Abstract
X chromosome structural abnormalities are relatively common in Turner Syndrome patients, in particular X isochromosomes. Reports over the last five decades examining asynchronous DNA replication between the normal X and isochromosome have clearly established that the structurally abnormal chromosome is the inactive X chromosome (Xi). Here the organization of chromatin at a deleted X chromosome, an Xq isochromosome and two isodicentric chromosomes were examined. Consistent with previous differential staining methods, at interphase the X isochromosome and isodicentric X chromosomes frequently formed bipartite Barr bodies, observed by fluorescence microscopy using numerous independent bona fide markers of Xi heterochromatin. At metaphase, with the exception of the pseudoautosomal region and the duplicated locus of the macrosatellite DXZ4 (if present on the abnormal X chromosome based on break-points), euchromatin markers were absent from the Xi, whereas histone variant macroH2A formed reproducible banded mirror-image chromosomes. Unexpectedly, the isodicentric chromosome in 46,X,idic(X)(q28) cells, that carry a near full-length q-arm-to-q-arm fused chromosome, showed at interphase very rare instances of Xi chromatin bodies that were separated by large distances in the nucleus. Further examination using immunofluorescence and FISH support the possibility that these rare cells may represent ones in which one half of the isodicentric chromosome is active and the other half inactive.
Keywords: X chromosome inactivation, Dicentric chromosomes, Heterochromatin, Dosage compensation
Introduction
X chromosome inactivation (XCI) is the mammalian process whereby X-linked gene expression is balanced between 46,XX females and 46,XY males (Lyon 1961). This balance is attained by shutting down most gene expression (Carrel and Willard 2005) from an X chromosome early during female development (Lyon 2002), and is achieved by repackaging the chromatin of the chosen inactive X chromosome (Xi) into facultative heterochromatin which can be readily observed as a densely staining mass, or Barr body, in female nuclei (Barr and Bertram 1949).
In addition to providing a mechanism to equalize the dose of X-linked gene products between the sexes, XCI protects against the devastating impact of aneuploidy such that 46,XXX females, are afflicted with relatively few major phenotypic effects (Otter et al. 2010). The reason behind the protective affect is that XCI shuts down all but one X, resulting in two Barr bodies in 47,XXX cells and three in 48,XXXX (Barr 1966). In addition to the occurrence of supernumerary X, monosomy X is viable resulting in Turner syndrome (Ford et al. 1959; Turner 1938). A substantial proportion of Turner patients are actually mosaic with cells lacking part of the second X (Jacobs et al. 1997). Among the structurally abnormal X’s, the most common that is observed in greater than 15% of Turner patients (Held et al. 1992; Hook and Warburton 1983; Palmer and Reichmann 1976; Ranke et al. 1983), is an isochromosome composed of two copies of Xq fused at the centromere, or just into Xp (Wolff et al. 1996) resulting in a mirror image of the long arm of the chromosome or i(Xq) (De la Chapelle et al. 1966; Ockey et al. 1966). Other abnormal X’s can be end-to-end fusions (Disteche et al. 1972), with near complete copies of the X. Most X isochromosomes or X fusions possess more than one centromere, with as many as three in one rare instance (Caine et al. 1993). Chromosomes with two centromeres are referred to as dicentric, and with the exception of physically close centromeres (Sullivan and Willard 1998), one centromere is silenced in those cells that stably segregate the chromosome in order to prevent breakage that would result were both centromeres active and being pulled to opposite poles of the cell during division (Stimpson et al. 2012).
One of the well-established hallmarks of the Xi, first reported more than 50 years ago (Gilbert et al. 1962; Morishma et al. 1962), is that the DNA of the Xi replicates later during S-phase than the active X chromosome (Xa). Xi DNA replication does not occur concurrently, but particular chromosomal regions replicate earlier than others in late S-phase (Willard and Latt 1976), although the precise replication pattern can vary slightly between different cell types (Willard 1977). With the exception of balanced X-autosome translocations, a common feature of cells harboring a structurally abnormal X is that this chromosome is invariably the Xi (Latt et al. 1976). While delayed replication timing has been reported extensively for X isochromosomes ( i(X)) and isodicentric X chromosomes (idic(X)) (Artifoni et al. 1983; Baranovskaya et al. 1976; Camargo and Cervenka 1984; Dalton et al. 1998; Dewald et al. 1978; Gaal et al. 1981; Lin and Wilson 1983; Mattei et al. 1977; Mutchinik et al. 1981; Petkovic et al. 2003; Pettigrew et al. 1991; Rack et al. 1994; Sarto and Therman 1980; Takenaka et al. 1988; Yu et al. 1980), few studies have examined chromatin organization at the Xi. With the large number of robust Xi chromatin markers presently available, the current study sought to investigate the organization of chromatin at several structurally abnormal Xi.
Materials and Methods
Cells and Culture
T-3352 are 46,XX normal female primary fibroblasts that were obtained from Dr. Stuart Schwartz at Case Western Reserve University. hTERT-RPE1 are 46, X,t(X:10) female retinal pigment epithelial cells derived from telomerase immortalization of primary RPE-340 cells (Bodnar et al. 1998) and were originally obtained from Clontech Laboratories Inc., (Cat. No. C4000–1). The X:10 translocation is the Xa in all cells (Darrow et al. 2016). hTERT-RPE1 cells were cultured as recommended by the supplier. These cells are no longer sold by Clontech, but are available from ATCC (CRL-4000). Primary fibroblasts GM03827, GM00735, GM06960 and GM07213 were all obtained from Coriell Institute for Medical Research. All primary fibroblasts were maintained in high glucose DMEM media (Genesee Scientific Cat. No.25–500 ) supplemented with 1x Penicillin-Streptomycin-Glutamine (Thermo Fisher Scientific Cat. No. 10378–016), 1x Non-Essential Amino Acids (Thermo Fisher Scientific Cat. No. 11140–050) and 20% (v/v) fetal bovine serum (Thermo Fisher Scientific Cat. No. 16000–069).
Antibodies
Antibodies raised against human macroH2A1 and macroH2A2 were described previously (Chadwick and Willard 2001; Chadwick and Willard 2002). Mouse monoclonals to human HP1 isoforms HP1-alpha (Cat. No. MAB3584), HP1-beta (Cat. No. MAB3448) and HP1-gamma (Cat. No. MAB3450) were all originally obtained from Chemicon International, but are now available from Millipore Sigma. Mouse monoclonal anti-histone H1 (Cat. No. Sc-8030) and rabbit polyclonal to HMG-I/Y (Cat. No. Sc-1564) were obtained from Santa Cruz Biotechnology. Rabbit polyclonal antibodies to H3K4me2 (Cat. No. 07–030), H3K27me3 (Cat. No. 07–449) and H3K9me3 (Cat. No. 07–523) were obtained from Millipore Sigma. Mouse monoclonal antibodies to H3K27me2/3 (Cat. No. 39535) were obtained from Active Motif. Images generated using the mouse H3K27me3 or rabbit H3K27me3 are indicated in the captions. Rabbit monoclonal antibodies to H2AK119Ub1 (Cat. No. 8240S) was obtained from Cell Signaling Technology. Rabbit polyclonal antibodies to SMCHD1 (Cat. No. A302–872A) were obtained from Bethyl Laboratories Inc. Alexa Fluor 555 direct-labeled goat anti-rabbit (A21429) and goat anti-mouse (A21424), and Alexa Fluor 488 direct-labeled goat anti-rabbit (A11034) and goat anti-mouse (A11029) were obtained from Thermo Fisher Scientific.
