Abstract
Objective:
Prior studies investigating hospital mechanical ventilation (MV) volume-outcome associations have had conflicting findings. Volume-outcome relationships within contemporary MV practices are unclear. We sought to determine associations between hospital MV volume and patient outcomes.
Design:
Retrospective cohort study.
Setting:
The California Patient Discharge Database 2016.
Patients:
Adult non-surgical patients receiving MV.
Interventions:
The primary outcome was hospital death with secondary outcomes of tracheostomy and 30-day readmission. We used multivariable generalized estimating equations to determine the association between patient outcomes and hospital MV volume quartile.
Measurements and Main Results:
We identified 51,689 patients across 274 hospitals who required MV in California in 2016. 38.2% of patients died in the hospital with 4.4% receiving a tracheostomy. Among survivors, 29.5% required readmission within 30 days of discharge. Patients admitted to high vs low volume hospitals had higher odds of death (Quartile 4 vs Quartile 1 adjusted OR=1.40, 95% CI 1.17–1.68) and tracheostomy (Quartile 4 vs Quartile 1 adjusted OR=1.58, 95% CI 1.21–2.06). However, odds of 30-day readmission among survivors was lower at high vs low volume hospitals (Quartile 4 vs Quartile 1 adjusted OR=0.77, 95% CI 0.67–0.89). Higher hospital MV volume was weakly correlated with higher hospital risk-adjusted mortality rates (ρ=0.16, p=0.008). These moderately strong observations were supported by multiple sensitivity analyses.
Conclusions:
Contrary to previous studies, we observed worse patient outcomes at higher MV volume hospitals. In the setting of increasing use of MV and changes in MV practices, multiple mechanisms of worse outcomes including resource strain are possible. Future studies investigating differences in processes of care between high and low volume hospitals are necessary.
Keywords: mechanical ventilation, hospital volume, mortality, tracheostomy, health services, respiratory failure, hospital readmission
Introduction
Hospital volume–outcome relationships have garnered significant attention based on the theory that ‘practice makes perfect’. Researchers have demonstrated improved outcomes at high volume hospitals for several surgical procedures and several critical care conditions.1–5 Positive hospital volume relationships have driven regionalization of complex surgical care, but debate continues regarding the benefits of regionalization of critical care practices.6–10
Invasive mechanical ventilation (MV) has been a particular case of interest for volume-outcome relationships. MV is a common yet complex process of care that involves coordination across multiple specialties (physicians, nurses, respiratory therapists, etc.). Greater experience may lead to improved outcomes or greater numbers can strain limited hospital resources. Studies analyzing hospital MV volume relationships, many of which used data more than a decade old, have arrived at conflicting conclusions about the volume-outcome relationship. Although several studies demonstrated improved outcomes at higher volume hospitals, others suggested no volume-outcome association for MV.11–14
MV practices have changed significantly since the studies evaluating MV volume-outcome. In the setting of shifts in MV practices towards lung protective ventilation, decreasing MV mortality, increasing utilization of long-term ventilation, and large increases in advanced directives limiting aggressive care, we sought to determine contemporary associations between hospital MV volume and patient-centered outcomes.15–19 Changes in the nature of volume-outcome relationships for such a fundamental critical care process as MV would have profound implications for discussions on regionalization of critical care. As MV mortality has been decreasing over time for a variety of reasons, we hypothesize that hospital volume will not be associated with patient outcomes.16, 17, 20
Materials and Methods
Please see the Online Supplemental Methods for full details on the study design and statistical analysis.
Patients:
We conducted a retrospective cohort study of the California Office of Statewide Health Planning and Development Patient Discharge Database (PDD) from 2016.21 The PDD contains administrative discharge data for 100% of non-federal hospital discharges in California as well as patient linkage data to track hospital readmissions.22 We identified adult, nonsurgical patients admitted to an acute care hospital who required MV using International Classifications of Disease, 10th edition (ICD-10) billing codes (Table E1).23 We excluded patients who were transferred to or from another hospital, patients with unknown vital status, and patients admitted to hospitals with fewer than 25 cases of nonsurgical MV in 2016. For individuals with multiple admissions requiring MV in 2016, we selected a random admission for inclusion.
Exposures and Outcomes:
The primary exposure was hospital MV volume divided into quartiles in order to account for non-linear volume-outcome relationships. Our primary outcome was hospital mortality with the secondary outcomes of tracheostomy and unplanned 30-day readmission among hospital survivors. We additionally investigated the association of hospital MV volume on hospital risk-adjusted rates of the primary and secondary outcomes.
