Abstract
Background
Residence in an ethnic enclave may be associated with survival among Latinas with breast cancer, but findings from prior studies are inconsistent.
Methods
We conducted parallel analyses of California and Texas cancer registry data for adult (≥18 years of age) Latinas diagnosed with invasive breast cancer from 1996 to 2005, with follow-up through 2014. We used existing indices applied to tract-level 2000 US Census data to measure Latinx enclaves and neighborhood socioeconomic status (nSES). We fitted multivariable Cox Proportional Hazard models for all-cause and breast cancer specific mortality adjusted for diagnosis year, patient age, nativity (with multiple imputation), tumor stage, histology, grade, size, and clustering by census tract.
Results
Among 38,858 Latinas, the majority (61.3% in CA, 70.5% in TX) lived in enclaves. In fully adjusted models for both states, foreign-born women, compared to US-born women, were more likely to die from breast cancer and all causes. Living in enclaves and in neighborhoods with higher SES were independently associated with improved survival from both causes. When combined into a four-level variable, compared to those living in low nSES enclaves, those in low nSES non-enclaves had worse survival for both causes; and, in the all-cause but not breast-cancer specific models, those in high nSES neighborhoods, regardless of enclave status, had improved survival from all-causes.
Conclusion
Applying the same methods across two states eliminated previously published inconsistent associations between enclave residence and breast cancer survival. Future studies should identify specific protective effects of enclave residence to inform interventions.
Keywords: Latino, ethnic enclave, nativity, neighborhood socioeconomic status, breast cancer survival
Precis:
Among Latinas with breast cancer living in California and Texas, foreign birthplace (vs. US birthplace) was associated with worse survival. Latinas living in more ethnically distinct neighborhoods and in higher SES neighborhoods had improved survival.
INTRODUCTION
Among US Latinas, breast cancer is the most commonly diagnosed cancer and is the leading cause of cancer death.1,2 Once diagnosed with breast cancer, most studies, but not all, demonstrate that Latinas do not survive as long as non-Latina white women.3–6 While there is growing recognition that neighborhoods play some role on outcomes across the cancer continuum, the extent to which features of a residential neighborhood may influence survival among Latinas with breast cancer is unclear.
Many Latinxs live in ethnic enclaves – culturally distinct neighborhoods with high concentrations of individuals of the same ethnic origin, high linguistic isolation, a large share of recent immigrants, and ethnic specific businesses and resources. Ethnic enclaves are hypothesized to contribute to outcomes across the cancer continuum through multiple pathways, some positively and some negatively.7,8 Co-ethnic residents within an enclave often maintain cultural norms and behaviors (e.g., diet and physical activity, social support) that may be health-promoting. Enclaves may facilitate communication and information sharing due to greater access to linguistic resources; they may also reduce exposure to discrimination and thus limit use of unhealthy coping behaviors (e.g., smoking, drinking) and reduce stress.9–13
In contrast, some features of enclaves could contribute to worse health. For example, neighborhoods with large Latinx and/or foreign-born populations face disproportionately higher poverty.14–16 Residence in neighborhoods with low socioeconomic status (nSES) is associated with worse survival among cancer patients.17,18 Low nSES may influence unhealthy behaviors and worse health through pathways associated with adverse social, built, and physical environments. For example, low nSES neighborhoods may have high crime and poor safety, greater social isolation, and low walkability resulting from high traffic density and poor street conditions, poor food environments with high concentrations of fast food restaurants, tobacco outlets or liquor stores, and greater proximity to environmental pollutants. Thus, to elucidate the association of ethnic enclave residence and cancer survival, nSES must be considered.19,20
Prior studies on the association of enclave residence and cancer survival demonstrated mixed results, underscored in a recent literature review on ethnic density and cancer outcomes.21 Five of the reviewed studies examined associations of Latinx ethnic density with survival of Latinas with breast cancer. Associations varied, with 2 null studies and others documenting both increased (n=1 study) and decreased (n=2 studies) survival among Latinas residing within ethnic enclaves compared to Latinas in non-enclave areas.19,22–25 These studies applied varying measures of neighborhood ethnic density or ethnic enclave residence and different analytic strategies, including adjustments for patient nativity and nSES. Because all 5 studies were limited to single states or metropolitan areas, it is unclear whether observed inconsistencies are a result of different analytic methods or true regional differences in enclave effects.
As nativity is often missing in cancer registry data, it can be imputed using varying approaches.23,25 Perhaps as a result, findings are varied and demonstrate that foreign-born Latinas, compared to US-born Latinas with breast cancer, have worse survival, no difference in survival, or improved survival.19,22,26
Given published inconsistencies, an improved understanding of the role of enclaves can help to inform neighborhood-level interventions designed to improve survival among Latinas. In this study, we aimed to investigate the independent associations of ethnic enclaves on survival after breast cancer among Latinas, accounting for patient nativity and nSES. We applied consistent measures and analytic methods to parallel analyses of California and Texas cancer registry data to compare and contrast ethnic enclave effects.
METHODS
Data
The data for these analyses are from two population-based cancer registries—the California Cancer Registry (CCR) and the Texas Cancer Registry. These two states have two of the largest U.S. Latinx populations. Both registries collect demographic and clinical data on incident cancers diagnosed in the state in accordance with North American Association of Central Cancer Registries standards.