Metaphase Chromosomes
Cytospun metaphase chromosomes for immunofluorescence and FISH were prepared essentially as described previously (Chadwick and Willard 2002). Briefly, a 1:400 dilution of KaryoMAX Colcemid (Cat. No. 15212–012, Thermo Fisher Scientific) was added to media of actively dividing cells and incubated for one hour at 37oC 5% CO2. The media was collected and transferred to a 50ml tube. Cells were washed with phosphate buffered saline (PBS) which was added to the media in the 50ml tube. Cells were detached with trypsin and collected using the media/PBS mixture, before counting followed by briefly pelleting the cells. The pellet was gently resuspended in hypotonic solution (75mM KCl, 0.8% (w/v) Na3C6H5O7) at 5×104 cells/ml for 13 minutes. Cells and chromosomes were attached to slides by centrifugation using a Shandon Cytospin 4 (Thermo Fisher Scientific), spinning at 1,900 x rpm for 10 minutes. Post-spin, samples were fixed for 10 minutes in 1x PBS supplemented with 0.1% (v/v) Triton X-100 and 3.7% formaldehyde, before washing twice in 1x PBS. Samples were then subjected to immunofluorescence followed by FISH.
Dropped metaphase chromosomes for FISH were prepared from an actively dividing ~80% confluent T75 culture vessel initially as described above. At the stage of hypotonic treatment, cells were resuspended in 25ml of 37°C hypotonic solution (75mM KCl) for 15 minutes. To this, 5ml of fresh 3:1 methanol:acetic acid fixing solution was added, the cell suspension was mixed and cells collected by centrifugation. The cell pellet was resuspended in 30ml of 3:1 fixative and pelleted once more. The cell pellet was resuspended in 1ml of 3:1 fixative solution and transferred to a 1.7ml microcentrifuge tube. The cells were pelleted and washed three times with 3:1 fixative before finally resuspending in 0.5ml of 3:1 fixative. Microscope slides were washed in 100% methanol and air dried before placing on a damp paper towel on an inverted 37°C heat block. Droplets of fixed cells were dropped onto the slide from ~30cm. After 2 minutes the slide was dehydrated through 70% and 100% ethanol for 2 minutes each before air-drying.
Immunofluorescence and Fluorescence In Situ Hybridization
For interphase analysis, cells were grown on standard microscope slides overnight, whereas samples for metaphase chromosome analysis were prepared as described above. Media was removed from cells before rinsing briefly with 1x PBS. Cells were fixed for 10 minutes in 1x PBS supplemented with 0.1% (v/v) Triton X-100 and 3.7% formaldehyde, before washing twice in 1x PBS. Samples were blocked for 15–30 minutes in a humidified chamber with 3% bovine serum albumin (BSA) in 1x PBS containing 0.1% (v/v) Tween-20 (PBS-T) overlaid with cover glass. Cover glass were tipped off before rinsing for 2 minutes in 1x PBS. An appropriate dilution of primary antibody/antibodies in PBS-T containing 1% (v/v) BSA were applied to samples followed by cover glass and incubation in a humidified chamber for at least 1 hour. Samples were washed three times for 2 minutes each in 1x PBS before applying a 1:200 dilution of direct-labeled secondary antibodies in PBS-T supplemented with 1% (v/v) BSA. Samples were washed three times for 2 minutes each in 1x PBS before fixing once more as described. For FISH, samples were dehydrated through 70% and 100% ethanol (v/v) for 2 minutes each before air drying. For samples not subjected to FISH, Vectashield with DAPI (Cat. No. H-1200, Vector Laboratories) was applied along with cover glass. SpectrumOrange direct-labeled CEP X human X alpha satellite FISH probe (Cat. No. 32–110023) and WCP-X FISH probe were obtained from Vysis Inc. The CEP-X probe is now available from Abbott Laboratories, but the WCP-X probe is discontinued. FISH was performed according to the manufacturer’s recommendation.
Bacterial artificial chromosomes (BACs) were obtained from the BACPAC Resources Center (Children’s Hospital Oakland Research Institute). Direct-labeled BAC probes were prepared using the Nick translation DNA labeling system 2.0 from Enzo Life Sciences (Ca. No. ENZ-GEN111–0050), according to the manufacturers recommendations. Probes were ethanol precipitated with 25 micrograms of human Cot-1 DNA (Cat. No. 15279011, Thermo Fisher Scientific) before resuspending in Hybrisol VII (Cat. No. 11RIST13901, MP Biomedicals). For interphase immunofluorescence combined with BAC FISH, cells were first immunostained as described above before an additional 15-minute incubation at room temperature in 3:1 fixative followed by 2 minutes each in 70% and 100% ethanol and air drying. Samples were denatured in 70% formamide, 2x SSC [7.0] at 83°C for 10 minutes before incubating at room temperature in 70% and 100% ethanol for 2 minutes each and air-drying. BAC probes were denatured at 70°C for 5 minutes followed by incubation at 37°C for 45 minutes. Probes were applied to the slides with coverglass, sealed with rubber cement and incubated overnight at 37°C in a humidified chamber. Samples were washed at 42°C twice in 50% formamide, 2xSSC for 8 minutes and once in 2xSSC for 8 minutes before applying Vectashield with DAPI. For dropped metaphase chromosome FISH, samples were denatured in 70% formamide, 2x SSC [7.0] at 72°C for 1 minute before incubating at room temperature in 70% and 100% ethanol for 2 minutes each and air-drying. Probes were prepared, applied and washed as above.
Fluorescence Microscopy
Images were collected using one of two different systems. The first used a Zeiss Axiovert 200M fitted with an AxioCam MRm using AxioVision 4.4 software (Carl Zeiss microimaging). The second was a Delta Vision pDV deconvolution system controlled by softWoRx 6.5.2 (GE Healthcare). Images were exported from either system and assembled into figures using Adobe Photoshop CC 2018 (Adobe Systems). Images in Figures 6, 7 and 8 were all taken using the Delta Vision pDV and consist of 1–4 merged 200 nm z-stacks.
Fig 6.
Further characterization of the Xi/Xi’s in GM07213 idic(X) cells. a Distribution of SMCHD1 (green) relative to DAPI (blue). The Xi appears as one large staining body, two adjacent staining bodies or two bodies greater than 5μm apart. b Distribution of H2AK119Ub1 (green) relative to DAPI (blue). c Distribution of H3K4me2 (green) relative to H3K27me3 (mouse monoclonal, red) as well as the merged image of the two (bottom row). The nuclei are counterstained with DAPI (blue). d Additional representative examples of images as described for part-c, in which the intense H3K4me2 foci within the hypo-methylated territory of the Xi is indicated by the white arrow heads. The white bar in each DAPI image is 5μm.
Fig 7.
Location of Xi heterochromatin relative to X centromeres defined by X alpha satellite FISH. a Distribution of H3K27me3 (mouse monoclonal, green) or DAPI (blue) merged with CEP-X (direct-labeled X alpha satellite FISH signal, red). Xi associated FISH signals are indicated by white arrow heads, whereas the white arrow indicates the Xa associated signal. b Examples of tetraploid cells showing the distribution of H3K27me3 (mouse monoclonal, green) or DAPI (blue) merged with CEP-X signals. The white bar in each DAPI image is 5μm.
Fig 8.
