Abstract
Background
The most common abnormal spirometric pattern reported in WTC worker and volunteer cohorts has consistently been that of a nonobstructive reduced forced vital capacity (low FVC). Low FVC is associated with obesity, which is highly prevalent in these cohorts. We used quantitative CT (QCT) to investigate proximal and distal airway inflammation and emphysema in participants with stable low FVC pattern.
Methods
We selected study participants with at least two available longitudinal surveillance spirometries, and a chest CT with QCT measurements of proximal airway inflammation (wall area percent, WAP), end-expiratory air trapping, suggestive of distal airway obstruction (expiratory to inspiratory mean lung attenuation ratio, MLAEI), and emphysema (percentage of lung volume with attenuation below −950 HU, LAV%). The comparison groups in multinomial logistic regression models were participants with consistently normal spirometries, and participants with stable fixed obstruction (COPD).
Results
Compared to normal spirometry participants, and after adjusting for age, sex, race/ethnicity, BMI, smoking, and early arrival at the WTC disaster site, low FVC participants had higher WAP (ORadj 1.24, 95% CI 1.06, 1.45, per 5% unit), suggestive of proximal airway inflammation, but did not differ in MLAEI, or LAV%. COPD participants did not differ in WAP with the low FVC ones and were more likely to have higher MLAEI or LAV% than the other two subgroups.
Discussion
WTC workers with spirometric low FVC have higher QCT-measured WAP compared to those with normal spirometries, but did not differ in distal airway and emphysema measurements, independently of obesity, smoking, and other covariates.
Keywords: multidetector computed tomography; Computer Assisted Image Processing; Obesity; bronchial diseases; smoke inhalation injury; World Trade Center Attack, 2001; Spirometry; forced vital capacity; Obstructive airway disease; Occupational Airways and Lung Diseases
Introduction
The low FVC or “restrictive” (as opposed to obstructive) spirometric ventilatory impairment has consistently and by far been the predominant abnormal spirometric pattern in the World Trade Center (WTC) disaster occupationally exposed population. Reported prevalences have been in the order of 20.5 to 28.8%[1–4], which exceed the 8.2% and 7.1% estimates among participants aged 40–59 in the National Health and Nutrition Examination Survey (NHANES) in 1988–1994, and 2007–2010, respectively[5], and those reported in several other studies in countries with developed economies[6]. This type of impairment is defined by the presence of a reduced forced vital capacity (FVC) without obstruction (i.e., with a normal ratio of first-second forced expiratory volume to FVC, FEV1/FVC). Although describing it as “low FVC” would be most accurate (as we will do henceforth), this spirometric ventilatory impairment pattern has received over many years a variety of names and slightly different definitions, such as restrictive[7,8], GOLD-unclassified (GOLD-U)[9], preserved ratio-impaired spirometry(PRISM)[10,11], nonspecific[12,13], or pseudorestrictive[14,15] pattern.
Although low FVC is considered a manifestation of airway obstruction according to modern spirometry interpretative guidelines[7], this is controversial, as is not commonly considered per se typical or diagnostic of common chronic obstructive airway diseases such as asthma or COPD. Furthermore, low FVC can also result from a variety of factors, including obesity[16] (more prevalent in the WTC occupational cohorts[17–19,4] than in the general U.S. population[20]), poor expiratory effort, and true lung restriction (i.e., reduced total lung capacity)[21]. While low FVC is most often a stable pattern, it can also be a transitional stage to or from chronic obstructive pulmonary disease (COPD)[22], and, importantly, is associated with functional limitations similar to those of moderate COPD[10].
Quantitative chest CT (QCT) has provided tools to characterize airway and parenchymal lung disorders[23]. We have gathered a large number of chest CT studies in one of the main WTC occupational cohorts[4,24]. The aim of this study was thus to examine quantitative chest CT (QCT) metrics of proximal airway inflammation, end-expiratory air trapping (suggestive of distal airway obstruction[25]), and emphysema[26] in WTC exposed participants with low FVC spirometric pattern, in comparison to equally exposed participants with normal spirometries, and those stably meeting the spirometric definition of chronic obstructive pulmonary disease (COPD).
Methods
Study Population and Data Acquisition
Our study population consisted of workers who participated in the rescue, recovery, cleanup, and restoration of the WTC site from September 11, 2001 to June 2002, and were screened and enrolled in research at the Mount Sinai WTC Clinical Center in New York City[2,27]. The screening and recruitment tools have been reported previously[2]. Participants, enrolled as early as April 2002, were initially evaluated at the time of entry into the cohort, and subsequently at 12- to 18-month intervals. Details of the periodic evaluations have been reported elsewhere, but included review of symptoms, occupational exposures, diseases, physical examination, spirometry, laboratory tests, and biennial chest radiograph[2]. This study involved a sub-cohort of participants additionally evaluated by the WTC Pulmonary Evaluation Unit who also had chest CT imaging performed[4], and at least 2 periodic spirometries with bronchodilator response (BDR) testing.
