Abstract
Objective
The relationship between adipocyte size and ad libitum energy intake has not been previously examined. We hypothesized an inverse relationship between adipocyte size and daily energy intake (DEI).
Methods
Seventy healthy adults (39M/31F; BMI 30.0±6.3) underwent dual energy X-ray absorptiometry and subcutaneous fat biopsies from the abdomen and thigh. Osmium-fixed adipocytes were sized with a Coulter counter. Volunteers self-selected food from a vending machine paradigm as the only source of energy intake over three days as inpatients. Volunteers also had 24-h respiratory quotient (RQ) measured in a whole-room indirect calorimeter.
Results
In women, the large cell peak diameter of the thigh depot was greater than that of the abdominal depot (Δ = +15.8 μm, p<0.0001). In women, thigh peak diameter was inversely associated with DEI (β = −264.7 kcal/day per 10 μm-difference, p=0.03), adjusted for demographics and body composition. The thigh peak diameter in women was associated with 24-h RQ (r= −0.47, p=0.04), adjusted for demographics, body composition, and 24-h energy balance. These associations did not extend to men or the abdominal depot.
Conclusions
In women, thigh adipocyte size was associated with reduced DEI and 24-h RQ, indicating a special role for thigh fat in women. This depot-specific sexual dimorphism indicates common regulation of energy intake and adipocyte size in the thigh region of women.
Keywords: human adipose tissue, energy intake, adipocytes, modeling
1. Introduction
Obesity, resulting from a prolonged imbalance between energy expenditure and energy intake, is a substantial public health problem [1, 2]. Understanding the factors regulating energy intake is therefore crucial. Despite abundant evidence from animal models that signals derived from adipose tissue convey information about energy reserves (e.g. leptin and other adipokines) to provide negative feedback on energy intake [3–5], human evidence linking fat mass (FM) and energy intake has been neither strong nor consistent, with prior studies showing no associations [6, 7] or weak negative associations [8, 9]. FM is comprised primarily of adipocytes of varying size, and adipocyte size is associated with appetite-regulating adipokines in cultured adipocytes [10], at the gene expression level in tissue [11], and in circulation [12–14], indicating a role for adipose tissue cellularity in appetite regulation. To our knowledge, whether adipocyte size is a determinant of ad libitum daily energy intake (DEI) has not been studied in humans.
Average adipocyte size has been shown to be directly associated with in vitro basal and isoproterenol stimulated lipolysis per cell [15]. In the same study, adipocyte size was directly associated with whole-body lipid oxidation and negatively correlated with 24-h respiratory quotient (24-h RQ) which primarily reflects the ratio of carbohydrate to fat oxidation. In previous studies, 24-h RQ was associated with greater ad libitum overeating (as well as future weight gain) [9, 16]. However, it is unknown if adipocyte size mediates the relationship between 24-h RQ and ad libitum DEI.
In the current study, the primary aim was to investigate whether adipocyte sizes from two different subcutaneous depots (i.e. abdomen and thigh) are determinants of ad libitum DEI using a vending machine paradigm, an objective and reproducible measure of food intake [17]. We also aimed to confirm the association between adipocyte size and 24-h RQ using whole-room indirect calorimetry and to evaluate whether adipocyte size is a determinant of DEI independent of 24-h RQ. We hypothesized that larger adipocytes are associated with lower ad libitum DEI, reduced 24-h RQ, and greater 24-h lipid oxidation (24-h LIPOX).
2. Methods
2.1. Participants and study design
Male and female participants were recruited from the Phoenix Metropolitan Area, Arizona, USA, between 2009 and 2016 and admitted to the clinical research unit for participation in a larger observational inpatient study (clinicaltrials.gov identifier: NCT00342732) investigating risk factors for obesity as previously described [9]. The protocol was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Written informed consent was obtained prior to participation. The present analysis was performed in 70 adults without diabetes (age<65 years). All participants were not currently using drugs, prescription medications or nicotine products, and healthy by screening history, physical examination, and routine laboratory testing. Women were verified to be nonpregnant with a urine pregnancy test.
An overview of the inpatient study is shown in Supplementary Fig. S1. During the inpatient stay, physical activity was restricted to light activities (e.g. playing pool, television, crafts) for the entire time course of the study. A standard weight-maintaining diet (50% carbohydrate, 30% fat, and 20% protein) was provided to the volunteers from the day of the admission. Based on body weight and sex, a unit-specific equation [18] was used to calculate the weight-maintaining energy needs (WMEN) for each individual and then adjusted daily by a research dietician to maintain a body weight within 1% of the weight on the admission day. On day two, body composition was assessed using dual-energy x-ray absorptiometry (Lunar Prodigy or iDXA, GE Lunar Healthcare, Madison, WI, USA). To make absorptiometry data comparable across the two different machines, absorptiometry measures were converged using an equation previously derived from a separate study on the metabolic unit to calculate total body fat percentage, and trunk and leg fat (both expressed as a percentage of total mass in the anatomical region mass) [19]. Waist circumference was measured at the umbilicus in the supine position and thigh circumference at the gluteal fold while standing.
After at least three days on the weight-maintaining diet, glucose tolerance was assessed by a 75-g oral glucose tolerance test according to the 2003 American Diabetes Association criteria [20]. Participants with diabetes were excluded. Plasma glucose concentrations was measured by the glucose oxidase method (Glucose analyzer GM9, Analox Instruments; Lunenbertg, MA, USA). Before starting the 3-day of vending machine paradigm the blood samples were drawn for the measurement of fasting adiponectin and leptin concentrations. Adiponectin and leptin were measured using the human Quantikine ELISA kits from R&D Systems (Minneapolis, MN, USA). The intra-assay and inter-assay percent coefficient variation were 3.20% and 6.03% for adiponectin and 1.34% and 1.58% for leptin, respectively.
2.2. Adipose tissue sampling and cell size analysis
Participants underwent biopsies for subcutaneous adipose tissue of the abdomen and thigh under local anesthesia with 1% lidocaine after an overnight fast and after resting for two hours. Lateral mid-thigh subcutaneous adipose tissue was sampled using a modified Bergström needle under suction. Abdominal subcutaneous adipose tissue was also sampled via needle aspiration lateral to the umbilicus. Samples of subcutaneous adipose tissue were immediately incubated in osmium tetroxide-solution (Structure Probe, West Chester, PA, USA) containing collidine (Sigma-Aldrich, St. Louis, MO, USA) in a water bath at 37°C for 48 hours as previously described [21]. The solution containing osmium-fixed cells were filtered with a 25 μm and 250 μm nylon mesh (Sefar, Buffalo, NY, USA). Following this procedure, adipocyte size was measured using the Multisizer 3 Coulter Counter (Beckman Coulter, Brea, CA, USA) with a 400-μm aperture set to count 6000 cells per run. Cell suspensions were diluted so that coincident counting was less than 10%. After collection of pulse sizes, the data was expressed on a linear scale involving 300 bins, each 0.733 μm wide, spanning the effective cell size range of the aperture (20 to 240 μm). Each fixed cell sample was run in duplicate and the data averaged. Data from bins between 25 to 240 μm were included for subsequent analysis. Since adipocyte sizing with this method reveals a bimodal distribution, with overlapping populations of small and large adipocytes, cell size distributions were characterized using nonlinear regression implemented in the R package nlstools [22] to obtain peak cell diameter of the large adipocyte population, nadir diameter (defined as the mid-point between the large and small populations), and percent small adipocytes (defined as the percent of cells with diameter less than the nadir diameter), as previously described [23, 24]. A representative distribution is shown in Supplementary Fig. S2 to illustrate these adipocyte size parameters. Of the 70 participants, 52 participants had adipocyte measures from both abdominal and thigh depots, and 15 and 3 had valid measures from the abdomen only and thigh only, respectively. Missing cell size data was most often due to insufficient biopsy sample. In addition, uncommon distributions not matching the bimodal pattern, as previously reported [25], were excluded from analysis.