Mapping Xi heterochromatin relative to Xq28 of the karyotypically normal X. a Xq23 (green signal indicated by the white arrow-heads) and 154 Mb Xq28 (red signal indicated by the white arrows) FISH signals on metaphase karyotypically normal X and idic(X) counterstained with DAPI (blue). b Same as part-a, but with the 155 Mb Xq28 probe. c Distribution of H2AK119Ub1 (green) or DAPI (blue) merged with the 154 Mb Xq28 FISH signal (red). The white arrow head highlights the FISH signal. d Same as part-c, but with the 155 Mb Xq28 probe. The white bar in each DAPI image is 5μm.
Results and Discussion
Xi characterization
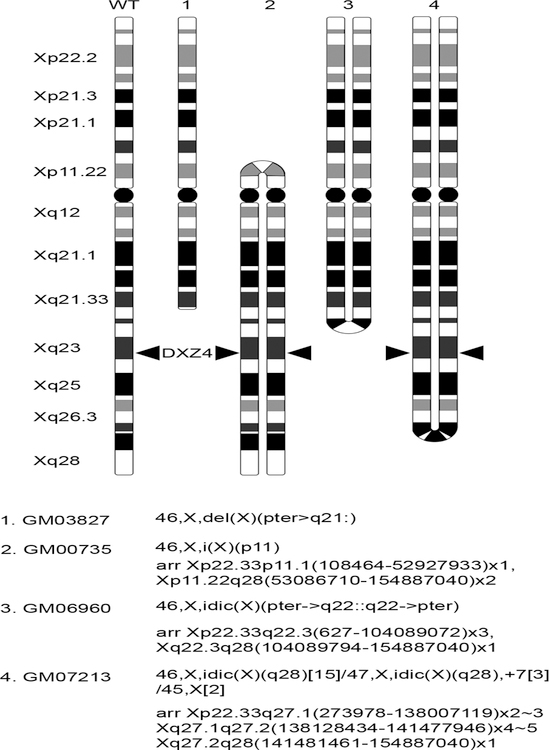
Four primary female cultures derived from individuals with an abnormal X chromosome were assessed. One lacks at least half of Xq (Su et al. 1984), one is a i(X) with duplicate copies of Xq and a small portion of Xp, and the other two are characterized by the presence of a idic(X) (Cohen et al. 1975), that have each been mapped by microarray hybridization to determine copy number variations and approximate break points (Tang et al. 2013) (Fig.1). Using delayed replication timing as a marker of the Xi (Gilbert et al. 1962; Morishma et al. 1962), previous reports of i(X) and idic(X) have found the abnormal X to be the Xi (Artifoni et al. 1983; Baranovskaya et al. 1976; Camargo and Cervenka 1984; Dalton et al. 1998; Dewald et al. 1978; Gaal et al. 1981; Lin and Wilson 1983; Mattei et al. 1977; Mutchinik et al. 1981; Petkovic et al. 2003; Pettigrew et al. 1991; Rack et al. 1994; Sarto and Therman 1980; Takenaka et al. 1988; Yu et al. 1980). Therefore, it was anticipated that the abnormal X’s examined here would all be Xi while the normal X would be the Xa.
Fig 1.
Ideograms representing the rearranged X chromosome in the human cells used in this study. The wild type (WT) X is shown for reference on the far left and major bands on Xp and Xq are indicated. Ideograms are drawn based on information obtained through copy number variation analysis by microarray characterization (Tang et al. 2013). The exception is GM03827 that was defined by cytogenetics (Riddell et al. 1986). The location at Xq23 of the macrosatellite DXZ4 (Giacalone et al. 1992) is indicated where retained or duplicated.
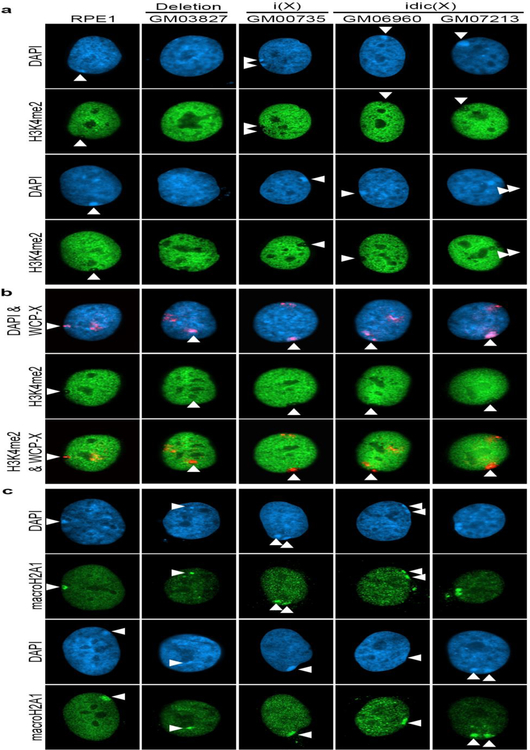
With the exception of a number of genes that escape X chromosome inactivation in humans (Carrel and Willard 2005), most X-linked genes on the chosen Xi are silent, and as such euchromatic chromatin features at the Xi are underrepresented. One such example is histone H3 di-methylated at lysine 4 (H3K4me2) that is largely absent from the Xi (Boggs et al. 2002). The notable exceptions are the pseudoautosomal region that is not dosage compensated (Graves et al. 1998), and the macrosatellite DXZ4, a large tandem repeat centered around Xq23/24 that adopts the opposite chromatin configuration to that of the flanking chromosome (Chadwick 2008; Chadwick and Willard 2002; Giacalone et al. 1992). As such, DXZ4 appears as an intensely staining H3K4me2 foci within the otherwise hypomethylated territory of the Xi (Boggs et al. 2002; Chadwick and Willard 2003). As expected, female cells carrying a normal Xi (RPE1) show a distinct void in the H3K4me2 pattern that encompasses the DAPI dense Barr body, within which is a single intensely-staining foci. Two of the three dicentric samples carry near complete copies of Xq and therefore have two copies of DXZ4 on the abnormal X. As expected, at interphase both samples showed either a single large void with two H3K4me2 foci or two immediately adjacent voids each with their own foci (Fig.2a). In contrast, the cells carrying the Xq deletion and the remaining isodicentric cells only have DXZ4 on their normal X chromosome (Fig.1). For the isodicentric sample, a void corresponding to the DAPI dense Barr body could be observed that lacked the DXZ4 H3K4me2 foci, whereas a void and Barr body were less obvious in the Xq deletion sample (Fig.2a). To validate that what was being interpreted as the Xi was indeed the case, cells were stained for H3K4me2 distribution followed by fluorescence in situ hybridization (FISH) with direct fluorescent labeled whole X chromosome paint (WCP-X). These data confirmed observations for the DXZ4 containing isodicentric chromosomes and highlighted the hypomethylated territory of the X in the cells lacking DXZ4 on the abnormal X (Fig.2b).
Fig 2.
Chromatin characterization of the Xi chromosome mutants. a Indirect immunofluorescence showing the distribution of H3K4me2 (green) in the cells indicated (label at top) relative to the nuclear counterstain DAPI (blue). White arrow heads indicate the location of the Xi. b Distribution of H3K4me2 (green) in the same cells compared to WCP-X FISH signals (red signal merged with the blue DAPI image), revealing the territories of the Xa and Xi. c Indirect immunofluorescence showing the distribution of macroH2A1 (green) relative to DAPI (blue).