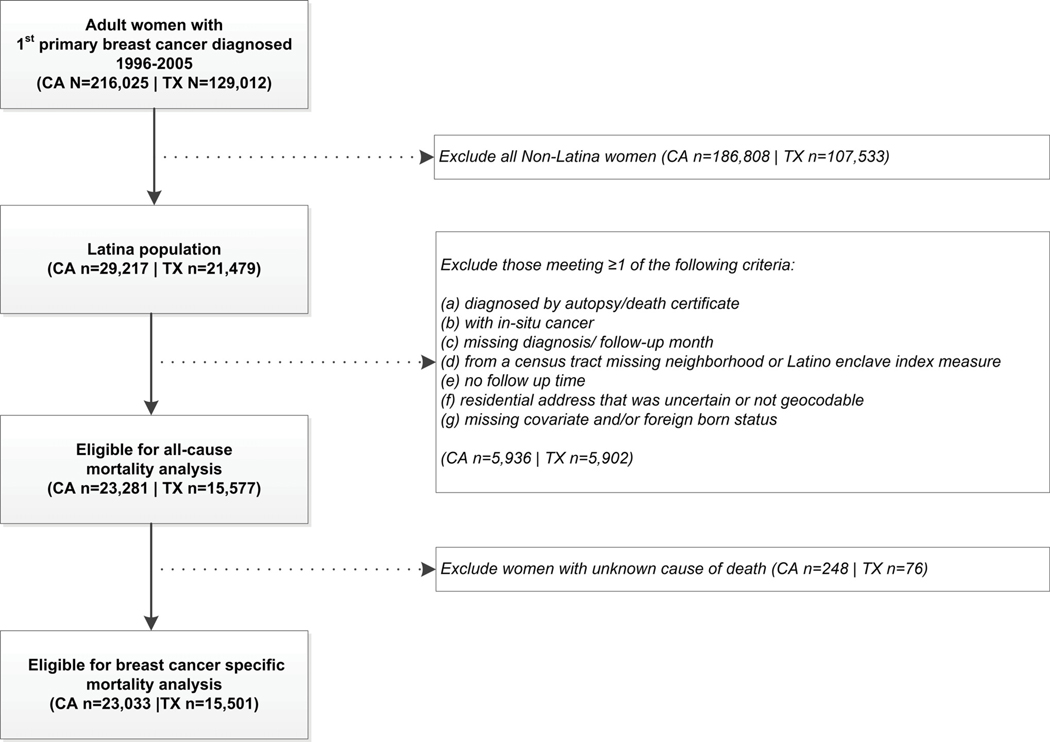
In all, n=50,696 Latina adults (age 18+) with a first primary breast cancer diagnosed between January 1, 1996 and December 31, 2005 were identified by the California (n=29,217) and Texas (n=21,479) Cancer Registries. The total number of Latinas eligible for analyses from California and Texas were 23,281 and 15,577, respectively (see Figure 1 for inclusion/exclusion criteria). We chose these years to anchor cancer diagnoses on 2000 Census data for our neighborhood variables and to have sufficient follow-up time to accrue enough events/deaths, given 5 year survival for breast cancer is approximately 90%.
Figure 1.
Inclusion and exclusion criteria and the final sample from the California and Texas Cancer Registries.
Registry geocodes were used to append Census 2000 tract-level data to ascertain nSES and Latinx enclave.8 The nSES index we used is a validated and well-established composite measure of 7 SES indicators, including education, occupation, employment, household income, poverty, rent and house values.5,6,27–32 We defined Latinx enclaves using an established multidimensional index of seven measures (percent of residents who are Latinx, foreign-born, recent immigrants, and linguistically isolated [general and of those who speak Spanish], with limited English proficiency [general and of those who speak Spanish]).33 We classified each index into state-specific quintiles. For nSES, Quintile 5 (Q5) represents the highest SES neighborhoods, while Q1 represent the lowest SES neighborhoods. For ethnic enclaves, Q5 represents most ethnically distinct neighborhoods while Q1 represents the least distinct.
We calculated follow-up time as number of days between diagnosis and either death or December 31, 2014. For breast cancer-specific survival, we censored follow-up at death date for those dying from another cause, and we excluded those with unknown cause of death.
Imputation
We imputed missing birthplace data (22% California; 44% Texas) to US-born or foreign-born using multiple imputation separately by state. We used maximum likelihood logistic regression to impute nativity using variables available from both states including age at diagnosis, year of diagnosis, tumor stage, tumor grade, tumor histology, tumor size (continuous with an indicator for missing data), reporting source, diagnosis and/or treatment at reporting facility (versus elsewhere), microscopic tumor confirmation, Hispanic origin, quintile categories of nSES and Latinx enclave and all component continuous census-level measures, time from diagnosis to death or December 31, 2014, and status at study end (alive, died of breast cancer, died of another cause, unknown cause of death).34 We fit imputation models 20 times, creating 20 datasets of imputed nativity. We excluded those missing data for any covariate (except for tumor size or grade) from imputation models (CA n=1,257; TX n=4,272). For descriptive analyses, we defined patients missing birthplace as foreign-born if nativity was imputed to foreign-born in more than 10 imputation runs, and US-born otherwise.
Statistical analysis
Separately by state, we fit multivariable Cox regression models on each of the 20 imputation datasets and combined regression results across all 20 imputed datasets to estimate hazard ratios (HR) and 95% confidence intervals for associations with mortality risk, using the rules by Rubin.35 The proportional hazards assumption did not hold for stage and tumor grade. Therefore, stage was included as a stratifying variable in all Cox regressions, allowing baseline hazards to vary by stage. Additionally stratifying by grade did not meaningfully change HRs for nativity, nSES, or enclave, so grade was included as a covariate. Minimally adjusted models included age (continuous) and diagnosis year (continuous). Fully adjusted models also included histology (ductal, lobular, other, unknown), grade (I, II, III/IV, unknown), tumor size (continuous in cm; with an indicator variable for other/missing), and census tract clustering (i.e., by using a sandwich estimator of the covariance structure that accounts for intracluster dependence). In CA and TX there was a median of 3 cases per census tract with interquartile ranges of 2–5 and 1–6, respectively. We performed Wald tests for trend across quintile categories.
We initially allowed the variables of interest, nSES and Latinx enclave, categorized by quintiles, to enter into models separately. Given high correlation as continuous measures (r=−0.76 in CA and r=−0.72 in TX) and an observed statistically significant interaction (CA overall survival, p-interaction=0.004; TX breast cancer-specific survival, p-interaction=0.013), we created a 4-level combined variable of nSES (low/high) and enclave residence (no/yes). Based on sample distributions, we defined high nSES as the top three state-specific quintiles and Latinx enclave as the top two state-specific quintiles. We did not observe statistically significant interactions between nativity and nSES nor enclave in both states.