All spirometries for this study were performed using the EasyOne® portable flow device (ndd Medizintechnik, Zurich, Switzerland). Bronchodilator response (BDR) was assessed by repeating spirometry 15 minutes after administration of 180 mcg of albuterol via metered dose inhaler and a disposable spacer. This was done most often at the baseline examination, and independently from clinical status. Predicted values for spirometric measurements were calculated for all participants’ acceptable tests, based on reference equations from the third phase of the NHANES[28]. All testing, quality assurance, ventilatory impairment pattern definitions, bronchodilator response presence, and interpretative approaches followed American Thoracic Society recommendations[29,30,7,31]. Spirometries in this study were selected if performance had been acceptable, and they had a good quality, based both on computer quality grade (a measure of reproducibility across trials)[31] A or B, or C if at least 5 trials had been obtained, and, importantly, the forced exhalatory time was at least 6 seconds[30,6].
As previously reported[4,24], all chest CT studies were performed at Mount Sinai Hospital in General Electric® or Siemens® multidetector row chest CT scanners. Chest CT studies were performed using a protocol[25] with a radiation dose at 120 kVp, and a mean of 146 (SD 69) mAs, with subjects in the supine position, from the lung apices to the bases in a single breath hold at maximum inspiration, with no contrast, and with section thickness not exceeding 3 mm. All deidentified and coded chest CT images were stored and catalogued during the past 7 years in the WTC PEU Chest CT Image Archive (ClinicalTrials.gov identifier NCT03295279). CT scans were selected to have adequate quality study for quantitative chest CT scan (QCT) measurements performed with the Simba system (http://www.via.cornell.edu/simba/simba)[32,33,24,34].
Measurements
Our outcomes of interest were three groups of participants selected and classified according to their spirometric pattern: (1) participants who had consistently normal spirometry (no restriction or obstruction, or evidence of bronchodilator response at any time, BDRany); (2) participants with FVC below their individual lower limit of normal (FVC<LLN) and no obstruction (i.e., their FEV1/FVC>LLN) in all of their spirometries[7,8]; and (3) participants with stable COPD spirometry pattern[35], defined as 2 or more spirometries with fixed obstruction (i.e., post-bronchodilator FEV1/FVC ratio <0.7), and no record of a normal or a low FVC spirometry. Spirometric grade was defined as recommended[35] for descriptive purposes.
Our primary predictors of interest were three QCT measures of proximal and distal conducting airway disease, and emphysema[26] in each participant’s first available CT, measured by the Simba system (http://www.via.cornell.edu/simba/simba)[33,32]. As an indicator of emphysema, we used the percent of lung volume with attenuation <−950 HU (low attenuation volume percent, LAV%). We used 2.5% as a cut point, based on previously published QCT findings in a nonsmoking healthy multiethnic population[36]. As an indicator of end-expiratory air trapping, and presumably distal airway disease, we used the expiratory to inspiratory chest CT ratio of mean lung attenuation ratio (MLAEI), with cut point of 87.4%, based on previously published data[37]. Finally, as an indicator of proximal airway disease, we used wall area percent (WAP) of the 3rd generation (segmental) bronchus of the right upper lobe, as a continuous variable in units of 5%[24]. A higher WAP suggests airway wall thickening, in relation to the lumen, which is in turn suggestive of airway inflammatory changes.
Covariates of interest included age at baseline examination, sex, race/ethnicity, body mass index (BMI), early arrival at WTC site (an indicator of WTC occupational exposure indicator, and defined dichotomously as arrival within 48 hours of the disaster[3,38]), baseline smoking status and intensity (in pack-years), pre-WTC occupational exposures, and occupation on 9/11/2001, as described previously[4]. Race/ethnicity was categorized as Latino of any race, non-Latino black/other ethnicity, English speaking and Polish speaking non-Latino White[4]. For descriptive purposes, we estimated the annual prevalence of current smoking for the study population for the years ending in June 2003 and June 2018.
Statistical analyses
Descriptive statistics included mean and standard deviation (SD), or median and interquartile ranges (IQR) for normally, and non-normally distributed continuous variables, respectively, and counts and proportions for categorical variables. Since some variables were missing >10% of the data, the latter were imputed using the fully conditional specification method (chained equations), to create 15 imputations of the original dataset. Unadjusted analyses were performed using simple logistic regression. Multinomial logistic regression was then performed with the three spirometrically defined categories as outcomes, and models created for each of the three primary QCT predictors (WAP, MLAEI, and LAV%), respectively. We chose adjusting covariates based on clinical relevance and previous work. Collinearity was excluded by the variance inflation factor method. Covariates were eliminated to prevent model overfitting by testing for evidence of effect measure modification (by change in estimate of primary predictor >10%) and removing those by smallest effect-estimate first, and testing for change in model fit. Model fit was examined using deviance/Pearson’s goodness of fit and average c statistic among the 15 imputations. All analyses were performed using SAS v.9.4 (SAS institute, Cary, NC).