To further characterize the Xi, cells were examined for the nuclear distribution of the histone variant macroH2A1 which is enriched at the Xi territory (Costanzi and Pehrson 1998). A clear macrochromatin body (MCB) overlapping the DAPI dense Barr body was observed in all samples, including the Xq deletion cells (Fig.2c). Furthermore, the Xi frequently appeared bipartite in the i(X) and idic(X) samples, with two adjacent MCBs as has been reported previously for other large abnormal X chromosomes using differential staining techniques (Dewald et al. 1978; Maeda et al. 1979; Mutchinik et al. 1981; Sarto and Therman 1980; Therman et al. 1974; Wielie et al. 1964; Yu et al. 1980).
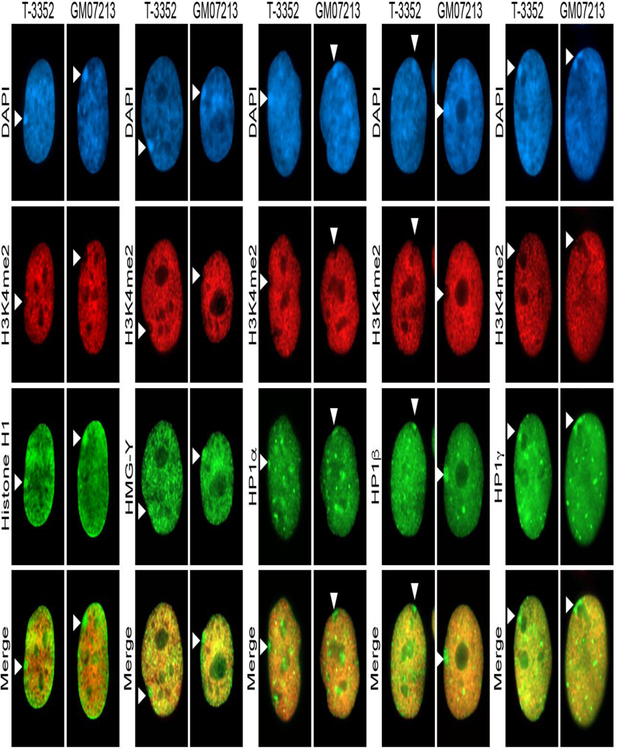
The abnormal X in GM07213 is close to a complete duplicated mirror-image copy of the X with a long arm-to-long arm fusion, and is only monosomy for material absent from Xq28 resulting in a nearly three hundred megabase (Mb) chromosome (Fig.1) accounting for the large Barr body observed in the cells. As such, these cells were characterized for other known markers of the Xi territory. Consistent with observations made in other human female cells (Chadwick and Willard 2003) and for normal primary female cells T-3352 that are examined here, the Xi in GM07213 was clearly enriched for histone H1, high mobility group Y (HMG-Y) and all three human heterochromatin protein isoforms: HP1α, HP1β and HP1γ with the distribution of each largely not aligning with the nuclear distribution of H3K4me2 and obvious enrichment within the Xi void (Fig.3).
Fig 3.
Further characterization of Xi chromatin in the idic(X) cells GM07213 relative to the Xi in wild type cells (T-3352). Indirect immunofluorescence shows the distribution of H3K4me2 (red, second row) relative to the indicated chromatin protein (green, third row), both of which are merged in the bottom row. The nuclear DNA is counterstained with DAPI (blue, top row). White arrow heads indicate the location of the Xi.
Metaphase Xi analysis
The H3K4me2 hypomethylation at the Xi is most obvious at metaphase (Boggs et al. 2002; Chadwick and Willard 2002). Therefore, the distribution of H3K4me2 at the Xi was examined in the i(X) and idic(X) cells. As expected, the large metaphase abnormal hypomethylated X was readily detected in both GM06960 and GM07213 (Fig.4a), with the obvious difference being that GM07213 that extends as far as Xq28 was characterized by bands of H3K4me2 at its’ two DXZ4 loci (Fig.4b), whereas GM06960 that lacks DXZ4 on the abnormal X as it only extends into Xq as far as Xq22 (Fig.1), lacks these signals (Fig. 4a). Unlike idic(X) where centromeres are physically close and can both remain active (Sullivan and Willard 1998), the centromeres in GM06960 and GM07213 are physically distant (Fig.1). Consistent with inactivation of one of the two centromeres for stable retention of the intact chromosome (Stimpson et al. 2012), only one primary constriction was observed (Fig.4b) despite the distortion cytocentrifugation has on metaphase chromosome appearance, consistent with the idic(X) chromosomes being pseudodicentric.
Fig 4.
Metaphase chromosome analysis of the i(X) (GM00735) and idic(X) (GM06960 and GM07213) samples. a Metaphase chromosome spreads showing the banding pattern of H3K4me2 (green) merged with DAPI (blue) for the i(X) and idic(X) cells indicated. The white arrows indicate the location of the Xi. b Additional examples of the idic(X) Xi chromosome from GM07213 showing H3K4me2 banding (green) merged with DAPI (blue). c Banding pattern of macroH2A1 (green) merged with DAPI (blue) at the Xi in the i(X) (GM00735) and idic(X) (GM06960 and GM07213) samples. To the right of each pair of images is a schematic drawing of the expected banding pattern based on the Xi structure as predicted from the expected WT pattern (far left) (Chadwick 2007; Chadwick and Willard 2002; Chadwick and Willard 2004). d Metaphase chromosome spreads of GM07213 showing the banding of H3K9me3 (green) merged with DAPI (blue) and the X alpha satellite hybridizing signal (red). The location of the normal Xa and idic(X) Xi are indicated. e Examples of H3K9me3 banding (green) merged with DAPI (blue) for the idic(X) Xi in GM07213. The red signals indicate the location of both centromeres (X alpha satellite). f Examples of H3K9me3 banding (green) merged with DAPI (blue) for the karyotypically normal Xa in GM07213. The red signal indicated the location of the X alpha satellite. g Metaphase idic(X) showing the banding pattern of H3K9me3 (green) merged with DAPI (blue) and centromeres (red). The banding pattern on the top half appears to more closely resemble the Xa pattern, whereas the pattern on the bottom half more closely resembles the Xi pattern.