Finally, to facilitate comparison of survival across the multiple independent and joint associations of interest, we calculated five-year survival probabilities and associated 95% confidence intervals from the fully adjusted Cox models with covariates set to their reference level or mean value and stage entered into the model as a covariate. Survival probability estimates were first normalized using the complementary log-log transformation before combining results across the 20 multiple imputation runs, and then the combined results were back-transformed.36
RESULTS
Table 1 shows the percent of Latina breast cancer cases who were foreign-born increased slightly after imputation. Table 2 shows patient characteristics. Texas had higher percent of cases living in ethnic enclaves than California. In both states, more foreign-born compared to US-born Latinas lived in ethnic enclaves and in low SES neighborhoods.
Table 1.
Distribution of patient nativity with and without multiple imputation among Latinas with breast cancer diagnosed in California or Texas, 1996–2005, by state.
| US-Born | Foreign-Born | Missing | Total | |||||
|---|---|---|---|---|---|---|---|---|
| N | Row Percent | N | Row Percent | N | Row Percent | N | Row Percent | |
| CA-no imputation | 7,876 | 33.83 | 10,217 | 43.89 | 5,188 | 22.28 | 23,281 | 100 |
| CA-with imputation | 12,621 | 54.21 | 10,660 | 45.79 | - | - | 23,281 | 100 |
| TX-no imputation | 5,965 | 38.29 | 2,735 | 17.56 | 6,877 | 44.15 | 15577 | 100 |
| TX-with imputation | 11,897 | 76.38 | 3,680 | 23.62 | - | - | 15577 | 100 |
Table 2.
Characteristics of Latinas with breast cancer diagnosed in California or Texas, 1996–2005, by state.
| Texas | California | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| US-Born (n=11,897) n (column %) | Foreign-Born (n=3,680) n (column %) | All (n=15,577) n (column %) | US-Born (n=12,621) n (column %) | Foreign-Born (n=10,660) n (column %) | All (n=23,281) n (column %) | |||||||
| Age at diagnosis | ||||||||||||
| <40 | 1302 | (10.9) | 456 | (12.4) | 1758 | (11.3) | 1386 | (11.0) | 1381 | (13.0) | 2767 | (11.9) |
| 40–49 | 2983 | (25.1) | 908 | (24.7) | 3891 | (25.0) | 3217 | (25.5) | 2971 | (27.9) | 6188 | (26.6) |
| 50–59 | 3058 | (25.7) | 861 | (23.4) | 3919 | (25.2) | 3094 | (24.5) | 2644 | (24.8) | 5738 | (24.6) |
| 60–69 | 2201 | (18.5) | 702 | (19.1) | 2903 | (18.6) | 2463 | (19.5) | 1892 | (17.7) | 4355 | (18.7) |
| 70+ | 2353 | (19.8) | 753 | (20.5) | 3106 | (19.9) | 2461 | (19.5) | 1772 | (16.6) | 4233 | (18.2) |
| Diagnosis year | ||||||||||||
| 1996–2000 | 4863 | (40.9) | 1627 | (44.2) | 6490 | (41.7) | 5593 | (44.3) | 4713 | (44.2) | 10306 | (44.3) |
| 2001–2005 | 7034 | (59.1) | 2053 | (55.8) | 9087 | (58.3) | 7028 | (55.7) | 5947 | (55.8) | 12975 | (55.7) |
| Summary stage | ||||||||||||
| Local | 6150 | (51.7) | 1565 | (42.5) | 7715 | (49.5) | 7419 | (58.8) | 5331 | (50.0) | 12750 | (54.8) |
| Regional | 4522 | (38.0) | 1493 | (40.6) | 6015 | (38.6) | 4578 | (36.3) | 4523 | (42.4) | 9101 | (39.1) |
| Distant | 716 | (6.0) | 299 | (8.1) | 1015 | (6.5) | 509 | (4.0) | 562 | (5.3) | 1071 | (4.6) |
| Unknown | 509 | (4.3) | 323 | (8.8) | 832 | (5.3) | 115 | (0.9) | 244 | (2.3) | 359 | (1.5) |
| Histology | ||||||||||||
| Ductal | 9440 | (79.3) | 2918 | (79.3) | 12358 | (79.3) | 10271 | (81.4) | 8456 | (79.3) | 18727 | (80.4) |
| Lobular | 768 | (6.5) | 196 | (5.3) | 964 | (6.2) | 885 | (7.0) | 630 | (5.9) | 1515 | (6.5) |
| Other | 1531 | (12.9) | 482 | (13.1) | 2013 | (12.9) | 1439 | (11.4) | 1539 | (14.4) | 2978 | (12.8) |
| Unknown | 158 | (1.3) | 84 | (2.3) | 242 | (1.6) | 26 | (0.2) | 35 | (0.3) | 61 | (0.3) |
| Grade | ||||||||||||
| I | 1253 | (10.5) | 296 | (8.0) | 1549 | (9.9) | 1991 | (15.8) | 1231 | (11.5) | 3222 | (13.8) |
| II | 3506 | (29.5) | 1109 | (30.1) | 4615 | (29.6) | 4333 | (34.3) | 3549 | (33.3) | 7882 | (33.9) |
| III/IV | 5221 | (43.9) | 1566 | (42.6) | 6787 | (43.6) | 4976 | (39.4) | 4679 | (43.9) | 9655 | (41.5) |
| Unknown | 1917 | (16.1) | 709 | (19.3) | 2626 | (16.9) | 1321 | (10.5) | 1201 | (11.3) | 2522 | (10.8) |
| Tumor Size (cm) | ||||||||||||
| Mean | 2.3 | 2.3 | 2.3 | 2.2 | 2.5 | 2.3 | ||||||
| Standard deviation | 3.2 | 4.1 | 3.5 | 2.1 | 2.5 | 2.3 | ||||||
| Missing: N (%) | 2422 | 20.4 | 1005 | 27.3 | 3427 | 22.0 | 1243 | (9.9) | 1265 | (11.9) | 2508 | (10.8) |
| Neighborhood socioeconomic status (nSES) quintile | ||||||||||||