Results
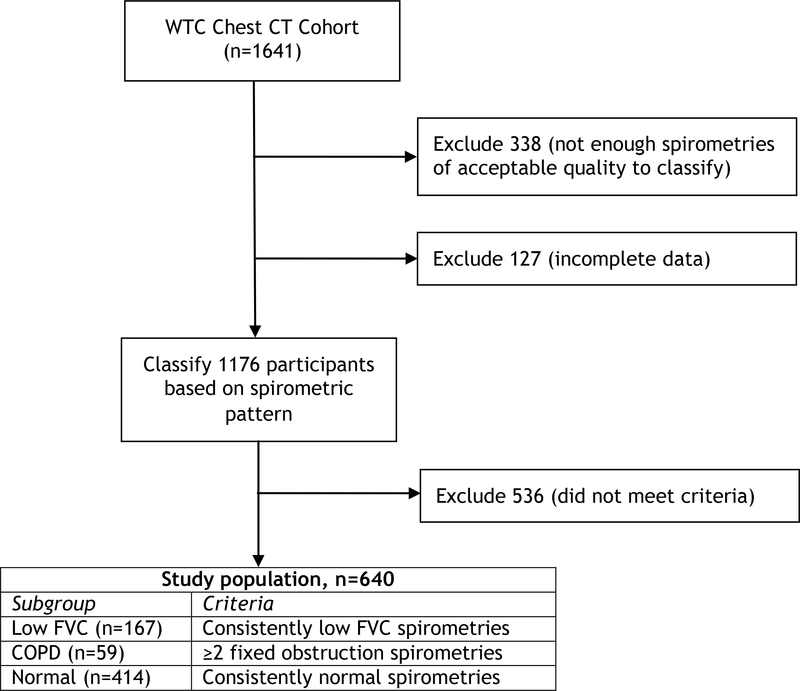
Figure 1 shows the study flow chart. Of the 1641 participants with images in the WTC Chest Imaging Archive, 1592 had at least 2 spirometries as of June 30, 2018, and 640 patients fit our above described stable spirometric pattern outcome definitions, and constituted our final study population. Each study participant had a median of 6 (IQR 4–7) spirometries, and their chest CT scan was performed a median of 8.75 (IQR 7.1–10.25) years after 9/11/2001. As shown in Table OS1, and compared to those excluded, participants included in the study had very slightly larger proportions of never smokers, men, and law enforcement workers and laborers, and substantially less evidence of BDR at any time.
Figure 1.
Study flow chart.
Of our study sample, 59 participants met our spirometric definition of COPD, 414 participants had consistently normal spirometries, and 167 participants consistently had low FVC. Table 1 shows the characteristics of our study population and the three subgroups. As in previous reports, participants were predominantly (84%) male, aged 45 years (SD 9 years) at baseline, and had an average BMI of 29.1 (SD 4.5) kg/m2, consistent with previous reports[17–19,4] on WTC occupational cohorts of prevalence of overweight and obesity exceeding contemporary national averages[20]. Only 17.5% were current smokers at their baseline evaluation, and the yearly prevalence of current smoking declined fairly steadily from 20.8% in the year ending in June 2003, to 7.3% in the year ending in June 2018. Bronchodilator response was evident at any given time in 48 (29%) of the low FVC, and 44 (75%) of the COPD subgroup participants. The spirometric severity of the COPD participants was mild in 20, moderate in 36, severe in 3, and very severe in none of them. Compared to the normal subgroup, the low FVC subgroup tended to have a higher BMI and proportion of nonsmokers, of workers arriving early at the WTC site, of Latino ethnicity, and of participants with law enforcement pre-WTC occupations. Participants with COPD tended to be older, more likely to be English speaking non-Latino/White, have a higher proportion of ever smokers and more smoking pack-years but, notably, approximately one third denied having ever smoked before 9/11/2001.
Table 1:
Characteristics of the study population, and the three spirometrically defined subgroups.
