Abstract
The present study aimed to investigate whether acute pain associated with castration and tail docking of male piglets may modulate the expression of salivary microRNAs (miRNAs) and to explore their potential use as biomarkers. Thirty-six healthy 4-d-old piglets (Hermitage × Duroc) were randomly assigned to three groups: the first group (12 piglets) has been pretreated with anesthetic and anti-inflammatory drugs (ANA) and then castrated and tail docked; the second one (12 piglets) has been castrated and tail docked without any drugs (CONV); the third one (12 piglets) has been only handled (SHAM). Saliva was collected 10 min before (control group) and 30 to 45 min after the procedures. Salivary cortisol has been quantified. The expression concentrations of seven miRNAs, namely miR-19b, miR-27b-3p, miR-215, miR-22-3p, miR-155-5p, hsa-miR-365-5p, and hsa-miR-204, were measured and assessed as potential biomarkers of pain by quantitative Polimerase Chain Reaction using TaqMan probes. The area under the receiver operating curve (AUC) was used to evaluate the diagnostic performance of miRNAs. The concentration of salivary cortisol increased after treatment in CONV and ANA, while no significant variation was observed in the SHAM group. The comparative analysis demonstrated that the concentrations of salivary miR-19b (P = 0.001), miR-27b (P = 0.042), and miR-365 (P < 0.0001) were significantly greater in CONV as compared with pretreatment. The AUC of pretreatment vs. CONV and CONV vs. ANA were excellent for miR-19b and miR-365 and fair for miR-27b. Combining two miRNAs, namely miR-19b and miR-365, in a panel increased the efficiency of distinguishing between pre- and post-treatment groups. No differences have been identified between SHAM and ANA groups. mRNA potential targets of differentially expressed-miRNA were investigated, and genes related to pain and inflammation were identified: miR-19b potentially modulates TGF-beta and focal adhesion pathways, miR-365 regulates cytokines expression (i.e., IL-1, Tumor Necross Factor-alpha, and IL-8 cytokine), and miR-27b regulates macrophage inflammatory protein pathways (i.e., MIP1-beta). In conclusion, we demonstrated that the abundance of miR-19b, miR-27b, and miR-365 increases in the saliva of piglets castrated and tail docked without the administration of pain-relieving drugs. Further studies are needed to assess their potential during routine husbandry procedures and to extend their assessment in other stressful events, such as weaning or chronic pain.
Keywords: microRNA, noninvasive biomarkers, pain, piglets
Introduction
Tail docking and castration of male piglets are routine husbandry procedures, which aim to reduce the damage caused by tail biting and the undesirable sexual behavior, making males less aggressive and easier to manage. In addition, castration avoids the boar taint in typical products, such as Italian protected designation of origin hams, which is produced with heavy mature pigs. These procedures are traditionally performed without anesthesia or analgesia, which are expensive and time consuming. Tail docking and castration have become a significant animal welfare concern in recent years causing stress, pain, and discomfort for the piglets. The European Declaration on the Alternatives to Pig Castration (European Declaration, 2010) (https://ec.europa.eu/food/animals/welfare/practice/farm/pigs/castration_alternatives_en), establishing that from 2012 surgical castration of pigs should be performed with prolonged analgesia, and/or anesthesia, was voluntarily signed by many organizations. A wide range of pain assessment measures has been investigated in pigs, such as behavioral indicators, including posture change (Edwards et al., 2009), behavior score, Piglet Grimace Scale (Di Giminiani et al., 2016), and physiological parameters, such as cortisol/adrenocorticotrophic hormone (Prunier et al., 2005). Multidisciplinary approaches based on the simultaneous measuring of both behavioral and physiological indicators are the most promising and wider procedures used to assess the impact of the painful condition in piglets (Ison et al., 2016).
The microRNAs (miRNAs) collected from body fluids, such as saliva, have potential as diagnostic molecules ( Wang et al., 2017a). MicroRNAs play a pivotal role in the orchestration of cellular homeostasis and their dysregulation was also associated with increased stress and pain (Lecchi et al., 2016, 2018). Several studies have been carried out on blood miRNA in pigs, to study the pathogenesis of infectious diseases (Huang et al., 2011; Bao et al., 2015; Hansen et al., 2016; Fleming and Miller, 2018) or stressful events, such as weaning, for example (Tao et al., 2016; Zhang et al., 2019), for investigating pathogenesis or in pigs, targeting infectious diseases.
The knowledge about the expression of salivary miRNAs in livestock saliva is limited. Saliva is a complex fluid, the composition of which is not constant and changes follow the daily cycle, diet, and health status. Saliva is considered the mirror of the body because it is a biofluid that filters and processes itself from the vasculature, nourishing the salivary glands into the oral cavity and reflecting virtually the entire spectrum of normal and disease states (Lee and Wong, 2009). Approximately, 40% of biomarkers suggested for diseases, such as cancers, cardiovascular disease, and stroke, are found in the whole saliva as well (Loo et al., 2010). Collecting saliva is a less invasive procedure than blood collection. Therefore, exploring the diagnostic potential of saliva should be a priority to improve pain mitigation strategies, thus improving animal welfare. To the best of authors’ knowledge, no information about microRNA presence in pig saliva is available.
Considering the diagnostic potential of miRNAs and the evidence of their identification in the porcine genome (Martini et al., 2014), tissues (Hou et al., 2016; Wang et al., 2017b), and serum (Hansen et al., 2016, 2018), the aims of the present study were to 1) ascertain whether acute pain associated with castration and tail docking may modulate the expression of salivary miRNAs; 2) investigate the potential use of differentially expressed (DE)-miRNAs as biomarkers to measure pain in pigs aiming to provide parameters capable to measure as finely as possible the pain that this procedure causes to the animals; and 3) integrate miRNAs to their target genes and their relative biological processes.
Materials and Methods
Animals husbandry and procedures
The procedures were carried out during routine surgical castration and tail docking. The protocol for care, handling, and sampling of animals defined in the present study was reviewed and approved by the Università degli Studi di Milano Animal Care and Use Committee (protocol no. 97/18).
The study was carried out at a sow farrow commercial pig farm, following a batch management production system. Thirty-six 4-d-old piglets (Hermitage × Duroc), clinically healthy and with homogeneous weight, were enrolled. Piglets were randomly assigned to three experimental groups (Table 1): group ANA including 12 piglets castrated and tail docked with pretreatment with anesthetic and anti-inflammatory drugs; group CONV including 12 piglets castrated and tail docked without any drugs; group SHAM including 12 piglets only handled. The experimental design included a control group, consisting of all 36 piglets before performing the procedures. The anesthetic approach has been selected based on the previous studies (Keita et al., 2010; Hansson et al., 2011; Kluivers-Poodt et al., 2013; Bonastre et al., 2016). Anesthetic procaine hydrochloride (40.0 mg and adrenaline tartrate 0.036 mg/mL) and meloxicam (0.4 mg/kg) were administrated according to the information sheets. Briefly, meloxicam was administered intra-muscular in the neck, while the anesthetic was administered with an insulin syringe in each testicle (0.125 mL/testicle). Piglets assigned to the SHAM group were handled in the same way than others, without castration and tail docking.