| Quintile 1: Low SES | 3793 | (31.9) | 1544 | (42.0) | 5337 | (34.3) | 2973 | (23.6) | 3950 | (37.1) | 6923 | (29.7) |
| Q2 | 2730 | (22.9) | 799 | (21.7) | 3529 | (22.7) | 3117 | (24.7) | 2706 | (25.4) | 5823 | (25.0) |
| Q3 | 1772 | (14.9) | 440 | (12.0) | 2212 | (14.2) | 2765 | (21.9) | 1850 | (17.4) | 4615 | (19.8) |
| Q4 | 1895 | (15.9) | 473 | (12.9) | 2368 | (15.2) | 2201 | (17.4) | 1229 | (11.5) | 3430 | (14.7) |
| Q5- High SES | 1707 | (14.3) | 424 | (11.5) | 2131 | (13.7) | 1565 | (12.4) | 925 | (8.7) | 2490 | (10.7) |
| Ethnic enclave quintile | ||||||||||||
| Quintile 1: Least ethnically distinct | 662 | (5.6) | 102 | (2.8) | 764 | (4.9) | 1405 | (11.1) | 525 | (4.9) | 1930 | (8.3) |
| Q2 | 1365 | (11.5) | 255 | (6.9) | 1620 | (10.4) | 2015 | (16.0) | 943 | (8.8) | 2958 | (12.7) |
| Q3 | 1935 | (16.3) | 338 | (9.2) | 2273 | (14.6) | 2602 | (20.6) | 1518 | (14.2) | 4120 | (17.7) |
| Q4 | 2998 | (25.2) | 786 | (21.4) | 3784 | (24.3) | 3352 | (26.6) | 2778 | (26.1) | 6130 | (26.3) |
| Q5: Most ethnically distinct | 4937 | (41.5) | 2199 | (59.8) | 7136 | (45.8) | 3247 | (25.7) | 4896 | (45.9) | 8143 | (35.0) |
| Joint nSES/enclave measure | ||||||||||||
| No Enclave, Low SES | 529 | (4.4) | 85 | (2.3) | 614 | (3.9) | 1108 | (8.8) | 465 | (4.4) | 1573 | (6.8) |
| Enclave, Low SES | 6900 | (58.0) | 2511 | (68.2) | 9411 | (60.4) | 4982 | (39.5) | 6191 | (58.1) | 11173 | (48.0) |
| No Enclave, High SES | 2306 | (19.4) | 428 | (11.6) | 2734 | (17.6) | 4914 | (38.9) | 2521 | (23.6) | 7435 | (31.9) |
| Enclave, High SES | 2162 | (18.2) | 656 | (17.8) | 2818 | (18.1) | 1617 | (12.8) | 1483 | (13.9) | 3100 | (13.3) |
| Vital Status | ||||||||||||
| Alive | 7033 | (59.1) | 1764 | (47.9) | 8797 | (56.5) | 8399 | (66.5) | 6256 | (58.7) | 14655 | (62.9) |
| Dead | 4864 | (40.9) | 1916 | (52.1) | 6780 | (43.5) | 4222 | (33.5) | 4404 | (41.3) | 8626 | (37.1) |
In minimally- and fully-adjusted models, nativity, Latinx enclave residence, and nSES were independently associated with both outcomes in both states. Given the similarity in findings across models, we present associations for fully adjusted models in Table 3 (see Supplemental Table 1 for minimally adjusted results). For all-causes, foreign-born Latinas had worse survival compared to US-born Latinas. Compared to those residing in the most ethnically distinct neighborhoods, those in the least distinct neighborhoods had worse survival. Compared to those residing in the highest SES neighborhoods, those in the lowest SES neighborhoods had worse survival. Results were similar for breast-cancer specific mortality.
Table 3.
Independent associations (Hazard Ratios, 95% Confidence Intervals; 5-year survival probabilities) of nativity, ethnic enclave and neighborhood socioeconomic status (nSES) and survival after breast cancer, CA and TX, 1996–2005
| All-cause mortality | Breast cancer-specific mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| Fully Adjusted HR (95% CI) | 5-year survival Probability (95% CI) | Fully Adjusted HR (95% CI) | 5-year survival Probability (95% CI) | |||||
| CALIFORNIA | ||||||||
| Nativity | ||||||||
| US Born (ref) | 1.00 | 0.953 | (0.946–0.959) | 1.00 | 0.986 | (0.983–0.989) | ||
| Foreign Born | 1.13 | (1.08–1.19) | 0.947 | (0.940–0.953) | 1.19 | (1.11–1.26) | 0.984 | (0.980–0.987) |
| Ethnic enclave quintile | ||||||||
| Quintile 1: Least ethnically distinct | 1.18 | (1.06–1.31) | 0.944 | (0.937–0.950) | 1.16 | (1.01–1.34) | 0.984 | (0.981–0.987) |
| Q2 | 1.21 | (1.10–1.34) | 0.942 | (0.935–0.948) | 1.21 | (1.07–1.38) | 0.983 | (0.980–0.986) |
| Q3 | 1.11 | (1.02–1.21) | 0.947 | (0.941–0.954) | 1.03 | (0.92–1.16) | 0.986 | (0.983–0.988) |
| Q4 | 1.06 | (1.00–1.14) | 0.950 | (0.943–0.956) | 1.05 | (0.96–1.15) | 0.986 | (0.982–0.988) |
| Q5: Most ethnically distinct (ref) | 1.00 | 0.953 | (0.946–0.959) | 1.00 | 0.986 | (0.983–0.989) | ||
| p-trend | <0.001 | 0.008 | ||||||
| nSES quintile | ||||||||
| Q1: Low SES | 1.58 | (1.41–1.76) | 0.926 | (0.919–0.932) | 1.47 | (1.27–1.70) | 0.980 | (0.976–0.983) |
| Q2 | 1.48 | (1.34–1.64) | 0.929 | (0.921–0.935) | 1.42 | (1.24–1.62) | 0.980 | (0.977–0.983) |
| Q3 | 1.32 | (1.20–1.46) | 0.937 | (0.930–0.944) | 1.29 | (1.14–1.47) | 0.982 | (0.979–0.985) |
| Q4 | 1.16 | (1.06–1.28) | 0.944 | (0.937–0.950) | 1.24 | (1.10–1.41) | 0.983 | (0.979–0.986) |
| Q5: High SES (ref) | 1.00 | 0.953 | (0.946–0.959) | 1.00 | 0.986 | (0.983–0.989) | ||