2.3. Metabolic chamber measures
Participants spent approximately 23.5 hours inside a whole-room indirect calorimeter to measure 24-hour energy expenditure (24-h EE) and substrate oxidation in eucaloric conditions as previously described (Supplementary Fig. S1) [26]. Prescribed energy intake in the metabolic chamber was based on previously developed equations [27] and was approximately 80% of the weight maintaining diet provided outside the chamber to account for restricted physical activity inside the chamber. For an accurate calculation of intake, all unconsumed food was returned to the metabolic kitchen for weighing. Carbon dioxide production and oxygen consumption were extrapolated to 24 hours to calculate 24-h EE and 24-h RQ. The 24-hour energy balance (ENBAL) was calculated as energy intake minus 24-h EE. For better accuracy of metabolic measures, only individuals with energy balance within ± 20% were included for analysis. From the 24-h RQ, 24-h carbohydrate oxidation (24-h CARBOX) and 24-h LIPOX rates were calculated accounting for 24-h protein oxidation (24-h PROTOX) obtained by the measurement of 24-h urinary nitrogen excretion as previously described [28]. Spontaneous physical activity (SPA) was detected by radar sensors and expressed as the percentage of time over the 24-h period in which activity was detected. Sleeping metabolic rate (SMR) was defined as the average energy expenditure between the hours of 2330 and 0500 during which SPA was < 1.5%. Sleep RQ was calculated during the same time points as above.
2.4. Ad libitum food intake measures
The measurement of ad libitum food intake over three days (Supplementary Fig. S1) was assessed using a vending machine paradigm, as previously described [17]. Upon admission, a food selection questionnaire in which a 9-point Likert scale (1=dislike extremely, 5=neutral, 9=like extremely) was given to assess the food preferences of each subject to rate each food item. Based on ratings of 80 items, 40 items (scores: 4–8) were stocked in an individual-specific, computer-operated vending machines during this period. The participants had unrestricted access to food for 23.5 hours. Prior to placing the food items in the vending machines, all food was weighed by the metabolic kitchen staff. Furthermore, any uneaten food was also weighed at the end of the vending day to calculate the actual intake. The CBORD Professional Diet Analyzer Program (CBORD, Inc., Ithaca, NY, USA) and the Food Processor database (ESHA version 10.0.0, ESHA Research, Salem, OR, USA) was used to calculate the daily total and individual macronutrient kilocalories consumed. Over three days, the average total ad libitum DEI was calculated and expressed as total kilocalories consumed daily. Similar calculations were performed for macronutrient intake (carbohydrate, fat, and protein). The DEI was also expressed as percentage of the WMEN determined prior to the three-day vending period.
2.5. Statistical Analysis
Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) and R 3.5.2 (The R Foundation for Statistical Computing, Vienna, Austria). Data are expressed as mean ± standard deviation. Categorical variables were compared with the χ2 test. As no differences in adipocyte parameters were observed between races using ANOVA, non-Native American individuals (n=38) were combined into one group (other races) and compared to Native Americans (n=32). Unpaired t-test were used to assess difference by sex and race, and the paired t-test was used to assess differences in adipocyte size between the abdominal and thigh depots. Correlations between normally distributed data were assessed by the Pearson’s correlation coefficient. Similar analyses were performed to assess the association between adipocyte size and ad libitum energy intake measures (i.e. DEI and macronutrient intake). Analyses were conducted by sex and depot to address the known influence of these variables on adipocyte morphology and function [29, 30]. To correct for multiple comparisons, a Bonferroni-corrected threshold for significance equal to 0.0125 was obtained by dividing the nominal threshold for significance (0.05) by the number of comparison groups (4 = 2 sexes + 2 depots). Linear regression analysis was used to calculate residuals of total ad libitum daily energy intake and residuals of macronutrient intake, adjusting for age, race, FM, and fat-free mass (FFM). Additionally, in a sensitivity analysis, height-normalized indices of both fat mass and fat-free mass (kg/m2) were used instead in place of the original covariates. Linear regression analysis was also performed to calculate residuals of 24-h RQ and substrate oxidation (adjusting for age, race, body fat percentage, and ENBAL) and to calculate residual 24-h EE and its components after adjustment for age, race, FM, and FFM. To increase the precision of regression estimates, residuals were calculated using the entire cohort of volunteers who completed the study which included participants who did not have adipose tissue biopsies (n=135).
3. Results
Demographic and anthropometric characteristics are reported in Table 1. Participants ranged in age from 19–65 years (mean 39.0±11.9) and Native American race was the most common racial group (46%). BMI ranged from 20.2–47.2 kg/m2 (mean 30.0 ±6.3). As expected, women had greater body fat percentage (40.2 ± 5.3%), and FM (34.2 ± 11.7 kg), and BMI (32.8 ± 7.4) compared to men (26.8 ± 5.1%; 23.5 ± 8.4 kg; 27.8 ± 4.1, respectively).
Table 1.
Demographic and anthropometric characteristics of the study cohort.
| Whole group (n=70) |
Men (n=39) |
Women (n=31) |
p value | |
|---|---|---|---|---|
| Age (years) | 39.0 ± 11.9 | 41.8 ± 11.8 | 35.5 ± 11.3 | 0.03 |
| Race | 32 NA, 38 O | 13 NA, 26 O | 19 NA, 12 O | 0.02 |
| Height (cm) | 168.2 ± 10.8 | 175.2 ± 8.3 | 159.4 ± 6.0 | <0.0001 |
| Body weight (kg) | 84.7 ± 17.8 | 85.7 ± 16.4 | 83.3 ± 19.5 | 0.58 |
| BMI (kg/m2) | 30.0 ± 6.3 | 27.8 ± 4.1 | 32.8 ± 7.4 | 0.0007 |
| FM (kg) | 28.3 ± 11.2 | 23.5 ± 8.4 | 34.2 ± 11.7 | <0.0001 |
| FFM (kg) | 56.4 ± 11.1 | 62.2 ± 9.1 | 49.1 ± 8.9 | <0.0001 |
| Body fat (%) | 32.7 ± 8.5 | 26.8 ± 5.1 | 40.2 ± 5.3 | <0.0001 |
| Trunk fat (%) | 31.7± 9.9 | 24.1 ± 5.2 | 40.9 ± 5.2 | <0.0001 |
| Leg fat (%) | 36.3 ± 8.2 | 31.3 ± 6.2 | 42.5 ± 6.0 | <0.0001 |
| Waist circumference (cm) | 100.6 ± 15.5 | 97.0 ± 11.9 | 104.9 ± 18.5 | 0.04 |
| Thigh circumference (cm) | 62.7 ± 8.1 | 59.9 ± 6.1 | 66.0 ± 8.9 | 0.001 |
| Waist-thigh-ratio | 1.6 ± 0.20 | 1.6 ± 0.16 | 1.6 ± 0.24 | 0.62 |
| Fasting glucose (mg/dL) | 92.8 ± 6.2 | 93.6 ± 6.7 | 91.8 ± 5.3 | 0.25 |
| 2-h glucose (mg/dL) | 128.4 ± 27.2 | 126.1 ± 28.5 | 131.2 ± 25.6 | 0.44 |
| Leptin (ng/mL)a | 19.9 ± 20.5 | 7.1 ± 6.2 | 36.1 ± 20.9 | <0.0001 |
| Adiponectin (μg/mL)b | 5.3 ± 4.1 | 5.8 ± 5.2 | 4.8 ± 1.9 | <0.0001 |
Data are presented as mean ± standard deviation.
NA: Native American; O: Other; BMI: Body mass index; FM: Fat mass; FFM: Fat free mass
Difference between men and women determined by unpaired t-test or χ2 test as appropriate.
Leptin and adiponectin measurements were available in 28 men had 22 in women.
3.1. Determinants of adipocyte measurements
Adipocyte sizes are reported in Table 2. Compared with women, men had lower peak and nadir diameters in the abdominal depot (Δ = −9.2 μm, p<0.011 and Δ = −6.5 μm p=0.015, respectively). Similarly, in the thigh depot, the peak and nadir diameters were lower in men than in women (Δ = −20.5 μm, p<0.0001 and Δ = −12.4 μm, p<0.0001, respectively). After adjustment for BMI, the significant difference in peak and nadir diameters in the thigh depot between men and women remained unchanged (Δ = −15.5 μm, p<0.0001 and Δ = −9.8 μm, p<0.0001, respectively), whereas there were no differences in either size parameter in the abdomen (all p>0.48). No differences in percent small cell between men and women in either depot was observed (p>0.4). No differences in the adipocyte size (peak diameter, nadir point, and percent small cells) in abdomen and thigh locations were observed between different races (Native American vs. other races, all p>0.1). Adipocyte size was not associated with age in either anatomical region (all p>0.3).
Table 2.