Statistical Analysis:
We performed univariate testing for differences in patient-level variables with ANOVA testing, linear regression, Chi-Square tests, and Cochran-Armitage tests for trends across hospital MV quartiles as indicated. We used generalized estimating equations with a compound symmetry covariance structure to account for correlation within hospitals to determine the association of the patient outcomes with hospital MV volume quartile. We calculated hospital risk-adjusted outcome rates using hierarchical regression models with the hospital as a random intercept. We compared hospital MV volume quartile with hospital risk-adjusted outcome rates using ANOVA testing with Tukey’s multiple comparison test for pairwise comparison and Spearman correlation tests. We adjusted statistical models for patient demographics including race/ethnicity and primary insurance payer, comorbidities, acute organ failures present on admission (markers of severity of illness) (Table E2), common causes of respiratory failure (pneumonia, COPD, asthma, heart failure, severe sepsis, septic shock, and acute respiratory distress syndrome (ARDS)) (Table E3), and early do-not resuscitate (DNR) status.24–26 Risk-adjustment with administrative data within this dataset had similar performance characteristics to physiologic predictors such as APACHE and SOFA. We included demographics such as race/ethnicity and primary insurance payer as both have been shown to be associated with MV outcomes and can be viewed as surrogates of social determinants of health that are associated with multiple patient outcomes.16 However, the association of primary payer may be unique to the United States as insurance and healthcare payment structures differ greatly in other countries.
Sensitivity Analyses:
We performed multiple sensitivity analyses the details of which can be found in the Online Supplement. (1) In order to assess the total impact of MV volume, we included surgical patients in determining the volume-outcome relationship. (2) We restricted the MV cohort to those patients who were intubated within 48 hours of admission and to patients who received MV for greater than 24 hours. (3) As MV quartiles can be defined in multiple ways, we determined volume-outcome associations with MV quartiles defined by equal numbers of patients as opposed to equal numbers of hospitals. (4) We selected a random hospitalization from 2016 for patients with multiple hospitalizations. We conducted 2 additional sensitivity analyses in which we selected the first hospitalization for each patient in 2016 and another in which we selected the last hospitalization in 2016. (5) In order to reduce the possible risk of bias from differential transfer practices across quartiles (e.g. if all low MV volume hospitals transfer their sickest patients to high volume hospitals leading to artificially lower mortality at low volume hospitals) we conducted a sensitivity analysis where patient outcomes for transfer patients were attributed to the originating hospitals. (6) To reduce misclassification bias solely related to newer ICD-10 codes, we conducted the same analyses with the PDD 2014 which used ICD-9-CM codes. (7) In order to address the potential competing risks between hospital death and tracheostomy, we conducted an additional sensitivity analysis in which we analyzed the volume-outcome association for the composite outcome of hospital death or tracheostomy. From a patient-centered perspective, both tracheostomy and death are often considered to be ‘negative outcomes’.27
In order to further address unmeasured confounding, we calculated the ‘e-value’ for our primary analysis and for our 2014 analysis.28, 29 The e-value is new method to address the issue of unmeasured confounding in observational research and describes the strength an unmeasured confounder would need to have over and above adjustment for measured confounders to shift the observed effect to the null.30 Finally, in order to test the association of hospital volume and outcomes in the presence of unmeasured confounding, we conducted an instrumental variable analysis with patient differential distance to the nearest high volume hospital (distance to the nearest high volume hospital minus distance to the nearest hospital) as the instrument.13, 31 Instrumental variable analyses use a variable strongly associated with the exposure (hospital MV volume) but only associated with the outcome through its association with exposure to quasi-randomize patients to the exposure of interest.32, 33 The result represents the strength of the association for the ‘marginal patient’ who would experience different outcomes based on the hospital to which they are admitted.34 We used a 2 step least squares regression approach as well as a less conventional 2 step logistic regression to determine the absolute risk difference for the marginal patient between high and low volume hospitals as well as the adjusted odds ratio (aOR).32, 33, 35, 36 Details of the instrumental variable analysis can be found in the Online Supplemental Methods.
All statistical testing was two-tailed and performed with a critical alpha=0.05 threshold with SAS v9.4 (SAS Institute, Cary, NC). The study was approved by the California Committee for Protection of Human Subjects (Sacramento, CA) and deemed exempt by the National Jewish Health Institutional Review Board (Denver, CO).
Results
We identified 51,689 patients across 274 hospitals meeting the inclusion criteria and requiring MV in California in 2016 (Figure 1). In this cohort of mechanically ventilated patients, 38.2% died in the hospital, 4.4% received a tracheostomy, and 29.5% of survivors were readmitted within 30 days of hospital discharge.
