Abstract
Background:
The objective of this study was to characterize prevalence and risk for pain, pain interference, and recurrent pain in adult survivors of childhood cancer, compared to siblings.
Methods:
This study analyzed longitudinal data from survivors (n=10,012; 48.7% female; median[range] age 31[17–57] years; median time since diagnosis 23 years) and siblings (n=3,173) from the Childhood Cancer Survivor Study. Survivors were diagnosed between 1970 and 1986 at one of 26 participating sites. Associations between risk factors (demographics, cancer-related, psychological symptoms) with pain, pain interference, and recurrent pain (5 years apart) were assessed using multinomial logistic regression. Path analyses examined cross-sectional associations between risk factors and pain outcomes.
Results:
Twenty-nine percent of survivors reported moderate-to-severe pain; 20% reported moderate to extreme pain interference; and 9% reported moderate-to-severe recurrent pain. Female sex, sarcoma/bone tumor diagnosis and severe/life-threatening chronic medical conditions were associated with recurrent pain. Depression and anxiety were associated with increased risk on all pain outcomes. Poor vitality mediated the effects of anxiety on high pain and pain interference (RMSEA 0.002).
Conclusions:
A large proportion of adult survivors report moderate-to-severe pain and pain interference more than 20 years post-diagnosis. Increased screening and early intervention for pain interference and recurrent pain is warranted.
Keywords: pain, childhood cancer, survivors, depression, anxiety, fatigue, longitudinal
Precis:
In this longitudinal cohort analysis, compared to siblings, a large proportion of adult survivors of childhood cancer continue to report significant pain and pain interference decades after diagnosis. Enhanced screening and early intervention is warranted.
Introduction
There are over 420,000 survivors of childhood cancer in the United States,1 many of whom are at increased risk of developing physical and psychosocial late effects following cancer therapy.2 Cancer therapy (chemotherapy, radiation, surgery) is associated with higher risk of serious or life-threatening late effects.1.2 Many survivors report experiencing pain3 and clinically significant interference in daily activities due to pain (pain interference) in adulthood.4 However, small sample sizes, limited follow-up, and variable pain assessment5 have resulted in variability in prevalence estimates and predictors of pain outcomes.
In a previous cross-sectional study, the prevalence of pain or migraine/headache was reported at 12.3–20.5% in long-term survivors.6 Increased risk of pain is associated with female sex and minority race/ethnicity,7,8 while younger age at diagnosis has been associated with both higher6 and lower risk of pain in long-term survivors.9 Treatment with vincristine and platinum agents,10 central nervous system radiation11 and amputation12 has been associated with increased risk for neuropathic pain.
The current study examined clinically significant (moderate to severe) pain at two time points, 5 years apart. Current literature to guide hypotheses focuses on chronic pain (≥ 3 months) in adults, as literature on pain across a 5-year interval is limited.13 Chronic pain has biological, psychological, and social effects and origins and is therefore viewed from a biopsychosocial perspective.14 The association between chronic pain and emotional distress (depression and anxiety) is well-established among adult non-cancer populations15 and appears to be bidirectional.16 Chronic pain can decrease mood and increase anxiety, which in turn, can lead to restriction of activities and maintain pain. Within the general population, chronic pain increases sleep disturbance and fatigue.17 which are associated with further reduced functioning, both directly and indirectly via depression and anxiety. Similar relationships are described in adult survivors of childhood cancer.5,18 Thus, we examined a potential pathway of increased emotional distress being associated with increased fatigue, and increased pain and pain interference.
The prevalence of pain, pain interference, and recurrent pain (5 years apart), as well as longitudinal associations with emotional distress and fatigue, has not been examined in long-term childhood cancer survivors. The current study aims to address these gaps. We hypothesized that: adult survivors of childhood cancer would report higher frequency of pain than siblings; demographics (female, minority race), cancer-related factors (older age at diagnosis, platinum chemotherapy, radiation, amputation, chronic medical conditions), and emotional distress would be associated with worse pain outcomes; and emotional distress would contribute to worse pain outcomes through lowered vitality (proxy for fatigue).
Methods
Participants
The Childhood Cancer Survivor Study (CCSS) is a retrospective cohort with longitudinal follow-up of survivors diagnosed before 21 years of age, ≥5 years from diagnosis, treated at one of 26 North American institutions between 1970 and 1986.19 Current participants completed questionnaires at follow-up 2 (FU2, beginning in 2002) and/or follow-up 4 (FU4, beginning in 2007) allowing longitudinal assessment of pain outcomes. The final study sample included 10,012 survivors and 3,173 siblings. The CCSS study was approved by the institutional review board at each institution and informed consent was obtained.
Primary Pain Outcomes
Three primary pain outcomes were derived using the bodily pain scale of the Short-Form 36 (SF-36).20 Participants answered the item “How much bodily pain have you had during the past 4 weeks” (None to Very Severe). Late-occurrence pain was defined as moderate, severe or very severe pain at either FU2 or FU4 (highest pain severity level reported at either time). Participants answered the item “During the past 4 weeks, how much did body pain interfere with your normal work” (Not at all to Extremely). Pain interference was defined as pain that moderately, quite a bit or extremely interferes with normal work at either FU2 or FU4. Recurrent pain was defined as moderate, severe or very severe pain at both FU2 and FU4 (yes/no).