Measurements of adipocyte size
| Whole group | Men | Women | p value | |
|---|---|---|---|---|
| Abdomen | n=67 | n=37 | n=30 | |
| Peak diameter, (μm) | 116.5 ± 15.0 | 112.3 ± 14.1 | 121.6 ± 14.7 | 0.01 |
| Nadir diameter, (μm) | 76.5 ± 11.0 | 73.6 ± 10.3 | 80.1 ± 11.0 | 0.02 |
| Small cells, (%) | 53.1 ± 14.9 | 53.4 ± 14.3 | 52.7 ± 15.9 | 0.80 |
| Thigh | n=55 | n=27 | n=28 | |
| Peak diameter, (μm) | 126.5 ± 17.5 | 116.1 ± 17.1 | 136.6 ± 11.0 | <0.0001 |
| Nadir diameter, (μm) | 83.8 ± 11.4 | 77.5 ± 10.4 | 89.8 ± 8.8 | <0.0001 |
| Small cells, (%) | 56.1 ± 12.5 | 54.8 ± 9.7 | 57.5 ± 14.8 | 0.40 |
Data are presented as mean ± standard deviation.
Difference between men and women determined by unpaired t-test.
In participants with adipocyte size measurements in both the abdomen and thigh, intrasubject correlation between thigh and abdominal regions for peak diameter, nadir diameter, and percent small cells was assessed. In men, peak diameter of the abdomen was correlated with peak diameter of the thigh (r=0.45, p=0.02, Supplemental Fig. S3A), whereas there was no correlation between nadir diameter and percent small cells between the two regions (all p>0.1, Supplemental Fig S3B and Supplemental Fig. S3C). In women, peak and nadir diameters in abdomen were moderately correlated with the same adipocyte size measure in the thigh (peak cell diameter, r=0.57, p=0.002; nadir diameter r=0.52, p=0.006, Supplemental Fig. S3D–E), whereas no correlation was observed between abdomen and thigh percent small cell (p=0.8, Supplemental Fig. S3C).
In women (n=27), the thigh peak and nadir diameters were greater compared to the abdominal region (Δ = +15.8 μm, 95% CI: 10.0 to 17.5, p=0.0001, Fig. 1B; Δ = +9.6 μm, 95% CI: 8.7 to 15.1, p<0.0001, Fig. 1D) whereas no differences were observed in men (all p>0.4, n =25, Fig. 1A–C–E). No difference was observed in percent small cell between the two fat depots in either sex (p>0.2, Fig 1E–F).
Fig. 1. Adipocyte size by sex.
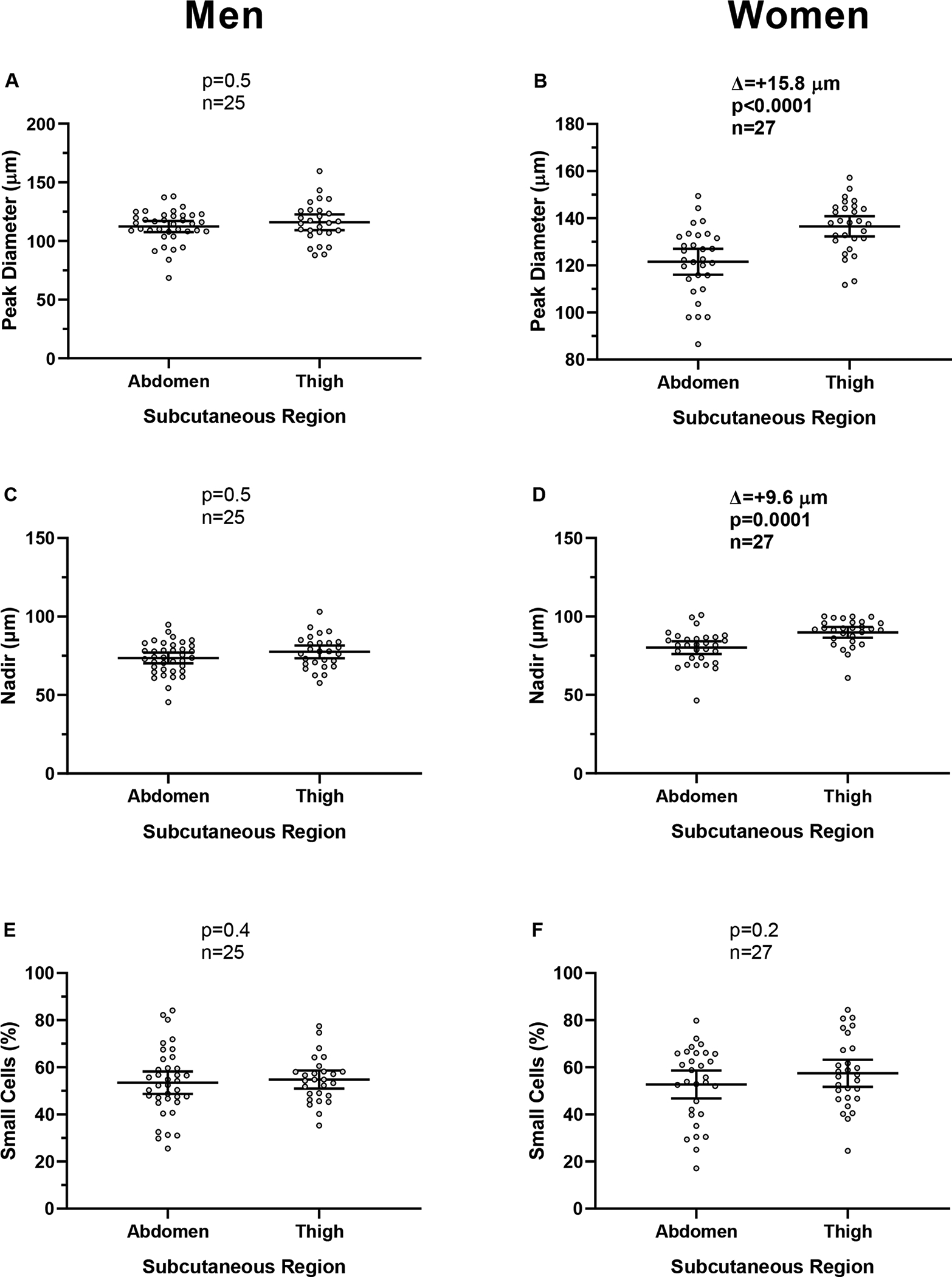
Panel A-C-E. Average difference (Δ) between abdominal and thigh regions for peak diameter (panel A), nadir diameter (panel C), and percent small cells (panel E) in men.
Panel B-D-F. Average difference (Δ) between abdominal and thigh regions for peak diameter (panel B), nadir diameter (panel D) and percent small cells (panel F) in women.
Paired t-test was used to assess differences between regions.
In both abdominal and thigh regions of men, peak diameter was positively correlated with FM (r=0.53, p=0.0007, Fig. 2A; r=0.64, p=0.0003, Fig. 3A), body fat percentage (r= 0.56, p=0.0002, Fig.2C; r=0.57, p=0.002, Fig. 3C), and BMI (r=0.47, p =0.0035, Fig 2E; r= 0.64, p=0.0003, Fig. 3E) respectively. In men, the peak diameter of the thigh region was directly associated with FFM (r= 0.58, p=0.0013) whereas no association was observed in the abdominal region (p=0.1). In women, the peak diameter of the abdominal region was directly associated with FM (r=0.50, p=0.005, Fig. 2B), FFM (r=0.36, p=0.04), %fat (r= 0.46, p=0.009, Fig. 2D), and BMI (r=0.55, p =0.001, Fig 2F). Furthermore, the peak diameter of the thigh was positively associated BMI (r=0.40, p=0.03, Fig. 3F) and showed a trend with FM (r=0.35, p=0.06, Fig. 3B), but no association was observed with body fat percentage (p=0.21, Fig. 3D).
Fig. 2. Relationships between peak diameter of adipocytes (abdomen) and adiposity measures in men and women.