The human Xi at metaphase is characterized by reproducible bands of macroH2A1 (Chadwick 2007; Chadwick and Willard 2002; Chadwick and Willard 2004). Knowing the structure of the i(X) and two idic(X) (Fig.1), how these bands might appear at metaphase can be predicted, and were generally consistent in all three cultures, generating near mirror-imaged banding patterns (Fig.4c). In some human cells, the inter-macroH2A1 bands are characterized by the presence of histone H3 trimethylated at lysine 9 (H3K9me3) (Chadwick and Willard 2004), although this heterochromatin marker is not as consistent between cell lines and cell types as macroH2A1 (Chadwick 2007). Metaphase GM07213 were stained with H3K9me3 followed by FISH using an X alpha satellite probe (CEP-X) to allow identification of the normal X in addition to the idic(X) chromosome. Consistent with what was previously observed for primary fibroblasts (Chadwick 2007), the abnormal Xi showed major bands of H3K9me3 at distal Xp and coincident with the CEP-X signal, with relatively little signal along the chromosome arms (Fig.4d and e), whereas the normal X, and assumed Xa, showed an additional major H3K9me3 band on Xq with occasionally a minor more distal band and signal at the telomeric region of Xq (Fig.4d and f). These immunofluorescence data are consistent with the abnormal X in GM07213 being the Xi and the normal X being the Xa (Carrel and Willard 1999). Interestingly, the H3K9me3 signal at the abnormal X occasionally appeared less symmetrical, with the signal on the arm with the primary constriction more intense than the opposite end of the chromosome (See left panel in Fig.4e). This observation is consistent with numerous reports in which late replication patterns on idic(X) are not always symmetrical (Baranovskaya et al. 1976; Gaal et al. 1981; Latt et al. 1976; Lin and Wilson 1983; Mutchinik et al. 1981; Yu et al. 1980) with bias of late replication in such cases more toward the primary constriction (Lin and Wilson 1983). Heterochromatin bands on the Xi align remarkably well with the timing at which the underlying DNA replicates, with bands of macroH2A1 replicating in mid to late S-phase followed by the interbands (or H3K9me3 bands in some case) replicating in late S-phase (Chadwick and Willard 2004) and therefore the presence or absence of heterochromatin bands likely impacts the replication timing/banding of the underlying DNA. Most unexpected was that one GM07213 metaphase idic(X) chromosome showed bands of H3K9me3 on one side of the chromosome, that more closely resembled those seen for the normal Xa (Fig.4g). This could simply reflect banding heterogeneity. However, a not completely inconceivable interpretation could be that half the chromosome is active and the other half inactive and the normal X also inactive in this cell. As such, cells were examined more closely for this possibility. For most idic(X), regions of monosomy X are extensive and therefore selecting the normal X and half of the abnormal X to be inactive would likely be inviable, but given how little of the X is monosomic in GM07213, this was worthy of further exploration.
Interphase bipartite Xi analysis in GM07213
The appearance of a bipartite Barr body (defined as visually separate heterochromatin masses), is not uncommon in abnormal large Xi (Dewald et al. 1978; Maeda et al. 1979; Mutchinik et al. 1981; Sarto and Therman 1980; Therman et al. 1974; Wielie et al. 1964; Yu et al. 1980). Indeed, in one X isochromosome study of 45,X/46,X,idic(X)(p22.3::p22.3) cells, almost 25% of Barr bodies were bipartite and sometimes separated by a distance at or greater that the width of one half of the Barr body (Mutchinik et al. 1981). This X is comparable in size to the abnormal X in GM07213. GM07213 cells were re-stained with other robust Xi markers, examining H3K27me3 distribution (Plath et al. 2003; Silva et al. 2003) (Fig.5a) and macroH2A2 (Chadwick and Willard 2001; Costanzi and Pehrson 2001) (Fig.5b). Based on nuclear size and number of Xi signals, 3.8% of cells were deemed to be polyploid, whereas 67.9% of cells showed a single large Xi mass, and 28.3% showed a bipartite signal (n=1463 nuclei). However, in less than 0.1% of cells, the distance between the two Xi signals in a bipartite cell was greater than 5μm (See bottom right macroH2A2 example in Fig.5b). Such a phenomenon where the center of the two halves of a bipartite Barr body were separated by greater than 5μm, could be reproduced using other known markers of the Xi including structural maintenance of chromosomes flexible hinge domain containing 1 (SMCHD1) (Blewitt et al. 2008) (Fig.6a) or histone H2A monoubiquitylated at lysine 119 (H2AK119Ub1) (de Napoles et al. 2004; Fang et al. 2004; Smith et al. 2004) (Fig.6b). Most often the Xi appeared as one large mass or when bipartite, the two halves were relatively close consistent with what was observed for H3K27me3 and macroH2A2 (Fig.5). The appearance of well separated Xi signals remained very rare (<0.1%: n=3000 SMCHD1, n=4000 H2AK119Ub1).
Fig 5.
Dual Barr body formation in GM07213 idic(X) cells. a Distribution of H3K27me3 (red, rabbit polyclonal), and b macroH2A2 (red) relative to DAPI (blue). The two intensely staining macrochromatin bodies show the formation of heterochromatin territories for the Xi.
The presence of cells that have selected the normal X and one half of the abnormal X as Xi would be highly unusual. An alternative explanation for what was being observed at interphase could be that these are simply tetraploid cells and the distant Barr bodies, reflect two spatially separate idic(X) heterochromatin masses. To address this, several complementary approaches were taken. First, cells were stained with H3K27me3 and H3K4me2 (Fig.6c). The H3K27me3 defines the location of the Xi territory (Plath et al. 2003; Silva et al. 2003) and clearly distinguishes bipartite from a singular heterochromatin body. The H3K4me2 defines the void of the X and the DXZ4 intense foci assists in determining if a cell is tetraploid depending on the ratio of foci to Barr bodies. Using this approach, the vast majority of cells showed either a single large H3K27me3 heterochromatin body with two H3K4me2 foci, or two H3K27me3 bodies the centers of which were within 5μm and were accompanied by a lone H3K4me2 foci each. H3K27me3 heterochromatin bodies separated by greater than 5μm defined by a single H3K4me2 foci were identified but at very low frequency. Tetraploid cells were obvious based on nuclear size and H3K4me2 foci number (data not shown). In an attempt to better quantitate the incidence of the well separated Xi heterochromatin masses, cells were stained two additional times for the distribution of H3K4me2 and H3K27me3. Out of 5,153 nuclei, five showed heterochromatin bodies separated by at least 5μm that resided within the hypo-H3K4me2 territory characterized by the presence of a single intense foci, confirming the rare appearance of the cells (<0.1%; Fig.6d).
As a complement to this approach, H3K27me3 immunofluorescence was coupled with CEP-X FISH (Fig.7a). Like the H3K7me3 and H3K4me2 immunofluorescence (Fig.6c,d), most cells possessed a large H3K27me3 heterochromatin body coincident with two CEP-X FISH signals and a third spatially distinct CEP-X signal, or two closely apposed heterochromatin bodies, each associated with a CEP-X signal and a spatially separated CEP-X signal elsewhere in the nucleus (Fig.7a, left panels). On rare occasion, nuclei were identified that showed two H3K27me3 heterochromatin bodies separated by at least 5μm from their centers, each associated with a CEP-X signal, but the third CEP-X signal was closely associated with one of the two heterochromatin bodies (Fig.7a, right panels). Once again, tetraploid cells were easy to recognize based on nuclear size and the number of CEP-X signals (Fig.7b).
To strengthen these observations, a second immuno-FISH approach was used. The idic(X) in GM07213 lacks Xq28, whereas the structurally normal X does not (Tang et al. 2013) (Fig.1). To confirm this, two Xq28 specific BAC probes were prepared. The first probe consists of a single BAC clone (RP13–824O4) that contains H2AFB1, F8 and F8A1 at 154 Mb (hg38). The second probe consists of a contig of three overlapping BACs (RP13–905N17, RP13–933C9 andRP13–968D14) centered at 155 Mb (hg38) that contain the H2AFB2 and/or H2AFB3. FISH was performed on dropped metaphase chromosomes using the 154 Mb or 155 Mb probe alongside a BAC probe (RP11–1066D24) from Xq23 containing the RTL4 gene at 112 Mb (hg38). The expectation was that the normal X would show signals for the Xq23 and Xq28 probes, whereas the idic(X) would only show two hybridizing bands for the Xq23 probe, which was indeed the case (Fig.8a,b). As with the cytospun metaphase chromosomes, the dropped metaphase idic(X) shows a single primary construction, consistent with being pseudodicentric. While the CEP-X FISH probes could not distinguish between the normal and abnormal X chromosomes, the Xq28 probe would be specific to the normal X. Therefore, cells were stained for H2AK119Ub1 distribution, followed by FISH with the 154 Mb or the 155 Mb probes.