Two secondary pain outcomes were also derived using the SF-3620 bodily pain scale. Pain trajectory characterized change across pain levels: (a) minimal pain (none to mild at both FU2 and FU40; (b) worsened pain (none to mild at FU2 and moderate to very severe at FU4); (c) improved pain (moderate to very severe at FU2 and none to mild at FU4). Pain interference trajectory characterized change across interference levels: (a) minimal interference (not at all to a little bit at both FU2 and FU4); (b) worsened interference (not at all to a little bit at FU2 and moderately to extremely at FU4); (c) improved interference (moderately to extremely at FU2 and not at all to a little bit at FU4); (d) persistent interference (moderately to extremely at both FU2 and FU4).
Risk factors
Demographic predictors included sex, race/ethnicity, age at survey. Descriptive socioeconomic variables included marital status, personal annual income, employment status, educational attainment. Cancer-related predictors included primary diagnosis, age at diagnosis, chemotherapy, cranial radiation therapy (CRT) dose, amputation surgery (yes/no). CRT dose was determined based on radiotherapy records review,21 considered as a continuous variable (per 10 Gy units). Total platinum chemotherapy dose (mg/m2) was grouped into a tertile score of 1 to 3, or assigned 0 for non-exposure.22 Chronic medical conditions were categorized and graded based on Common Terminology Criteria for Adverse Events, version 4.03.23 Unless otherwise noted, demographic, socioeconomic, and cancer-related predictors measured at FU2 were used in analyses.
Depression and anxiety were measured using the 18-item Brief Symptom Inventory-18 (BSI-18),24 which utilizes a five-point Likert scale and is validated in adult survivors of childhood cancer.25 Raw scores are converted to sex-specific T-scores, with higher scores representing greater depression and anxiety. Change in depression and change in anxiety variables were calculated by subtracting the FU2 T-score from the FU4 T-score. Four items on the SF-3620 measured vitality (the degree of feeling energetic and full of life versus feeling tired and worn out) on a six-point Likert scale at FU2. Raw scores are converted to T-scores, with lower scores representing lower vitality. Vitality was used as a proxy for fatigue as it correlated 0.77 (P<0.001) with the Functional Assessment of Chronic Illness Therapy- Fatigue subscale in cancer survivors and provided a larger sample.18
At both FU2 and FU4 participants were provided a list of common medications and asked to write the name of all prescribed medications they took for more than one month or for a total of 30 days in one year during the previous two-years. For this study, antidepressants, non-opioid analgesics, and opioid analgesics were classified using the American Hospital Formulary Service Drug Information online database.26
Statistical Analysis
Means and standard deviations were calculated for continuous variables and two-sample t-tests were conducted to compare survivors and siblings. Frequencies and Chi-Square tests were conducted for categorical variables. Multiple imputation accounted for imputation variability under the assumption that data are missing at random.27 Imputation was made more efficient by first imputing a missing CRT dose for 2034 (14.2%) survivors using Hot Deck Imputation approach28 based on treatment era, age at diagnosis, and diagnosis. BSI T-scores were then imputed for 2,287 (22.8%) survivors FU2 and 2,096 (20.9%) at FU4 data simultaneously using the fully conditional approach (FCS option) in SAS (SAS 9.4, SAS Institute, Cary NC), with race, sex, and CRT dose as covariates. All analyses were conducted on the 20 imputed data sets.27 Parameter estimates and SEs were summarized using MIANALYZE procedure with MODELEFFECTs statement in SAS.
Comparisons of pain outcomes between survivors and siblings were conducted using Multinomial Logistic Regression (GENMOD procedure, with Poisson distribution and LOG link),29 adjusted for age at survey, sex, and chronic medical condition grade. Among survivors, separate multinomial logistic regressions and log-binomial models examined demographics, cancer-related factors, and emotional distress on pain outcomes, adjusting for age at diagnosis, years of follow-up, sex, race, depression, anxiety, antidepressant and analgesic use. Relative risk (RR) are provided to indicate the increased risk of an exposed group compared to a non-exposed group, along with the precision of the estimate (95% CI).
Path analysis was used to examine the direct and indirect effects (through vitality) of depression and anxiety on pain and pain interference using weighted least squares estimates probit regressions from Mplus 7.4 (Los Angeles, CA).30 Demographics, antidepressant and analgesic medication use, and chronic medical condition grade were also considered as risk factors in the path analysis. The correlation between depression and anxiety (Spearman r = 0.61 p<0.001), as well as pain and pain interference (Cramer’s V = 0.54), was modeled in path analyses.
Results
Survivors versus Siblings
Survivors were roughly 7 years old (median=6.7, range=0–21) at diagnosis and 23 years (median=23.0, range=15–35) post-diagnosis at FU2. The most common cancer diagnoses were leukemia (34.0%), Hodgkin Lymphoma (12.7%) and CNS malignancy (12.5%). Compared to siblings, survivors were slightly younger, more likely to be single/never married, and have a lower annual income (Table 1).
Table 1.
