Abstract
Background:
CD14 is a membrane glycoprotein primarily expressed by myeloid cells that plays a key role in inflammation. Soluble CD14 (sCD14) levels carry a poor prognosis in chronic heart failure (HF), but whether elevations in sCD14 precede HF is unknown. We tested the hypothesis that sCD14 is associated with HF incidence and its subtypes independent of major inflammatory biomarkers among older adults.
Methods and Results:
We included participants in the Cardiovascular Health Study without preexisting HF and available baseline sCD14. We evaluated the associations of sCD14, high-sensitivity C-reactive protein (hsCRP), interleukin-6 (IL-6), and white blood cell count (WBC) with incident HF and subtypes using Cox regression. Among 5217 participants, 1878 had incident HF over 13.6 years (609 classifiable as preserved [HFpEF] and 419 as reduced ejection fraction [HFrEF]). After adjusting for clinical and laboratory covariates, sCD14 was significantly associated with incident HF (HR 1.56 per doubling, 95% CI 1.29–1.89), an association that was numerically stronger than for hsCRP (HR per doubling 1.10, 95% CI 1.06–1.15), IL-6 (HR 1.18, 95% CI 1.10–1.25), and WBC (HR 1.24, 95% CI 1.09–1.42), and that remained significant after adjustment for the other markers of inflammation. This association for sCD14 was observed with HFpEF (HR 1.50, 95% CI 1.07–2.10) but not HFrEF (HR 0.99, 95% CI 0.67–1.49).
Conclusions:
Plasma sCD14 was associated with incident HF independently and numerically more strongly than other major inflammatory markers. This association was only observed with HFpEF in the subset with classifiable HF subtypes. Pending replication, these findings have potentially important therapeutic implications.
Introduction
Inflammation has long been recognized as a risk factor for heart failure (HF).1 Experimental studies have shown a mechanistic role for monocyte activation and pro-inflammatory cytokine secretion in the pathogenesis of myocardial dysfunction and adverse remodeling.1 Further, population-based studies have shown a relationship between various inflammatory biomarkers and incident HF.2 Additionally, it has been observed that patients with immune dysregulation and chronic inflammation, such as those with autoimmune diseases3, have significantly increased risk of HF.
The lack of efficacy for anti-inflammatory agents in HF highlights the need to better understand upstream mechanisms. One little-explored area concerning the inflammatory determinants of HF is the potential contribution of reduced intestinal barrier function and chronic low-grade endotoxemia.4 Such endotoxemia has been implicated in aging, obesity, and glucose dysregulation,5 but clinical studies are limited by challenges in measurement of plasma endotoxin (lipopolysaccharide [LPS]).6 Yet, LPS both binds and stimulates release into the circulation of cluster of differentiation 14 (CD14), a pattern-recognition receptor that promotes LPS engagement of toll-like receptors on myeloid and other cells to activate secretion of pro-inflammatory cytokines.7 Experimental studies have in fact implicated CD14 in LPS-induced myocardial inflammation and dysfunction, a well-recognized consequence of septicemia.8 Detectable in plasma in a soluble form (sCD14) arising from proteolytic cleavage of the membrane-bound molecule or direct cellular secretion,7 this molecule therefore affords the opportunity to indirectly assess immune activation stemming from microbial translocation and its potential impact on HF risk.
Previous studies have linked levels of sCD14 to atherosclerotic cardiovascular disease and mortality in older adults,9 in people living with human immunodeficiency virus infection10 and in patients with chronic kidney disease.11 Small clinical studies have also shown sCD14 levels to be higher in patients with HF than healthy controls;12 to possibly relate to peripheral edema in this setting;13 and to associate with mortality.14 However, whether the elevations in sCD14 precede HF and its subtypes remains unknown. Here, we parlayed available measurement of sCD14, as well as the inflammatory markers white blood cell count (WBC), high-sensitivity C-reactive protein (hsCRP) and interleukin (IL)-6, in a population-based study of older adults to test the hypothesis that higher sCD14 is associated with incident HF and, secondarily, its subtypes (reduced [HFrEF] and preserved ejection fraction [HFpEF]), and that such associations are independent of these other inflammatory measures.
Methods
Study population
The design and rationale of the Cardiovascular Health Study (CHS) are detailed elsewhere.15 Briefly, CHS is a prospective population-based cohort study of older Medicare-eligible adults (65–100 years) recruited from 4 field centers in the United States: Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Allegheny County, Pennsylvania. The main objective of the study is to evaluate traditional risk factors and identify novel determinants of cardiovascular disease in older adults. Eligible persons had to be non-institutionalized, be expected to remain in the area for the next 3 years, and be able to provide informed consent without a proxy respondent. Wheelchair-bound individuals, and those receiving hospice treatment, radiation therapy or chemotherapy for cancer were excluded. The original cohort included 5201 participants that were recruited in 1988–1989. A supplemental cohort of 687 black individuals was recruited in 1992–1993 for a total of 5888 participants. After exclusion of participants with prevalent HF, there were 5613 participants, of whom 5217 had available sCD14 determinations performed on retrospectively identified stored specimens, and were included in this study. At enrollment, participants underwent a detailed evaluation, which included medical and social histories, physical examination, medication use, and blood collection. Patients were followed for cardiovascular events and death.
Measurement of circulating biomarkers
Blood was obtained from participants at enrollment via phlebotomy after an 8-hour fast and specimens aliquoted for storage at −80°C in the CHS central blood laboratory, where the assays were eventually performed. At the central laboratory, control specimens are added routinely (three different controls per microtiter plate) and run with study specimens during each analytical batch, allowing calculation of within-run and between-run coefficients of variation (CVs), as well as adjustment for assay standardization drift over time. In addition, control sets comprising specimens from n=20 subjects are run to maintain longitudinal stability of the assays over longer measurements periods. The stability of peptides to long-term storage under deep freezing has been previously documented. Soluble CD14 was measured from baseline EDTA-plasma in two separate runs in 2010 and 2011 using ELISA (R&D Systems, Minneapolis, MN). The interassay CVs were 5.3–12.4% at control concentrations of 1170–1336 ng/ml. hsCRP was measured from EDTA-plasma specimens in a single large batch in 1997–98 by use of a high-sensitivity enzyme-linked immunosorbent assay developed at the CHS central blood laboratory.16 The interassay CVs have been reported at 5.5–6.8% at control concentrations 1.05–2.52 mg/l,16 and at 6.2% averaged overall.17 IL-6 was measured in EDTA-plasma in three separate batches from 1999 to 2003 using commercial ELISA kits (Quantikine IL-6, R&D Systems, Minneapolis, MN, USA). The interassay CVs were 4.1–35.8% at control values of 0.5–4.2 pg/ml, with an overall average interassay CV of 12.1%. Complete blood counts (including white blood cell counts) were measured at each of the participating clinical centers using the following instruments: Coulter Stack S cell counter (Coulter, Inc., Hialeah, FL) (the University of Pittsburgh and the University of California at Davis) and the Sysmex NE8000 counter (Toa Electronics, Inc., Chicago, Illinois) (Wake Forest University and the Johns Hopkins University).