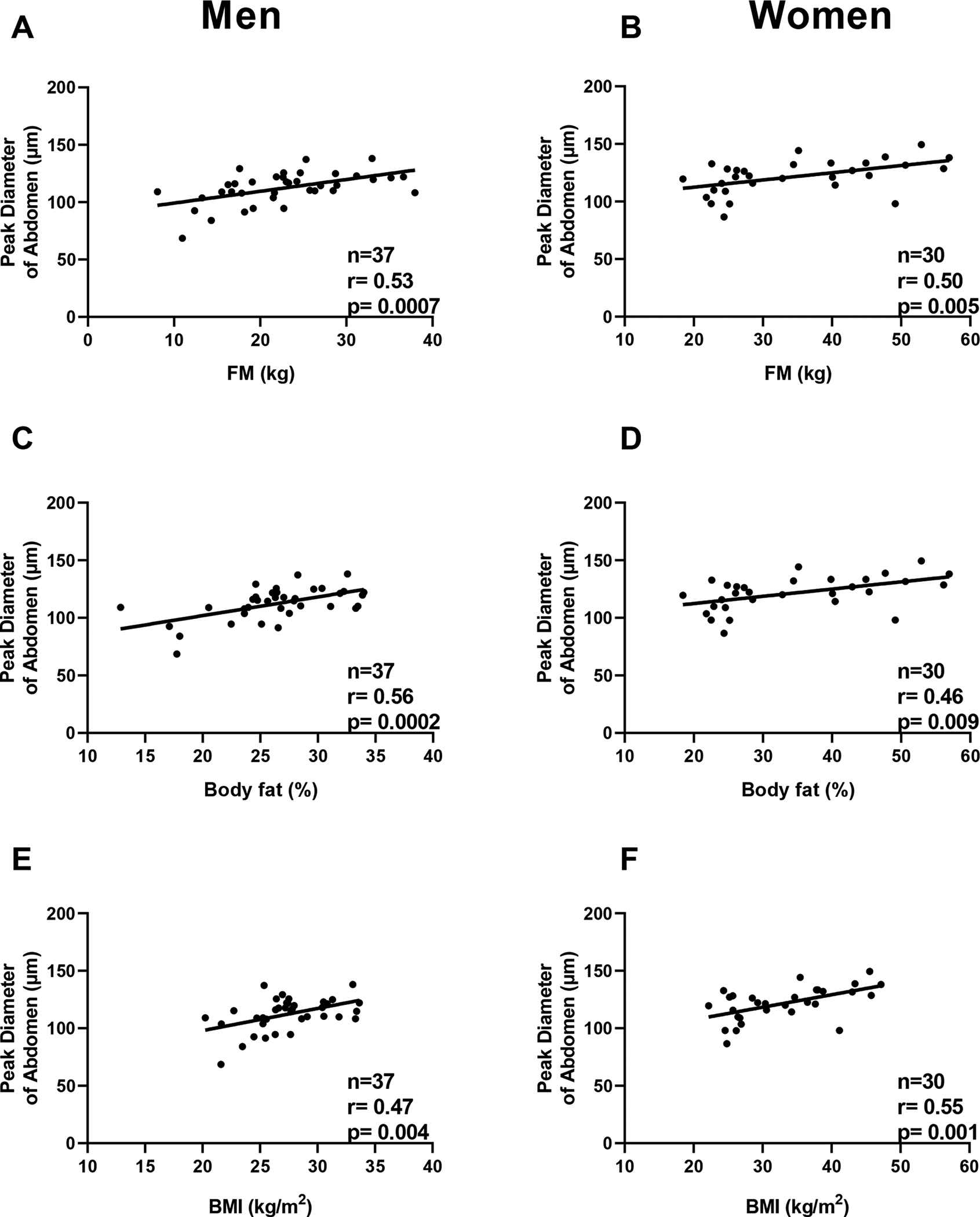
Panel A-C-E. Relationships between peak diameter of adipocyte size in the abdomen and fat mass (panel A), body fat percentage (panel C), and body mass index (panel E) in men.
Panel B-D-F. Relationships between peak diameter of the thigh adipocyte size and fat mass (panel B), body fat percentage (panel D), and body mass index (panel F) in women.
Pearson’s correlation coefficient (r) is reported along with its significance (p).
Fig. 3. Relationships between peak diameter of adipocytes (thigh) and adiposity measures in men and women.
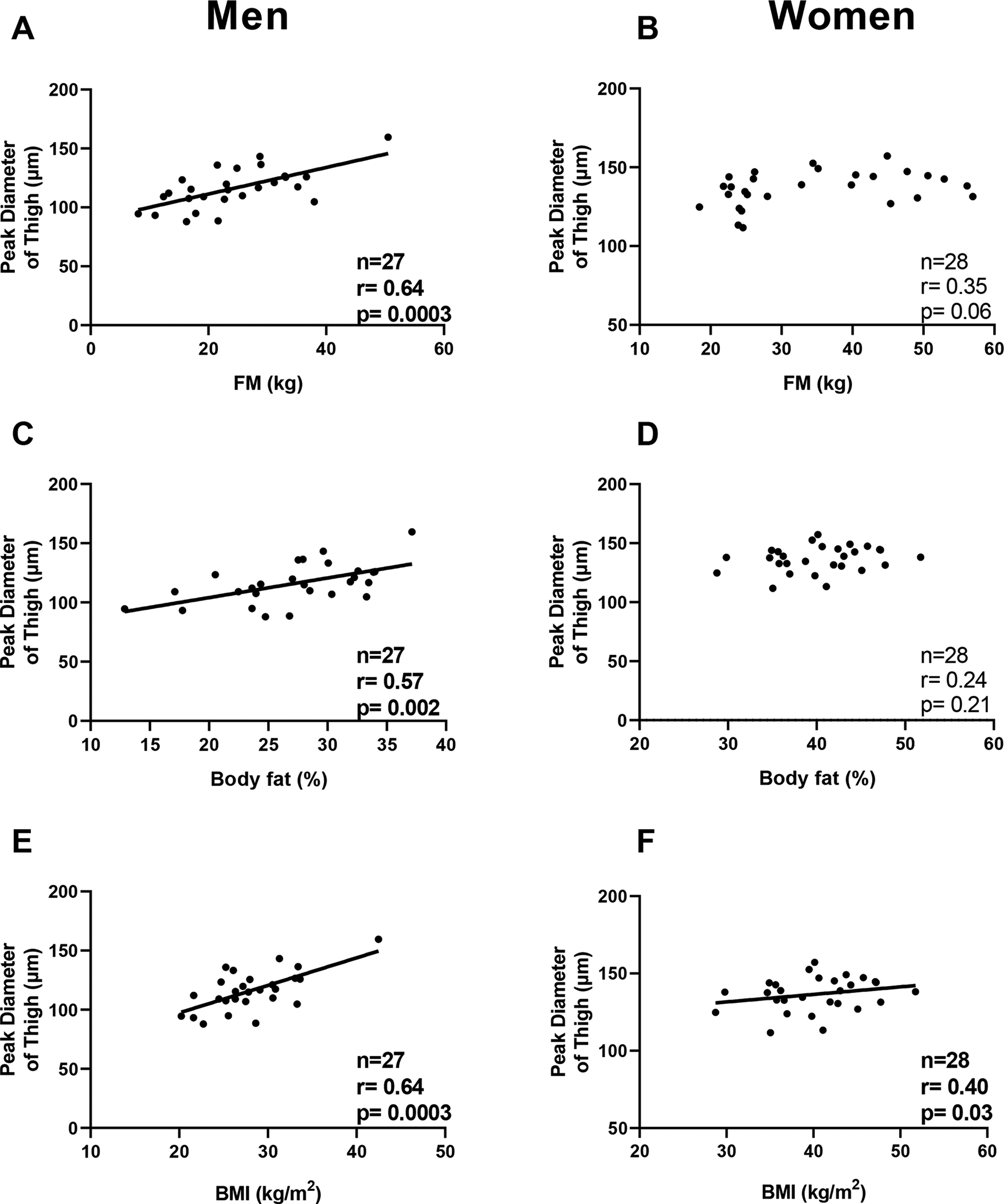
Panel A-C-E. Relationships between peak diameter of adipocyte size in the thigh and fat mass (panel A), body fat percentage (panel C), and body mass index (panel E) in men. Panel B-D-F. Relationships between peak diameter of the thigh adipocyte size and fat mass (panel B), body fat percentage (panel D), and body mass index (panel F) in women.
Pearson’s correlation coefficient (r) is reported along with its significance (p).
In women, waist circumference and trunk fat percentage were associated with peak diameter of the abdomen (r=0.54, p=0.002 and r=0.37, p=0.04, respectively). Thigh circumference was associated with peak diameter of the thigh (r=0.40, p=0.03). No association was observed between leg fat percentage and peak diameter of the thigh (p=0.3). In men, peak diameter of the abdomen was associated with waist circumference (r=0.42, p=0.009) but not trunk fat percentage (p=0.09). Both thigh circumference and leg fat percentage were associated with peak diameter of the thigh (r=0.67, p=0.0001 and r=0.51, p=0.008, respectively). Adipocyte size was not significantly associated with circulating leptin or adiponectin levels independent of age, race, and body composition in either sex or depot (all p>0.35).