| Variable* | All Participants | Normal | Low FVC | COPD | |
|---|---|---|---|---|---|
| n | 640 | 414 | 167 | 59 | |
| LAV% | median | 0.05 (0–0.42) | 0.05 (0–0.45) | 0.02 (0–0.10) | 0.87 (0.14–4.11) |
| >2.5% | 37 (6) | 20 (5) | 3 (2) | 14 (24) | |
| WAP | median | 61.9 (56–41) | 60.5 (56–66) | 64.6 (59–68) | 64.9 (60–72) |
| MLAEI | median | 83.0 (78–87) | 83.0 (78–86) | 81.0 (75–85) | 88.2 (85–92) |
| >87.4% | 45 (7) | 25 (6) | 7 (4) | 13 (22) | |
| Age | years | 45 (9) | 45 (9) | 46 (9) | 50 (9) |
| Sex | male | 538 (84) | 339 (82) | 146 (87) | 53 (90) |
| female | 102 (16) | 87 (18) | 21 (13) | 6 (10) | |
| BMI | kg/m2 | 29.1 (4.5) | 28.6 (4.0) | 30.5 (5.5) | 28.3 (4.4) |
| Race/ethnicity | non-Latino white | 342 (53) | 223 (54) | 79 (47) | 40 (68) |
| Latino | 238 (37) | 159 (38) | 67 (40) | 12 (20) | |
| non-Latino black/other | 60 (9) | 32 (8) | 21 (13) | 7 (12) | |
| Occupation | Management/services | 108 (18) | 59 (15) | 35 (21) | 14 (24) |
| Construction trades | 114 (19) | 69 (18) | 35 (21) | 10 (17) | |
| Laborers/cleaners | 206 (34) | 170 (44) | 22 (13) | 14 (24) | |
| Production/Transportation | 28 (5) | 16 (4) | 9 (5) | 3 (5) | |
| Law enforcement | 156 (25) | 75 (19) | 63 (38) | 18 (31) | |
| Arrival at | < 48 hours | 308 (48) | 164 (41) | 106 (63) | 38 (64) |
| WTC site | > 48 hours | 332 (52) | 250 (60) | 61 (37) | 21 (36) |
| BDR | at any visit | 92 (14) | 0 (0) | 48 (29) | 44 (75) |
| Smoking | Never | 350 (55) | 233 (56) | 99 (59) | 18 (31) |
| Current | 112 (18) | 67 (16) | 26 (16) | 19 (32) | |
| Former | 178 (28) | 114 (28) | 42 (27) | 22 (37) | |
| Smoking | pack-years | 7 (13) | 5 (11) | 6 (11) | 21 (24) |
Presented as n (%), mean (SD), or median (IQR), as appropriate
Table 2 presents the unadjusted and adjusted comparisons of the three groups, by each of the three QCT metrics. Compared to normal spirometry participants, low FVC participants had higher WAP (ORadj 1.25, 95% CI 1.08, 1.46, per 5% unit), suggestive of proximal airway inflammation, but did not differ in end-expiratory air trapping (MLAEI), or emphysema (LAV%). WAP did not differ between the low FVC and the COPD subgroups. On the other hand, and as expected, participants with COPD spirometries were significantly more likely to have higher MLAEI and LAV% than both the low FVC and normal spirometry participants.
Table 2.
Unadjusted and adjusted comparisons of the three QCT measurements of emphysema (LAV%, >2.5%) and proximal (WAP, per 5% units), and distal (MLAEI, >87.4%) airway disease, among three spirometrically defined groups. The multinomial logistic regression models were adjusted for age, sex, race/ethnicity, BMI, baseline smoking status and smoking pack-years, and early arrival (within 48 hours) at the WTC disaster site.
| QCT metric | Comparison | OR | 95% CI | ORadj | 95% CI |
|---|---|---|---|---|---|
| WAP | Low FVC vs. Normal | 1.32 | 1.15, 1.52 | 1.25 | 1.08, 1.46 |
| COPD vs. Low FVC | 1.11 | 0.87, 1.42 | 1.13 | 0.87, 1.47 | |
| COPD vs. Normal | 1.47 | 1.17, 1.85 | 1.41 | 1.10, 1.80 | |
| MLAEI | Low FVC vs. Normal | 0.74 | 0.37, 1.48 | 0.85 | 0.40, 1.79 |
| COPD vs. Low FVC | 6.56 | 2.69, 16.02 | 5.64 | 2.02, 15.76 | |
| COPD vs. Normal | 4.83 | 2.43, 9.60 | 4.77 | 2.03, 11.20 | |
| LAV% | Low FVC vs. Normal | 0.48 | 0.14, 1.61 | 0.53 | 0.15, 1.87 |
| COPD vs. Low FVC | 10.36 | 2.95, 36.43 | 7.42 | 1.94, 28.31 | |
| COPD vs. Normal | 4.98 | 2.40, 10.32 | 3.94 | 1.58, 9.82 |
Discussion
To our knowledge, this study is the first to focus on low FVC, uniformly the most common spirometric abnormality described in the WTC occupational cohorts[1,3,24], and to use QCT measurements for its characterization. The focus on low FVC pattern demands stringent quality criteria for spirometry, including forced exhalatory times of at least 6 seconds[6], which is virtually unprecedented in previous studies with surveillance spirometry data from the largest WTC occupational cohorts. We demonstrated an association of proximal airway inflammation (as suggested by WAP) with a persistent low FVC pattern, even after adjustment for important and relevant covariates, like BMI[39] and smoking. WAP did not seem different between the low FVC and the COPD subgroups, and was higher in both than in the normal subgroup. As expected, COPD participants were more likely to have higher QCT measurements of distal airway inflammation, and emphysema than those of both normal and low FVC spirometry participants.
Our low FVC subgroup had similar characteristics to the GOLD unclassified (GOLD-U) smokers subgroup of COPDGene®[9], including increased BMI and a higher proportion of nonwhite participants, and an increased subsegmental airway wall thickness compared to smoking control participants. Our findings are similar, in a cohort with a >50% proportion of never smokers and steadily declining current smoking prevalence, and adjusting for both smoking and BMI in our models. The predominance of proximal airway abnormalities over more distal involvement suggests chronic bronchitis, is similar to what has been described in subjects with COPD and chronic bronchitis, compared to those without it[40], and lends further support to the clinical diagnosis of nonspecific or nonobstructive chronic bronchitis for a substantial proportion of WTC workers with lower airway disease[3,38]. Of note, our results are independent of obesity, which has a well documented excess prevalence in the WTC occupational cohorts[17–19,4], and a well-known negative association with FVC[16], and positive with WAP[39]. Although pleural thickening is prevalent in about 1 in 5 WTC worker in this cohort[4], there is no consensus on the effect of that finding on FVC in the absence of asbestosis, and our larger previous study did not suggest it[4]. We excluded participants with evidence of bronchodilator response (BDR) at any time from the normal subgroup, because finding BDR would more readily lead, given consistent symptoms, to a clinical classification of asthma. The requirement of at least two spirometries with fixed obstruction to define COPD is in accordance with the known instability of that diagnosis, particularly in mild disease[22,41] like that of our cohort participants, and allowed a confident estimation of the cumulative incidence of COPD in this cohort with chest CT imaging data at 3.6%.