| p-trend | <0.001 | <0.001 | ||||||
| TEXAS | ||||||||
| Nativity | ||||||||
| US Born (ref) | 1.00 | 0.937 | (0.927–0.945) | 1.00 | 0.982 | (0.977–0.985) | ||
| Foreign Born | 1.18 | (1.10–1.26) | 0.917 | (0.905–0.928) | 1.27 | (1.16–1.38) | 0.975 | (0.969–0.980) |
| Ethnic enclave quintile | ||||||||
| Quintile 1: Least ethnically distinct | 1.28 | (1.12–1.47) | 0.918 | (0.905–0.929) | 1.19 | (1.00–1.42) | 0.978 | (0.972–0.982) |
| Q2 | 1.11 | (1.00–1.24) | 0.929 | (0.920–0.938) | 0.97 | (0.84–1.12) | 0.982 | (0.977–0.986) |
| Q3 | 1.15 | (1.06–1.25) | 0.926 | (0.916–0.935) | 1.16 | (1.04–1.29) | 0.978 | (0.973–0.983) |
| Q4 | 1.12 | (1.05–1.19) | 0.929 | (0.919–0.938) | 1.07 | (0.98–1.17) | 0.980 | (0.975–0.984) |
| Q5: Most ethnically distinct (ref) | 1.00 | 0.937 | (0.927–0.945) | 1.00 | 0.982 | (0.977–0.985) | ||
| p-trend | <0.001 | 0.115 | ||||||
| nSES quintile | ||||||||
| Q1: Low SES | 1.44 | (1.29–1.60) | 0.908 | (0.899–0.917) | 1.24 | (1.08–1.43) | 0.977 | (0.972–0.981) |
| Q2 | 1.31 | (1.18–1.46) | 0.917 | (0.908–0.925) | 1.15 | (1.00–1.31) | 0.979 | (0.974–0.983) |
| Q3 | 1.30 | (1.17–1.45) | 0.918 | (0.908–0.927) | 1.17 | (1.02–1.35) | 0.978 | (0.973–0.983) |
| Q4 | 1.14 | (1.03–1.27) | 0.927 | (0.918–0.935) | 1.06 | (0.93–1.21) | 0.980 | (0.976–0.984) |
| Q5: High SES (ref) | 1.00 | 0.937 | (0.927–0.945) | 1.00 | 0.982 | (0.977–0.985) | ||
| p-trend | <0.001 | 0.001 | ||||||
Fully adjusted models include age at diagnosis, tumor grade, tumor size, year of diagnosis, histology, underlying stratification by stage and clustering by census tract.
In fully-adjusted models with enclave and nSES defined as a 4-category combination variable (Table 4; see Supplemental Table 1 for minimally adjusted results), foreign-born Latinas had worse all-cause and breast-cancer specific survival compared to US-born Latinas. In comparison to Latinas residing in low-nSES enclaves, those in high-SES neighborhoods had improved all-cause survival in both states regardless of enclave status. Latinas residing in low–nSES non-enclave neighborhoods had higher all-cause mortality (compared to low-nSES enclaves). For breast cancer-specific survival, results were similar, but statistical significance was only observed in CA for those residing in high-SES non-enclave neighborhoods.
Table 4.
Independent association of nativity and joint associations (Hazard Ratios, 95% Confidence Intervals; 5-year survival probabilities) of neighborhood socioeconomic status (nSES)/ethnic enclave and survival after breast cancer, CA and TX, 1996–2005
| All-cause mortality | Breast cancer-specific mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| Fully Adjusted HR (95% CI) | 5-year survival Probability (95% CI) | Fully Adjusted HR (95% CI) | 5-year survival Probability (95% CI) | |||||
| CALIFORNIA | ||||||||
| Nativity | ||||||||
| US Born (ref) | 1.00 | 0.926 | (0.919–0.932) | 1.00 | 0.980 | (0.976–0.983) | ||
| Foreign Born | 1.12 | (1.07–1.17) | 0.918 | (0.911–0.924) | 1.17 | (1.10–1.25) | 0.976 | (0.973–0.980) |
| Joint nSES/ enclave measure | ||||||||
| High SES, Enclave | 0.89 | (0.84–0.95) | 0.934 | (0.927–0.940) | 0.95 | (0.87–1.03) | 0.981 | (0.977–0.984) |
| High SES, Non-Enclave | 0.85 | (0.81–0.89) | 0.936 | (0.931–0.941) | 0.89 | (0.83–0.95) | 0.982 | (0.979–0.984) |
| Low SES, Enclave (ref) | 1.00 | 0.926 | (0.919–0.932) | 1.00 | 0.980 | (0.976–0.983) | ||
| Low SES, Non-Enclave | 1.15 | (1.06–1.26) | 0.914 | (0.904–0.922) | 1.13 | (1.00–1.28) | 0.977 | (0.972–0.981) |
| TEXAS | ||||||||
| Nativity | ||||||||
| US Born (ref) | 1.00 | 0.909 | (0.900–0.917) | 1.00 | 0.977 | (0.972–0.981) | ||
| Foreign Born | 1.17 | (1.09–1.25) | 0.895 | (0.884–0.905) | 1.26 | (1.16–1.37) | 0.971 | (0.965–0.977) |
| Joint nSES/ enclave measure | ||||||||
| High SES, Enclave | 0.91 | (0.85–0.97) | 0.917 | (0.909–0.925) | 0.94 | (0.86–1.02) | 0.979 | (0.974–0.982) |
| High SES, Non-Enclave | 0.90 | (0.85–0.96) | 0.917 | (0.909–0.925) | 0.94 | (0.87–1.02) | 0.978 | (0.974–0.982) |
| Low SES, Enclave (ref) | 1.00 | 0.909 | (0.900–0.917) | 1.00 | 0.977 | (0.972–0.981) | ||
| Low SES, Non-Enclave | 1.09 | (0.97–1.23) | 0.900 | (0.885–0.913) | 1.06 | (0.92–1.22) | 0.975 | (0.969–0.980) |
Fully adjusted models include age at diagnosis, tumor grade, tumor size, year of diagnosis, histology, underlying stratification by stage and clustering by census tract.