Adipocyte size (peak diameter and nadir) was also analyzed in relation to fat distribution expressed as the ratio of trunk or leg fat mass to the total body fat mass or the waist-thigh-ratio. In unadjusted and adjusted analysis (for BMI), the adipocyte size in the abdomen of men was associated with the ratio of trunk fat mass to total body fat mass (both p=0.02). There was no similar association observed in women (p>0.20). Furthermore, in either sex, there was no association between adipocyte size in the thigh and the ratio of leg fat mass to total body fat mass (both p>0.4). Furthermore, there were no observed associations between thigh or abdomen adipocyte size with the waist-to-thigh ratio in either sex (all p>0.10).
3.2. Relationship between adipocyte size and ad libitum food intake
Ad libitum food intake measurements are described in Table 3. For the entire cohort, the average DEI was 3229.4±1103.8 kcal/day (or 125.4 ± 38.7% when expressed as percentage of WMEN).
Table 3.
Ad Libitum food intake and metabolic chamber measurements.
| Whole study group | Men | Women | p value | |
|---|---|---|---|---|
| Vending machine system | ||||
| Daily energy intake (kcal/day)a | 3229.4 ± 1103.8 | 3723.0 ± 1078.0 | 2608.4 ± 784.3 | <0.0001 |
| WMEN (kcal/day) | 2459.2 ± 862.9 | 2829.7 ± 869.6 | 1993.0 ± 592.9 | <0.0001 |
| Daily energy intake (% WMEN) | 125.4 ± 38.7 | 139.7± 39.8 | 107.4 ± 29.2 | 0.002 |
| CHO intake (kcal/day) | 1700.0 ± 585.2 | 1950.9 ± 580.5 | 1384.4 ± 419.6 | <0.0001 |
| FAT intake (kcal/day) | 1180.7 ± 512.8 | 1366.0 ± 541.6 | 947.5 ± 363.9 | 0.0004 |
| PRO intake (kcal/day) | 379.2 ± 146.2 | 442.7 ± 143.1 | 299.6 ± 106.8 | <0.0001 |
| Metabolic chamber measures | ||||
| Energy balance (kcal/day) | 139.8 ± 194.0 | 172.1 ± 220.8 | 91.25 ± 135.1 | 0.11 |
| EE in energy balance (kcal/day) | 2049.9 ± 341.2 | 2174.6 ±338.6 | 1862.92 ± 251.63 | 0.0003 |
| Sleeping metabolic rate (kcal/day) | 1589.7 ± 257.4 | 1680.0 ±247.4 | 1454.1 ± 212.0 | 0.0005 |
| RQ in energy balance (ratio) | 0.86 ± 0.05 | 0.87 ± 0.04 | 0.85 ± 0.06 | 0.08 |
| Non-protein RQ (ratio) | 0.88 ± 0.07 | 0.89 ± 0.05 | 0.86 ± 0.08 | 0.07 |
| RQ Sleep (ratio) | 0.82 ± 0.06 | 0.83 ± 0.04 | 0.80 ± 0.08 | 0.043 |
| CARBOX, (kcal/day) | 999.0 ± 386.3 | 1118.5 ± 308.1 | 819.8 ± 427.3 | 0.003 |
| LIPOX (kcal/day) | 663.8 ± 425.7 | 631.7 ± 405.3 | 712.1 ± 459.2 | 0.48 |
| PROTOX (kcal/day) | 361.5 ± 125.5 | 396.5 ± 132.0 | 309.1 ± 95.4 | 0.007 |
| SPA (%) | 6.7 ± 4.0 | 7.0 ± 4.1 | 6.2 ± 3.9 | 0.48 |
Data are presented as mean ± standard deviation.
Difference between men and women determined by unpaired t-test.
Ad libitum food intake measures are reported as the average of three days on the vending machines.
WMEN: weight maintaining energy needs; CHO: carbohydrate; PRO: protein; EE: energy expenditure; RQ: respiratory quotient;
CARBOX: carbohydrate oxidation; LIPOX: lipid oxidation; PROTOX: protein oxidation; SPA: spontaneous physical activity
3.2.1. Thigh Depot
The peak diameter of thigh adipocytes in women was negatively associated with residuals of DEI (β = −264.7 kcal/day per 10 μm-difference in peak diameter, CI: −508.2 to −21.2, p=0.03, Fig. 4B, Supplementary Table 1), daily protein intake (β = −31.3 kcal/day per 10 μm-difference in peak diameter, CI: −60.5 to −2.1, p=0.04, Fig. 4H) and showed a trend with carbohydrate intake (β = −137.5 kcal/day per 10 μm-difference in peak diameter, CI: −278.0 to 2.9, p=0.054, Fig. 4D). There was no association with residual fat intake (p=0.1, Fig. 4F). Results were unchanged in sensitivity analysis using fat-free mass index and fat mass index in place of FFM and FM, respectively. In contrast to women, no associations between adipocyte cell size and DEI or macronutrient intake were observed in men (all p>0.5, Fig. 4A–C–E–G). In either sex, neither leptin nor adiponectin were associated with residual DEI (all p>0.30). The independent effects of thigh adipocyte size in women and adipokines on DEI were not discernable as the multivariable model including these two variables was not overall significant (p>0.30).
Fig. 4. Relationships between peak diameter of thigh adipocytes and ad libitum food intake, by sex.
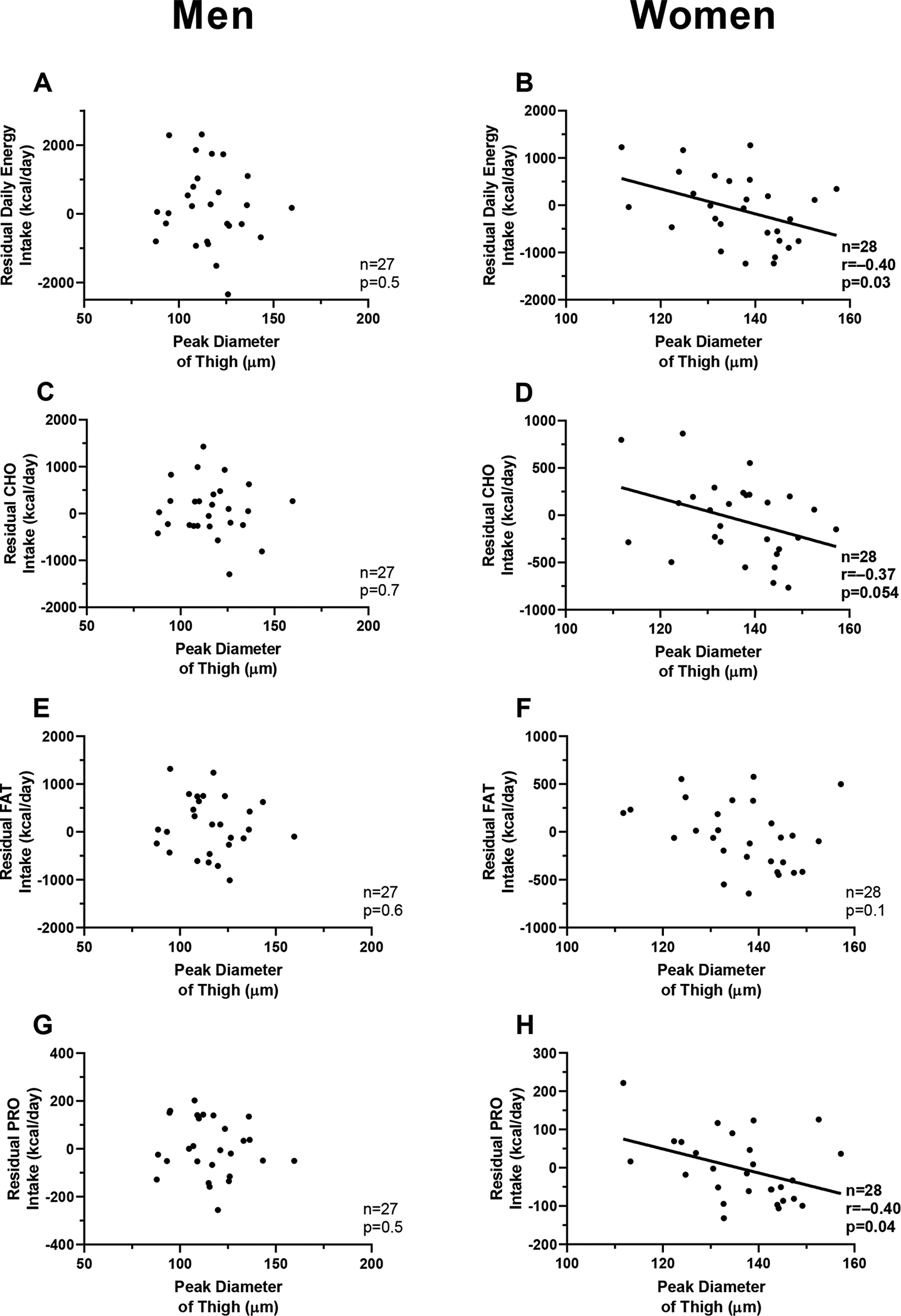
Panel A-C-E-G. Relationships between peak diameter of the thigh adipocytes and residuals of daily energy intake (panel A), carbohydrate intake (panel C), fat intake (panel E), and protein intake (panel G) in men.