We did not find that QCT-measured emphysema or end-expiratory air trapping were increased in the low FVC compared to the normal spirometry participants. As the distal airways are the main site of the obstruction in COPD[42,43], it is not surprising that low FVC with proximal airway disease, but without substantial evidence (even if indirect) of small airway disease, or of emphysema, is not associated with spirometric obstruction. Other lines of evidence have suggested, however, that low FVC without obstructive spirometric impairment [44] or in response to bronchial challenge testing[45] may result from loss of lung units in parallel distally to obstructed bronchioles. Unadjusted impulse oscillometric data in a WTC-exposed community resident and worker case series reported evidence of increased distal airway resistance in association with restrictive ventilatory impairment[46].
The strengths of this study relate to the fairly extensive availability of functional and QCT measurements, in well-defined subgroups with stable spirometric patterns, and the high prevalence of the main pattern under study (low FVC).
Our study also had some limitations. QCT measurements lack normative data, but our choices of criteria are reasonable based on reports from selected large studies, and our group definitions allowed the intended comparisons. Categorizations were necessary for MLAEI and LAV%, given the distribution of the data, with large number of participants (even in the COPD subgroup) with normal or very mild abnormalities. Distal airway inflammation is likely to be present in at least some subgroups of individuals with low FVC. Our inability to demonstrate significantly increased QCT end-expiratory air trapping in low FVC vs. normal spirometry participants does not exclude distal airway inflammation, as increased WAP has been demonstrated to predict it[47], and current QCT tools do not measure it directly[26]. We defined our subgroups strictly, based on consistent longitudinal functional data, and excluding many participants who made subgroup transitions. The study of those transitions requires a different methodologic approach[22]. Lastly, we lacked total lung capacity to exclude true restriction in our low FVC subgroup, but aside from infrequent and heterogenous cases, interstitial lung disease (ILD) has not been demonstrated thus far in the large occupational WTC cohorts. Interstitial lung abnormalities are, however, present in some of our workers with chest CT images[4], and long term surveillance will help identify incident cases of ILD.
In conclusion, we demonstrated QCT evidence of proximal airway inflammation in WTC workers with persistent low FVC spirometry pattern, compared to participants with normal spirometries, and independently from obesity and tobacco smoking. This finding supports the plausibility of low FVC as resulting from a chronic inflammatory airway disease presumably resulting of inhaled toxicant exposures. As these cohorts are followed up for years to come, it will be important to appraise the impact of this condition on general health, given the reported associations of low FVC pattern not only with increased prevalence of respiratory symptoms, but with a variety of respiratory[10] and multisystemic functional limitations, medical co-morbidities[48,9,49,8,50], and even mortality risk[51–54].
Supplementary Material
Acknowledgements
The authors would like to thank all participants in this study, and the staff of the WTC Health Program Clinical Center of Excellence at the Mount Sinai Selikoff Centers for Occupational Health, and the WTC Data Center at Mount Sinai. The authors acknowledge the able research support of Ms. Lilliam Tirado, Mr. Raymond Mathews, and Mr. Horacio Romero. ClinicalTrials.gov identifier: NCT03295279 (WTC Chest CT Imaging Archive).
Funding sources and disclosure statement:
This project was funded by grant U1 OH011697 (RED, PI), and contract 200-2017-93325 (WTC General Responders Cohort Data Center), from the National Institute for Occupational Safety and Health of the U. S. Centers for Disease Control and Prevention. The contents of this article are the sole responsibility of the authors and do not necessarily represent the official views of the CDCP/NIOSH. The funding agency had no role in the study design, in the collection, analysis, or interpretation of the data, in the writing of the report, or in the decision to submit this article for publication. The authors have no other relevant financial disclosures to make.