Table 1.
Procedures and samples collection
| Time Nr of sampling | 10 min before first | 0 min | 30 to 45 min after second |
|---|---|---|---|
| ANA | Sampling and then drugs administration 1 and 2 | Castration, tail docking, iron, and antibiotic | Sampling |
| CONV | Sampling | Castration, tail docking, iron, and antibiotic | Sampling |
| SHAM | Sampling | Handling | Sampling |
1aesthetic: procaine and adrenaline.
2anti-inflammatory: meloxicam.
Castration was performed according to the farm practice. Briefly, the piglet was grabbed by the back legs and hold in an upside-down position. Holding the testicle, the scrotum was incised with a scalpel and the testicle was pushed out through the incision, then the testicles and attached cord were pulled and discarded. The operation was repeated for the other testicle. The incision was left open and sprayed with a disinfectant (oxytetracycline hydrochloride spray, 74 to 148 mg/capo/die) (Allen, 1992). Tail docking was performed at 12 to 18 mm from the base of the tail with a gas-heated iron for tail docking to seal the wound as soon as possible.
Saliva collection
Saliva was collected between 8 a.m. and 12:00 a.m., 10 min before and 30 to 45 min after castration and tail docking (Table 1). Sampling was carried out in the farrowing room to allow the piglets an olfactory and vocal contact with their mother. Saliva was collected using specialized salivary tubes (Salivette, Sarstedt, Nümbrecht, Germany) keeping the animals in standing position and promoting salivation with 2 to 3 drops of 5% citric acid with a plastic Pasteur pipette (Gallagher et al., 2002). The cotton roll was kept in the piglet mouth allowing the animal to chew it for 2 to 3 min followed by centrifugation of sponge-containing Salivette tubes at 1000 × g for 15 min to collect saliva. If during collection the sponge was spat out, a new one was used. Samples were kept at 4 to 8 °C and delivered to the laboratory within 8 h. Saliva was then transferred to cryovials, frozen in liquid nitrogen, and then stored at −80 °C.
Salivary cortisol quantification
The saliva’s cortisol concentration was measured using a solid-phase, competitive chemiluminescent enzyme immunoassay, the Immulite 1000 Cortisol (catalog number LKC01, Medical System, Genova, Italy), validated for determinations in porcine saliva (Escribano et al., 2012). An increase in concentration was interpreted as a positive stress response (Hellhammer et al., 2009).
MicroRNAs extraction and real-time quantitative PCR
Total RNA was extracted from saliva using miRNeasy Serum/Plasma Kit (Qiagen, catalog number 217184, Milan, Italy). An aliquot of 50 µL per sample was transferred to a new tube and 1 mL of Qiazol (Qiagen) was added. The Caenorhabditis elegans miRNA cel-miR-39 (Qiagen, catalog number 219610) was used as synthetic spike-in control because of a lack of sequence homology to swine miRNAs. After incubation at room temperature for 5 min, 3.75 µL (25 fmol final concentration) of spike-in control was added and the samples were vortexed to ensure complete mixing. The RNA extraction was then carried out according to the manufacturer’s instruction. The reverse transcription was performed using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, catalog number 4366596, Monza, Italy) using miRNA-specific stem-loop Reverse Transcription (RT) primers, according to the manufacturer’s instructions. Reverse transcription reactions were performed in 15 μL volume reactions containing 1.5 µL 10X miRNA RT buffer, 1 µL MultiScribe reverse transcriptase (50 U/µL), 0.30 µL 100 mM dNTP mix, 0.19 µL RNase Inhibitor (20 U/µL), 6 μL of custom RT primer pool, and 3.01 µL of nuclease-free water. The custom RT primer pool was prepared by combining 10 µL of each 5X RT primer in a final volume of 1,000 µL; the final concentration of each primer in the RT primer pool was 0.05X each; 3 μL of saliva RNA was added to each RT reaction. Every RT reaction mixture was incubated on ice for 5 min, 16 °C for 30 min, 42 °C for 30 min, and then 85 °C for 5 min.
The quantitative Polimerase Chain Reaction experiments were designed following the Minimum Information for Publication of Quantitative Real-Time PCR Experiments guidelines. Small RNA TaqMan assays were performed according to the manufacturer’s instruction. The selection of miRNAs was based on previous publications in which these miRNAs were found to be related to pain in humans and mice (Heyn et al., 2016; Pan et al., 2016; Wang et al., 2016; Sakai et al., 2017; Wu et al., 2018). The selected primer/probe assays (Life Technologies, Monza, Italy) included cel-miR-39-3p (assay ID000200), hsa-miR-19b (assay ID000396), hsa-miR-27b-3p (assay ID000409), hsa-miR-215 (assay ID000518), has-miR-22-3p (assay ID000398), has-miR-155-5p (assay ID000479), has-miR-204 (assay ID000508), and has-miR-365-3p (assay ID001020). Quantitative reactions were performed in duplicate in scaled-down (12 µL) reaction volumes using 6 µL TaqMan 2X Universal Master Mix II (Applied Biosystems, catalog number 4440044), 0.6 µL miRNA specific TaqMan Assay 20X, and 1µL of the RT product per reaction on Eco Real-Time PCR detection system (Illumina, San Diego, CA, USA). The standard cycling program was 50 °C for 2 min, 95 °C for 10 min, and 40 cycles of 95 °C for 15 s and 60 °C for 60 s. Data were normalized relative to the expression of cel-miR-39. MicroRNA expression concentrations are presented in terms of fold change normalized to cel-miR-39 expression using the formula 2−ΔΔCq. The targets of the significant miRNA were determined from the TargetScan database (http://www.targetscan.org/vert_71/), functional enrichment of the mRNA was performed using the DAVID bioinformatics resources (https://david.ncifcrf.gov/), and biological pathways in the Kyoto Encyclopedia of Genes and Genomes (KEGG) were examined for enrichment (http://www.genome.jp/kegg/).
Statistical analysis
Statistical analysis was performed using XLStat software (Addinsoft SARL, Paris, France). Statistical significance was accepted at P ≤ 0.05. Data were tested for normality and homogeneity of variance using the Kolmogorov–Smirnov and Levene test, respectively. As data were not normally distributed, nonparametric statistical tests were applied. The nonparametric Wilcoxon signed-rank test for paired samples and Kruskal–Wallis test were used to assess the differences in cortisol and miRNA concentrations between groups, respectively. Match paired Wilcoxon test was run to compare miRNA concentrations pre- and post-treatment. Receiver operating characteristic (ROC) analysis was performed to determine the diagnostic accuracy of targets. The diagnostic values were calculated for miR-19b, miR-27, and miR-365 alone and in combination. The ROC analysis was carried out by plotting the true positive (sensitivity) vs. the false positive (1-specificity). The definitions of the relationship between the area under the curve (AUC) and diagnostic accuracy were as previously reported (Šimundić, 2009).