Panel B-D-F-H. Relationships between peak diameter of the thigh adipocytes and residuals of daily energy intake (panel B), carbohydrate intake (panel D), fat intake (panel F) and protein intake (panel H) in women.
Pearson’s correlation coefficient (r) is reported along with its significance (p).
Residuals of food intake were calculated using linear regression adjusting for age, race, fat mass, and fat-free mass.
Linear regression analysis was used to calculate residuals of CARBOX and LIPOX after adjustment for age, sex, race, fat mass, and fat-free mass.
RQ: respiratory quotient; CARBOX: carbohydrate oxidation; LIPOX: lipid oxidation
The results in panels 4B, 4D, and 4H were not significant when considering a Bonferroni-corrected threshold for significance.
Neither thigh circumference nor body fat percentage in the leg were associated with residuals of DEI and macronutrient intake in either men or women (all p>0.6). Furthermore, in women, the association between peak diameter of thigh adipocytes on residuals of DEI remained independent of leg fat mass percentage (p=0.04) and the ratio of leg fat mass to total body fat mass (p=0.02).
3.2.2. Abdominal Depot
The abdominal adipocyte size measurements (peak diameter, nadir, and percent small cells) were not associated with DEI, carbohydrate intake, fat intake or protein intake either in unadjusted analyses or after adjustment for their known determinants in either sex (all p>0.7).
3.3. Relationship between adipocyte size and metabolic chamber measurements
3.3.1. Metabolic fuel selection in the thigh
In men, thigh peak diameter was not associated with residual 24-h RQ (p=0.2, Fig. 5A), CARBOX (p=0.1, Fig. 5C), LIPOX (p=0.2, Fig. 5E), and PROTOX (p=0.3). Conversely, in women, peak diameter in the thigh was negatively associated with lower residual 24-h RQ (β = −0.022 per 10 μm-difference in peak diameter, CI: −0.04 to −0.001, p=0.04, Fig 5B, Supplementary Table 2), lower non-protein RQ (β = −0.027 per 10 μm-difference in peak diameter, CI: −0.054 to −0.00029, p=0.02), lower 24-h RQ sleep (β = −0.031 per 10 μm-difference in peak diameter, CI: −0.054 to −0.007, p=0.004), lower residual 24-h CARBOX (β = −15.3 kcal/day, CI: −28.5 to −2.1, p=0.02, Fig. 5D), and higher residual 24-h LIPOX (β = +16.3 kcal/day, CI: 0.56 to 32.0, p=0.04, Fig. 5F). The association between peak diameter in the thigh and residual 24-h RQ was independent of leg fat mass percentage (p=0.01) and the ratio of leg fat mass to total body fat mass.
Fig. 5. Relationships between peak diameter of thigh adipocytes and metabolic fuel selection near energy balance, by sex.
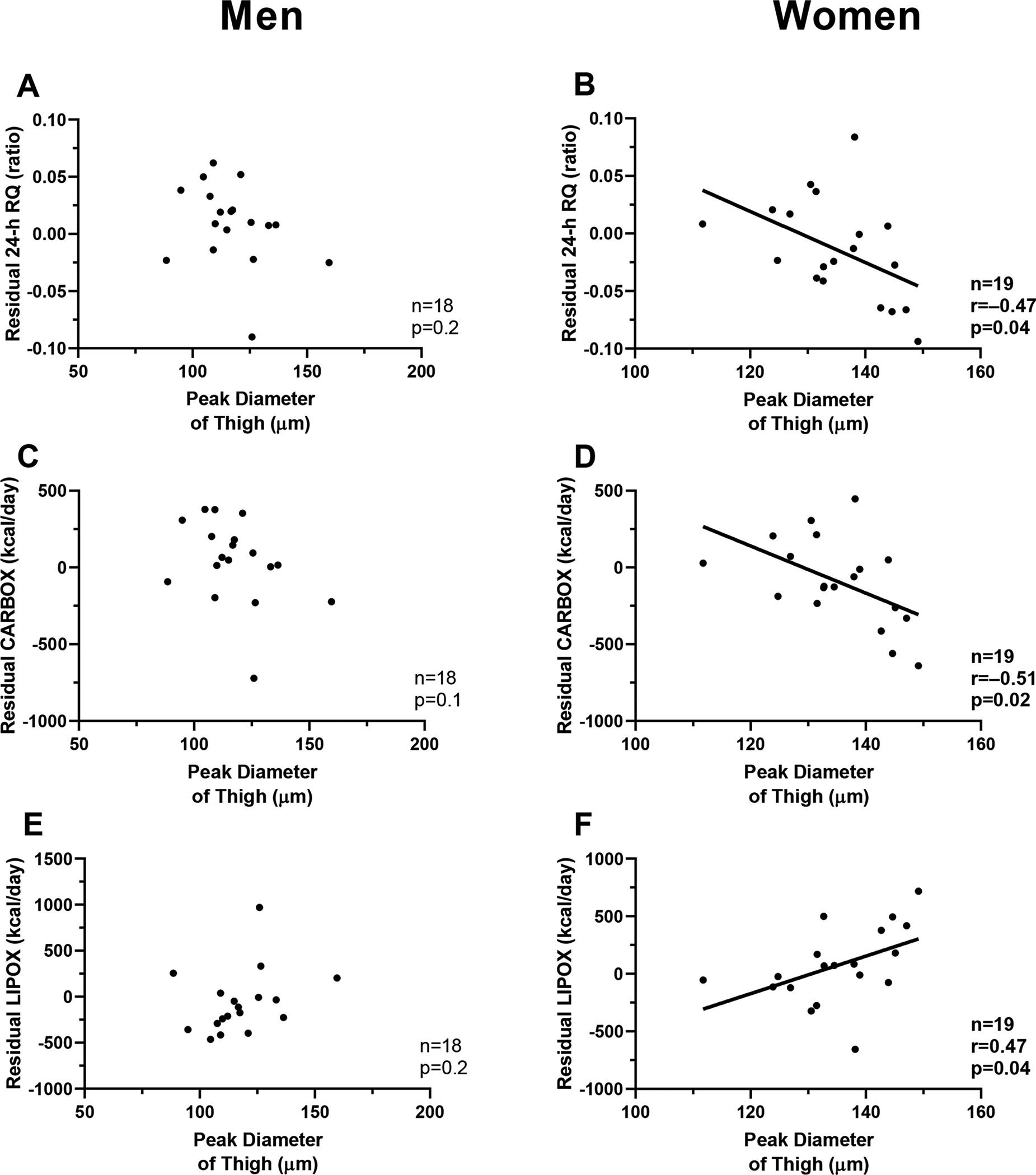
Panel A-C-E. Relationships between peak diameter of the thigh adipocytes and residuals of 24-h respiratory quotient (panel A), carbohydrate oxidation (panel C), and lipid oxidation (panel E) in men.
Panel B-D-F. Relationships between peak diameter of the thigh adipocytes and residuals of 24-h respiratory quotient (panel B), carbohydrate oxidation (panel D), and lipid oxidation (panel F) in women.
Pearson’s correlation coefficient (r) is reported along with its significance (p).
Linear regression analysis was used to calculate residuals of 24-h RQ after adjustment for age, race, body fat percentage and energy balance.