Demographics and cancer-related characteristics of adult childhood cancer survivors and siblings
| Survivorsa N = 10,012 |
Siblingsa
N = 3,173 |
||||||
|---|---|---|---|---|---|---|---|
| Med | Range | Med | Range | P-value | |||
| Age at diagnosis | Years | 6.7 | 0–20.99 | ||||
| Time since diagnosis | Years | 23 | 15–35 | ||||
| Age at FU2 follow-up | Years | 31 | 17–57 | 33 | 9–58 | <0.001 | |
| 24 | 0.3–106 | ||||||
| n | % | n | % | P-value | |||
| Marital status | Single/Never married | 4519 | 45.6 | 999 | 31.7 | ||
| Married or Living as married | 4615 | 46.5 | 1862 | 59.1 | |||
| Widowed/Divorced/Separated | 782 | 7.9 | 291 | 9.2 | <0.001 | ||
| Education | Non-college graduate | 5371 | 53.8 | 1489 | 46.9 | ||
| College graduate | 4621 | 46.2 | 1683 | 53.1 | <0.001 | ||
| Sex | Male | 5139 | 51.3 | 1487 | 46.9 | ||
| Female | 4873 | 48.7 | 1686 | 53.1 | <0.001 | ||
| Race/Ethnicity | Non-Hispanic White | 8557 | 85.5 | 2797 | 88.1 | ||
| Non-Hispanic Black | 356 | 3.6 | 75 | 2.4 | |||
| Hispanic | 426 | 4.3 | 98 | 3.1 | |||
| Others | 673 | 6.7 | 203 | 6.4 | <0.001 | ||
| Annual household income | <$20,000 | 1150 | 13.4 | 212 | 7.5 | ||
| $20,000-$59,999 | 3768 | 43.9 | 1054 | 37 | |||
| >=$60,000 | 3657 | 42.7 | 1580 | 55.5 | <0.001 | ||
| Health insurance | Yes/Canadian resident | 8703 | 87.7 | 2855 | 90.4 | ||
| No | 1222 | 12.3 | 303 | 9.6 | <0.001 | ||
| Diagnosis | Leukemia | 3408 | 34.0 | ||||
| CNS tumor | 1254 | 12.5 | |||||
| Hodgkin lymphoma | 1273 | 12.7 | |||||
| Non-Hodgkin lymphoma | 768 | 7.7 | |||||
| Wilm’s tumor | 943 | 9.4 | |||||
| Neuroblastoma | 686 | 6.9 | |||||
| Soft Tissue sarcoma | 872 | 8.7 | |||||
| Bone tumor | 809 | 8.1 | |||||
| Amputation surgery | No | 9482 | 94.7 | ||||
| Yes | 526 | 5.3 | |||||
| Chemotherapy platinum agents | No | 8711 | 95.5 | ||||
| Yes | 415 | 4.5 | |||||
| Total platinum score (tertiles) | 0 | 8711 | 95.8 | ||||
| 1 | 144 | 1.58 | |||||
| 2 | 73 | 0.8 | |||||
| 3 | 162 | 1.78 | |||||
| Chronic medical condition | Grade 0–2c | 7281 | 72.7 | 2955 | 93.1 | ||
| Grade 3–4d | 2731 | 27.3 | 218 | 6.9 | <0.001 | ||
Demographic information is presented from FU2 survey, unless missing, then FU4 was used.
Total cranial radiation dose among those who received CRT (n=3296).
Chronic medical condition grade 0–2 at both FU2 and FU4.
Chronic medical condition grade 3–4 at eigher FU2 or FU4.
Compared to siblings, survivors reported more severe/very severe pain (RR=1.28, 95%CI=1.07–1.54; Table 2), quite a bit/extreme pain interference (RR=1.26, 95%CI=1.06–1.51) and moderate-to-severe recurrent pain (RR=1.23, 95%CI=1.01–1.45). Trajectories of pain (RR=1.21, 95%CI=1.01–1.45) and pain interference (RR=1.33, 95%CI=1.09–1.63) were more likely to worsen from FU2 to FU4 for survivors compared to siblings (Table 2).
Table 2.
Prevalence and relative risk of pain outcomes in adult survivors and siblings
| Survivor n(%) |
Sibling n(%) |
RR(95% CI) | |
|---|---|---|---|
| Late-occurrence paina | |||
| Moderate | 2037(20.5) | 572(18.2) | 1.16(1.04–1.30) |
| Severe/very severe | 808(8.1) | 182(5.8) | 1.28(1.07–1.54) |
| None-mild | 7101(71.4) | 2393(76.0) | 1.0 |
| Moderate-to-severe recurrent painb | |||
| Yes | 639(9.1) | 136(6.5) | 1.23(1.02–1.49) |
| No | 6407(90.9) | 1959(93.5) | 1.0 |
| Pain interferencea | |||
| Moderate | 1061(11.0) | 267(8.8) | 1.28(1.10–1.49) |
| Quite a bit/extreme | 890(9.2) | 198(6.5) | 1.26(1.06–1.51) |
| Not at all/a little bit | 7691(79.8) | 2579(84.7) | 1.0 |
| Worsened pain trajectoryb | |||
| Worsened pain (none-mild to moderate-very severe) | 597(8.5) | 149(7.1) | 1.21(1.01–1.45) |
| Minimal pain | 4916(69.8) | 1557(74.3) | 1.0 |
| Worsened pain interference trajectoryb | |||
| Worsened interference (not at all/a little bit to moderate-extreme) | 516(11.3) | 116(8.5) | 1.33(1.09–1.62) |
| Minimal interference | 3227(70.8) | 1077(78.8) | 1.0 |
| Persistent pain interference trajectoryb | |||
| Persistent interference (moderate-extreme) | 361(7.9) | 54(4.0) | 0.94(0.89–0.98) |
| Improved interference (moderate-extreme to not at all/a little bit) | 457(10.0) | 120(8.8) | 1.0 |
Notes. RR(95% CI) bolded to indicate p<0.05.