Results
Salivary cortisol increased in CONV and ANA groups
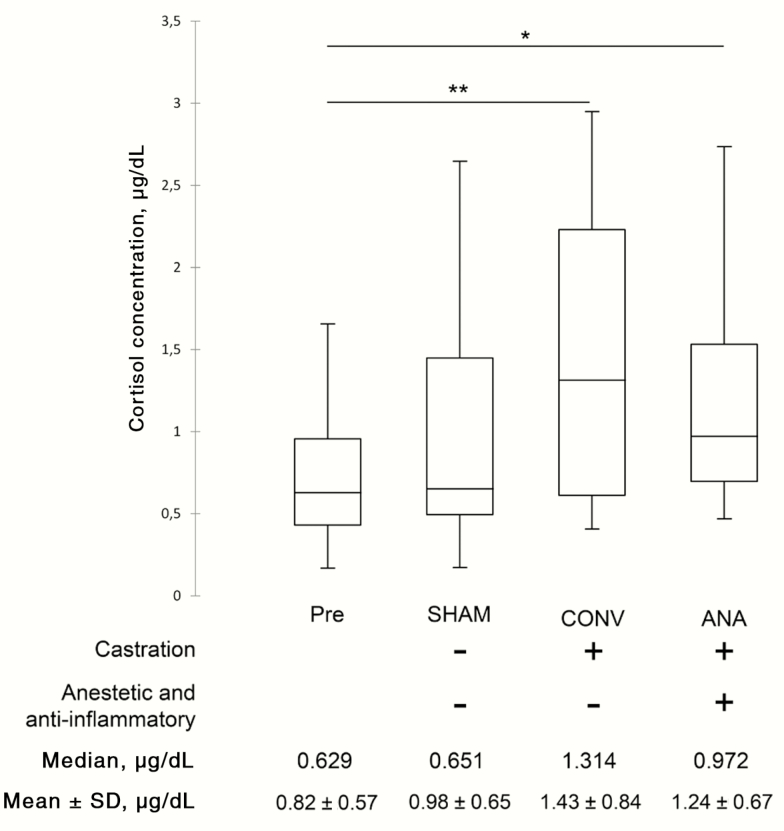
Large variability in cortisol concentration between individual piglets was measured ( Figure 1 ). The cortisol concentration in the saliva of piglets increased after treatment in all groups. In detail, the median values of CONV (1.312 μg/dL; P = 0.01) and ANA (0.972 μg/dL; P = 0.043) groups were significantly higher than median value of pretreated group (0.629 μg/dL). No significant variation was observed in SHAM group (0.651 μg/dL; P = 0.83) (Figure 1).
Figure 1.
Salivary cortisol concentrations in piglets change after castration and tail docking. The black lines inside the boxes mark the medians. *P < 0.05; **P < 0.01.
Castration and tail docking without drugs alter the expression amounts of miR-19b, miR-27b, and miR-365 in the saliva of piglets
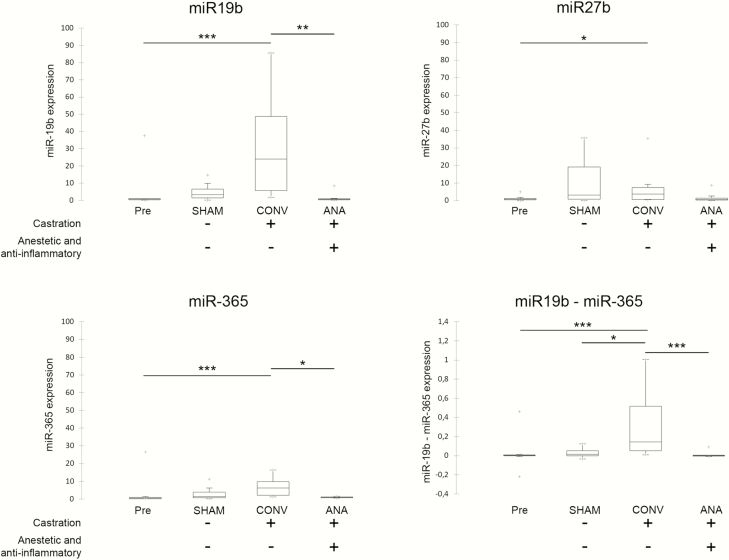
Small RNA was extracted from all collected samples, except for one saliva sample of the CONV group, the volume of which was not sufficient to carry out the extraction. MicroRNAs were normalized to the concentration of the artificial spike-in cel-miR-39, which was used as internal control and as reference miRNA. Four miRNAs, namely miRNA miR-215, miR-22-3p, miR-155-5p, and miR-204, were not found in saliva and thus were excluded from further analysis. Three miRNA, namely miR-19b, miR365p, and miR-27b, were detected in all samples and then subjected to further analysis. The comparative analysis demonstrated that three salivary miRNAs, namely miR-19b, miR-27b, and miR-365, had a significant DE in the saliva of piglets (Figure 2). CONV expressed a higher level of miR-19b, miR-27b, and miR-365 than pre-procedures. The amounts of miR-19b and miR-365 were also higher in CONV, as compared with ANA. In detail, the abundance of miR-19b was increased 10.7 (P = 0.001) and 19.9 (P = 0.002) folds compared to controls and ANA, respectively. The abundance of miR-365 was increased to 3.7 (P < 0.001) and 6.7 (P = 0.033) folds as compared with controls and ANA, respectively. Finally, miR-27b was increased in the CONV group compared with controls (6.9-folds; P = 0.042).
Figure 2.
Distribution charts of salivary miRNA concentrations. Saliva was collected from piglets 30 to 45 min after castration and tail docking or handling; samples were analyzed for the presence of pain-related miRNAs. Distribution charts of (A) miR-19b, (B) miR-27b, (C) miR-365, and (D) combination of miR-19b and miR-365 amounts. The black lines inside the boxes mark the medians. *P < 0.05; **P < 0.01; ***P < 0.001.