Assessment and definition of covariates
The study assessments for CHS are described elsewhere.15 Age, race, smoking status, educational level, and alcohol use were based on self-report collected at the time of enrollment. Physical activity, weight, and height were assessed at enrollment. Blood pressure were measured using standardized protocols. Glomerular filtration rate was estimated from serum cystatin C as previously described.18 N-terminal pro-B-type natriuretic peptide (NT-proBNP) and high-sensitivity cardiac troponin T (cTnT) were measured in samples after long-term storage at −70 to −80°C using an Elecsys 2010 system (Roche Diagnostics, Indianapolis, IN) as previously reported.19,20 Other laboratory measures included serum creatinine, glucose and lipid panels, which were measured soon after collection and shipment to the Core Laboratory in dry ice. Serum glucose and creatinine were measured using Kodak Ektachem 700 analyzer with reagents (Eastman Kodak, Rochester, NY). The Olympus Demand system (Olympus, Lake Success, NY) was used for cholesterol, high-density lipoprotein (HDL)-cholesterol, and triglyceride determinations.9 Diabetes was defined as fasting glucose 126 mg/dL or higher, random glucose 200 mg/dL or higher, or use of diabetes medication. Heavy alcohol use was defined as ≥14 drinks/week for males and ≥7 for females. Left ventricular ejection fraction (LVEF) was assessed qualitatively using 2-dimensional transthoracic echocardiography performed at baseline in the original, but not the supplemental, cohort.21 LVEF was classified as normal (>55%), borderline (45–54%), and impaired (<45%).21 Prevalent coronary heart disease (CHD) was determined by patient report of physician diagnosis of angina, myocardial infarction, coronary artery bypass graft surgery, or percutaneous coronary intervention prior to enrollment.22 This was validated by confirmatory evidence from the baseline examination, including medication inventory; surveys of treating physicians; and review of hospital records, including at follow-up.23 At follow-up, incident CHD, similarly defined, was adjudicated by the CHS Events Committee.22 Prevalent stroke and transient ischemic attack (TIA) was based on patient report of physician diagnosis for these conditions, and validated as for CHD.23 Prevalent HF was assessed using self-report, confirmed by use of HF medications, physician questionnaires and review of medical records.23 Prevalent atrial fibrillation was based on a combination of ECG and inpatient or outpatient diagnosis codes.24 The frailty score consisted of no frailty (score=0), pre-frailty (score=1) and frailty (score=2), defined, respectively, by presence of none, one or two, or three or more of the following: unintentional weight loss (≥10 lbs. in past year), self-reported exhaustion, weakness (grip strength), slow walking speed, and low physical activity25.
Outcome
The primary outcome of this study was incident HF. Adjudication of events is described in detail elsewhere.21,22 Potential HF events were identified during semiannual contacts with participants involving telephone interviews or in-person visits. Such HF events were referred to the CHS Events Committee for final adjudication based on review of hospital and outpatient medical records. Determination was based on a physician diagnosis of HF, presence of confirmatory symptoms (dyspnea, fatigue, orthopnea, paroxysmal nocturnal dyspnea) and signs (edema, rales, tachycardia, gallop, displaced left ventricular impulse), medical treatment for HF, or supportive findings on diagnostic testing, such as cardiomegaly and pulmonary edema on chest X-ray or reduced ventricular function by echocardiography or ventriculography. Each case was presented by a field center investigator, with adjudication made by consensus following review and discussion by the whole committee. In addition, records were reviewed for assessment of left ventricular (LV) function at the time of index HF presentation. Each index HF event was categorized as HFrEF (LV ejection fraction [EF] 45%) or HFpEF (LVEF 45%), or unclassified (no LV function assessment available). This was based on reported LVEF by echocardiography (most commonly) or radionuclide or contrast ventriculography performed up to 30 days from the index HF event. When LVEF was not reported, but a qualitative description was provided in the report, moderate or severely reduced LV function was classified as HFrEF (taken as LVEF <45%), and normal or mildly reduced LV function was classified as HFpEF (taken as LVEF 45%). In a secondary analysis, we examined associations with HFrEF (LVEF<40%), HF with mid-range EF (HFmEF) (LVEF 40–49%), and HFpEF (LVEF 50%) for events in which an LVEF value was available in the diagnostic test report.
Statistical analysis
We described the cohort and compared baseline participant characteristics across sCD14 quartiles using linear trend tests for continuous variables and chi-square tests for categorical variables. A trend test for a continuous variable across quartiles of biomarker is a linear regression test where the quartiles are used as the exposure, not the actual values of the biomarker. Correlations between base-2 logarithmically transformed biomarkers were assessed by computing Pearson coefficients. Time to event was calculated as the interval in years from the baseline visit in 1989–90 or 1992–93 to the date of first incident HF, death, loss to follow-up, or end of follow-up on June 30, 2014, whichever was earliest (i.e., participants were censored at the time of death or loss to follow-up if this preceded the incident HF event). Kaplan-Meier curves were generated to describe the association between sCD14 quartiles and time to incident HF. Log-rank tests were used to compare the curves across sCD14 quartiles. Incidence rates were computed per 100 persons-years by adding available time until the study endpoint, time of censoring or end of follow-up for all participants. We used Cox proportional hazards models to estimate the association between sCD14, WBC, hsCRP, IL-6 and incident HF. We computed hazard ratios for doubling of biomarkers along with their 95% confidence intervals. The proportional hazards assumption was examined using graphical and numerical methods based on scaled Schoenfeld residuals. Models were adjusted according to variables selected a priori based on known associations or biological plausibility. We first adjusted for age, sex, and race (Model 1), and subsequently adjusted additionally for education, body mass index (BMI), smoking, alcohol, physical activity score, systolic blood pressure, antihypertensive use, diabetes, estrogen use, low-density lipoprotein (LDL), high-density lipoprotein (HDL), estimated glomerular filtration rate (eGFR), prevalent CHD, prevalent stroke, prevalent TIA, and prevalent atrial fibrillation (Model 2). In separate sensitivity analyses, we additionally adjusted for NT-proBNP, which was available for a subset of patients (n=4,013); for cTnT, also available in a subset (n=4762), which was winsorized at the 99th percentile of its concentration to remove the influence of outliers; for frailty score, likewise available in a subset (n=4734) which may be partially on the causal pathway to HF; and for incident CHD as a time-varying covariate. To check the functional form of sCD14 and the other inflammatory markers in the regression we used generalized additive models with penalized splines and found no meaningful departures from a linear fit. We also tested for interaction of inflammatory biomarkers by age, sex, race and CHD in relation to incident HF. To assess the association of each inflammatory biomarker independent of the others, we constructed an additional model to include the 4 inflammatory markers (sCD14, hsCRP, IL-6, WBC) in addition to clinical covariates in Model 2. We also explored interactions of inflammatory biomarkers with age, sex, race, and prevalent CHD by including cross-product terms in the models. To evaluate the associations between inflammatory biomarkers and HF subtypes, we used cause-specific hazard models, censoring individuals at the time of the other type of event. Cause-specific hazard models were chosen over sub-distribution hazard models because adjudication of HF events in CHS is done only for first, and not for recurrent events. The former approach is preferred in studies focused on potential causal relationships, rather than risk prediction.26 As a sensitivity analysis, we evaluated whether associations differed when an LVEF cutoff of 50% was used for HFpEF vs. HFrEF. Additional analyses included categorization of HF by LVEF into 3 groups (<40%, 40–49%, ≥50%). All analyses were done using R (R Development Core Team; http://www.r-project.org). All tests are two sided, and P<0.05 was considered statistically significant. P values were not adjusted for multiple testing.