Multinomial Logistic Regression
Multivariable log-binomial model.
At FU2, survivors reported more symptoms of depression (M=49.2[11.9% clinical] vs. M=47.0[7.3% clinical]; p<0.001) and anxiety (M=47.8[7.8% clinical] vs. M=46.8[5.7% clinical]; p<0.001) compared to siblings. Survivors were more likely to have grade 3–4 chronic medical conditions than siblings (27.1% vs. 6.6%; p<0.001). At FU2, 13.02% of survivors reported using antidepressants, 3.90% non-opioid analgesics, and 3.96% opioid analgesics. Survivors reported greater total analgesic use than siblings (11.7% vs. 9.4%; p=0.01) but no difference in antidepressant use (Supplemental Table 1).
Demographic Predictors of Pain Outcomes
Female sex was consistently associated with higher risk of severe/very severe late-occurrence pain (RR=1.50, 95%CI=1.49–1.51), quite a bit/extreme pain interference (RR=1.51, 95%CI=1.49–1.53), moderate-to-severe recurrent pain (RR=1.33, 95%CI 1.32–1.34), and increased risk (19–29%) for worse pain and pain interference trajectories (Tables 3 and 4). Individuals older at follow-up and of minority race (Other than Non-Hispanic White) showed increased risk for poor pain outcomes (Table 3).
Table 3.
Risk factors for late-occurrence pain and pain interference outcomes in adult survivors.
| Late-occurrence pain | Pain interference | |||
|---|---|---|---|---|
| Moderatea | Severe/very severea | Moderateb | Quite a bit/extremeb | |
| RR(95%CI) | RR(95%CI) | RR(95%CI) | RR(95%CI) | |
| Age at diagnosis (per year) | 1.02(1.02–1.02) | 1.01(1.01–1.02) | 1.02(1.02–1.03) | 1.02(1.02–1.02) |
| Years follow-up (per year) | 1.09(1.09–1.09) | 1.11(1.11–1.12) | 1.10(1.10–1.10) | 1.12(1.11–1.12) |
| Female | 1.32(1.31–1.33) | 1.50(1.49–1.51) | 1.37(1.36–1.38) | 1.51(1.49–1.53) |
| Other than Non-Hispanic White | 1.00(0.99–1.01) | 1.17(1.15–1.18) | 1.12(1.11–1.14) | 1.37(1.34–1.39) |
| Amputation | 1.37(1.35–1.38) | 1.86(1.82–1.90) | 1.14(1.13–1.16) | 1.42(1.39–1.46) |
| Cranial radiation therapy (per 10Gy increase) | 1.02(1.01–1.02) | 1.00(0.99–1.01) | 1.02(1.01–1.02) | 1.02(1.00–1.03) |
| Total platinum score | 1.06(1.05–1.07) | 1.02(1.01–1.03) | 1.12(1.11–1.13) | 1.17(1.16–1.19) |
| Depression (per 0.5 SD) | 1.09(1.08–1.10) | 1.17(1.15–1.18) | 1.13(1.11–1.15) | 1.20(1.19–1.22) |
| Anxiety (per 0.5 SD) | 1.16(1.15–1.17) | 1.22(1.19–1.25) | 1.17(1.15–1.18) | 1.22(1.20–1.23) |
| Antidepressant use | 1.70(1.68–1.73) | 2.39(2.34–2.45) | 1.79(1.77–1.84) | 2.44(2.39–2.49) |
| Analgesic use | 3.09(3.06–3.13) | 8.00(7.88–8.17) | 3.48(3.45–3.54) | 7.25(7.13–7.39) |
| Model 2: Cancer diagnosis | RR(95%CI) | RR(95%CI) | RR(95%CI) | RR(95%CI) |
| CNS tumor | 1.06(1.04–1.07) | 1.02(1.00–1.04) | 1.03(1.01–1.04) | 1.10(1.08–1.12) |
| Lymphoma | 0.79(0.79–0.80) | 0.86(0.85–0.87) | 0.85(0.84–0.86) | 0.99(0.98–1.01) |
| Sarcoma/Bone tumor | 1.10(1.09–1.11) | 1.47(1.45–1.49) | 1.15(1.14–1.17) | 1.38(1.35–1.41) |
| Wilms’/Neuroblastoma | 0.89(0.88–0.89) | 0.96(0.95–0.97) | 0.91(0.90–0.92) | 0.74(0.73–0.76) |
| Leukemia | 1.0 | 1.0 | 1.0 | 1.0 |
| Model 3: Chronic medical condition | RR(95%CI) | RR(95%CI) | RR(95%CI) | RR(95%CI) |
| Grade 3–4 | 1.33(1.32–1.34) | 1.76(1.75–1.78) | 1.39(1.38–1.40) | 2.06(2.04–2.07) |
| Grade 0–2 | 1.0 | 1.0 | 1.0 | 1.0 |
Notes. RR(95% CI) bolded to indicate p<0.05.
Reference group is none to mild late-occurrence pain.
Reference group is not at all to a little bit of pain interference.
Table 4.