Figure 1:
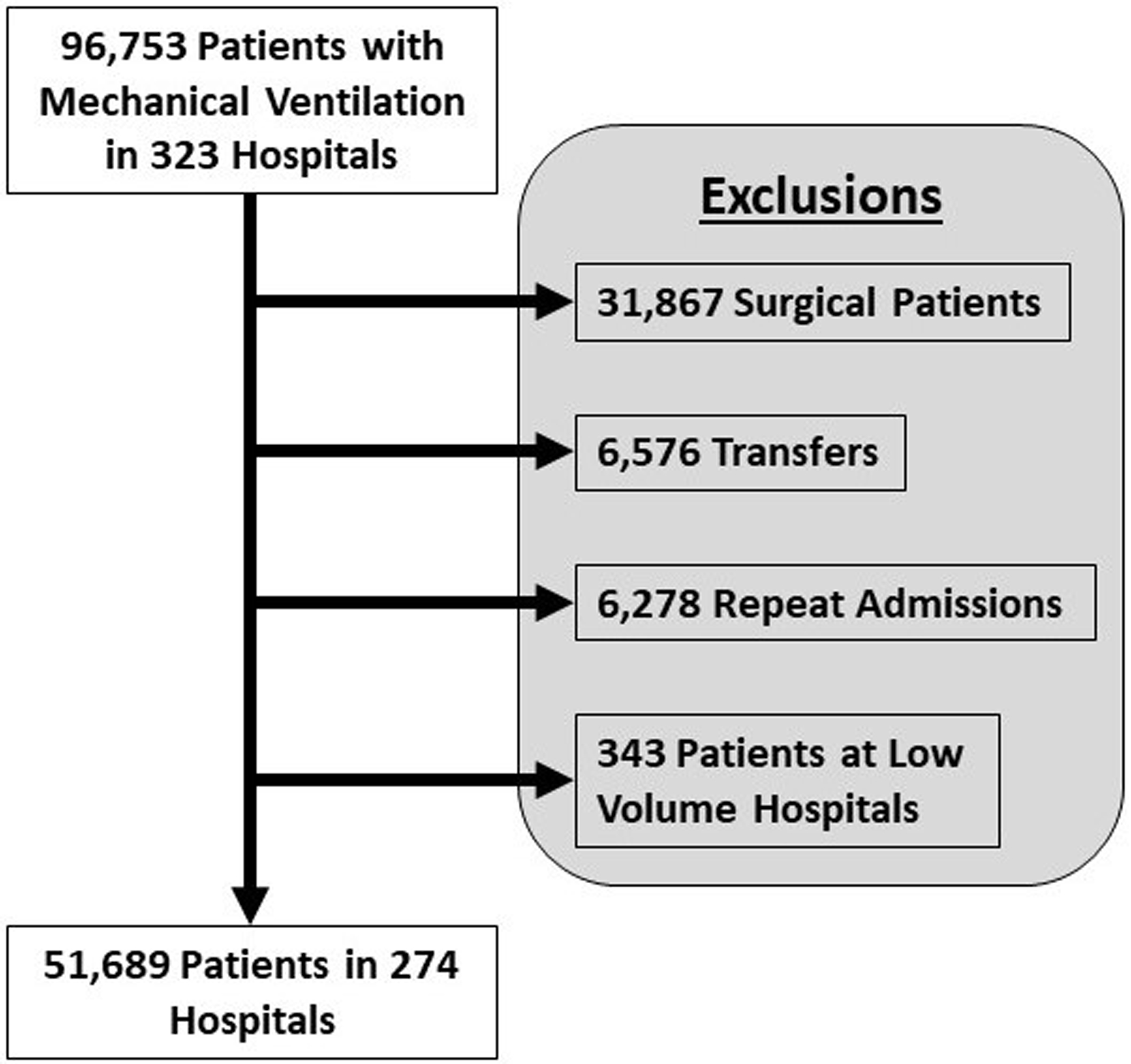
Study Design. We identified adult nonsurgical patients who required MV during their hospitalization. We excluded surgical patients, patients transferred to or from another acute care hospital, and patients admitted to low hospital MV volume hospitals (<25 cases in 2016). For patients with multiple admissions in which they required MV in 2016, we identified one hospitalization at random for analysis. Abbreviations: MV – mechanical ventilation.
Patient Characteristics:
Hospital MV volume varied widely between hospitals (median 170 cases, IQR=196 cases, range 25–953 cases). Table 1 presents key patient characteristics across hospital MV volume quartiles.25 High hospital MV volume hospitals tended to have fewer white patients, while middle quartile hospitals tended to have fewer Medicaid patients. Markers of severity of illness did not substantially differ between hospital MV volume quartiles. Slightly lower rates of organ failures at high volume hospitals may suggest a lower threshold to initiate MV in these hospitals. Higher quartile hospitals had lower rates of patients with any diagnosis of pneumonia and COPD exacerbation compared to lower quartile hospitals.