Results
Baseline characteristics
The baseline characteristics of the study population and their relationship with quartiles of sCD14 are shown in Table 1. Levels of sCD14 were positively associated with age, smoking, heavy alcohol consumption, hypertension, diabetes, prevalent CHD, prevalent stroke, frailty score, LDL, NT-proBNP, and cTnT. sCD14 levels were inversely related to male sex, black race, education, BMI, estrogen use, and eGFR. All three other inflammatory markers were weakly correlated with sCD14. Pearson’s correlation coefficients with log2sCD14 were r=0.18 (95% CI 0.15–0.20) for log2IL-6, r=0.24 (95% CI 0.21–0.26) for log2hsCRP, and r=0.12 (95% CI 0.10–0.15) for log2WBC.
Table 1:
Baseline Characteristics of the study population by quartile of sCD14
| Characteristic* | Total (n=5217) | Quartile 1 (n=1305) | Quartile 2 (n=1304) | Quartile 3 (n=1304) | Quartile 4 (n=1304) | P Value |
|---|---|---|---|---|---|---|
| sCD14, ng/ml | 1636±357 | 1244±134 | 1498±56 | 1703±64 | 2098±307 | |
| Age, years | 72.7±5.5 | 72.2±5.3 | 72.3±5.31 | 72.8±5.5 | 73.6±6.0 | <0.001 |
| Male, n (%) | 2200 (42.2) | 692 (53) | 615 (47.2) | 477 (36.6) | 416 (31.9) | <0.001 |
| Black race, n (%) | 771 (14.8) | 327 (25.1) | 175 (13.4) | 146 (11.2) | 123 (9.4) | <0.001 |
| Education ≥12th grade, n (%) | 2286 (43.9) | 630 (48.5) | 577 (44.4) | 560 (43) | 519 (40) | <0.001 |
| BMI, kg/m2 | 26.6±4.7 | 27.1±4.6 | 26.8±4.5 | 26.6±4.7 | 26.0±4.7 | <0.001 |
| Current smoking, n (%) | 636 (12.2) | 129 (9.9) | 138 (10.6) | 173 (13.3) | 196 (15) | <0.001 |
| Heavy alcohol use, n (%) | 554 (10.7) | 108 (8.3) | 145 (11.2) | 157 (12.1) | 144 (11.1) | 0.011 |
| Physical activity score, n (%) | <0.001 | |||||
| 0 | 1205 (23.5) | 256 (19.9) | 265 (20.6) | 297 (23.2) | 387 (30.3) | |
| 1 | 2689 (2.4) | 688 (53.5) | 691 (53.7) | 657 (51.3) | 653 (51.1) | |
| 2 | 237 (24.1) | 343 (26.7) | 331 (25.7) | 326 (25.5) | 237 (18.6) | |
| Systolic blood pressure, mmHg | 137±22 | 135±21 | 137±21 | 137±22 | 138±22 | 0.001 |
| Antihypertensive medication, n (%) | 2374 (46) | 534 (41) | 571(44) | 607 (47) | 662 (51) | <0.001 |
| Diabetes, n (%) | 805 (15.5) | 166 (12.8) | 197 (15.1) | 188 (14.5) | 254 (19.5) | <0.001 |
| Estrogen, n (%) | 371 (12.3) | 140 (22.9) | 88 (12.8) | 83 (10) | 60 (6.8) | <0.001 |
| Prevalent CHD, n (%) | 896 (17.2) | 219 (16.8) | 224 (17.2) | 212 (16.3) | 241 (18.5) | 0.479 |
| Prevalent atrial fibrillation, n (%) | 128 (2.5) | 35 (2.7) | 30 (2.3) | 40 (3.1) | 23 (1.8) | 0.168 |
| Prevalent stroke, n (%) | 197 (3.8) | 33 (2.5) | 44 (3.4) | 44 (3.4) | 76 (5.8) | <0.001 |
| Prevalent TIA, n (%) | 128 (2.5) | 23 (1.8) | 34 (2.6) | 31 (2.4) | 40 (3.1) | 0.186 |
| Frailty score, n (%) | <0.001 | |||||
| 0 | 2265 (47.8) | 596 (49.1) | 604 (50.3) | 593 (50.3) | 472 (41.4) | |
| 1 | 2182 (46.1) | 556 (45.8) | 542 (45.1) | 531 (45.0) | 553 (48.5) | |
| 2 | 287 (6.1) | 61 (5.0) | 55 (4.6) | 55 (4.7) | 116 (10.2) | |
| LDL, mg/dl | 130 ±36 | 126±33 | 130 ±33 | 132 ±36 | 133 ±39 | <0.001 |
| HDL, mg/dl | 54 ±16 | 54±16 | 54±16 | 55±15 | 55±16 | 0.534 |
| eGFR, ml/min/1.73 m2 | 78±20 | 85±18 | 82±18 | 77±18 | 70±21 | <0.001 |
| NT-proBNP, pg/ml | 242±694 | 204±398 | 198±373 | 218±362 | 346±1206 | <0.001 |
| cTnT, pg/ml | 8.16±7.58 | 7.60±6.77 | 7.67±6.78 | 7.84±7.14 | 9.56±9.2 | <0.001 |
| Interleukin-6, pg/ml | 0.81±0.88 | 0.64 ±0.84 | 0.74 ±0.82 | 0.85 ±0.9 | 1.03 ±0.93 | <0.001 |
| hsCRP, mg/l | 1.35 ±1.48 | 0.96 ±1.36 | 1.20 ±1.35 | 1.43 ±1.41 | 1.82 ±1.64 | <0.001 |
| WBC, ×1000/mm2 | 2.6 ±0.4 | 2.53 ±0.39 | 2.59 ±0.38 | 2.59 ±0.39 | 2.67 ±0.43 | <0.001 |
| LV ejection fraction† | 0.639 | |||||
| ≥55% | 4244 (92.2) | 960 (91.9) | 1076 (92.0) | 1106 (92.8) | 1102 (92.1) | |
| 45–54% | 236 (5.1) | 57 (5.5) | 61 (5.2) | 62 (5.2) | 56 (4.7) | |
| <45% | 123 (2.7) | 28 (2.7) | 32 (2.7) | 24 (2.0) | 39 (3.3) |
Continuous variables are presented as mean ± SD.