Risk factors for moderate-to-severe recurrent pain, pain and pain interference trajectory at two time points, FU2 to FU4, in adult survivors.
| Moderate-to-severe recurrent pain |
Pain trajectory | Pain interference trajectory | ||
|---|---|---|---|---|
| Yesa | Worsened painb | Worsened interferencec | Persistent interferenced | |
| RR(95%CI) | RR(95%CI) | RR(95%CI) | RR(95%CI) | |
| Age at diagnosis (per year) | 1.03(1.03–1.03) | 1.00(1.00–1.00) | 1.01(1.01–1.01) | 1.03(1.03–1.03) |
| Years follow-up (per year) | 1.03(1.03–1.03) | 1.03(1.02–1.03) | 1.00(1.00–1.00) | 1.08(1.08–1.08) |
| Female | 1.33(1.32–1.34) | 1.27(1.27–1.28) | 1.29(1.28–1.30) | 1.19(1.18–1.20) |
| Other than Non-Hispanic White | 0.89(0.88–0.90) | 0.99(0.99–1.00) | 1.11(1.11–1.12) | 0.78(0.77–0.78) |
| Amputation | 1.54(1.52–1.56) | 1.29(1.27–1.30) | 1.07(1.06–1.08) | 0.85(0.84–0.87) |
| Cranial radiation therapy (per 10Gy increase) | 1.02(1.01–1.02) | 0.98(0.98–0.99) | 1.06(1.05–1.07) | 0.97(0.96–0.98) |
| Total platinum score | 0.97(0.96–0.98) | 0.76(0.75–0.76) | 0.92(0.91–0.92) | 1.04(1.03–1.06) |
| Depression FU2 (per 0.5 SD) | 1.10(1.08–1.11) | 1.15(1.12–1.18) | 1.25(1.23–1.27) | 1.07(1.04–1.11) |
| Change in depression (per 0.5 SD) | 1.01(1.01–1.02) | 1.12(1.11–1.13) | 1.16(1.15–1.17) | 1.00(0.99–1.01) |
| Anxiety FU2 (per 0.5 SD) | 1.27(1.26–1.28) | 1.27(1.24–1.30) | 1.11(1.08–1.13) | 1.15(1.12–1.18) |
| Change in anxiety (per 0.5 SD) | 1.19(1.18–1.20) | 1.22(1.21–1.23) | 1.16(1.15–1.17) | 1.19(1.18–1.20) |
| Antidepressants use FU2 and FU4 | 1.43(1.41–1.46) | 1.50(1.49–1.53) | 1.22(1.21–1.25) | 2.10(2.07–2.12) |
| No antidepressant use | 1.0 | 1.0 | 1.0 | 1.0 |
| Analgesic use FU2 and FU4 | 5.42(5.34–5.53) | 2.26(2.23–2.30) | 4.53(4.45–4.63) | 3.29(3.19–3.37) |
| No analgesic use | 1.0 | 1.0 | 1.0 | 1.0 |
| Model 2: Primary cancer diagnosisᵗ | RR(95%CI) | RR(95%CI) | RR(95%CI) | RR(95%CI) |
| CNS tumor | 1.02(1.00–1.03) | 0.84(0.83–0.86) | 1.04(1.03–1.05) | 1.07(1.05–1.08) |
| Lymphoma | 1.00(0.99–1.01) | 0.76(0.75–0.76) | 1.01(1.00–1.02) | 1.61(1.58–1.65) |
| Sarcoma/Bone tumor | 1.33(1.32–1.34) | 1.01(1.01–1.02) | 1.01(1.01–1.02) | 1.44(1.42–1.47) |
| Wilms tumor/Neuroblastoma | 1.04(1.03–1.06) | 0.83(0.83–0.84) | 0.80(0.80–0.81) | 0.69(0.68–0.71) |
| Leukemia | 1.0 | 1.0 | 1.0 | 1.0 |
| Model 3: Chronic medical conditionᵗ | RR(95%CI) | RR(95%CI) | RR(95%CI) | RR(95%CI) |
| Grade 3–4 FU2 and FU4 | 1.50(1.49–1.51) | 1.25(1.25–1.26) | 1.32(1.31–1.32) | 1.53(1.52–1.53) |
| Grade 0–2 FU2 and FU4 | 1.0 | 1.0 | 1.0 | 1.0 |
Notes. RR(95% CI) bolded to indicate p<0.05.
Model adjusted for depression at FU2, change in depression, anxiety at FU2, change in anxiety, age at diagnosis, years follow-up, sex, race, antidepressant use, and analgesic use.
Reference group is no moderate-to-severe recurrent pain.
Reference group is no change in none/very mild/mild pain.
Reference group is no change in none/a little bit of pain interference.
Reference group is improved from moderate/quite a bit/extreme pain interference to none/a little bit of pain interference.
Cancer-Related Predictors of Pain Outcomes
Each year older at diagnosis was associated with a 1–3% increased risk for late-occurrence pain, pain interference, and moderate-to-severe recurrent pain (Tables 3 and 4). Compared to leukemia, survivors of sarcoma/bone tumor had increased risk for severe late-occurrence pain (RR=1.47, 95%CI 1.45–1.49), quite a bit/extreme pain interference (RR=1.38, 95%CI 1.35–1.41), and moderate-to-severe recurrent pain (RR=1.33, 95%CI 1.32–1.34).
