.2527/jas.2009-2169 [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Frances G., Dubois S., Gilbert H., and Noblet J.. . 2013. Effect of thermal heat stress on energy utilization in two lines of pigs divergently selected for residual feed intake. J. Anim. Sci. 91:1162–1175. doi: 10.2527/jas.2012-5689 [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Kerdoncuff M., Anaïs C., and Gourdine J. L.. . 2008. Effect of temperature level on thermal acclimation in Large White growing pigs. Animal 2:1619–1626. doi: 10.1017/S1751731108002814 [DOI] [PubMed] [Google Scholar]
- Safranski T. J., Lucy M. C., Rhoades J. N., Estienne M., Wiegert J. G., Rhoads M., Rhoads R. P., Baumgard L. H., and Ross J. W.. . 2015. Reproductive performance of gilts having developed in heat stressed dams. J. Anim. Sci. 93(Suppl 2):85. [Google Scholar]
- Sanz Fernandez M. V., Johnson J. S., Abuajamieh M., Stoakes S. K., Seibert J. T., Cox L., Kahl S., Elsasser T. H., Ross J. W., Clay Isom S., . et al. 2015. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol. Rep. 3(2):e12315. doi: 10.14814/phy2.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota K., and Kayamura T.. . 1989. Effects of prenatal heat stress on postnatal growth, behavior and learning capacity in mice. Biol. Neonate 56:6–14. doi: 10.1159/000242981 [DOI] [PubMed] [Google Scholar]
- Sinha R. A., Singh B. K., and Yen P. M.. . 2018. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat. Rev. Endocrinol. 14:259–269. doi: 10.1038/nrendo.2018.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibiel A. L., Peñagaricano F., Amorín R., Ahmed B. M., Dahl G. E., and Laporta J.. . 2018. In utero heat stress alters the offspring epigenome. Sci. Rep. 8:14609. doi: 10.1038/s41598-018-32975-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thureen P. J., Trembler K. A., Meschia G., Makowski E. L., and Wilkening R. B.. . 1992. Placental glucose transport in heat-induced fetal growth retardation. Am. J. Physiol. 263(3 Pt 2):R578–R585. doi: 10.1152/ajpregu.1992.263.3.R578 [DOI] [PubMed] [Google Scholar]
- Van Milgen J., and Noblet J.. . 2003. Partitioning of energy intake to heat, protein, and fat in growing pigs. J. Anim. Sci. 81(E-Suppl.): E86–E93. doi: 10.2527/2003.8114_suppl_2E86x [DOI] [Google Scholar]
- Welberg L. A., Thrivikraman K. V., and Plotsky P. M.. . 2005. Chronic maternal stress inhibits the capacity to up-regulate placental 11beta-hydroxysteroid dehydrogenase type 2 activity. J. Endocrinol. 186:R7–R12. doi: 10.1677/joe.1.06374 [DOI] [PubMed] [Google Scholar]
- Wilmoth T. A., Callahan Z. D., Safranski T. J., and Wiegand B. R.. . 2015. Effects of in utero heat stress on muscle development of barrows. J. Anim. Sci. 93(Suppl. 2):34. [Google Scholar]
- Yang P., Hao Y., Feng J., Lin H., Feng Y., Wu X., Yang X., and Gu X.. . 2014. The expression of carnosine and its effect on the antioxidant capacity of longissimus dorsi muscle in finishing pigs exposed to constant heat stress. Asian-Australas. J. Anim. Sci. 27:1763–1772. doi: 10.5713/ajas.2014.14063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C., Wang G., Gao S., Huang Y., Zhao R., and Yang X.. . 2018. Maternal restraint stress during pregnancy negatively affects behaviors and antioxidant capacity of offspring rats (Rattus norvegicus). Can. J. Zool. 96(8):882–887. doi: 10.1139/cjz-2017-0264 [DOI] [Google Scholar]
- Zhao W., Liu F., Cottrell J. J., Leury B. J., Bell A. W., and Dunshea F. R.. . 2019. Heat stress during early-mid gestation causes placental insufficiency and growth restriction in pigs. In: Book of Abstracts of the 70th Annual Meeting of the European Federation of Animal Science. Annual Meeting of the European Association for Animal Production, 25, Presented at 70. Annual Meeting of the European Federation of Animal Science (EAAP); Ghent, Belgium; August 26 to 30, 2019. Wageningen (The Netherlands): Wageningen Academic Publishers; p. 263. [Google Scholar]
- Zhao L., McMillan R. P., Xie G., Giridhar S. G. L. W., Baumgard L. H., El-Kadi S., Selsby J., Ross J., Gabler N., Hulver M. W., . et al. 2018. Heat stress decreases metabolic flexibility in skeletal muscle of growing pigs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315:R1096–R1106. doi: 10.1152/ajpregu.00404.2017 [DOI] [PubMed] [Google Scholar]