Adjusted survival probabilities demonstrate the differences between enclave and nSES quintiles, the four-level variable, and between nativity groups. Differences in survival probabilities allow for a more qualitative comparison among various categories showing little difference in probability of survival for those residing in high SES neighborhoods, regardless of enclave status, but for those residing in low SES neighborhoods, we observed lower survival probability for those in non-enclave neighborhoods compared to those residing in enclaves. Notably, differences appear larger for overall survival compared with breast cancer-specific survival.
DISCUSSION
To address inconsistent associations between ethnic enclave and breast cancer survival in the literature, we used the same multilevel measures and analytic methods and found similar associations across two states. We observed consistent associations between survival, nSES, ethnic enclave residence, and nativity among Latinas with breast cancer across both states. We also demonstrated that foreign-born Latinas are more likely to live in low-SES neighborhoods and in more ethnically distinct neighborhoods, compared to US-born Latinas, Taken together, these results provide a compelling rationale for continued attention to multilevel and place-based factors contributing to the survival of Latinas with breast cancer.
Enclave
When examined using quintiles, we observed associations of enclave residence after accounting for nativity and nSES and other covariates, with residence in more distinct ethnic neighborhoods associated with improved survival in both states. We observed statistically significant trends for quintiles of all-cause survival in both states; however for breast-cancer specific survival, while the direction of the point estimates was nearly always consistent, the trend across quintiles was significant in California but not Texas.
Neighborhood SES
Latinas living in neighborhoods with lower SES faced worse survival from both causes in both states. This finding is consistent with a large body of literature demonstrating worse survival among cancer patients living in neighborhoods with low-SES, regardless of how nSES is measured.17–19,22–24
Enclave and Neighborhood SES
When compared to those in low-nSES enclaves, Latinas in either type of high-SES neighborhood (enclave or non-enclave) had improved survival and Latinas in low-nSES non-enclaves had the worst survival, although this was not consistently statistically significant by cause of death or state. This demonstrates that enclaves may entail some benefits for residents that result in improved survival. Co-ethnic residents within enclaves often maintain lifestyles, cultural norms, and behaviors (e.g., diet and physical activity, social networks, social cohesion) that are health-promoting. Enclaves may facilitate communication and information sharing due to greater access to linguistic resources; they may also reduce exposure to discrimination and thus limit use of unhealthy coping behaviors (e.g., smoking, drinking) and reduce levels of individual stress.12,37–40 Enclave-survival associations for Texas, while trending in the same direction as results for California, are somewhat more attenuated, which may reflect differences in sample distribution, historical patterns related to immigration and settlement, or numerous other social, political, and physical environment differences between the states.
Comparison with Prior Research
Prior breast cancer studies from California using the same nSES and enclave measures as in our study also demonstrated worse survival for those in low-SES neighborhoods, regardless of enclave status.19,22 Prior studies demonstrated differing results, both positive and negative, regarding enclave residence. In two prior Texas studies, two different measures of neighborhood Latinx composition were associated with increased mortality.23,26 These prior studies may not be directly comparable to this study given different methods (e.g., differing lengths of follow-up, adjustment for different covariates, inclusion of other racial/ethnic groups, and in the Texas studies, use of a single indicator of enclave, i.e., neighborhood ethnic composition/segregation).
We demonstrated the importance of using the same methods across states to ensure findings are comparable and not artifacts of methodological differences. A multicomponent ethnic enclave index better captures the multiple dimensions of place that may be relevant for survival, going beyond single measures such as ethnic density. Our measure allows for identification of enclaves that are both culturally and ethnically concentrated and distinct from the remainder of the state in regard to race/ethnicity, language, nativity, and recency of immigration.33
Nativity
Foreign-born, compared to US-born Latinas in both states had worse survival from both causes. Prior studies demonstrated inconsistent findings. Foreign-born Latinas with breast cancer had worse survival in a Texas study,24 slightly improved all-cause (but not cause-specific) survival in one California study,19 and equivalent survival to US-born Latinas in another California study.22 Differences in methodology, including nativity imputation methods, may explain these discrepancies. Consistent with prior research, we also demonstrated that foreign-born Latinas are more likely to live in ethnic enclaves and low-SES neighborhoods.19,24
Implications
Future intervention and research should prioritize historically underserved populations and neighborhoods. More research on the pathways through which enclaves and neighborhood SES impact survival is needed to inform and tailor community interventions. Future research is needed in other states with Latinx populations who differ by race, country of origin, nativity, length of time in the US, and residential settlement patterns.
Limitations
Our study has several limitations. Birthplace is often missing in registry data.41–44 Imputation is necessary because dropping patients missing birthplace reduces generalizability and introduces bias due to the unique reasons why data are missing.34,45,46 Registry data are often missing at random (MAR), i.e., missing conditional to other observed variables. For example, missing nativity and ethnicity are conditional on vital status, among other variables, because these data are obtained from death certificates.34,41–44 Multiple imputation (MI) can be used to handle data that are MAR or missing completely at random and has been applied and validated to impute missing cancer registry data (e.g., stage).47–50 While we used a multiple imputation model validated in a study of cervical cancer,34 there may be some misclassification. We calculated sensitivity and specificity of our imputation using a sample of Latinas with known birthplace and found we could determine US-birthplace (93.5% in CA and 86.2%) and foreign-birthplace (90.7% in CA and 77.0%) with good accuracy. Notably, when we did not impute nativity and kept unknown as a category, direction of enclave and nSES variables remained unchanged. Our imputation approach is the best available method to examine nativity disparities in survival at this time because any interpretation of nativity is flawed without imputation (given reasons for missingness outlined above), and because we lacked gold-standard (i.e., self-report) birthplace data. Future studies should collect self-report birthplace to allow these validations. In addition, cancer registries should work with their reporting facilities to ensure that this information is collected in a systematic way given its importance in understanding patterns of cancer burden in rapidly growing populations in the US.
Data were unavailable for several prognostic factors and length of neighborhood residence. When we repeated analyses with CA treatment data (missing in TX data), findings were similar. Results may not be generalizable outside of Texas and California, where Latinx are predominantly white, many are US-born, many are multi-generational, and most foreign-born are from Mexico.51
Finally, we acknowledge that any potential beneficial or detrimental impacts of ethnic enclaves are likely highly contextualized and dependent on specific place-based historic and cultural patterns of immigration and assimilation. Despite these limitations, our study fills key gaps in the literature by using the same multilevel measures and imputation methods across two states with the largest U.S. Latinx populations.