Each 10Gy increase in CRT dose was associated with increased risk for moderate late-occurrence pain and pain interference (RRs=1.02; Table 3). Platinum-based chemotherapy increased the risk for moderate and severe late-occurrence pain and pain interference (RRs 1.02–1.17). Amputation increased risk for severe late-occurrence pain (RR=1.86, 95%CI=1.82–1.90), quite a bit/extreme pain interference (RR=1.42, 95%CI=1.39–1.46), and moderate-to-severe recurrent pain (RR=1.54, 95%CI=1.52–1.56). The impact of treatment variables on pain trajectory and pain interference trajectory was variable (Table 4).
Having a grade 3–4 chronic medical condition was associated with worse late-occurrence pain and pain interference outcomes (RRs 1.33–2.06; Table 3), as well as moderate-to-severe recurrent pain, worsened pain trajectory, and worsened/persistent pain interference trajectory (RRs 1.25–1.53; Table 4).
Supplemental Tables 2 and 3 report the prevalence of adult survivor pain outcomes by diagnosis and treatment. Supplemental Table 4 reports individual grade 3–4 chronic medical condition associations with pain outcomes.
Psychological Predictors of Pain Outcomes
Among survivors, depression was associated with a higher risk for worse pain outcomes (RRs 1.07–1.20; Tables 3 and 4). Every half standard deviation increase in depression from FU2 to FU4 was associated with a 12% higher risk for worsened pain trajectory and 16% higher risk for worsened pain interference trajectory. Anxiety showed the same pattern of results as depression (Table 4). Antidepressant use was associated with worse pain outcomes (RRs 1.22–2.44; Tables 3 and 4). Survivors who took analgesics showed the greatest risk for reporting worse pain outcomes with increased risk ranging from 126% to 700%.
Path Analysis of Depression, Anxiety, Vitality, and Pain Outcomes
Depression and anxiety showed significant direct and indirect effects associated with increased pain and pain interference (Figure 1). Increased depression had a significant direct effect on lowered vitality. Increased anxiety showed direct and indirect effects through lowered vitality on both pain and pain interference after adjusting for other risk factors. The indirect effect through vitality accounted for 43.2% and 43.7% of total effect of anxiety on pain and pain interference, respectively (Figure 1, Supplemental Table 5). Antidepressant and analgesic use similarly showed significant direct and indirect effects through vitality on pain and pain interference.
Figure 1. Direct and indirect effects on pain and pain interference in adult survivors at FU2.
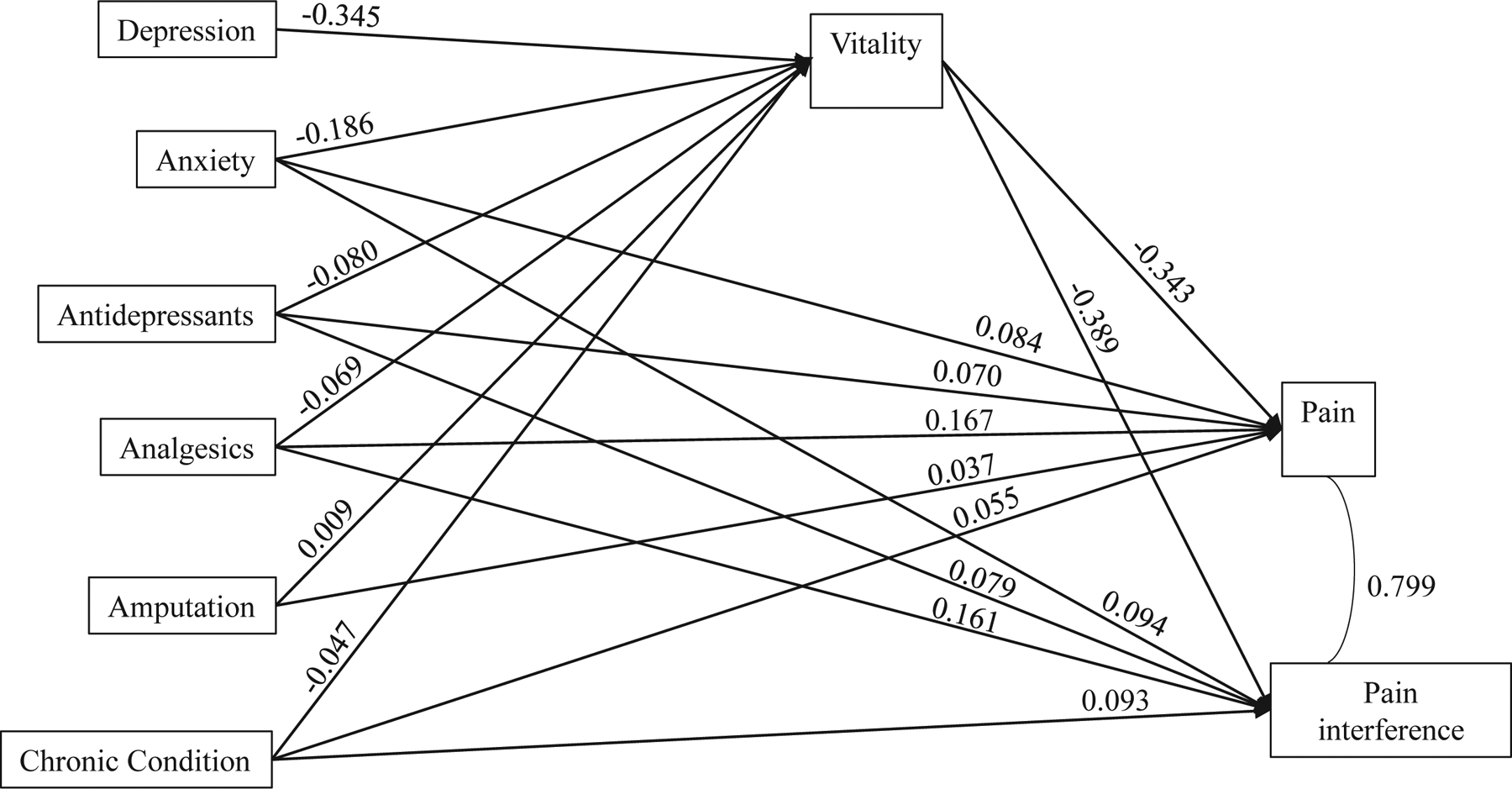
Notes. Model adjusted for race, sex, age at diagnosis, and follow-up time. Model Fit indices: Comparative fit index (CFI) = 1.000; Root means square error of approximation (RMSEA) = 0.002, (90% CI 0.000 – 0.014). All estimates are standardized estimates. All p’s < 0.001, except for p = 0.381 for amputation on vitality, p = 0.003 for amputation on pain, and p = 0.017 for race on pain interference.