Only available for the original cohort (n=4,603).
BMI = Body mass index; cTnT = Cardiac troponin T; CHD = Coronary heart disease; eGFR = Estimated glomerular filtration rate; HDL = High-density lipoprotein; hsCRP = High-sensitivity C-reactive protein; LDL = Low-density lipoprotein; LV = Left ventricular; NT-proBNP = N-terminal pro-B-type natriuretic peptide. TIA = Transient ischemic attack; WBC = White blood cell count.
Association of sCD14 and other inflammatory biomarkers with incident HF
Over a median follow-up of 13.6 (interquartile range 8.2–19.8; range 0.01–25.0) years, 1878 participants had incident HF over 71,834 person-years of follow-up, with an incidence rate of 2.9 (95% CI 2.7–3.2) per 100 person-years. During this period, 109 participants were lost to follow-up. HF incidence rate increased across higher quartiles of sCD14, with event rates per 100 person-years of 2.4 (95% CI 2.1–2.9) in the first quartile, 2.8 (95% CI 2.4–3.4) in the second, 3.0 (95% CI 2.5–3.7) in third, and 3.5 (95% CI 2.9–4.4) in fourth. Figure 1 shows the Kaplan-Meier curves for HF-free survival by quartiles of sCD14.
Figure 1.
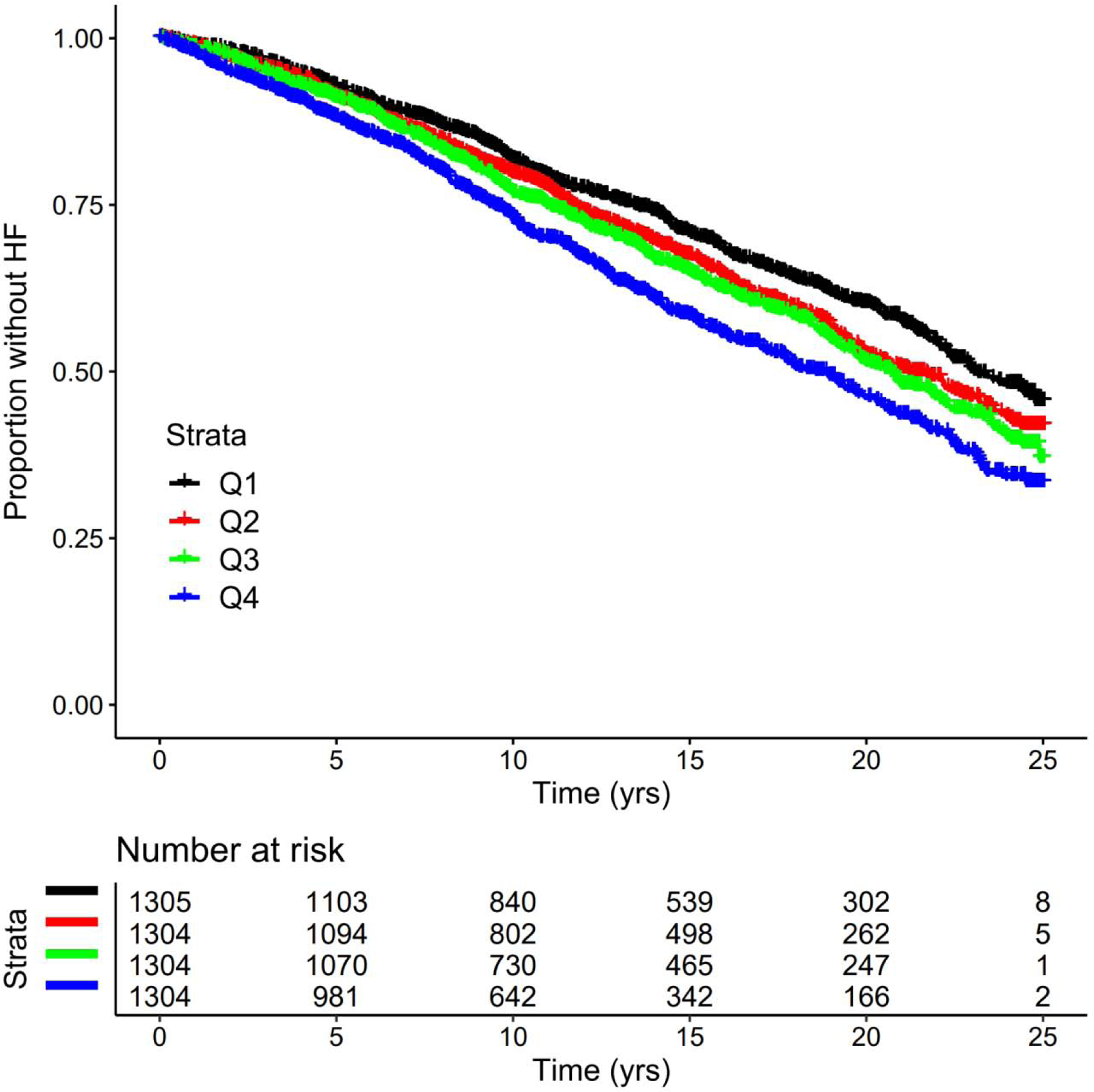
Kaplan-Meier plot of sCD14 quartiles and incident heart failure. Q = Quartile; yrs = years.
The associations of sCD14 with incident HF after accounting for potential confounders are presented in Table 2, as are those for the other inflammatory biomarkers. Generalized additive model plots for all inflammatory biomarkers and incident HF were consistent with a linear relationship. After adjusting for age, sex and race, sCD14 was associated with significantly increased risk of HF, an association that was attenuated but remained significant after additional adjustment for standard clinical and laboratory covariates (Model 2). For every doubling of sCD14 level, there was a 56% (95% CI 29–89%) higher hazard of a HF event during follow-up. For descriptive purposes, corresponding risk estimates for quartiles of sCD14 in relation to incident HF are presented in Supplemental Table 1. Sensitivity analyses did not show any meaningful change in the risk estimate after additional adjustment for NT-proBNP, cTnT, frailty or incident CHD as a time-varying covariate (Supplemental Table 2).