Linear regression analysis was used to calculate residuals of CARBOX and LIPOX after adjustment for age, race, fat mass, and fat-free mass.
RQ: respiratory quotient; CARBOX: carbohydrate oxidation; LIPOX: lipid oxidationThe results in panels 5B, 5D, and 5F were not significant when considering a Bonferroni-corrected threshold for significance.
Furthermore, a positive association between adjusted 24-h RQ and DEI in women was observed (r=0.47, p=0.04). In a multivariable model including both 24-h RQ and peak diameter of the thigh, the peak diameter was inversely associated with residual daily energy intake (β = −447.1 per 10 μm-difference in peak diameter, CI: −845.9 to −48.2, p=0.03) independent of 24-RQ (p=0.89) which was no longer a determinant of residual daily energy intake. Neither nadir diameter nor percent small cell in the thigh region was associated with 24-h RQ, CARBOX, LIPOX, or PROTOX (all p>0.08).
3.3.2. Metabolic fuel selection in the abdomen
The abdominal adipocyte size measurements (peak diameter, nadir, and percent small cells) were not associated with 24-h RQ, CARBOX, LIPOX, and PROTOX in unadjusted analyses nor after adjustment for their known determinants in either sex (all p>0.2).
3.3.3. Energy expenditure
Adipocyte size measurements (peak diameter, nadir, or percent small cell size) were not associated with 24-h EE, SMR, or SPA in either sex or depot (all p>0.07).
4. Discussion
In the current study, we evaluated whether adipocyte size from two different subcutaneous depots (abdomen and thigh) were determinants of ad libitum daily energy and macronutrient intake in a cohort of 70 healthy adults. To our knowledge, this relationship has not been previously been established. We identified a sexual dimorphism and a depot difference in the relationship between ad libitum food intake and adipocyte size. Adipocyte size (i.e. peak diameter of the large cell population) of the thigh in women was associated with reduced daily energy intake, carbohydrate intake, and protein intake independent of FM, FFM, and demographic variables. Adipocyte size from the abdomen of women or from both depots in men were not determinants of energy intake. Moreover, we found that in women, thigh adipocytes were larger than those from the abdomen, whereas there were no observed regional differences in men. We found that, in contrast to men, the thigh adipocyte size of women was associated with reduced 24-h RQ, reduced CARBOX, and greater LIPOX after adjustment of known determinants. Furthermore, thigh adipocyte size in women was inversely associated with ad libitum energy intake independent of 24-h RQ.
The results indicate that energy intake is linked to signals from the thigh depot and emphasizes the putative differences in adipose tissue as a function of sex and depot. The findings may be related to the differences in adipose tissue between men and women. Body fat percentage, at the same BMI, is higher in women compared to men [31, 32]. Furthermore, women preferentially store more adipose tissue in the thigh and gluteal regions (i.e. gynoid fat distribution) whereas men accumulate adipose tissue in the central region (i.e. android fat distribution) [31, 33]. It has been proposed that the gynoid fat distribution has a specialized function in women, supported by evidence that gluteofemoral fat is selectively mobilized for pregnancy and lactation [34]. The gluteofemoral depot in women is the primary source for long chain polyunsaturated fatty acids (LC-PUFAs), such as omega-3 docosahexaenoic acid (DHA), which is vital for fetal/infant brain development [35]. Compared to abdominal adipose tissue, outer thigh fat has been shown to be enriched in essential LC-PUFAs [36]. With each cycle of pregnancy and lactation, gluteofemoral fat is either reduced in absolute terms in poorly nourished population or in relative terms in well-nourished populations [37]. In the current study, women with larger adipocytes in the thigh had reduced ad libitum DEI, indicating that brain centers involved in food intake regulation may be sensing the adequacy of these fat reserves from this depot through a negative feedback signal linked to size that is vital to female reproduction and breast-feeding. In contrast to previous reports [10–14], we found no association with circulating levels of adipokines (i.e. leptin and adiponectin) and adipocyte size. This suggests that there may be other appetite-regulating signals linked to adipocyte size. The independent effects of adipocyte size and adipokines on DEI were not able to be assessed due to limited sample size (n=18 women).
Similar to prior evidence [34], our study confirmed that adipocyte size in the thigh is greater than in the abdomen of women but was of similar size in men, further indicating that these depots may be differentially regulated in women. The sex- and depot-specific differences in adipose tissue appear to extend to whole-body metabolic fuel selection. Women with greater thigh adipocyte diameter had reduced 24-h RQ, greater LIPOX, and reduced CARBOX, indicating that the femoral depot influences substrate oxidation in women as opposed to the abdominal depot. Since it is known that 24-h RQ is positively associated with energy intake in humans [9, 38], this study also examined 24-h RQ and adipocyte size in the same statistical model and found that thigh adipocyte diameter in women was a determinant of ad libitum DEI independent of 24-h RQ.
A strength of the study is the application of the Coulter counter method which has the advantage of being able to measure a large number of cells (> 10,000) and allows for the quantification of the small adipocyte population not as apparent using other methods (e.g. collagenase digestion or histology) [39]. In addition, an objective measure of food intake was used in contrast to less accurate methods based on questionnaires or self-report [40].
Our study has several limitations. First, although the study included both sexes and more than one fat depot, the sample size was relatively small. Second, some individuals did not have valid metabolic chamber measurements, reducing the sample size for the analysis of adipocyte size and metabolic fuel selection. Third, since the women were mostly obese, it is uncertain if these results are generalizable to lean women. Fourth, we did not have measurements of hormones in the hypothalamic-pituitary-gonadal axis that coordinate the female reproductive system (e.g. estradiol) which might affect the food intake regulation in women. This precludes assessment of how these reproductive hormones might influence the relationship between adipocyte size and energy intake [41, 42]. Lastly, we did not collect biomarkers related to fat oxidation which might have been helpful to further characterize the relationship of adipose tissue and metabolic fuel selection.
In summary, the thigh adipocyte size of healthy adult women was greater than the size in the abdomen, but size did not differ between the depots in men, indicating that this is privileged depot in women. Importantly, it was this depot (thigh) in women that was also found to be a determinant of ad libitum total energy intake and metabolic fuel selection (reduced 24-h RQ, higher 24-h LIPOX) independent of body composition. Moreover, thigh adipocyte size was inversely associated with DEI independent of 24-h RQ. These findings highlight sex- and depot-specific differences in adipose tissue and indicate a special role for thigh fat in women for energy-intake regulation.
Supplementary Material
Study Importance Questions.
What is already known about this subject?
Adipose-derived hormones, linked to adipocyte size, influence energy intake.
Osmium-fixation and high-throughput electronic sizing reveals a bimodal distribution of subcutaneous adipocyte size suggesting distinct large cell (mature) and small cell populations.
Sex-dependent depot differences are important mediators of both adipocyte development and function.
What are the new findings in your manuscript?
The diameter of the large adipocytes from the thigh of healthy adult women was greater compared to the abdomen.
In women, large cell adipocyte diameter from the thigh was independently associated with reduced ad libitum daily energy intake and reduced 24-hour respiratory quotient.
How might your results change the direction of research or the focus of clinical practice?
Thigh adipose tissue may be a privileged depot in women important for energy-intake regulation and warrants further investigation.
The present study emphasizes the need for detailed adipocyte sizing methods in metabolic research.
5. Acknowledgments
The authors thank the volunteers and clinical staff of the Phoenix Epidemiology and Clinical Research Branch.
Funding: This study was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations:
- 24-h EE
24-hour energy expenditure
- ENBAL
24-hour energy balance
- 24-h RQ
24-hour respiratory quotient
- 24-h CARBOX
24-hour carbohydrate oxidation
- 24-h LIPOX
24-hour lipid oxidation
- 24-h PROTOX
24-hour protein oxidation
- DEI
daily energy intake
- FFM
fat-free mass
- FM
fat mass
- SPA
spontaneous physical activity
Footnotes
Disclosure Statement: The authors declared no conflict of interest.