Using the 154 Mb probe, most cells showed a single FISH signal that was physically separated from the single or bipartite Xi heterochromatin masses (Fig.8c, top row). However, in a small number of nuclei, the FISH probe associated with one half of the bipartite Xi (Fig.8c, bottom row). Consistent with this observation, the same trend was observed when using the 155 Mb probe (Fig.8d). Notably, the FISH signal was generally observed immediately abutting or partially overlapping the heterochromatin mass. However, this was not unexpected, as Xq28 is not enriched for the cytologically obvious H2AK119Ub1 facultative heterochromatin bands (Fig.4c), and instead resides within the inter-band territory that is characterized by H3K9me3 in some female cells (Chadwick and Willard 2004). FISH probes from this interval remain physically close to the H2AK119Ub1 territory but do not typically overlap (Chadwick 2007).
Collectively, these data corroborate the frequent formation of bipartite Xi by large i(X) and idic(X) through the use of multiple well established Xi markers, and that almost exclusively the abnormal X is the Xi. Nevertheless, these data also raise the possibility that near full-length idic(X) have the potential to exist as a hybrid with one half as the Xa and the other as the Xi alongside inactivation of the karyotypically normal X. It is highly likely that i(X) and idic(X) that retain the X inactivation center (XIC) (Pettigrew et al. 1991) are dealt with in the same way that XIC are in supernumerary X cells (Skuse et al. 2018). At the time that X chromosome inactivation takes place, the number of X chromosomes are counted and a random choice is made as to which X will remain active and all others are subsequently silenced. In the case of idic(X), how much X material will be monosomic depends on where the break occurs. The more material that is monosomy the less viable cells will be that silence XIST at an XIC on half of the abnormal conjoined X, as well as silencing the remaining half of the abnormal X and the entire normal X. Conceivably, an idic(X) that consists of two complete or near complete copies of the X may not be at such a selective disadvantage that cells which choose an XIC on the isochromosome to be the Xa are not completely lost. The rarity of such potential cells described here and the lack of previous reports on their existence by others suggests that cells that do make such a choice are disadvantaged. Rare unexpected irregularities with dicentric X chromosome behavior is not new to this report, others have described a situation whereby at low frequency, the dicentric X DNA replication occurred simultaneously with the normal X (Lin and Wilson 1983). One alternative explanation for the rare observation reported here could reflect reactivation of the silenced centromere, resulting in chromosome breakage and that perhaps the two spatially distinct heterochromatin bodies reflect such an occurrence. However, why a second non-H3K27me3 CEP-X FISH signal would be juxtaposed to an Xi FISH signal is more fitting of a physical connection between the two. If the hybrid Xa-Xi interpretation of the data is indeed true, that an extremely low number of cells in GM07213 carry an idic(X) that is active on one half and inactive at the other, then one would predict that such an occurrence would be restricted to cells from individuals who carry an idic(X) that harbor a near complete mirror image of the X chromosome. The ramifications to those GM07213 cells that are derived from a ancestral cell that may have made this choice would be that by silencing half of the idic(X) and the normal X chromosome would mean that genes from Xq28 that are absent from the idic(X) would be silent, which is not an insignificant number of genes. However, it is possible that some cells may survive if silencing of Xq28 on the normal X is compromised which may reflect the low cell numbers observed here that appear to be descendants of a cell that made such a choice. However, if the rare cells observed here that show well separated heterochromatin masses are actually the two heterochromatic halves of the idic(X) and not a hybrid Xa-Xi idic(X), then the extreme distances between the physically attached bipartite chromosome territories are remarkable. Nevertheless, the data presented here does not rule out the intriguing possibility that these cells actually represent rare instances in which the idic(X) is an Xa-Xi hybrid.
Acknowledgements
I am grateful to Dr. Huntington Willard for continued use of the cells used in this study. I thank Dr. Stuart Schwartz for the provision of T-3352 cells. This work was supported by the National Institute of General Medical Sciences at the National Institutes of Health [grant number GM117003 to B.P.C.]
Abbreviations
- ATCC
American Type Culture Collection
- BSA
Bovine serum albumin
- BAC
Bacterial artificial chromosome
- CEP-X
X alpha satellite centromere probe
- DAPI
4,6-diaminidino-2-phenylindole
- FBS
Fetal bovine serum
- FISH
Fluorescence in situ hybridization
- H2AK119Ub1
Histone H2A mono ubiquitylated at lysine 119
- H3K4me2
Histone H3 di-methylated at lysine 4
- H3K9me3
Histone H3 tri-methylated at lysine 9
- H3K27me3
Histone H3 tri-methylated at lysine 27
- HMG-Y
High mobility group protein Y
- HP1
Heterochromatin protein 1
- idic(X)
Isodicentric X chromosome
- i(X)
Isochromosome X
- MCB
Macrochromatin body
- Mb
Megabase
- PBS
Phosphate buffered saline
- PBS-T
Phosphate buffered saline supplemented with Tween-20
- SMCHD1
Structural maintenance of chromosomes flexible hinge domain containing 1
- WCP-X
X whole chromosome paint
- WT
Wild type
- Xa
Active X chromosome
- Xi
Inactive X chromosome
- XIC
X inactivation center
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
The author declares that they do not have any conflicts of interest.