Diagnostic performance of miR-19b and miR-365 alone or in combination discriminated between groups with different treatments
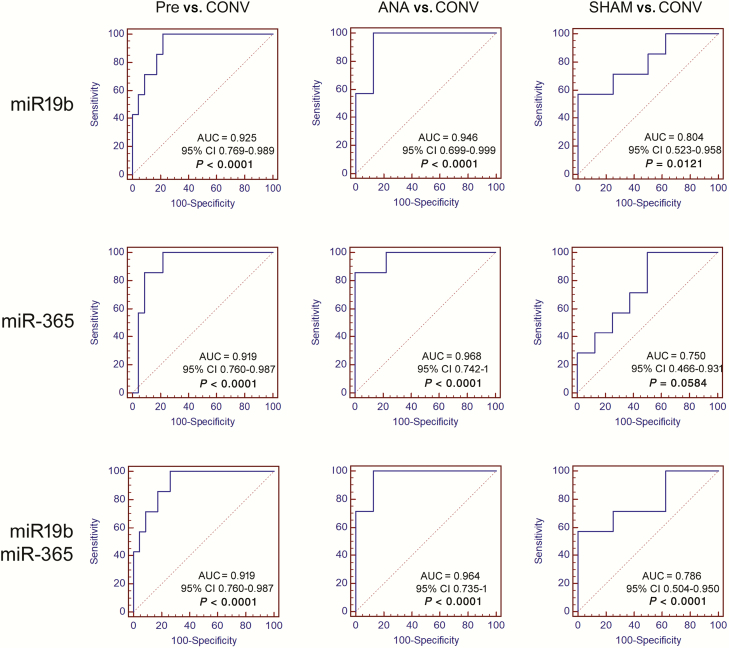
To analyze the diagnostic value of DE-miRNAs in saliva, ROC curve analysis was performed and the associated AUC was used to confirm the diagnostic potency of each miRNA. Cutoff points were set to maximize the sum of sensitivity and specificity (Table 2).
Table 2.
AUC and sensitivity and specificity of DE-miRNAs in piglet’s saliva
| AUC | 95% CI | P-value | Cutoff | Sensitivity–specificity | ||
|---|---|---|---|---|---|---|
| miR-19b | Pre-CONV | 0.925 | 0.769 to 0.989 | <0.0001 | 1.07 | 100–78.26 |
| Pre-ANA | 0.543 | 0.356 to 0.723 | 0.7261 | |||
| Pre-SHAM | 0.709 | 0.519 to 0.857 | 0.0904 | |||
| CONV–ANA | 0.946 | 0.699 to 0.999 | <0.0001 | 1.07 | 100–87.5 | |
| CONV–SHAM | 0.804 | 0.523 to 0.958 | 0.0121 | 5.59 | 71.4–75 | |
| ANA–SHAM | 0.742 | 0.468 to 0.923 | 0.0841 | |||
| miR-27b | Pre-CONV | 0.770 | 0.581 to 0.903 | 0.0149 | 0.83 | 71.43–60.87 |
| Pre-ANA | 0.511 | 0.326 to 0.694 | 0.9353 | |||
| Pre-SHAM | 0.723 | 0.533 to 0.868 | 0.1017 | |||
| CONV–ANA | 0.750 | 0.466 to 0.931 | 0.0639 | |||
| CONV–SHAM | 0.500 | 0.239 to 0.761 | 1 | |||
| ANA–SHAM | 0.734 | 0.460 to 0.918 | 0.0836 | |||
| miR-365 | Pre-CONV | 0.919 | 0.760 to 0.987 | <0.0001 | 1.43 | 85.7–91.3 |
| Pre-ANA | 0.698 | 0.508 to 0.849 | 0.0366 | 0.51 | 100–52.17 | |
| Pre-SHAM | 0.723 | 0.533 to 0.868 | 0.039 | 0.51 | 87.5–52.17 | |
| CONV–ANA | 0.968 | 0.742 to 1 | <0.0001 | 1.54 | 85.71–100 | |
| CONV–SHAM | 0.750 | 0.466 to 0.931 | 0.0584 | |||
| ANA–SHAM | 0.594 | 0.326 to 0.826 | 0.5765 | |||
| miR19b -miR-365 | Pre-CONV | 0.919 | 0.760 to 0.987 | <0.0001 | 0.012 | 85.71–82.61 |
| Pre-ANA | 0.592 | 0.402 to 0.764 | 0.4554 | |||
| Pre-SHAM | 0.609 | 0.418 to 0.778 | 0.4474 | |||
| CONV–ANA | 0.964 | 0.735 to 1 | <0.0001 | 0.0063 | 100–87.5 | |
| CONV–SHAM | 0.786 | 0.504 to 0.950 | 0.0275 | 0.0396 | 71.43–75 | |
| ANA–SHAM | 0.778 | 0.506 to 0.942 | 0.0309 | 0.0063 | 100–66.67 |
The ability of miRNAs to separate the tested samples into those with different treatments is defined as diagnostic accuracy and is measured by the AUC, where an area of 1 represents a perfect test; an area of 0.5 represents a worthless test. The ability to discriminate control group and CONV was excellent for miR-19b (AUC: 0.925; 95% confidence interval [CI] 0.769 to 0.989; P < 0.0001) and miR-365 (AUC: 0.919; 95% CI 0.760 to 0.987; P < 0.0001), and good for miR-27 (AUC: 0.770; 95% CI 0.581 to 0.903; P = 0.0149) (Table 2; Figure 3). Thus, miR-19b and miR-365 can well discriminate between animals castrated without analgesics and pre-castrated group. The AUC of CONV vs. ANA was excellent for miR-19b (AUC: 0.946; 95% CI 0.699 to 0.999; P < 0.0001) and miR-365 (AUC: 0.968; 95% CI 0.742 to 1; P < 0.0001), and good for miR-27b (AUC: 0.750; 95% CI 0.466 to 0.931; P = 0.0639) (Table 2; Figure 3). Thus, miR-19b and miR-365 can well discriminate between animals castrated without and with analgesics. The ability to discriminate the SHAM group and ANA was good or sufficient for all DE-miRNAs (Table 2).
Figure 3.
ROC curve analysis of candidate pain-related miRNA.
Further statistical analysis was performed considering the average relative quantification values of DE-miRNAs with excellent AUC, namely miR-19b and miR-365. The AUCs of control group vs. CONV and CONV vs. ANA were excellent, 0.919 (95% CI 0.760 to 0.987; P < 0.0001; sensitivity 85.71% and specificity 82.61%, cutoff 0.012) and 0.964 (95% CI 0.735 to 1; P < 0.0001; sensitivity 100% and specificity 87.5%, cutoff 0.0063), respectively (Figure 3).