Discussion
This is the first study to characterize the prevalence and risk factors for pain and pain interference using longitudinal assessment among adult survivors of childhood cancer. Approximately 29% of adult survivors reported moderate to serve pain at least once, more than 20 years post-diagnosis, 20% reported moderate to extreme pain interference, and 9% reported moderate-to-severe pain at both time points (5 years apart). Pain prevalence rates and analgesic use were significantly greater than those reported by siblings.
As hypothesized, older age at diagnosis and follow-up, female gender, and grade 3–4 chronic medical condition were consistently associated with increased risk for worse pain outcomes. Minority race, platinum-based chemotherapy, CRT, and CNS tumor were associated with increased risk for late-occurrence pain and pain interference; however, effects of these variables on moderate to severe recurrent pain, pain trajectory, and pain interference trajectory was variable. Further research is needed investigating the impact of chemotherapy and radiation treatment on longitudinal pain outcomes, as well as differences in diagnosis groups.
Survivors of soft-tissue sarcoma/bone tumors reported more moderate-to-severe recurrent pain and extreme pain interference than survivors of leukemia. Post-hoc analyses revealed survivors of soft-tissue sarcoma/bone tumors were more likely to have amputation (n=501, 29.8%) than other diagnoses (n=25, 0.3%; p<0.001), consistent with amputation post-surgical pain outcomes in the general population.12 Close monitoring for persisting pain or phantom limb pain,31 along with early treatment and rehabilitation should be considered for survivors of soft-tissue sarcomas/bone tumors.
Depression and anxiety were associated with increased risk for worse pain outcomes. The observed relationship between increased depression and anxiety symptoms over the 5-year follow-up period and worsened pain trajectory and pain interference trajectory is consistent with a bidirectional biopsychosocial model of chronic pain.14–16 Overlapping neurological pathways involving the corticolimbic system (in particular the medial prefrontal cortex, amygdala, and hippocampus) have been implicated in the robust and consistent relationships observed between negative emotions and persistent pain.32 Path analysis models demonstrated that higher emotional distress was associated with lower vitality, and lower vitality accounted for 43% of the association between anxiety and pain outcomes. These findings support our proposed pathway model and further emphasize the importance of emotional distress and fatigue in relation to pain in adult survivors.16–18
Antidepressant use and analgesic use were associated with worse pain outcomes. Individuals who used analgesics at both FU2 and FU4 (Table 4) had a 3.5-fold and 4.4-fold increased risk for worsened pain interference and moderate-to-severe recurrent pain, respectively, compared to no analgesics. While causation and directionality cannot be inferred, results may suggest that analgesic use does not normalize pain reports over time. Combined treatment modalities of pharmacologic, psychological, and exercise therapies appear most effective for reducing pain and pain interference in survivors of adult-onset cancer.33 Future work is needed to examine combined treatment modalities for chronic and/or recurrent pain in adult survivors of childhood cancer.
Limitations
Although our study is novel and the first to examine longitudinal patterns of pain in adult survivors of childhood cancer, there are several limitations. Measurement of pain at two time points five years apart may result in underestimation of the impact of pain and result in a mixed group of chronic and recurrent pain. Chronic pain is examined more than recurrent pain in the literature, with epidemiological studies suggesting chronic pain occurs in 20–31% of the general adult population.34 The availability of only two data points to describe pain trajectory and lack of specificity regarding pain origin may limit description of the true trajectory and pain interference. An additional limitation includes limited examination of other medications, such as anticonvulsants, sometimes used for pain management, as reason for prescription was not collected. Future research is needed to examine pain outcomes and the impact of psychological factors using more frequent assessment and advanced statistical models to directly assess the temporal relationships between treatment, psychological, and pain outcomes.
Conclusions
In conclusion, these results suggest that increased screening of pain and pain interference throughout survivorship of childhood cancer is warranted, particularly for survivors with identified risk factors such as female sex, sarcoma/bone tumor, amputation, grade 3–4 chronic medical conditions, and higher levels of emotional distress and fatigue. Early intervention may reduce long-term impacts of pain on functional outcomes.
Supplementary Material
Funding Support:
This work was supported by the National Cancer Institute (CA55727, Gregory T. Armstrong, M.D., Principal Investigator). Support to St Jude Children’s Research Hospital was also provided by the Cancer Center Support (CORE) grant (CA21765, Charles Roberts, M.D., Principal Investigator) and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Authors report no conflict of interests.