Footnotes
Statement of Ethics:
This study complied with the guidelines for human studies in accordance with the World Medical Association Declaration of Helsinki. The Mount Sinai Program for the Protection of Human Subjects (IRB 18 00603) reviewed and approved the study protocol, with waiver of informed consent.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Prezant DJ, Weiden M, Banauch GI, McGuinness G, Rom WN, Aldrich TK, Kelly KJ (2002) Cough and bronchial responsiveness in firefighters at the World Trade Center site. N Engl J Med 347 (11):806–815. doi: 10.1056/NEJMoa021300 [DOI] [PubMed] [Google Scholar]
- 2.Herbert R, Moline J, Skloot G, Metzger K, Barron S, Luft B, Markowitz S, Udasin I, Harrison D, Stein D, Todd AC, Enright P, Stellman JM, Landrigan PJ, Levin SM (2006) The World Trade Center disaster and the health of workers: five-year assessment of a unique medical screening program. Environ Health Perspect 114 (12):1853–1858. doi: 10.1289/ehp.9592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de la Hoz RE, Shohet MR, Chasan R, Bienenfeld LA, Afilaka AA, Levin SM, Herbert R (2008) Occupational toxicant inhalation injury: the World Trade Center (WTC) experience. Int Arch Occup Environ Health 81 (4):479–485. doi: 10.1007/s00420-007-0240-x [DOI] [PubMed] [Google Scholar]
- 4.de la Hoz RE, Weber J, Xu D, Doucette JT, Liu X, Carson DA, Celedón JC (2018) Chest CT scan findings in World Trade Center workers. Arch Environ Occup Health 74 (5):263–270. doi: 10.1080/19338244.2018.1452712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford ES, Mannino DM, Wheaton AG, Giles WH, Presley-Cantrell L, Croft JB (2013) Trends in the prevalence of obstructive and restrictive lung function among adults in the United States: findings from the National Health and Nutrition Examination surveys from 1988–1994 to 2007–2010. Chest 143 (5):1395–1406. doi: 10.1378/chest.12-1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godfrey MS, Jankowich MD (2016) The vital capacity is vital: epidemiology and clinical significance of the restrictive spirometry pattern. Chest 149 (1):238–251. doi: 10.1378/chest.15-1045 [DOI] [PubMed] [Google Scholar]
- 7.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CPM, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J (2005) Interpretative strategies for lung function tests. Eur Respir J 26 (5):948–968. doi: 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 8.Leone N, Courbon D, Thomas F, Bean K, Jego B, Leynaert B, Guize L, Zureik M (2009) Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med 179 (6):509–516. doi: 10.1164/rccm.200807-1195OC [DOI] [PubMed] [Google Scholar]
- 9.Wan ES, Hokanson JE, Murphy JR, Regan EA, Make BJ, Lynch DA, Crapo JD, Silverman EK (2011) Clinical and radiographic predictors of GOLD-unclassified smokers in the COPDGene study. Am J Respir Crit Care Med 184 (1):57–63. doi: 10.1164/rccm.201101-0021OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan ES, Castaldi PJ, Cho MH, Hokanson JE, Regan EA, Make BJ, Beaty TH, Han MK, Curtis JL, Curran-Everett D, Lynch DA, DeMeo DL, Crapo JD, Silverman EK (2014) Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res 15:89. doi: 10.1186/s12931-014-0089-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan ES, Fortis S, Regan EA, Hokanson J, Han MK, Casaburi R, Make BJ, Crapo JD, DeMeo DL, Silverman EK (2018) Longitudinal phenotypes and mortality in preserved ratio impaired spirometry in the COPDGene study. Am J Respir Crit Care Med 198 (11):1397–1405. doi: 10.1164/rccm.201804-0663OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyatt RE, Cowl CT, Bjoraker JA, Scanlon PD (2009) Conditions asociated with an abnormal nonspecific pattern of pulmonary function tests. Chest 135 (2):419–424 [DOI] [PubMed] [Google Scholar]
- 13.Chevalier-Bidaud B, Gillet-Juvin K, Callens E, Chenu R, Graba S, Essalhi M, Delclaux C (2014) Nonspecific pattern of lung function in a respiratory physiology unit: causes and prevalence: results of an observational cross-sectional and longitudinal study. BMC Pulm Med 14:148. doi: 10.1186/1471-2466-14-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prime FJ, Scadding JG (1968) Blood-gas and spirometric findings in chronic bronchitis and asthma. Lancet 291 (7556):1372. doi: 10.1016/S0140-6736(68)92069-2 [DOI] [Google Scholar]
- 15.Bouhuys A, van de Woestijne KP (1968) Restrictive versus obstructive ventilatory impairment. Lancet 292 (7563):352. doi: 10.1016/s0140-6736(68)90561-8 [DOI] [PubMed] [Google Scholar]
- 16.Jones RL, Nzekwu MM (2006) The effects of body mass index on lung volumes. Chest 130 (3):827–833. doi: 10.1378/chest.130.3.827 [DOI] [PubMed] [Google Scholar]
- 17.Skloot GS, Schechter CB, Herbert R, Moline JM, Levin SM, Crowley LE, Luft BJ, Udasin IG, Enright PL (2009) Longitudinal assessment of spirometry in the World Trade Center Medical Monitoring Program. Chest 135 (2):492–498 Erratum in Chest 2009; 2135(2004):1114, . doi: 10.1378/chest.08-1391 [DOI] [PubMed] [Google Scholar]