DE-miRNAs potentially modulate tumor growth factor-beta signaling pathway
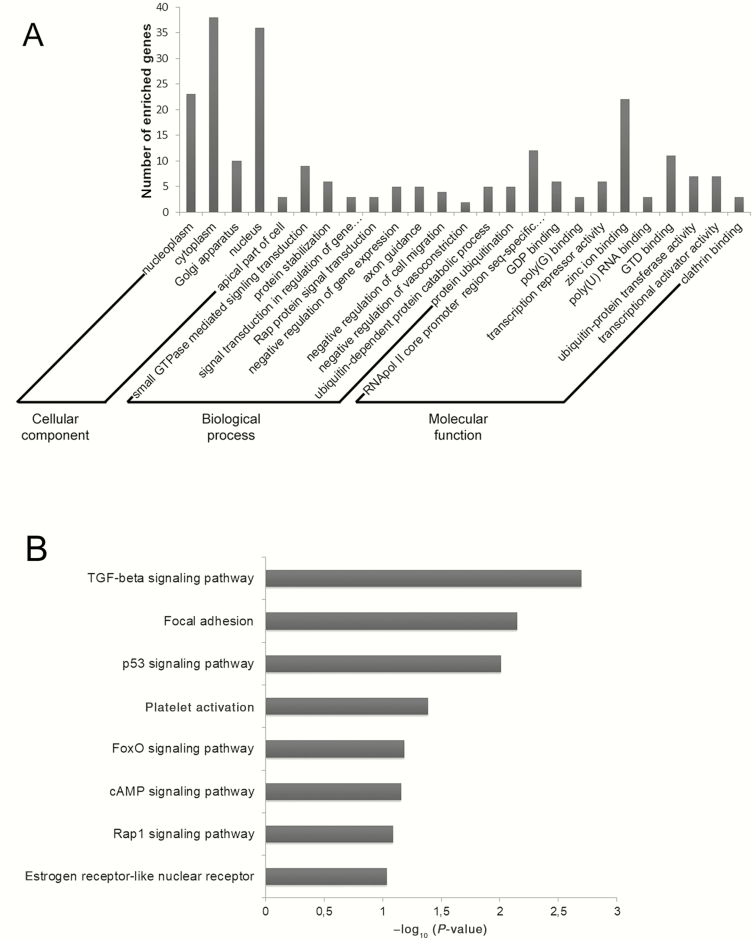
The predicted gene targets of the significant DE-miRNAs were computationally retrieved from the TargetScan database. Only gene targets with a cumulative weighted context score, which measures the target thresholds, of <−0.4 were included in the miRNA–gene interaction network. The mRNA enrichment was performed using the DAVID bioinformatics resources, to explore the function of these candidate biomarkers. The Gene Ontology analysis was carried out using DAVID at three different levels: Molecular Function, Cellular Component, and Biological Process. Figure 4A illustrates the top 10 items that were significantly enriched by target genes for each of the above Gene Ontology levels. The enriched Gene Ontology terms in Molecular Function mainly included genes involved in the binding of DNA and RNA, zinc ion, and clathrin. The Cellular Component items were associated with the hallmarks of a cell: nucleoplasm, cytoplasm, Golgi apparatus, nucleus, and apical part of the cell. Most Gene Ontology Biological Process items converged on the modulation of protein shelf life, gene expression, and axon guidance. The KEGG pathway analysis was performed on the whole targets of DE-miRNAs, and Figure 4B outlines the significantly enriched pathways, among which tumor growth factor (TGF)-beta signaling pathway, focal adhesion, and platelet activation were at the top.
Figure 4.
MicroRNA target prediction and pathway enrichment. (A) GO annotation of genes regulated by identified pain-related miRNAs. The targeted genes were annotated by DAVID tools at three levels, including Cellular Component, Biological Process, and Molecular Function. (B) Pathway enrichment for genes regulated by pain-related miRNAs. Genes were retrieved and enriched in the KEGG pathway with DAVID tools. The statistical significance level (P-value) was negative 10-based log transformed.
Discussion
To the best of the knowledge of the authors, the presence of microRNA in pig saliva had not been detected. The present study demonstrated for the first time the relationship between the abundance of salivary miRNA and the insurgency of pain and stress related to surgical castration and tail docking in piglets. We found that the concentration of miR-19b, miR-365, and miR-27b is significantly upregulated in castrated and docked tail piglets. These effects are mitigated by the preemptive administration of anesthetic drugs. The results reported in the present study suggest that salivary concentrations of miR-19b and miR-365 were effective in accurately differentiating piglets affected by acute pain from both controls and animals treated with local anesthetics (ANA group, as shown by ROC analysis). The KEGG analysis demonstrated that DE-miRNAs are involved in inflammation and pain development by directly targeting mRNAs coding for proteins involved in TGFβ pathways and focal adhesion. To assess stress responses in piglets, salivary cortisol, which reflects only the free active fraction of cortisol, has been quantified as well. Salivary cortisol concentration showed a large variability between piglets, as previously reported (Ott et al., 2014; Martínez-Miró et al., 2016). Our results are in line with cortisol level quantified using different stressor models (Escribano et al., 2012; Contreras-Aguilar et al., 2019), indicating that castration is a stressful event for piglets compared with handling alone and that analgesia/anesthesia did not affect cortisol level, as previously reported on blood cortisol concentration (Numberger et al., 2016).
Practices routinely carried out on piglets, such as castration and tail docking, are regarded as painful. Although many of European stakeholders committed themselves to stop surgical castration by 2018, however, 75% of male pigs are still surgically castrated in the EU (Backus et al., 2018; De Briyne et al., 2016). Moreover, the stakeholders of some countries, including Denmark and Eastern Europe countries (De Briyne et al., 2016), envisage that the surgical castration of male pigs without anesthesia or analgesia is not an issue (Bonneau and Weiler, 2019). The efficacy of current pain mitigation procedures is poorly understood and depends on an accurate measurement of pain, which has become a significant animal welfare concern in husbandry management. Surgical castration using analgesia and anesthesia includes all the advantages of surgical castration and also improved welfare, meaning no or less pain during and after surgery. Some disadvantages include lower feed efficiency, higher environmental impact, and increased costs associated with the application of pain relief (De Briyne et al., 2016). Saliva has been identified as a potential source of miRNAs, being not invasive and reflecting the physiopathological condition of the individual. By finding that microRNAs are differently abundant in animals subjected to stress related to castration and dock tailing, we provide information that will allow for a more accurate pain assessment during routine husbandry procedures. These results also provide the background for the use of miRNAs to assess the mitigation effect of pain management procedures and drugs in pigs, an issue that is still debated (Dzikamunhenga et al., 2014).
The finding that miR-19b, miR-365, and miR-27b were more abundant in animals where the pain related to surgery was not mitigated by the use of anesthetic is consistent with their physiological roles, as shown by the pathway enrichment of their predicted mRNA targets. The results provided evidence that DE-miRNAs target the expression of genes coding for proteins involved in pain-regulatory pathways and inflammation. MiR-19b is a member of the miR-17-92 cluster, whose role is to act as a powerful modulator of the TGFβ pathway during the immune response (Jiang et al., 2011).