ClinicalTrials.gov identifier: NCT00342732
References
- [1].Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9· 1 million participants. The Lancet. 2011;377:557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. The Lancet. 2011;378:815–25. [DOI] [PubMed] [Google Scholar]
- [3].Blundell J Biology of the drive to eat. Implications for un derstanding human appetite and obesity. Ernahrungs Umschau. 2018;65:126–33. [Google Scholar]
- [4].Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661. [DOI] [PubMed] [Google Scholar]
- [5].Stubbs RJ, Hopkins M, Finlayson GS, Duarte C, Gibbons C, Blundell JE. Potential effects of fat mass and fat-free mass on energy intake in different states of energy balance. Eur J Clin Nutr. 2018;72:698–709. [DOI] [PubMed] [Google Scholar]
- [6].Lissner L, Habicht J-P, Strupp BJ, Levitsky D, Haas JD, Roe D. Body composition and energy intake: do overweight women overeat and underreport? The American journal of clinical nutrition. 1989;49:320–5. [DOI] [PubMed] [Google Scholar]
- [7].Cameron JD, Sigal RJ, Kenny GP, Alberga AS, Prud’homme D, Phillips P, et al. Body composition and energy intake—skeletal muscle mass is the strongest predictor of food intake in obese adolescents: the HEARTY trial. Applied Physiology, Nutrition, and Metabolism. 2016;41:611–7. [DOI] [PubMed] [Google Scholar]
- [8].Weise CM, Hohenadel MG, Krakoff J, Votruba SB. Body composition and energy expenditure predict ad-libitum food and macronutrient intake in humans. International journal of obesity. 2014;38:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Piaggi P, Thearle MS, Krakoff J, Votruba SB. Higher daily energy expenditure and respiratory quotient, rather than fat-free mass, independently determine greater ad libitum overeating. The Journal of Clinical Endocrinology & Metabolism. 2015;100:3011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–33. [DOI] [PubMed] [Google Scholar]
- [11].Zhang Y, Zitsman JL, Hou J, Fennoy I, Guo K, Feinberg J, et al. Fat cell size and adipokine expression in relation to gender, depot, and metabolic risk factors in morbidly obese adolescents. Obesity. 2014;22:691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lundgren M, Svensson M, Lindmark S, Renstrom F, Ruge T, Eriksson JW. Fat cell enlargement is an independent marker of insulin resistance and ‘hyperleptinaemia’. Diabetologia. 2007;50:625–33. [DOI] [PubMed] [Google Scholar]
- [13].Wahlen K, Sjolin E, Lofgren P. Role of fat cell size for plasma leptin in a large population based sample. Exp Clin Endocrinol Diabetes. 2011;119:291–4. [DOI] [PubMed] [Google Scholar]
- [14].Meyer LK, Ciaraldi TP, Henry RR, Wittgrove AC, Phillips SA. Adipose tissue depot and cell size dependency of adiponectin synthesis and secretion in human obesity. Adipocyte. 2013;2:217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Frankl J, Piaggi P, Foley JE, Krakoff J, Votruba SB. In Vitro lipolysis is associated with whole-body lipid oxidation and weight gain in humans. Obesity. 2017;25:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba B, Raz I, Saad M, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. American Journal of Physiology-Endocrinology And Metabolism. 1990;259:E650–E7. [DOI] [PubMed] [Google Scholar]
- [17].Venti CA, Votruba SB, Franks PW, Krakoff J, Salbe AD. Reproducibility of ad libitum energy intake with the use of a computerized vending machine system. The American journal of clinical nutrition. 2010;91:343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ferraro R, Boyce VL, Swinburn B, De Gregorio M, Ravussin E. Energy cost of physical activity on a metabolic ward in relationship to obesity. The American Journal of Clinical Nutrition. 1991;53:1368–71. [DOI] [PubMed] [Google Scholar]
- [19].Reinhardt M, Piaggi P, DeMers B, Trinidad C, Krakoff J. Cross calibration of two dual energy X densitometers and comparison of visceral adipose tissue measurements by iDXA and MRI. Obesity. 2017;25:332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Genuth S, Alberti KG, Bennett P, et al. ; Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–3167 [DOI] [PubMed] [Google Scholar]
- [21].Hirchi J Cellularity of obese and nonobese human adipose tissue. Fed Proc1970. p. 1516–21. [PubMed] [Google Scholar]
- [22].Baty F, Ritz C, Charles S, Brutsche M, Flandrois J-P, Delignette-Muller M-L. A toolbox for nonlinear regression in R: the package nlstools. Journal of Statistical Software. 2015;66:1–21. [Google Scholar]
- [23].McLaughlin T, Lamendola C, Coghlan N, Liu T, Lerner K, Sherman A, et al. Subcutaneous adipose cell size and distribution: relationship to insulin resistance and body fat. Obesity. 2014;22:673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].McLaughlin T, Sherman A, Tsao P, Gonzalez O, Yee G, Lamendola C, et al. Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia. 2007;50:1707–15. [DOI] [PubMed] [Google Scholar]
- [25].Yang J, Eliasson B, Smith U, Cushman SW, Sherman AS. The Size of Large Adipose Cells Is a Predictor of Insulin Resistance in First-Degree Relatives of Type 2 Diabetic Patients. Obesity. 2012;20:932–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. Journal of Clinical Investigation. 1986;78:1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Abbott W, Howard BV, Christin L, Freymond D, Lillioja S, Boyce VL, et al. Short-term energy balance: relationship with protein, carbohydrate, and fat balances. American Journal of Physiology-Endocrinology and Metabolism. 1988;255:E332–E7. [DOI] [PubMed] [Google Scholar]
- [28].Jequier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Annual review of nutrition. 1987;7:187–208. [DOI] [PubMed] [Google Scholar]
- [29].Karastergiou K, Fried SK. Cellular mechanisms driving sex differences in adipose tissue biology and body shape in humans and mouse models. Sex and gender factors affecting metabolic homeostasis, diabetes and obesity: Springer; 2017. p. 29–51. [DOI] [PubMed] [Google Scholar]
- [30].Fried SK, Lee MJ, Karastergiou K. Shaping fat distribution: new insights into the molecular determinants of depot-and sex-dependent adipose biology. Obesity. 2015;23:1345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Blaak E Gender differences in fat metabolism. Current Opinion in Clinical Nutrition & Metabolic Care. 2001;4:499–502. [DOI] [PubMed] [Google Scholar]
- [32].Jackson AS, Stanforth PR, Gagnon J, Rankinen T, Leon AS, Rao DC, et al. The effect of sex, age and race on estimating percentage body fat from body mass index: The Heritage Family Study. Int J Obes Relat Metab Disord. 2002;26:789–96. [DOI] [PubMed] [Google Scholar]
- [33].Fried SK, Kral JG. Sex differences in regional distribution of fat cell size and lipoprotein lipase activity in morbidly obese patients. International journal of obesity. 1987;11:129–40. [PubMed] [Google Scholar]
- [34].Rebuffe-Scrive M, Enk L, Crona N, Lönnroth P, Abrahamsson L, Smith U, et al. Fat cell metabolism in different regions in women. Effect of menstrual cycle, pregnancy, and lactation. The Journal of clinical investigation. 1985;75:1973–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lassek WD, Gaulin SJ. Waist-hip ratio and cognitive ability: is gluteofemoral fat a privileged store of neurodevelopmental resources? Evolution and Human Behavior. 2008;29:26–34. [Google Scholar]
- [36].Phinney SD, Stern JS, Burke KE, Tang AB, Miller G, Holman RT. Human subcutaneous adipose tissue shows site-specific differences in fatty acid composition. The American journal of clinical nutrition. 1994;60:725–9. [DOI] [PubMed] [Google Scholar]
- [37].Lassek WD, Gaulin SJ. Changes in body fat distribution in relation to parity in American women: a covert form of maternal depletion. Am J Phys Anthropol. 2006;131:295–302. [DOI] [PubMed] [Google Scholar]
- [38].Longo KA, Charoenthongtrakul S, Giuliana DJ, Govek EK, McDonagh T, DiStefano PS, et al. The 24-hour respiratory quotient predicts energy intake and changes in body mass. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2009;298:R747–R54. [DOI] [PubMed] [Google Scholar]
- [39].Stenkula KG, Erlanson-Albertsson C. Adipose cell size: importance in health and disease. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2018;315:R284–R95. [DOI] [PubMed] [Google Scholar]
- [40].Shim JS, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiol Health. 2014;36:e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kim K, Wactawski-Wende J, Michels KA, Plowden TC, Chaljub EN, Sjaarda LA, et al. Dairy food intake is associated with reproductive hormones and sporadic anovulation among healthy premenopausal women. The Journal of nutrition. 2016;147:218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philosophical Transactions of the Royal Society B: Biological Sciences. 2006;361:1251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