- 18.Webber MP, Lee R, Soo J, Gustave J, Hall CB, Kelly K, Prezant D (2011) Prevalence and incidence of high risk for obstructive sleep apnea in World Trade Center-exposed rescue/recovery workers. Sleep Breath 15 (3):283–294. doi: 10.1007/s11325-010-0379-7 [DOI] [PubMed] [Google Scholar]
- 19.Flegal KM, Carroll MD, Ogden CL, Curtin LR (2010) Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303 (3):235–241. doi: 10.1001/jama.2009.2014 [DOI] [PubMed] [Google Scholar]
- 20.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM (2006) Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295 (13):1549–1555 [DOI] [PubMed] [Google Scholar]
- 21.Venkateshiah SB, Ioachimescu OC, McCarthy K, Stoller JK (2008) The utility of spirometry in diagnosing pulmonary restriction. Lung 186 (1):19–25. doi: 10.1007/s00408-007-9052-8 [DOI] [PubMed] [Google Scholar]
- 22.Sood A, Petersen H, Qualls C, Meek PM, Vazquez-Guillamet R, Celli BR, Tesfaigzi Y (2016) Spirometric variability in smokers: transitions in COPD diagnosis in a five-year longitudinal study. Respir Res 17 (1):147. doi: 10.1186/s12931-016-0468-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.San José Estépar R, Reilly JJ, Silverman EK, Washko GR (2008) Three-dimensional airway measurements and algorithms. Proc Am Thorac Soc 5 (9):905–909. doi: 10.1513/pats.200809-104QC [DOI] [PubMed] [Google Scholar]
- 24.de la Hoz RE, Liu X, Doucette JT, Reeves AP, Bienenfeld LA, Wisnivesky JP, Celedón JC, Lynch DA, San José Estépar R (2018) Increased airway wall thickness is associated with adverse longitudinal first-second forced expiratory volume trajectories of former World Trade Center workers. Lung 196 (4):481–489. doi: 10.1007/s00408-018-0125-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendelson DS, Roggeveen M, Levin SM, Herbert R, de la Hoz RE (2007) Air trapping detected on end-expiratory high-resolution computed tomography in symptomatic World Trade Center rescue and recovery workers. J Occup Environ Med 49 (8):840–845. doi: 10.1097/JOM.0b013e3180d09e87 [DOI] [PubMed] [Google Scholar]
- 26.Coxson HO (2012) Lung parenchyma density and airwall thickness in airway diseases. Breathe (Sheffield, England) 9 (1):36–45. doi: 10.1183/20734735.018912 [DOI] [Google Scholar]
- 27.Woskie SR, Kim H, Freund A, Stevenson L, Park BY, Baron S, Herbert R, Siegel de Hernandez M, Teitelbaum S, de la Hoz RE, Wisnivesky JP, Landrigan P (2011) World Trade Center disaster: assessment of responder occupations, work locations, and job tasks. Am J Ind Med 54 (9):681–695. doi: 10.1002/ajim.20997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hankinson JL, Odencratz JR, Fedan KB (1999) Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 159 (1):179–187. doi: 10.1164/ajrccm.159.1.9712108 [DOI] [PubMed] [Google Scholar]
- 29.American Thoracic Society (1995) Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 152 (3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792 [DOI] [PubMed] [Google Scholar]
- 30.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J (2005) Standardisation of spirometry. Eur Respir J 26 (2):319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 31.Enright PL, Skloot GS, Cox-Ganser JM, Udasin IG, Herbert R (2010) Quality of spirometry performed by 13,599 participants in the World Trade Center Worker and Volunteer Medical Screening Program. Respir Care 55 (3):303–309 [PubMed] [Google Scholar]
- 32.Keller BM, Reeves AP, Apostolakos MJ, Wang J, Yankelevitz DF, Henschke CI (2009) Quantitative assessment of emphysema from whole lung CT scans: comparison with visual grading. In: SPIE International Symposium on Medical Imaging (February 2009), vol 7260 SPIE, Bellingham, WA, p 726008 [Google Scholar]
- 33.Lee J, Reeves AP, Fotin SV, Apananosovich TV, Yankelevitz DF (2008) Human airway measurement from CT Images. In: SPIE International Symposium on Medical Imaging (February 2008), vol 6915 SPIE, Bellingham, WA, p 691518 [Google Scholar]
- 34.de la Hoz RE, Jeon Y, Reeves, San José Estépar R, Liu X, Doucette JT, Celedón JC, Nolan A (2019) Increased pulmonary artery diameter is associated with reduced FEV1 in former World Trade Center workers. Clin Respir J 2019 (13):614–623. doi: 10.1111/crj.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, Frith P, Halpin DM, Lopez Varela MV, Nishimura M, Roche N, Rodríguez-Roisin R, Sin DD, Singh D, Stockley R, Vestbo J, Wedzicha JA, Agustí A (2017) Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Arch Bronconeumol 53 (3):128–149. doi: 10.1016/j.arbres.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 36.Hoffman EA, Ahmed FS, Baumhauer H, Budoff M, Carr JJ, Kronmal R, Reddy S, Barr RG (2014) Variation in the percent of emphysema-like lung in a healthy, nonsmokingmultiethnic sample - The MESA Lung Study. Ann Am Thorac Soc 11 (6):898–907. doi: 10.1513/AnnalsATS.201310-364OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mets OM, Zanen P, Lammers JWJ, Isgum I, Gietema HA, van Ginneken B, Prokop M, de Jong PA (2012) Early identification of small airways disease on lung cancer screening CT: comparison of current air trapping measures. Lung 190:629–633. doi: 10.1007/s00408-0129422-8 [DOI] [PubMed] [Google Scholar]