TGF-β belongs to a superfamily with more than 30 member proteins that are released after injury within the inflamed area and amplifies peripheral nociceptor transduction, evoking functional plasticity and increasing the excitability of nociceptors, aiming to protect the injured area by increasing pain sensitivity (Schaible et al., 2011). TGF-β family members exert a protective role against nerve-injury-induced neuropathic pain (Echeverry et al., 2009) and maintain the integrity of the blood–brain barrier, preventing the development of pathological pain following peripheral inflammatory (Ronaldson et al., 2009) or neural injuries (Echeverry et al., 2011). TGF-β interacts with their cell targets through two receptors, TGF β receptor (TGFβR) I and TGFβRII. TGFβR activation recruits SMAD proteins that are also involved in the miRNAs biogenesis at both transcriptional and post-transcriptional levels (Blahna and Hata, 2012). Remarkably, Mothers Against Decapentaplegic Homolog 3 (SMAD 3) transcriptionally regulates the miR-17-92 cluster, to which miR-19b belongs (Luo et al., 2014). The authors may, therefore, speculate that miR-19b increased abundance induced by castration and tail docking in piglets may be driven by TGFβ upregulation, which in turn may be feedback regulated by miR-19b.
MicroRNA-365 is involved in pathways involved in the regulation of inflammation. An analog investigation carried out in a rat model of morphine analgesic tolerance (Wu et al., 2018) demonstrated that the upregulation of miR-365 was related to morphine tolerance by targeting the mRNA expressions of β-arrestin2, ERK, and CREB and decreasing the contents of IL-1β, Tumor Necross Factor-alpha TNF-α, and IL-18. Similarly, to what demonstrated for TGFβ, also the expression of miR-365 is upregulated by pro-inflammatory cytokines, such as IL1β, providing a further example of the feedback-regulatory loop (Yang et al., 2016). Therefore, miR-365 may also be involved in a complex regularity network linking pain to inflammation, contributing to explain at the molecular level the decrease of inflammatory defenses during statuses where animals are stressed. The last miRNA whose abundance was found to be increased in animals subjected to dock tailing and castration without anesthesia was miR-27b. Finally, as miR-19b and miR-365, miR-27b is also related to the dampening of inflammation, which is carried out by targeting the pro-inflammatory protein MIP1-β (Li et al., 2017).
Conclusions
The present study demonstrated that salivary miRNAs were found to be more abundant in animals exposed to the surgical removal of tail docking and castration. This effect is reduced by the use of anesthesia. The finding of these miRNAs in saliva and their good diagnostic values, as demonstrated by ROC analysis, suggests the use of miR-19b and miR-365 as potential noninvasive biomarkers to assess pain and stress in pigs, although validation on a larger number of animals, and comparison with other physiological and behavioral parameters are required. Measurements of miRNAs might also be applied to identify potential sex differences in pain sensitivity. No significant differences in behavior, facial grimacing, or emitted vocalizations between male and female piglets were found in a model of pain caused by tail docking (Viscardi and Turner, 2019). Given the background that miRNA measurement identified sex differences in pig adipose tissue (Mentzel et al., 2015), miRNA measure may address the issue of measuring pain in husbandry procedure in sows, providing information useful to the development of targeted pain mitigation strategies.
Glossary
Abbreviations
- ANA
piglets castrated and tail docked with pretreatment with anesthetic and anti-inflammatory drugs
- AUC
area under the curve
- CONV
piglets castrated and tail docked without any drugs
- DE-miRNA
differentially expressed microRNA
- KEGG
Kyoto encyclopedia of genes and genomes
- miRNA
microRNA
- ROC
receiver operating characteristic
- RT
reverse transcription
- SHAM
piglets only handled
- TGF
tumor growth factor
- TGFβR
TGF β receptor
Conflict of interest statement
The authors declare there are no competing interests.
Literature Cited
- Allen D. L. 1992. Diseases of swine. 7th ed. Ames: Iowa State University Press. [Google Scholar]
- Backus G., Higuera M., Juul N., Nalon E., and De Briyne. N.. 2018. Second progress report 2015–2017 on the European declaration on alternatives to surgical castration of pigs. Available from https://www.boarsontheway.com/wp-content/uploads/2018/08/Second-progress-report-2015-2017-final-1.pdf
- Bao H., Kommadath A., Liang G., Sun X., Arantes A. S., Tuggle C. K., Bearson S. M., Plastow G. S., Stothard P., and Guan L. E. L.. . 2015. Genome-wide whole blood microRNAome and transcriptome analyses reveal miRNA-mRNA regulated host response to foodborne pathogen Salmonella infection in swine. Sci. Rep. 5:12620. doi: 10.1038/srep12620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blahna M. T., and Hata A.. . 2012. Smad-mediated regulation of microRNA biosynthesis. FEBS Lett. 586:1906–1912. doi: 10.1016/j.febslet.2012.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonastre C., Mitjana O., Tejedor M. T., Calavia M., Yuste A. G., Úbeda J. L., and Falceto M. V.. . 2016. Acute physiological responses to castration-related pain in piglets: the effect of two local anesthetics with or without meloxicam. Animal 10:1474–1481. doi: 10.1017/S1751731116000586 [DOI] [PubMed] [Google Scholar]
- Bonneau M., and Weiler U.. . 2019. Pros and cons of alternatives to piglet castration: welfare, boar taint, and other meat quality traits. Animals (Basel). 9:884. doi: 10.3390/ani9110884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Aguilar M. D., Escribano D., Martínez-Miró S., López-Arjona M., Rubio C. P., Martínez-Subiela S., Cerón J. J., and Tecles F.. . 2019. Application of a score for evaluation of pain, distress and discomfort in pigs with lameness and prolapses: correlation with saliva biomarkers and severity of the disease. Res. Vet. Sci. 126:155–163. doi: 10.1016/j.rvsc.2019.08.004 [DOI] [PubMed] [Google Scholar]