References
- 1.Robison LL, Hudson MM., Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer Volume:2014;14(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang IC, Brinkman TM, Kenzik K, et al. Association between the prevalence of symptoms and health-related quality of life in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort study. J Clin Oncol. 2013;31(33):4242–4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowers DC, Griffith T, Gargan L, et al. Back pain among long-term survivors of childhood leukemia. J Pediatr Hematol Oncol. 2012;34(8):624–629. [DOI] [PubMed] [Google Scholar]
- 5.Alberts NM, Gagnon MM, Stinson JN. Chronic pain in survivors of childhood cancer: a developmental model of pain across the cancer trajectory. Pain. 2018;159(10):1916–1927. [DOI] [PubMed] [Google Scholar]
- 6.Lu Q, Krull KR, Leisenring W, et al. Pain in long-term adult survivors of childhood cancers and their siblings: a report from the Childhood Cancer Survivor Study. Pain. 2011;152(11):2616–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miser AW, Dothage JA, Wesley RA, Miser JS. The prevalence of pain in a pediatric and young adult cancer population. Pain. 1987;29(1):73–83. [DOI] [PubMed] [Google Scholar]
- 8.Anghelescu DL, Faughnan LG, Popenhagen MP, Oakes LL, Pei D, Burgoyne LL. Neuropathic pain referrals to a multidisciplinary pediatric cancer pain service. Pain Manag Nurs. 2014;15(1):126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA. 2003;290(12):1583–1592. [DOI] [PubMed] [Google Scholar]
- 10.Ness KK, Jones KE, Smith WA, et al. Chemotherapy-related neuropathic symptoms and functional impairment in adult survivors of extracranial solid tumors of childhood: results from the St. Jude Lifetime Cohort Study. Arch Phys Med Rehabil. 2013;94(8):1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeltzer LK, Recklitis C, Buchbinder D, et al. Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2396–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother. 2009;9(5):723–744. [DOI] [PubMed] [Google Scholar]
- 13.Treede RD, Rief W, Barke A, et al. A classification of chronic pain for ICD-11. Pain. 2015;156(6):1003–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gatchel RJ, Peng YB, Peters ML, et al. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133(4), 581–624. [DOI] [PubMed] [Google Scholar]
- 15.McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106(1–2):127–133. [DOI] [PubMed] [Google Scholar]
- 16.Lerman SF, Rudich Z, Brill S, et al. Longitudinal associations between depression, anxiety, pain, and pain-related disability in chronic pain patients. Psychosom Med. 2015;77(3):333–41. [DOI] [PubMed] [Google Scholar]
- 17.Burgess HJ, Burns JW, Buvanendran A, Gupta R, Chont M, Kennedy M, Bruehl S. Associations between sleep disturbance and chronic pain intensity and function. Clin J Pain. 2019;35(7):569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clanton NR, Klosky JL, Li C, et al. Fatigue, vitality, sleep, and neurocognitive functioning in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2011;117(11):2559–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ware JE, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: Quality Metric Incorporated; 2003. [Google Scholar]
- 21.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166(1 Pt 2):141–157. [DOI] [PubMed] [Google Scholar]
- 22.Nagarajan R, Kamruzzaman A, Ness KK, et al. Twenty years of follow-up of survivors of childhood osteosarcoma: a report from the Childhood Cancer Survivor Study. Cancer. 2011;117(3):625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Cancer Institute. Common Terminology Criteria for Adverse Events v4.0. NCI, NIH, DHHS. May 29, 2009. NIH publication #09–7473.
- 24.Derogatis LR. BSI-18: Administration, scoring and procedures manual. Baltimore, MD: Clinical Psychometric Research; 2000. [Google Scholar]
- 25.Recklitis CJ, Parsons SK, Shih MC, Mertens A, Robison LL, Zeltzer L. Factor structure of the brief symptom inventory–18 in adult survivors of childhood cancer: results from the childhood cancer survivor study. Psychol Assess. 2006;18(1):22–32. [DOI] [PubMed] [Google Scholar]
- 26.American Society of Health-System Pharmacists: AHFS Drug Information, 2015. http://www.ahfsdruginformation.com/product-ahfs-di.aspx
- 27.van Buuren S. Flexible imputation of missing data: Chapman & Hall/CRC, Interdisciplinary Statistics Series. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2012 [Google Scholar]
- 28.Andridge RR, Little RJ. A review of hot deck imputation for survey non-response. Int Stat Rev. 2010;78(1):40–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramezani N. Analyzing non-normal binomial and categorical response variables under varying data conditions. University of Northern Colorado: Paper 11702–2016. [Google Scholar]
- 30.Muthén LK, Muthén BO. Mplus User’s Guide. Seventh Edition Los Angeles, CA: Muthén & Muthén; 1998-2012. [Google Scholar]
- 31.Demoss P, Ramsey LH, Karlson CW. Phantom limb pain in pediatric oncology. Front Neurol. 2018;9:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vachon-Presseau E, Tétreault P, Petre B, et al. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain. 2016;139(Pt 7):1958–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moryl N, Coyle N, Essandoh S, Glare P. Chronic pain management in cancer survivors. J Natl Compr Canc Netw. 2010;8(9):1104–1110. [DOI] [PubMed] [Google Scholar]
- 34.Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017;4:CD011279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