Table 2:
Association between inflammatory markers and incident HF
| Models | HR (95% CI) of HF per doubling of biomarker, P value | |||
|---|---|---|---|---|
| sCD14 | IL-6 | hsCRP | WBC | |
| Model 1 | 1.95 (1.66–2.29) P<0.001 | 1.34 (1.27–1.41) P<0.00i | 1.20 (1.16–1.24) P<0.001 | 1.75 (1.57–1.95) P<0.001 |
| Model 2 | 1.56 (1.29–1.89) P<0.001 | 1.18 (1.10–1.25), P<0.001 | 1.10 (1.06–1.15), P<0.001 | 1.24 (1.09–1.42), P<0.001 |
| Model 3 | 1.34 (1.09–1.64) P<0.001 | 1.12 (1.04–1.20) P<0.001 | 1.05 (1.00–1.10) P=0.040 | 1.12 (0.96–1.29) P=0.142 |
CI = Confidence interval; HF = Heart failure; HR = Hazard ratio.
Model 1. Adjusted for age, sex, and race. (Participants in analysis: sCD14, n=5217; IL-6, n=4909; hsCRP, n=5192; WBC, n=5187.)
Model 2. Adjusted for covariates in Model 1 plus education, BMI, smoking, alcohol, physical activity score, systolic blood pressure, antihypertensive use, diabetes, estrogen use, LDL, HDL, eGFR, prevalent coronary heart disease, prevalent stroke, prevalent TIA, prevalent atrial fibrillation. (Participants in analysis: sCD14, n=4413; IL-6, n=4151; CRP, n=4399; WBC, n=4397.)
Model 3. Adjusted for covariates in Model 2 plus all other inflammatory biomarkers. (Participants in analysis: n=4142.)
Similarly, levels of the inflammatory biomarkers IL-6, hsCRP, and WBC count were significantly associated with increased HF incidence after minimal adjustment, and such associations were attenuated but persisted after full adjustment for standard clinical and laboratory covariates (Table 2). For each doubling in biomarker level, the hazard of HF increased by 18% (95% CI 10–25%) for IL-6, 10% (95% CI 6–15%) for hsCRP, and 24% (95% CI 6–44%) for WBC after accounting for covariates in Model 2.
Table 2 also presents the risk estimates of HF for each inflammatory marker after all were added simultaneously to Model 2. After such mutual adjustment, the associations were further attenuated, but continued to be significant for sCD14, IL-6, and CRP. sCD14 exhibited the numerically highest risk estimate, with a 34% (95% CI 9–64%) hazard increase per doubling, as compared with 12% (95% CI 4–20%) for IL-6 and 5% (95% CI 0–10%) for hsCRP.
There was no interaction between age, sex, race or prevalent CHD with any of the inflammatory biomarkers in relation to incident HF (all P>0.10), with three exceptions. There was evidence of effect modification by age for sCD14 and WBC, wherein older age was associated with smaller magnitudes of association with HF (age log2sCD14: HR 0.97, 95% CI 0.94–1.00, P=0.046; and age log2WBC: HR 0.98, 95% CI 0.96–1.00, P=0.041). There was also evidence of interaction between race and hsCRP, such that the association was weaker among black participants (black race log2hsCRP: HR 0.89; 95% CI: 0.81–0.98, P=0.014).
Relationship of sCD14 and Other Inflammatory Markers with HF Subtypes
Of the 1878 incident HF events, 609 had preserved EF (HFpEF), 419 had reduced EF (HFrEF), and the remainder could not be classified. Among the 1000 incident HF cases with a documented LVEF value (excluding 28 cases subclassified based on qualitative descriptions of LV function), 145 (14.5%) had an LVEF<30%, 178 (17.8%) LVEF=30–39%, 95 (9.5%) LVEF=40–44%, 85 (8.5%) LVEF=45–49%, and the remainder an LVEF 50%. As shown in Table 3, after full adjustment for standard clinical and laboratory covariates (Model 2), sCD14 was significantly associated with increased risk of incident HFpEF, but not HFrEF. The remaining three inflammatory markers were each significantly associated with both HFpEF and HFrEF. After additional simultaneous adjustment for all inflammatory biomarkers, the observed associations were negligibly to moderately attenuated with the exception of the relationship of hsCRP with HFrEF, which was the only one to remain statistically significant. The associations of sCD14 and WBC with HFpEF were modestly attenuated in the model adjusting concurrently for all inflammatory markers, and became non-significant. Findings were consistent when HFpEF was defined as LVEF 50% and HFrEF as LVEF<50%. As shown in Supplemental Table 3, further subclassification into HFpEF, HFmEF and HFrEF yielded similar biomarker associations for the HFpEF and HFrEF subtypes, but no significant associations were observed with HFmEF, which had a more modest number of events.
Table 3:
Association between sCD14 and HF subtypes
| Models | n | HR (95% CI) per doubling of biomarker, P value | |
|---|---|---|---|
| HF with preserved EF | HF with reduced EF | ||
| sCD14 | |||
| Model 1 | 5217 | 1.70 (1.27–2.26), P<0.001 | 1.51 (1.07–2.11), P=0.018 |
| Model 2 | 4413 | 1.50 (1.07–2.10), P=0.019 | 0.99 (0.67–1.49), P=0.978 |
| Model 3 | 4142 | 1.37 (0.96–1.97), P=0.084 | 0.76 (0.50–1.17), P=0.215 |
| IL-6 | |||
| Model 1 | 4909 | 1.27 (1.16–1.40), P<0.001 | 1.35 (1.21–1.51), P<0.001 |
| Model 2 | 4151 | 1.13 (1.01–1.27), P=0.038 | 1.18 (1.02–1.36), P=0.022 |
| Model 3 | 4142 | 1.05 (0.92–1.19), P=0. 500 | 1.07 (0.90–1.25), P=0.451 |
| hsCRP | |||
| Model 1 | 5192 | 1.15 (1.08–1.21), P<0.001 | 1.26 (1.18–1.35), P<0.001 |
| Model 2 | 4399 | 1.09 (1.02–1.17), P=0.014 | 1.16 (1.07–1.26), P<0.001 |
| Model 3 | 4142 | 1.05 (0.96–1.13), P=0.285 | 1.16 (1.04–1.28), P=0.006 |
| WBC | |||
| Model 1 | 5187 | 1.78 (1.45–2.18), p<0.001 | 2.15 (1.72–2.70), P<0.001 |
| Model 2 | 4397 | 1.37 (1.08–1.75), P=0.010 | 1.38 (1.05–1.82), P=0.023 |
| Model 3 | 4142 | 1.27 (0.98–1.64), P=0.069 | 1.21 (0.89–1.64), P=0.234 |
CI = Confidence interval; EF = Ejection fraction; HF = Heart failure; HR = Hazard ratio. Model 1. Adjusted for age, sex, and race.