Conclusions
We demonstrated consistently harmful effects of low-nSES residence, but some evidence that enclaves may have small protective effects. We also found synergistic effects between enclaves and nSES, and worse survival for foreign-born Latinas with breast cancer, compared to US-born Latinas. Future place-based, mixed-methods research and intervention within ethnic enclaves is warranted given enclaves’ high concentration of underserved populations and overall lower nSES compared to non-enclave areas. By engaging community members in future research, cancer prevention and control efforts can better leverage local assets, such as ethnic-specific businesses and social networks, to improve cancer outcomes within enclaves.
Supplementary Material
Acknowledgments:
Cancer data have been provided by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services, https://www.dshs.texas.gov/tcr/.
Funding: This work was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP006344; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute, Cancer Registry of Greater California. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors. Dr. Hughes was funded through a postdoctoral fellowship at the University of Texas School of Public Health Cancer Education and Career Development Program, NCI/NIH Grant R25 CA57712. Cancer incidence data were provided by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services, 1100 West 49th Street, Austin, TX 78756, https://www.dshs.texas.gov/tcr/.
Footnotes
Conflict of interest: Authors do not have any conflicts of interest to disclose.
REFERENCES
- 1.American Cancer Society. Cancer facts & figures for Hispanics/Latinos 2012–2014. Atlanta: American Cancer Society, 2012. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-034778.pdf Accessed on August 19, 2015. [Google Scholar]
- 2.Siegel RL, Fedewa SA, Miller KD, et al. Cancer statistics for Hispanics/Latinos, 2015. Ca-a Cancer Journal for Clinicians. 2015;65(6):457–480. [DOI] [PubMed] [Google Scholar]
- 3.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67(6):439–448. [DOI] [PubMed] [Google Scholar]
- 4.Li C, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Archives of internal medicine. 2003;163. [DOI] [PubMed] [Google Scholar]
- 5.Martinez ME, Gomez SL, Tao L, et al. Erratum to: Contribution of clinical and socioeconomic factors to differences in breast cancer subtype and mortality between Hispanic and non-Hispanic white women. Breast cancer research and treatment. 2017;166(1):195. [DOI] [PubMed] [Google Scholar]
- 6.Martinez ME, Gomez SL, Tao L, et al. Contribution of clinical and socioeconomic factors to differences in breast cancer subtype and mortality between Hispanic and non-Hispanic white women. Breast cancer research and treatment. 2017;166(1):185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warnecke RB, Oh A, Breen N, et al. Approaching health disparities from a population perspective: The National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98(9):1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez SL, Shariff-Marco S, DeRouen M, et al. The impact of neighborhood social and built environment factors across the cancer continuum: Current research, methodological considerations, and future directions. Cancer. 2015;121(14):2314–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roux AVD, Mair C. Neighborhoods and health. Ann Ny Acad Sci. 2010;1186:125–145. [DOI] [PubMed] [Google Scholar]
- 10.Yen IH, Michael YL, Perdue L. Neighborhood Environment in Studies of Health of Older Adults A Systematic Review. Am J Prev Med. 2009;37(5):455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meijer M, Rohl J, Bloomfield K, Grittner U. Do neighborhoods affect individual mortality? A systematic review and meta-analysis of multilevel studies. Soc Sci Med. 2012;74(8):1204–1212. [DOI] [PubMed] [Google Scholar]
- 12.Krieger N Theories for social epidemiology in the 21st century: an ecosocial perspective. International journal of epidemiology. 2001;30(4):668–677. [DOI] [PubMed] [Google Scholar]
- 13.Gallo LC, Penedo FJ, de los Monteros KE, Arguelles W. Resiliency in the Face of Disadvantage: Do Hispanic Cultural Characteristics Protect Health Outcomes? J Pers. 2009;77(6):1707–1746. [DOI] [PubMed] [Google Scholar]
- 14.Jargowsky PA. Immigrants and neighborhoods of conentrated poverty: Assimilation or stagnation? . 2006. [Google Scholar]
- 15.Osypuk TL, Diez Roux AV, Hadley C, Kandula NR. Are immigrant enclaves healthy places to live? The Multi-ethnic Study of Atherosclerosis. Social science & medicine. 2009;69(1):110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Logan JR, Turner RN. Hispanics in the United States: Not only Mexicans. March 2013. Available at: https://www.russellsage.org/research/reports/hispanics-segregation Downloaded on June 11, 2018.