- De Briyne N., Berg C., Blaha T., and Temple D.. . 2016. Pig castration: will the EU manage to ban pig castration by 2018? Porcine Health Manag. 2:29. doi: 10.1186/s40813-016-0046-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giminiani P., Brierley V. L., Scollo A., Gottardo F., Malcolm E. M., Edwards S. A., and Leach M. C.. . 2016. The assessment of facial expressions in piglets undergoing tail docking and castration: toward the development of the piglet grimace scale. Front. Vet. Sci. 3:100. doi: 10.3389/fvets.2016.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzikamunhenga R. S., Anthony R., Coetzee J., Gould S., Johnson A., Karriker L., McKean J., Millman S. T., Niekamp S. R., and O’Connor A. M.. . 2014. Pain management in the neonatal piglet during routine management procedures. Part 1: a systematic review of randomized and non-randomized intervention studies. Anim. Health Res. Rev. 15:14–38. doi: 10.1017/S1466252314000061 [DOI] [PubMed] [Google Scholar]
- Echeverry S., Shi X. Q., Haw A., Liu H., Zhang Z. W., and Zhang J.. . 2009. Transforming growth factor-beta1 impairs neuropathic pain through pleiotropic effects. Mol. Pain 5:16. doi: 10.1186/1744-8069-5-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverry S., Shi X. Q., Rivest S., and Zhang J.. . 2011. Peripheral nerve injury alters blood-spinal cord barrier functional and molecular integrity through a selective inflammatory pathway. J. Neurosci. 31:10819–10828. doi: 10.1523/JNEUROSCI.1642-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. A., von Borell E., and Bonneau M.. . 2009. Guest Editorial: Scientific and practical issues associated with piglet castration. Animal 3:1478–1479. doi: 10.1017/S1751731109990760 [DOI] [PubMed] [Google Scholar]
- Escribano D., Fuentes-Rubio M., and Cerón J. J.. . 2012. Validation of an automated chemiluminescent immunoassay for salivary cortisol measurements in pigs. J. Vet. Diagn. Invest. 24:918–923. doi: 10.1177/1040638712455171 [DOI] [PubMed] [Google Scholar]
- European Declaration. 2010. European declaration on alternatives to surgical castration of pigs. Available from https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwjl5-aH1P7oAhVVysQBHdoyAjsQFjAAegQIARAB&url=https%3A%2F%2Fec.europa.eu%2Ffood%2Fsites%2Ffood%2Ffiles%2Fanimals%2Fdocs%2Faw_prac_farm_pigs_cast-alt_declaration_en.pdf&usg=AOvVaw2TNEmh2FQhzsX-9whvECpS
- Fleming D. S., and Miller L. C.. . 2018. Identification of small non-coding RNA classes expressed in swine whole blood during HP-PRRSV infection. Virology 517:56–61. doi: 10.1016/j.virol.2018.01.027 [DOI] [PubMed] [Google Scholar]
- Gallagher N. L., Giles L. R., and Wynn P. C.. . 2002. The development of a circadian pattern of salivary cortisol secretion in the neonatal piglet. Biol. Neonate 81:113–118. doi: 10.1159/000047195 [DOI] [PubMed] [Google Scholar]
- Hansen E. P., Kringel H., Thamsborg S. M., Jex A., and Nejsum P.. . 2016. Profiling circulating miRNAs in serum from pigs infected with the porcine whipworm, Trichuris suis. Vet. Parasitol. 223:30–33. doi: 10.1016/j.vetpar.2016.03.025 [DOI] [PubMed] [Google Scholar]
- Hansen E. P., Kringel H., Thamsborg S. M., Jex A., and Nejsum P.. . 2018. Corrigendum to “Profiling circulating miRNAs in serum from pigs infected with the porcine whipworm, Trichuris suis’’” [Vet. Parasitol. 223 (2016) 30–33], (S0304401716300772), (10.1016/j.vetpar.2016.03.025)). Vet. Parasitol. 249:1. doi: 10.1016/j.vetpar.2017.11.001 [DOI] [PubMed] [Google Scholar]
- Hansson M., Lundeheim N., Nyman G., and Johansson G.. . 2011. Effect of local anaesthesia and/or analgesia on pain responses induced by piglet castration. Acta Vet. Scand. 53:34. doi: 10.1186/1751-0147-53-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellhammer D. H., Wüst S., and Kudielka B. M.. . 2009. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 34:163–171. doi: 10.1016/j.psyneuen.2008.10.026 [DOI] [PubMed] [Google Scholar]
- Heyn J., Luchting B., Hinske L. C., Hübner M., Azad S. C., and Kreth S.. . 2016. miR-124a and miR-155 enhance differentiation of regulatory T cells in patients with neuropathic pain. J. Neuroinflammation 13:248. doi: 10.1186/s12974-016-0712-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X., Yang Y., Zhu S., Hua C., Zhou R., Mu Y., Tang Z., and Li K.. . 2016. Comparison of skeletal muscle miRNA and mRNA profiles among three pig breeds. Mol. Genet. Genomics 291:559–573. doi: 10.1007/s00438-015-1126-3 [DOI] [PubMed] [Google Scholar]
- Huang T. H., Uthe J. J., Bearson S. M., Demirkale C. Y., Nettleton D., Knetter S., Christian C., Ramer-Tait A. E., Wannemuehler M. J., and Tuggle C. K.. . 2011. Distinct peripheral blood RNA responses to Salmonella in pigs differing in Salmonella shedding levels: intersection of IFNG, TLR and miRNA pathways. PLoS One. 6:e28768. doi: 10.1371/journal.pone.0028768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison S. H., Clutton R. E., Di Giminiani P., and Rutherford K. M.. . 2016. A review of pain assessment in pigs. Front. Vet. Sci. 3:108. doi: 10.3389/fvets.2016.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S., Li C., Olive V., Lykken E., Feng F., Sevilla J., Wan Y., He L., and Li Q. J.. . 2011. Molecular dissection of the miR-17-92 cluster’s critical dual roles in promoting Th1 responses and preventing inducible Treg differentiation. Blood 118:5487–5497. doi: 10.1182/blood-2011-05-355644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keita A., Pagot E., Prunier A., and Guidarini C.. . 2010. Pre-emptive meloxicam for postoperative analgesia in piglets undergoing surgical castration. Vet. Anaesth. Analg. 37:367–374. doi: 10.1111/j.1467-2995.2010.00546.x [DOI] [PubMed] [Google Scholar]
- Kluivers-Poodt M., Zonderland J. J., Verbraak J., Lambooij E., and Hellebrekers L. J.. . 2013. Pain behaviour after castration of piglets; effect of pain relief with lidocaine and/or meloxicam. Animal 7:1158–1162. doi: 10.1017/S1751731113000086 [DOI] [PubMed] [Google Scholar]
- Lecchi C., Dalla Costa E., Lebelt D., Ferrante V., Canali E., Ceciliani F., Stucke D., and Minero M.. . 2018. Circulating miR-23b-3p, miR-145-5p and miR-200b-3p are potential biomarkers to monitor acute pain associated with laminitis in horses. Animal 12:366–375. doi: 10.1017/S1751731117001525 [DOI] [PubMed] [Google Scholar]
- Lecchi C., Marques A. T., Redegalli M., Meani S., Vinco L. J., Bronzo V., and Ceciliani F.. . 2016. Circulating extracellular miR-22, miR-155, and miR-365 as candidate biomarkers to assess transport-related stress in turkeys. Animal 10:1213–1217. doi: 10.1017/S1751731115003043 [DOI] [PubMed] [Google Scholar]