Model 2. Adjusted for covariates in Model 1 plus education, BMI, smoking, alcohol, physical activity score, systolic blood pressure, antihypertensive use, diabetes, estrogen use, LDL, HDL, eGFR, prevalent coronary heart disease, prevalent stroke, prevalent TIA, prevalent atrial fibrillation.
Model 3. Adjusted for covariates in Model 2 plus all other inflammatory biomarkers.
Discussion
In this study of community-dwelling older adults, we found that sCD14 was significantly associated with an increased incidence of HF independent of clinical and laboratory covariates, as well as the major inflammatory markers hsCRP, IL-6, and WBC. The association for sCD14 was also numerically the strongest compared to the three other markers examined, each of which was itself significantly associated with an increased risk of HF after adjustment for clinical and laboratory covariates and, excepting WBC, in the model simultaneously adjusting for all inflammatory markers. We also found that, after adjustment for clinical and laboratory covariates, sCD14 was significantly associated with HFpEF but not HFrEF, in contrast to the other inflammatory markers, which were significantly associated with both HFpEF and HFrEF. This association of sCD14 with HFpEF was modestly attenuated and became non-significant after simultaneous adjustment for the other inflammatory markers, whose associations with HF subtypes were variably attenuated in this model and also became non-significant, with the exception of hsCRP and HFrEF.
To our knowledge, this is the first study to evaluate the longitudinal association between sCD14 and HF or its subtypes in a population-based sample. The present findings newly document an association between plasma sCD14 and incident HF, and particularly HFpEF, in a cohort of older adults, the segment of the population at highest risk of HF. That the relationship of sCD14 with HF was independent of other major inflammatory markers implicated previously2,27 and again here, and that it showed the numerically strongest risk estimate suggest that sCD14 could be a particularly relevant measure, whether as a risk factor for or mediator of pathways leading to HF in elders.
Laboratory studies have shown that, in addition to monocytes/macrophages, both animal and human cardiomyocytes can express membrane-bound CD14,28,29 and that this co-receptor is crucial for development of inflammation and myocardial depression in response to LPS in animal models of sepsis-induced cardiac dysfunction.8 It is plausible that a similar mechanism might be involved in the context of metabolic endotoxemia, the chronic translocation of microbial products that has been documented in preclinical and clinical studies in the context of intestinal dysbiosis,4 such as occurs in obesity5 and aging-related metabolic dysregulation30, as well as HIV infection.31 Indeed, higher sCD14 levels were associated with older age, diabetes, lower physical activity score, and reduced eGFR, suggesting greater age-related glucose dysregulation and potentially greater impairment to intestinal barrier function. In our previous work in CHS, however, sCD14 showed associations with insulin resistance that did not persist after adjustment for IL-6, hsCRP and WBC.32 Furthermore, unlike the latter inflammatory biomarkers, sCD14 did not show an association with incident diabetes, albeit with a more moderate number of incident events. The basis for these divergent findings for glucose dysregulation and HF awaits further study.
Notably, sCD14 levels were associated with lower, and not higher, BMI in our cross-sectional analyses. This may be consistent with lower BMI being a marker of poorer health (e.g., wasting, sarcopenia) among elders, which would be accompanied by greater intestinal barrier dysfunction in this age group.4 Nevertheless, while sCD14 was cross-sectionally associated with higher frailty, additional adjustment for frailty score did not meaningfully affect the risk estimate for sCD14 and HF. Furthermore, prevalent HF, which apart from leading to wasting and lower BMI can also produce congestion of the intestinal mucosa, has been linked to endotoxemia and immune activation.13 To assess whether unrecognized HF at baseline may have influenced our findings, we undertook adjustment for NT-proBNP in the subset with available measures, but its lack of appreciable influence on the findings argues against this possibility.
In our cohort free of prevalent HF, direct stimulation by circulating LPS of membrane-bound CD14 on monocytes/macrophages, cardiomyocytes and other cells, including hepatocytes and adipocytes, leading to increased shedding of sCD14 extracellularly,7 could account for the association observed. Yet, gut-derived endotoxin can also bind sCD14, which can quench endotoxin by transferring it to lipoprotein particles,33 but can also deposit on endothelial cell membranes to stimulate pro-inflammatory pathways therein.34 Thus, both direct and indirect stimulation of different cell types in the vasculature may drive LPS-induced atherosclerosis, which could explain the relationship between sCD14 and incident atherosclerotic CVD documented previously in CHS.9 We did not find that adjustment for CHD as a time-varying covariate had an impact on this relationship, however, arguing against atherosclerosis as a mediating factor.
Indeed, the finding that sCD14 was associated only with incident HFpEF is consistent with potential underlying mechanisms being distinct from CHD-induced LV systolic dysfunction. But sCD14 has also been linked to aortic stiffness, a clear determinant of HFpEF.35 Moreover, the finding that the association only held for HFpEF suggests that the association could substantially relate to sCD14 effects on microvascular endothelial function.34 Such microvascular effects foster cardiomyocyte stiffness and collagen deposition with expansion of the extracellular matrix, and are deemed central to the pathogenesis of HFpEF.36 Still, the extent to which these mechanisms explain the observed association cannot be determined here. Indeed, CD14 is not entirely specific for LPS, and sCD14 levels have also been determined to be an acute phase reactant,37 suggesting that other pathways or mechanisms could also be involved.
If supported by further research, our findings could offer new therapeutic approaches for the prevention of HF and, particularly, HFpEF, a subtype that lacks proven effective therapies. A monoclonal antibody for human CD14 is currently in phase 2 trial in acute respiratory distress syndrome (clinicaltrials.gov NCT: NCT03017547), and could offer a testable strategy for prevention and treatment of HF/HFpEF in individuals with high sCD14 levels in the future. The sCD14-HFpEF association identified here is also notable because a recent multi-cohort analysis documented that although various biomarkers, including the inflammatory biomarker CRP (as seen here), were able to predict HFrEF, few predicted HFpEF.38 Our findings therefore raise the possibility that sCD14 might be usefully incorporated in risk-prediction schemes for this difficult-to-forecast disorder, but this will require separate study. But the implications of our findings, if replicated, would be to shine a light on CD14 and related pathways as potentially of pathophysiologic relevance to HF, and especially HFpEF, onset, offering new targets for prevention of this disorder.