References
- Artifoni L, Baccichetti C, Piovan E, Anglani F, Lenzini E, Goppion G, Tenconi R (1983) Replication patterns of human X isochromosomes by high-resolution banding Cytogenet Cell Genet 36:649–651 doi: 10.1159/000131989 [DOI] [PubMed] [Google Scholar]
- Baranovskaya LI, Egolina NA, Zakharov AF, Tsvetkova TG (1976) Isochromosome X in man: different DNA replication patterns in the long arms Hum Genet 33:55–60 doi: 10.1007/bf00447286 [DOI] [PubMed] [Google Scholar]
- Barr ML (1966) Correlations between sex chromatin patterns and sex chromosome complexes in man. In: Moore KL (ed) The Sex Chromatin. W. B. Saunders Company, Philadelphia, PA, pp 129–161 [Google Scholar]
- Barr ML, Bertram EG (1949) A morphological distinction between neurones of the male and female, and the behaviour of the nucleolar satellite during accelerated nucleoprotein synthesis Nature 163:676–677 [DOI] [PubMed] [Google Scholar]
- Blewitt ME et al. (2008) SmcHD1, containing a structural-maintenance-of-chromosomes hinge domain, has a critical role in X inactivation Nat Genet 40:663–669 doi: 10.1038/ng.142 [DOI] [PubMed] [Google Scholar]
- Bodnar AG et al. (1998) Extension of life-span by introduction of telomerase into normal human cells Science 279:349–352 [DOI] [PubMed] [Google Scholar]
- Boggs BA, Cheung P, Heard E, Spector DL, Chinault AC, Allis CD (2002) Differentially methylated forms of histone H3 show unique association patterns with inactive human X chromosomes Nat Genet 30:73–76. [DOI] [PubMed] [Google Scholar]
- Caine A, Mason G, Daly HA, Ricketts SM (1993) An unusual tricentric X chromosome detected prenatally Prenat Diagn 13:1061–1065 [DOI] [PubMed] [Google Scholar]
- Camargo M, Cervenka J (1984) DNA replication and inactivation patterns in structural abnormality of sex chromosomes. I.X-A translocations, rings, fragments, isochromosomes, and pseudo-isodicentrics Hum Genet 67:37–47 doi: 10.1007/bf00270556 [DOI] [PubMed] [Google Scholar]
- Carrel L, Willard HF (1999) Heterogeneous gene expression from the inactive X chromosome: an X-linked gene that escapes X inactivation in some human cell lines but is inactivated in others Proc Natl Acad Sci U S A 96:7364–7369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel L, Willard HF (2005) X-inactivation profile reveals extensive variability in X-linked gene expression in females Nature 434:400–404 [DOI] [PubMed] [Google Scholar]
- Chadwick BP (2007) Variation in Xi chromatin organization and correlation of the H3K27me3 chromatin territories to transcribed sequences by microarray analysis Chromosoma 116:147–157 [DOI] [PubMed] [Google Scholar]
- Chadwick BP (2008) DXZ4 chromatin adopts an opposing conformation to that of the surrounding chromosome and acquires a novel inactive X-specific role involving CTCF and antisense transcripts Genome Res 18:1259–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick BP, Willard HF (2001) Histone H2A variants and the inactive X chromosome: identification of a second macroH2A variant Hum Mol Genet 10:1101–1013. [DOI] [PubMed] [Google Scholar]
- Chadwick BP, Willard HF (2002) Cell cycle-dependent localization of macroH2A in chromatin of the inactive X chromosome J Cell Biol 157:1113–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick BP, Willard HF (2003) Chromatin of the Barr body: histone and non-histone proteins associated with or excluded from the inactive X chromosome Hum Mol Genet 12:2167–2178 [DOI] [PubMed] [Google Scholar]
- Chadwick BP, Willard HF (2004) Multiple spatially distinct types of facultative heterochromatin on the human inactive X chromosome Proc Natl Acad Sci U S A 101:17450–17455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MM, Rosenmann A, Hacham-Zadeh S, Dahan S (1975) Dicentric X-isochromosome (Xqi dic) and pericentric inversion of No. 2 [inv(2) (p15 q21)] in a patient with gonadal dysgenesis Clin Genet 8:11–17 doi: 10.1111/j.1399-0004.1975.tb01948.x [DOI] [PubMed] [Google Scholar]
- Costanzi C, Pehrson JR (1998) Histone macroH2A1 is concentrated in the inactive X chromosome of female mammals Nature 393:599–601. [DOI] [PubMed] [Google Scholar]
- Costanzi C, Pehrson JR (2001) MacroH2A2, a new member of the MacroH2A core histone family J Biol Chem 276:21776–21784. [DOI] [PubMed] [Google Scholar]
- Dalton P, Coppin B, James R, Skuse D, Jacobs P (1998) Three patients with a 45,X/46,X,psu dic(Xp) karyotype J Med Genet 35:519–524 doi: 10.1136/jmg.35.6.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow EM et al. (2016) Deletion of DXZ4 on the human inactive X chromosome alters higher-order genome architecture Proc Natl Acad Sci U S A 113:E4504–4512 doi: 10.1073/pnas.1609643113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Chapelle A, Wennstrom J, Hortling H, Ockey CH (1966) Isochromosome-X in man. I Hereditas 54:260–276 doi: 10.1111/j.1601-5223.1966.tb02021.x [DOI] [PubMed] [Google Scholar]
- de Napoles M et al. (2004) Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation Dev Cell 7:663–676 [DOI] [PubMed] [Google Scholar]
- Dewald G, Spurbeck JL, Gordon H (1978) Replication patterns of three isodicentric X chromosomes and an X isochromosome in human lymphocytes Am J Med Genet 1:445–460 doi: 10.1002/ajmg.1320010407 [DOI] [PubMed] [Google Scholar]
- Disteche C, Hagemeijer A, Frederic J, Progneaux D (1972) An abnormal large human chromosome identified as an end-to-end fusion of two X’s by combined results of the new banding techniques and microdensitometry Clin Genet 3:388–395 doi: 10.1111/j.1399-0004.1972.tb01472.x [DOI] [PubMed] [Google Scholar]
- Fang J, Chen T, Chadwick B, Li E, Zhang Y (2004) Ring1b-mediated H2A ubiquitination associates with inactive X chromosomes and is involved in initiation of X inactivation J Biol Chem 279:52812–52815 [DOI] [PubMed] [Google Scholar]
- Ford CE, Jones KW, Polani PE, De Almeida JC, Briggs JH (1959) A sex-chromosome anomaly in a case of gonadal dysgenesis (Turner’s syndrome) Lancet 1:711–713 doi: 10.1016/s0140-6736(59)91893-8 [DOI] [PubMed] [Google Scholar]
- Gaal M, Laszlo J, Bosze P (1981) Cytogenetic investigation of six patients with X isochromosomes, i(Xq), and of two subjects with isodicentric X chromosomes, idic (Xq) Hum Genet 58:362–365 doi: 10.1007/bf00282816 [DOI] [PubMed] [Google Scholar]
- Giacalone J, Friedes J, Francke U (1992) A novel GC-rich human macrosatellite VNTR in Xq24 is differentially methylated on active and inactive X chromosomes Nat Genet 1:137–143. [DOI] [PubMed] [Google Scholar]
- Gilbert CW, Muldal S, Lajthal LG, Rowley J (1962) Time-sequence of human chromosome duplication Nature 195:869–873. [DOI] [PubMed] [Google Scholar]
- Graves JA, Wakefield MJ, Toder R (1998) The origin and evolution of the pseudoautosomal regions of human sex chromosomes Hum Mol Genet 7:1991–1996 doi:ddb250 [pii] [DOI] [PubMed] [Google Scholar]
- Held KR, Kerber S, Kaminsky E, Singh S, Goetz P, Seemanova E, Goedde HW (1992) Mosaicism in 45,X Turner syndrome: does survival in early pregnancy depend on the presence of two sex chromosomes? Hum Genet 88:288–294 doi: 10.1007/bf00197261 [DOI] [PubMed] [Google Scholar]