- Lee Y. H., and Wong D. T.. . 2009. Saliva: an emerging biofluid for early detection of diseases. Am. J. Dent. 22:241–248 [PMC free article] [PubMed] [Google Scholar]
- Li W., Chang N., Tian L., Yang J., Ji X., Xie J., Yang L., and Li L.. . 2017. miR-27b-3p, miR-181a-1-3p, and miR-326-5p are involved in the inhibition of macrophage activation in chronic liver injury. J. Mol. Med. (Berl). 95:1091–1105. doi: 10.1007/s00109-017-1570-0 [DOI] [PubMed] [Google Scholar]
- Loo J. A., Yan W., Ramachandran P., and Wong D. T.. . 2010. Comparative human salivary and plasma proteomes. J. Dent. Res. 89:1016–1023. doi: 10.1177/0022034510380414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo T., Cui S., Bian C., and Yu X.. . 2014. Crosstalk between TGF-β/Smad3 and BMP/BMPR2 signaling pathways via miR-17-92 cluster in carotid artery restenosis. Mol. Cell. Biochem. 389:169–176. doi: 10.1007/s11010-013-1938-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Miró S., Tecles F., Ramón M., Escribano D., Hernández F., Madrid J., Orengo J., Martínez-Subiela S., Manteca X., and Cerón J. J.. . 2016. Causes, consequences and biomarkers of stress in swine: an update. BMC Vet. Res. 12:171. doi: 10.1186/s12917-016-0791-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini P., Sales G., Brugiolo M., Gandaglia A., Naso F., De Pittà C., Spina M., Gerosa G., Chemello F., Romualdi C., . et al. 2014. Tissue-specific expression and regulatory networks of pig microRNAome. PLoS One. 9:e89755. doi: 10.1371/journal.pone.0089755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentzel C. M., Anthon C., Jacobsen M. J., Karlskov-Mortensen P., Bruun C. S., Jørgensen C. B., Gorodkin J., Cirera S., and Fredholm M.. . 2015. Gender and obesity specific microRNA expression in adipose tissue from lean and obese pigs. PLoS One. 10:e0131650. doi: 10.1371/journal.pone.0131650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numberger J., Ritzmann M., Übel N., Eddicks M., Reese S., and Zöls S.. . 2016. Ear tagging in piglets: the cortisol response with and without analgesia in comparison with castration and tail docking. Animal 10:1864–1870. doi: 10.1017/S1751731116000811 [DOI] [PubMed] [Google Scholar]
- Ott S., Soler L., Moons C. P., Kashiha M. A., Bahr C., Vandermeulen J., Janssens S., Gutiérrez A. M., Escribano D., Cerón J. J., . et al. 2014. Different stressors elicit different responses in the salivary biomarkers cortisol, haptoglobin, and chromogranin A in pigs. Res. Vet. Sci. 97:124–128. doi: 10.1016/j.rvsc.2014.06.002 [DOI] [PubMed] [Google Scholar]
- Pan Z., Zhang M., Ma T., Xue Z. Y., Li G. F., Hao L. Y., Zhu L. J., Li Y. Q., Ding H. L., and Cao J. L.. . 2016. Hydroxymethylation of microRNA-365-3p regulates nociceptive behaviors via Kcnh2. J. Neurosci. 36:2769–2781. doi: 10.1523/JNEUROSCI.3474-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunier A., Mounier A. M., and Hay M.. . 2005. Effects of castration, tooth resection, or tail docking on plasma metabolites and stress hormones in young pigs. J. Anim. Sci. 83:216–222. doi: 10.2527/2005.831216x [DOI] [PubMed] [Google Scholar]
- Ronaldson P. T., Demarco K. M., Sanchez-Covarrubias L., Solinsky C. M., and Davis T. P.. . 2009. Transforming growth factor-beta signaling alters substrate permeability and tight junction protein expression at the blood-brain barrier during inflammatory pain. J. Cereb. Blood Flow Metab. 29:1084–1098. doi: 10.1038/jcbfm.2009.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai A., Saitow F., Maruyama M., Miyake N., Miyake K., Shimada T., Okada T., and Suzuki H.. . 2017. MicroRNA cluster miR-17–92 regulates multiple functionally related voltage-gated potassium channels in chronic neuropathic pain. Nat. Commun. 8:16079. doi: 10.1038/ncomms16079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible H. G., Ebersberger A., and Natura G.. . 2011. Update on peripheral mechanisms of pain: beyond prostaglandins and cytokines. Arthritis Res. Ther. 13:210. doi: 10.1186/ar3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimundić A.-M. 2009. Measures of diagnostic accuracy: basic definitions. EJIFCC. 19:203–211. [PMC free article] [PubMed] [Google Scholar]
- Tao X., Xu Z., and Men X.. . 2016. Analysis of serum microRNA expression profiles and comparison with small intestinal microRNA expression profiles in weaned piglets. PLoS One. 11:e0162776. doi: 10.1371/journal.pone.0162776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscardi A., and Turner P.. . 2019. Use of meloxicam, buprenorphine, and Maxilene® to assess a multimodal approach for piglet pain management, part 2: tail-docking. Anim. Welf. 28:499–510. doi: 10.7120/09627286.28.4.499 [DOI] [Google Scholar]
- Wang X., Kaczor-Urbanowicz K. E., and Wong D. T.. . 2017a. Salivary biomarkers in cancer detection. Med. Oncol. 34:7. doi: 10.1007/s12032-016-0863-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Qi R., Liu H., Wang J., Huang W., Yang F., and Huang J.. . 2017b. Effects of conjugated linoleic acid supplementation on the expression profile of miRNAs in porcine adipose tissue. Genes (Basel). 8(10):71. doi: 10.3390/genes8100271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Xu W., Zhong T., Song Z., Zou Y., Ding Z., Guo Q., Dong X., and Zou W.. . 2016. miR-365 targets β-arrestin 2 to reverse morphine tolerance in rats. Sci. Rep. 6:38285. doi: 10.1038/srep38285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. P., She R. X., Yang Y. P., Xing Z. M., Chen H. W., and Zhang Y. W.. . 2018. MicroRNA-365 alleviates morphine analgesic tolerance via the inactivation of the ERK/CREB signaling pathway by negatively targeting β-arrestin2. J. Biomed. Sci. 25:10. doi: 10.1186/s12929-018-0405-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Guan Y., Tian S., Wang Y., Sun K., and Chen Q.. . 2016. Mechanical and IL-1β responsive miR-365 contributes to osteoarthritis development by targeting histone deacetylase 4. Int. J. Mol. Sci. 17:436. doi: 10.3390/ijms17040436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. Z., Chen D. W., He J., Zheng P., Yu J., Mao X. B., Huang Z. Q., Luo Y. H., Luo J. Q., and Yu B.. . 2019. Long-term dietary resveratrol supplementation decreased serum lipids levels, improved intramuscular fat content, and changed the expression of several lipid metabolism-related miRNAs and genes in growing-finishing pigs1. J. Anim. Sci. 97:1745–1756. doi: 10.1093/jas/skz057 [DOI] [PMC free article] [PubMed] [Google Scholar]