- 17.Lian M, Schootman M, Doubeni CA, et al. Geographic variation in colorectal cancer survival and the role of small-area socioeconomic deprivation: a multilevel survival analysis of the NIH-AARP Diet and Health Study Cohort. American journal of epidemiology. 2011;174(7):828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh GK, Miller BA, Hankey BF, Edwards BK. Area socioeconomic variations in U.S. cancer incidence, mortality, stage, treatment, and survival, 1975–1999. Bethesda, MD: National Cancer Institute;2003. NIH Publication No. 03–0000 [Google Scholar]
- 19.Keegan TH, Quach T, Shema S, Glaser SL, Gomez SL. The influence of nativity and neighborhoods on breast cancer stage at diagnosis and survival among California Hispanic women. BMC cancer. 2010;10:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy AL, Hughes D, Yoshikawa H. Intersections between nativity, ethnic density, and neighborhood SES: using an ethnic enclave framework to explore variation in Puerto Ricans’ physical health. American journal of community psychology. 2013;51(3–4):468–479. [DOI] [PubMed] [Google Scholar]
- 21.Fang CY, Tseng M. Ethnic density and cancer: A review of the evidence. Cancer. 2018;124(9):1877–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banegas MP, Tao L, Altekruse S, et al. Heterogeneity of breast cancer subtypes and survival among Hispanic women with invasive breast cancer in California. Breast cancer research and treatment. 2014;144(3):625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pruitt SL, Lee SJ, Tiro JA, Xuan L, Ruiz JM, Inrig S. Residential racial segregation and mortality among black, white, and Hispanic urban breast cancer patients in Texas, 1995 to 2009. Cancer. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pruitt SL, Tiro JA, Xuan L, Lee SJC. Hispanic and Immigrant Paradoxes in U.S. Breast Cancer Mortality: Impact of Neighborhood Poverty and Hispanic Density. International Journal of Environmental Research and Public Health. 2016;13(12):1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bemanian A, Beyer KM. Measures Matter: The Local Exposure/Isolation (LEx/Is) Metrics and Relationships between Local-Level Segregation and Breast Cancer Survival. Cancer Epidemiol Biomarkers Prev. 2017;26(4):516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pruitt SL, Tiro JA, Xuan L, Lee SJ. Hispanic and Immigrant Paradoxes in U.S. Breast Cancer Mortality: Impact of Neighborhood Poverty and Hispanic Density. Int J Environ Res Public Health. 2016;13(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703–711. [DOI] [PubMed] [Google Scholar]
- 28.Keegan TH, John EM, Fish KM, Alfaro-Velcamp T, Clarke CA, Gomez SL. Breast cancer incidence patterns among California Hispanic women: differences by nativity and residence in an enclave. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1208–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Froment MA, Gomez SL, Roux A, DeRouen MC, Kidd EA. Impact of socioeconomic status and ethnic enclave on cervical cancer incidence among Hispanics and Asians in California. Gynecol Oncol. 2014;133(3):409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao L, Ladabaum U, Gomez SL, Cheng I. Colorectal cancer mortality among Hispanics in California: differences by neighborhood socioeconomic status and nativity. Cancer. 2014;120(22):3510–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu M, Tatalovich Z, Gibson JT, Cronin KA. Using a composite index of socioeconomic status to investigate health disparities while protecting the confidentiality of cancer registry data. Cancer Causes Control. 2014;25(1):81–92. [DOI] [PubMed] [Google Scholar]
- 32.Von Behren J, Abrahao R, Goldberg D, Gomez SL, Setiawan VW, Cheng I. The influence of neighborhood socioeconomic status and ethnic enclave on endometrial cancer mortality among Hispanics and Asian Americans/Pacific Islanders in California. Cancer Causes Control. 2018;29(9):875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez SL, Glaser SL, McClure LA, et al. The California Neighborhoods Data System: a new resource for examining the impact of neighborhood characteristics on cancer incidence and outcomes in populations. Cancer causes & control : CCC. 2011;22(4):631–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montealegre JR, Zhou R, Amirian ES, Scheurer ME. Uncovering nativity disparities in cancer patterns: Multiple imputation strategy to handle missing nativity data in the Surveillance, Epidemiology, and End Results data file. Cancer. 2014;120(8):1203–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- 36.Moscovici JL, Ratitch B. Combining Survival Analysis Results after Multiple Imputation of Censored Event Times. PharmaSUG 2017 - Paper SP05. https://www.pharmasug.org/proceedings/2017/SP/PharmaSUG-2017-SP05.pdf Accessed on 5-28-2018. [Google Scholar]
- 37.Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010;1186(1):125–145. [DOI] [PubMed] [Google Scholar]
- 38.Yen IH, Michael YL, Perdue L . Neighborhood environment in studies of health of older adults: a systematic review. American journal of preventive medicine. 2009;37(5):455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meijer M, Rohl J, Bloomfield K, Grittner U. Do neighborhoods affect individual mortality? A systematic review and meta-analysis of multilevel studies. Social science & medicine (1982). 2012;74(8):1204–1212. [DOI] [PubMed] [Google Scholar]
- 40.Gallo LC, Penedo FJ, Espinosa de los Monteros K, Arguelles W. Resiliency in the face of disadvantage: do Hispanic cultural characteristics protect health outcomes? J Pers. 2009;77(6):1707–1746. [DOI] [PubMed] [Google Scholar]
- 41.Clegg LX, Reichman ME, Hankey BF, et al. Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies. Cancer Causes Control. 2007;18(2):177–187. [DOI] [PubMed] [Google Scholar]
- 42.Gomez SL, Glaser SL. Quality of cancer registry birthplace data for Hispanics living in the United States. Cancer Causes Control. 2005;16(6):713–723. [DOI] [PubMed] [Google Scholar]
- 43.Lin SS, Clarke CA, O’Malley CD, Le GM. Studying cancer incidence and outcomes in immigrants: methodological concerns. Am J Public Health. 2002;92(11):1757–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin SS, O’Malley CD, Lui SW. Factors associated with missing birthplace information in a population-based cancer registry. Ethnicity & disease. 2001;11(4):598–605. [PubMed] [Google Scholar]
- 45.Gomez SL, Quach T, Horn-Ross PL, et al. Hidden breast cancer disparities in Asian women: disaggregating incidence rates by ethnicity and migrant status. Am J Public Health. 2010;100 Suppl 1:S125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montealegre JR, Zhou R, Amirian ES, Follen M, Scheurer ME. Nativity disparities in late-stage diagnosis and cause-specific survival among Hispanic women with invasive cervical cancer: an analysis of Surveillance, Epidemiology, and End Results data. Cancer Causes Control. 2013;24(11):1985–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Howlader N, Noone AM, Yu M, Cronin KA. Use of imputed population-based cancer registry data as a method of accounting for missing information: application to estrogen receptor status for breast cancer. American journal of epidemiology. 2012;176(4):347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eisemann N, Waldmann A, Katalinic A. Imputation of missing values of tumour stage in population-based cancer registration. BMC medical research methodology. 2011;11:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Falcaro M, Nur U, Rachet B, Carpenter JR. Estimating excess hazard ratios and net survival when covariate data are missing: strategies for multiple imputation. Epidemiology. 2015;26(3):421–428. [DOI] [PubMed] [Google Scholar]
- 50.Luo Q, Egger S, Yu XQ, Smith DP, O’Connell DL. Validity of using multiple imputation for “unknown” stage at diagnosis in population-based cancer registry data. PloS one. 2017;12(6):e0180033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.American Fact Finder. Table QT-P3, 2010 Census Summary File 1 (race); and Table B05006, 2006–2010 American Community Survey (foreign-born place of birth). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.