This study has limitations that need to be acknowledged. The sCD14 measure had an interassay CV with a variability in the upper range that exceeded 10%. This suboptimal assay precision may have led to an underestimation of the true magnitude of the associations of interest. Likewise, measurement of sCD14, along with the other inflammatory biomarkers, at a single point in time is also likely to have underestimated the association observed with long-term HF risk. Although the present study did use the high-sensitivity CRP assay, it did not employ the more recent high-sensitivity assay for IL-6. Hence, the relative association of high-sensitivity IL-6 with incident HF will require further study. Next, CHS did not systematically collect information on infectious or inflammatory diseases, and cannot account for their potential contributions here. To the extent that this was a generally healthy community-dwelling population, serious disorders of this type are unlikely. Further, although the associations of interest were independent of a large panel of potential confounders, the possibility of residual confounding cannot be excluded. In addition, approximately half of the incident HF events did not have available LVEF, such that the analyses of HF subtypes must be interpreted with caution. Because this was an elderly cohort, findings may not be generalizable to younger populations.
In conclusion, the present analyses show that higher sCD14 levels are associated with an increased incidence of HF among older adults, an association that is both independent and numerically stronger than that for other major inflammatory biomarkers also associated with HF risk. This relationship was apparent for HFpEF, but not for HFrEF, raising the possibility that CD14-mediated inflammation could be of particular importance for this HF subtype that currently lacks interventions of proven benefit. Pending independent replication, these findings have potentially important implications for both treatment and risk prediction of this prominent aging-related disorder.
Supplementary Material
Acknowledgements
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). JRK was supported by K24 HL135413 from the National Heart, Lung, and Blood Institute. A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: SGA has no conflicts of interest. JRK reports stock ownership in Amgen, Bristol-Myers Squibb, Gilead Sciences, Johnson & Johnson, Medtronic, Merck, and Pfizer. BMP serves on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson.
References
- 1.Mann DL. Innate Immunity and the Failing Heart. Circ Res 2015;116:1254–1268. Available at: http://circres.ahajournals.org/lookup/doi/10.1161/CIRCRESAHA.116.302317. Accessed January 15, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boer RA de, Nayor M, deFilippi CR, Enserro D, Bhambhani V, Kizer JR, Blaha MJ, Brouwers FP, Cushman M, Lima JAC, Bahrami H, Harst P van der, Wang TJ, Gansevoort RT, Fox CS, Gaggin HK, Kop WJ, Liu K, Vasan RS, Psaty BM, Lee DS, Hillege HL, Bartz TM, Benjamin EJ, Chan C, Allison M, Gardin JM, Januzzi JL, Shah SJ, Levy D, Herrington DM, Larson MG, Gilst WH van, Gottdiener JS, Bertoni AG, Ho JE, Ho JE. Association of Cardiovascular Biomarkers With Incident Heart Failure With Preserved and Reduced Ejection Fraction. JAMA Cardiol 2018;3:215–224. Available at: http://www.ncbi.nlm.nih.gov/pubmed/29322198. Accessed March 2, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim CH, Al-Kindi SG, Jandali B, Askari AD, Zacharias M, Oliveira GH. Incidence and risk of heart failure in systemic lupus erythematosus. Heart 2017;103:227–233. Available at: http://heart.bmj.com/lookup/doi/10.1136/heartjnl-2016-309561. Accessed December 9, 2017. [DOI] [PubMed] [Google Scholar]
- 4.König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, Whyte J, Troost F, Brummer R-J. Human Intestinal Barrier Function in Health and Disease. Clin Transl Gastroenterol 2016;7:e196 Available at: http://www.ncbi.nlm.nih.gov/pubmed/27763627. Accessed March 2, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti J-F, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007;56:1761–1772. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17456850. Accessed March 2, 2019. [DOI] [PubMed] [Google Scholar]
- 6.Boutagy NE, McMillan RP, Frisard MI, Hulver MW. Metabolic endotoxemia with obesity: Is it real and is it relevant? Biochimie 2016;124:11–20. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26133659. Accessed March 2, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanoni I, Granucci F. Role of CD14 in host protection against infections and in metabolism regulation. Front Cell Infect Microbiol 2013;3:32 Available at: http://journal.frontiersin.org/article/10.3389/fcimb.2013.00032/abstract. Accessed March 2, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knuefermann P, Nemoto S, Misra A, Nozaki N, Defreitas G, Goyert SM, Carabello BA, Mann DL, Vallejo JG. CD14-deficient mice are protected against lipopolysaccharide-induced cardiac inflammation and left ventricular dysfunction. Circulation 2002;106:2608–15. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12427659. Accessed January 15, 2018. [DOI] [PubMed] [Google Scholar]
- 9.Reiner AP, Lange EM, Jenny NS, Chaves PHM, Ellis J, Li J, Walston J, Lange LA, Cushman M, Tracy RP. Soluble CD14: Genomewide Association Analysis and Relationship to Cardiovascular Risk and Mortality in Older Adults. Arterioscler Thromb Vasc Biol 2013;33:158–164. Available at: http://atvb.ahajournals.org/cgi/doi/10.1161/ATVBAHA.112.300421. Accessed January 15, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, Neaton JD, Brenchley JM, Deeks SG, Sereti I, Douek DC, INSIGHT SMART Study Group. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011;203:780–90. Available at: https://academic.oup.com/jid/article-lookup/doi/10.1093/infdis/jiq118. Accessed January 15, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poesen R, Ramezani A, Claes K, Augustijns P, Kuypers D, Barrows IR, Muralidharan J, Evenepoel P, Meijers B, Raj DS. Associations of Soluble CD14 and Endotoxin with Mortality, Cardiovascular Disease, and Progression of Kidney Disease among Patients with CKD. Clin J Am Soc Nephrol 2015;10:1525–1533. Available at: http://cjasn.asnjournals.org/cgi/doi/10.2215/CJN.03100315. Accessed January 15, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anker SD, Egerer KR, Volk HD, Kox WJ, Poole-Wilson PA, Coats AJ. Elevated soluble CD14 receptors and altered cytokines in chronic heart failure. Am J Cardiol 1997;79:1426–30. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9165177. Accessed January 15, 2018. [DOI] [PubMed] [Google Scholar]
- 13.Niebauer J, Volk HD, Kemp M, Dominguez M, Schumann RR, Rauchhaus M, Poole-Wilson PA, Coats AJ, Anker SD. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet (London, England) 1999;353:1838–42. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10359409. Accessed January 15, 2018. [DOI] [PubMed] [Google Scholar]