- 38.de la Hoz RE (2011) Occupational lower airway disease in relation to World Trade Center inhalation exposure. CurrOpin Allergy Clin Immunol 11 (2):97–102. doi: 10.1097/ACI.0b013e3283449063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de la Hoz RE, Liu X, Celedón JC, Doucette JT, Jeon Y, Reeves AP, San José Estépar R (2019) Association of obesity with quantitative chest CT measured airway wall thickness in WTC workers with lower airway disease. Lung 197 (4):517–522. doi: 10.1007/s00408-01900246-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orlandi I, Moroni C, Camiciottoli G, Bartolucci M, Pistolesi M, Villari N, Mascalchi M (2005) Chronic obstructive pulmonary disease: thin-section CT measurement of airway wall thickness and lung attenuation. Radiology 234 (2):604–610. doi: 10.1148/radiol.2342040013 [DOI] [PubMed] [Google Scholar]
- 41.Aaron SD, Tan WC, Bourbeau J, Sin DD, Loves RH, MacNeil J, Whitmore GA (2017) Diagnostic instability and reversals of chronic obstructive pulmonary disease diagnosis in individuals with mild to moderate airflow obstruction. Am J Respir Crit Care Med 196 (3):306–314. doi: 10.1164/rccm.201612-2531OC [DOI] [PubMed] [Google Scholar]
- 42.Hogg JC, Macklem PT, Thurlbeck WM (1968) Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med 278 (25):1355–1360. doi: 10.1056/NEJM196806202782501 [DOI] [PubMed] [Google Scholar]
- 43.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Paré PD (2004) The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 350 (26):2645–2653. doi: 10.1056/NEJMoa032158 [DOI] [PubMed] [Google Scholar]
- 44.Briscoe WA, Dubois AB (1958) The relationship between airway resistance, airway conductance, and lung volumes in subjects of different age and body size. J Clin Invest 37 (9):1279–1285. doi: 10.1172/JCI103715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gibbons WJ, Sharma A, Lougheed D, Macklem PT (1996) Detection of excessive bronchoconstriction in asthma. Am J Respir Crit Care Med 153 (2):582–589. doi: 10.1164/ajrccm.153.2.8564102 [DOI] [PubMed] [Google Scholar]
- 46.Berger KI, Reibman J, Oppenheimer BW, Vlahos I, Harrison D, Goldring RM (2013) Lessons from the World Trade Center disaster: airway disease presenting as restrictive dysfunction. Chest 144 (1):249–257. doi: 10.1378/chest.12-1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, Elliott WM, Hogg JC, Pare PD (2005) The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med 171 (2):142–146. doi: 10.1164/rccm.200407-874OC [DOI] [PubMed] [Google Scholar]
- 48.Johnston AK, Mannino DM, Hagan GW, Davis KJ, Kiri VA (2008) Relationship between lung function impairment and incidence or recurrence of cardiovascular events in a middle-aged cohort. Thorax 63 (7):599–605. doi: 10.1136/thx.2007.088112 [DOI] [PubMed] [Google Scholar]
- 49.Mannino DM, McBurnie MA, Tan W, Kocabas A, Anto J, Vollmer WM, Buist AS (2012) Restricted spirometry in the Burden of Lung Disease Study. Int J Tuberc Lung Dis 16 (10):1405–1411. doi: 10.5588/ijtld.12.0054 [DOI] [PubMed] [Google Scholar]
- 50.Jankowich M, Elston B, Liu Q, Abbasi S, Wu WC, Blackshear C, Godfrey M, Choudhary G (2018) Restrictive spirometry pattern, cardiac structure and function, and incident heart failure in African Americans - the Jackson Heart Study. Ann Am Thorac Soc 15 (10):1186–1196. doi: 10.1513/AnnalsATS.201803-184OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Engstrom G, Hedblad B, Nilsson P, Wollmer P, Berglund G, Janzon L (2003) Lung function, insulin resistance and incidence of cardiovascular disease: a longitudinal cohort study. J Intern Med 253 (5):574–581 [DOI] [PubMed] [Google Scholar]
- 52.Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD (2010) Morbidity and mortality associated with the restrictive spirometric pattern: a longitudinal study. Thorax 65 (6):499–504. doi: 10.1136/thx.2009.126052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee HM, Le H, Lee BT, Lopez VA, Wong ND (2010) Forced vital capacity paired with Framingham Risk Score for prediction of all-cause mortality. Eur Respir J 36 (5):1002–1006. doi: 10.1183/09031936.00042410 [DOI] [PubMed] [Google Scholar]
- 54.Hickson DA, Burchfiel CM, Liu J, Petrini MF, Harrison K, White WB, Sarpong DF (2011) Diabetes, impaired glucose tolerance, and metabolic biomarkers in individuals with normal glucose tolerance are inversely associated with lung function: the Jackson Heart Study. Lung 189 (4):311–321. doi: 10.1007/s00408-011-9296-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.