- Hook EB, Warburton D (1983) The distribution of chromosomal genotypes associated with Turner’s syndrome: livebirth prevalence rates and evidence for diminished fetal mortality and severity in genotypes associated with structural X abnormalities or mosaicism Hum Genet 64:24–27 doi: 10.1007/bf00289473 [DOI] [PubMed] [Google Scholar]
- Jacobs P, Dalton P, James R, Mosse K, Power M, Robinson D, Skuse D (1997) Turner syndrome: a cytogenetic and molecular study Ann Hum Genet 61:471–483 doi: 10.1046/j.1469-1809.1997.6160471.x [DOI] [PubMed] [Google Scholar]
- Latt SA, Willard HF, Gerald PS (1976) BrdU-33258 Hoechst analysis of DNA replication in human lymphocytes with supernumerary or structurally abnormal X chromosomes Chromosoma 57:135–153 [DOI] [PubMed] [Google Scholar]
- Lin MS, Wilson MG (1983) The sequence of DNA replication in an iso-dicentric X-chromosome in peripheral blood lymphocytes and skin fibroblasts from the same individual Hum Genet 65:139–143 doi: 10.1007/bf00286650 [DOI] [PubMed] [Google Scholar]
- Lyon MF (1961) Gene action in the X-chromosome of the mouse (Mus musculus L.) Nature 190:372–373. [DOI] [PubMed] [Google Scholar]
- Lyon MF (2002) X-chromosome inactivation and human genetic disease Acta Paediatr Suppl 91:107–112 [DOI] [PubMed] [Google Scholar]
- Maeda T, Ohno M, Takada M, Nishida M, Tsukioka K, Tomita H (1979) Turner’s syndrome with a duplication-deficiency X chromosome derived from a maternal pericentric inversion X chromosome Clin Genet 15:259–266 doi: 10.1111/j.1399-0004.1979.tb00977.x [DOI] [PubMed] [Google Scholar]
- Mattei JF, Taramasco H, Mattei MG, Lucas C, Aubert L, Giraud F (1977) A girl with mosaicism for a dicentric X chromosome (45,X/46,X,dic(X) (Xqter to p22::p22 to qter)) Hum Genet 38:39–48 doi: 10.1007/bf00295806 [DOI] [PubMed] [Google Scholar]
- Morishma A, Grumbach MM, Taylor JH (1962) Asynchronous duplication of human chromosomes and the origin of sex chromatin Proc Natl Acad Sci USA 48:756–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutchinik O, Casas L, Ruz L, Lisker R, Lozano O (1981) Symmetrical replication patterns and sex chromatin bodies formation of an idic(X)(p22.3::p22.3) chromosome Hum Genet 57:261–264 doi: 10.1007/bf00278940 [DOI] [PubMed] [Google Scholar]
- Ockey CH, Wennstrom J, De la Chapelle A (1966) Isochromosome-X in man. II Hereditas 54:277–292 doi: 10.1111/j.1601-5223.1966.tb02022.x [DOI] [PubMed] [Google Scholar]
- Otter M, Schrander-Stumpel CT, Curfs LM (2010) Triple X syndrome: a review of the literature Eur J Hum Genet 18:265–271 doi: 10.1038/ejhg.2009.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CG, Reichmann A (1976) Chromosomal and clinical findings in 110 females with Turner syndrome Hum Genet 35:35–49 doi: 10.1007/bf00295617 [DOI] [PubMed] [Google Scholar]
- Petkovic I, Barisic I, Bago R (2003) Cytogenetic evaluation, fluorescence in situ hybridization, and molecular study of psu idic(X)(pter-->q22.3::q22.3-->pter) chromosome abberation in a girl with moderate growth retardation Croat Med J 44:494–499 [PubMed] [Google Scholar]
- Pettigrew AL, McCabe ER, Elder FF, Ledbetter DH (1991) Isodicentric X chromosome in a patient with Turner syndrome--implications for localization of the X-inactivation center Hum Genet 87:498–502 doi: 10.1007/bf00197176 [DOI] [PubMed] [Google Scholar]
- Plath K et al. (2003) Role of histone H3 lysine 27 methylation in X inactivation Science 300:131–135 [DOI] [PubMed] [Google Scholar]
- Rack KA et al. (1994) Absence of the XIST gene from late-replicating isodicentric X chromosomes in leukaemia Hum Mol Genet 3:1053–1059 doi: 10.1093/hmg/3.7.1053 [DOI] [PubMed] [Google Scholar]
- Ranke MB, Pfluger H, Rosendahl W, Stubbe P, Enders H, Bierich JR, Majewski F (1983) Turner syndrome: spontaneous growth in 150 cases and review of the literature Eur J Pediatr 141:81–88 doi: 10.1007/bf00496795 [DOI] [PubMed] [Google Scholar]
- Riddell DC et al. (1986) Regional localization of 18 human X-linked DNA sequences Cytogenet Cell Genet 42:123–128 doi: 10.1159/000132264 [DOI] [PubMed] [Google Scholar]
- Sarto GE, Therman E (1980) Replication and inactivation of a dicentric X formed by telomeric fusion Am J Obstet Gynecol 136:904–911 doi: 10.1016/0002-9378(80)91049-2 [DOI] [PubMed] [Google Scholar]
- Silva J et al. (2003) Establishment of histone h3 methylation on the inactive x chromosome requires transient recruitment of eed-enx1 polycomb group complexes Dev Cell 4:481–495 [DOI] [PubMed] [Google Scholar]
- Skuse D, Printzlau F, Wolstencroft J (2018) Sex chromosome aneuploidies Handb Clin Neurol 147:355–376 doi: 10.1016/B978-0-444-63233-3.00024-5 [DOI] [PubMed] [Google Scholar]
- Smith KP, Byron M, Clemson CM, Lawrence JB (2004) Ubiquitinated proteins including uH2A on the human and mouse inactive X chromosome: enrichment in gene rich bands Chromosoma 113:324–335 [DOI] [PubMed] [Google Scholar]
- Stimpson KM, Matheny JE, Sullivan BA (2012) Dicentric chromosomes: unique models to study centromere function and inactivation Chromosome research : an international journal on the molecular, supramolecular and evolutionary aspects of chromosome biology 20:595–605 doi: 10.1007/s10577-012-9302-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TS, Nussbaum RL, Airhart S, Ledbetter DH, Mohandas T, O’Brien WE, Beaudet AL (1984) Human chromosomal assignments for 14 argininosuccinate synthetase pseudogenes: cloned DNAs as reagents for cytogenetic analysis Am J Hum Genet 36:954–964 [PMC free article] [PubMed] [Google Scholar]
- Sullivan BA, Willard HF (1998) Stable dicentric X chromosomes with two functional centromeres Nat Genet 20:227–228 doi: 10.1038/3024 [DOI] [PubMed] [Google Scholar]
- Takenaka M, Teramoto H, Ueda K, Fujiwara A, Ohama K, Nishi Y (1988) A case of 45,X/46,X,dic(X)(qter----p22::p22----qter) with Turner’s phenotype in a Japanese girl Hiroshima J Med Sci 37:51–55 [PubMed] [Google Scholar]
- Tang Z et al. (2013) A dynamic database of microarray-characterized cell lines with various cytogenetic and genomic backgrounds G3 3:1143–1149 doi: 10.1534/g3.113.006577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therman E, Sarto GE, Patau K (1974) Center for Barr body condensation on the proximal part of the human Xq: a hypothesis Chromosoma 44:361–366 [DOI] [PubMed] [Google Scholar]
- Turner HH (1938) A syndrome of infantilism, congenital webbed neck, and cubitus valgus Endocrinology 23:566–574 [PubMed] [Google Scholar]
- Wielie G, Coenegracht JM, Stalder G (1964) A Very Large Metacentric Chromosome in a Woman with Symptoms of Turner’s Syndrome Cytogenetics 3:427–440 [DOI] [PubMed] [Google Scholar]
- Willard HF (1977) Tissue-specific heterogeneity in DNA replication patterns of human X chromosomes Chromosoma 61:61–73 [DOI] [PubMed] [Google Scholar]
- Willard HF, Latt SA (1976) Analysis of deoxyribonucleic acid replication in human X chromosomes by fluorescence microscopy Am J Hum Genet 28:213–227 [PMC free article] [PubMed] [Google Scholar]
- Wolff DJ, Miller AP, Van Dyke DL, Schwartz S, Willard HF (1996) Molecular definition of breakpoints associated with human Xq isochromosomes: implications for mechanisms of formation Am J Hum Genet 58:154–160 [PMC free article] [PubMed] [Google Scholar]
- Yu CW, Chen H, Morrison J (1980) Kinetics of DNA replication in a dicentric X chromosome formed by long arm to long arm fusion Hum Genet 56:71–79 doi: 10.1007/bf00281572 [DOI] [PubMed] [Google Scholar]