- 14.Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, Niebauer J, Hooper J, Volk HD, Coats AJ, Anker SD. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation 2000;102:3060–7. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11120695. Accessed January 15, 2018. [DOI] [PubMed] [Google Scholar]
- 15.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–76. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1669507. Accessed January 15, 2018. [DOI] [PubMed] [Google Scholar]
- 16.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem 1997;43:52–8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8990222. Accessed January 15, 2018. [PubMed] [Google Scholar]
- 17.Cushman M, Arnold AM, Psaty BM, Manolio TA, Kuller LH, Burke GL, Polak JF, Tracy RP. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: The cardiovascular health study. Circulation 2005;112:25–31. [DOI] [PubMed] [Google Scholar]
- 18.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Lente F, Bruce RD, Zhang YL, Greene T, Levey AS. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 2008;51:395–406. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18295055. Accessed October 26, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.deFilippi CR, Lemos JA de, Tkaczuk AT, Christenson RH, Carnethon MR, Siscovick DS Gottdiener JS, Seliger SL. Physical activity, change in biomarkers of myocardial stress and injury, and subsequent heart failure risk in older adults. J Am Coll Cardiol 2012;60:2539–47. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23158528. Accessed May 9, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeFilippi CR, Lemos JA De, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA - J Am Med Assoc 2010;304:2494–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ, Aurigemma GP, Manolio TA. Outcome of congestive heart failure in elderly persons: Influence of left ventricular systolic function. The cardiovascular health study. Ann Intern Med 2002;137:631–639. [DOI] [PubMed] [Google Scholar]
- 22.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol 1995;5:278–85. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8520709. Accessed January 15, 2018. [DOI] [PubMed] [Google Scholar]
- 23.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol 1995;5:270–7. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8520708. Accessed October 27, 2019. [DOI] [PubMed] [Google Scholar]
- 24.Macheret F, Bartz TM, Djousse L, Ix JH, Mukamal KJ, Zieman SJ, Siscovick DS, Tracy RP, Heckbert SR, Psaty BM, Kizer JR. Higher circulating adiponectin levels are associated with increased risk of atrial fibrillation in older adults. Heart 2015;101:1368–1374. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25855796. Accessed May 9, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, Mcburnie MA. Frailty in Older Adults: Evidence for a Phenotype. J Gerontol 2001;56:146–156. Available at: http://biomedgerontology.oxfordjournals.org/. Accessed February 29, 2020. [DOI] [PubMed] [Google Scholar]
- 26.Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation 2016;133:601–609. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26858290. Accessed March 2, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PWF, D’Agostino RB, Framingham Heart Study. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation 2003;107:1486–91. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12654604. Accessed January 15, 2018. [DOI] [PubMed] [Google Scholar]
- 28.Cowan DB, Poutias DN, Nido PJ Del, McGowan FX. CD14-independent activation of cardiomyocyte signal transduction by bacterial endotoxin. Am J Physiol Circ Physiol 2000;279:H619–H629. Available at: http://www.physiology.org/doi/10.1152/ajpheart.2000.279.2.H619. Accessed March 2, 2019. [DOI] [PubMed] [Google Scholar]
- 29.Yücel G, Zhao Z, El-Battrawy I, Lan H, Lang S, Li X, Buljubasic F, Zimmermann W-H, Cyganek L, Utikal J, Ravens U, Wieland T, Borggrefe M, Zhou X-B, Akin I. Lipopolysaccharides induced inflammatory responses and electrophysiological dysfunctions in human-induced pluripotent stem cell derived cardiomyocytes. Sci Rep 2017;7:2935 Available at: http://www.nature.com/articles/s41598-017-03147-4. Accessed January 15, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh S, Lertwattanarak R, Garduno J d. J, Galeana JJ, Li J, Zamarripa F, Lancaster JL, Mohan S, Hussey S, Musi N. Elevated Muscle TLR4 Expression and Metabolic Endotoxemia in Human Aging. Journals Gerontol Ser A Biol Sci Med Sci 2015;70:232–246. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24846769. Accessed March 2, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006;12:1365–71. Available at: http://www.nature.com/articles/nm1511. Accessed March 2, 2019. [DOI] [PubMed] [Google Scholar]
- 32.Shitole SG, Biggs ML, Reiner AP, Mukamal KJ, Djousse L, Ix JH, Barzilay JI, Tracy RP, Siscovick D, Kizer JR. Soluble CD14 and CD14 variants, other inflammatory markers, and glucose dysregulation in older adults: The cardiovascular health study In: Diabetes Care.Vol 42 American Diabetes Association Inc; 2019:2075–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitchens RL, O’keefe GE, Munford RS. Plasma CD14 decreases monocyte responses to LPS by transferring cell-bound LPS to plasma lipoproteins. J Clin Invest 2001;108 Available at: https://dm5migu4zj3pb.cloudfront.net/manuscripts/13000/13139/cache/13139.1-20150302092800-covered-253bed37ca4c1ab43d105aefdf7b5536.pdf. Accessed March 2, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pugin J, Schürer-Maly CC, Leturcq D, Moriarty A, Ulevitch RJ, Tobias PS. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci U S A 1993;90:2744–8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7681988. Accessed March 2, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amar J, Ruidavets JB, Bal Dit Sollier C, Bongard V, Boccalon H, Chamontin B, Drouet L, Ferrières J. Soluble CD14 and aortic stiffness in a population-based study. J Hypertens 2003;21:1869–77. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14508193. Accessed January 15, 2018. [DOI] [PubMed] [Google Scholar]
- 36.Paulus WJ, Tschöpe C. A Novel Paradigm for Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol 2013;62:263–271. Available at: http://linkinghub.elsevier.com/retrieve/pii/S0735109713018901. Accessed January 15, 2018. [DOI] [PubMed] [Google Scholar]
- 37.Bas S, Gauthier BR, Spenato U, Stingelin S, Gabay C. CD14 is an acute-phase protein. J Immunol 2004;172:4470–9. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15034063. Accessed March 2, 2019. [DOI] [PubMed] [Google Scholar]
- 38.Boer RA de, Nayor M, deFilippi CR, Enserro D, Bhambhani V, Kizer JR, Blaha MJ, Brouwers FP, Cushman M, Lima JAC, Bahrami H, Harst P van der, Wang TJ, Gansevoort RT, Fox CS, Gaggin HK, Kop WJ, Liu K, RS, Psaty BM, Lee DS, Hillege HL, Bartz TM, Benjamin EJ, Chan C, Allison M, Gardin JM, Januzzi JL, Shah SJ, Levy D, Herrington DM, Larson MG, Gilst WH van, Gottdiener JS, Bertoni AG, Ho JE. Association of Cardiovascular Biomarkers With Incident Heart Failure With Preserved and Reduced Ejection Fraction. JAMA Cardiol 2018. Available at: http://cardiology.jamanetwork.com/article.aspx?doi=10.1001/jamacardio.2017.4987. Accessed January 15, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