Table 1:
Patient Characteristics Across Hospital Mechanical Ventilation Quartiles
| Variable | Quartile 1 n=3,620 | Quartile 2 n=8,230 | Quartile 3 n=13,976 | Quartile 4 n=25,863 | p-value |
|---|---|---|---|---|---|
| MV Episodes - (number of cases) | <94 | 94–170 | 171–289 | ≥290 | N/A |
| Hospitals (n) | 68 | 69 | 68 | 69 | |
| Mean Age (SD) | 63.6 (17.1) | 65.2 (16.9) | 65.4 (16.9) | 63.3 (17.3) | <0.0001 |
| Female (%) | 44.0 | 45.2 | 45.3 | 43.6 | 0.02 |
| Other | 3.9 | 4.1 | 4.4 | 5.3 | |
| Other | 1.9 | 2.2 | 1.5 | 1.8 | |
| Early DNR Order (%) | 15.6 | 14.2 | 14.2 | 14.5 | 0.46 |
| Mean Elixhauser Comorbidity Score (SD)* | 10.6 (8.8) | 10.8 (8.9) | 11.1 (8.8) | 10.4 (8.9) | <0.0001 |
| Shock (%)** | 29.1 | 31.1 | 31.2 | 31.0 | 0.17 |
| Renal Failure (%)** | 39.0 | 39.9 | 40.6 | 37.4 | <0.0001 |
| Neurologic Failure (%)** | 4.1 | 5.1 | 4.8 | 4.7 | 0.74 |
| Hepatic Failure (%)** | 7.3 | 8.0 | 7.9 | 7.5 | 0.55 |
| Hematologic Failure (%)** | 16.3 | 18.1 | 18.2 | 16.9 | 0.17 |
| Acidosis (%)** | 27.0 | 25.2 | 27.3 | 27.0 | 0.09 |
| Pneumonia (%) | 37.2 | 34.8 | 34.3 | 33.3 | <0.0001 |
| COPD (%) | 16.2 | 13.9 | 13.1 | 12.4 | <0.0001 |
| Asthma (%) | 2.0 | 2.4 | 2.2 | 2.0 | 0.14 |
| Heart Failure (%) | 14.9 | 16.0 | 16.0 | 15.8 | 0.47 |
| Severe Sepsis (%) | 8.0 | 7.7 | 8.8 | 6.7 | <0.0001 |
| Septic Shock (%) | 28.9 | 29.2 | 30.0 | 29.5 | 0.43 |
| ARDS (%) | 2.6 | 2.3 | 2.0 | 1.8 | 0.0002 |
Abbreviations: MV – mechanical ventilation. SD – standard deviation. DNR – do-not-resuscitate. COPD – chronic obstructive pulmonary disease. ARDS – acute respiratory distress syndrome.
Individual comorbidities were used in multivariable modeling. The comorbidity score was calculated without the cardiac arrhythmia comorbidity. See reference 29
Present on admission
Patient Outcomes:
Patients admitted to hospitals with higher vs lower hospital MV volume had higher risk-adjusted hospital mortality (Quartile 4=38.6% vs Quartile 1=34.2%, aOR=1.40, 95% CI 1.17–1.68) and tracheostomy (Quartile 4=4.5% vs Quartile 1=3.5%, aOR=1.56, 95% CI 1.20–2.03) (Table 2). Conversely, patients admitted to higher MV volume hospitals had lower odds of 30-day readmission (Quartile 4=35.1% vs Quartile 1=28.7%, aOR=0.77, 95% CI 0.67–0.89).
Table 2:
Risk-Adjusted Patient Outcome Rates Across Hospital Mechanical Ventilation Quartiles
| Outcome | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | aOR (95% CI) Quartile 4 vs Quartile1 |
|---|---|---|---|---|---|
| Hospital Death (%) | 34.2 | 37.5 | 37.3 | 38.7 | 1.39 (1.17 – 1.67) |
| Tracheostomy (%) | 3.5 | 4.4 | 4.2 | 4.5 | 1.56 (1.20 – 2.03) |
| 30-Day Readmission (%)* | 35.1 | 30.9 | 30.7 | 28.7 | 0.77 (0.67 – 0.89) |
Abbreviations: aOR – adjusted odds ratio. CI – confidence interval.
Readmission was determined among hospital survivors.
Hospital Outcomes:
When comparing hospital risk-adjusted outcome rates across hospital MV volume quartiles, we observed significant differences in mean hospital risk-adjusted rates of hospital death (p=0.03), tracheostomy (p=0.01), and 30-day readmission (p=0.01) across hospital quartiles (p-values indicate difference in means using the ANOVA test) (Table E8). In pairwise comparisons between quartiles, there was only a statistically significant difference in hospital risk-adjusted outcome rates for all outcomes between Quartile 1 and Quartile 4 (Figure 2). We observed a weak positive correlation between hospital risk-adjusted outcome rates of death (ρ=0.16, p=0.008), and tracheostomy (ρ=0.15, p=0.01), but negatively correlated with 30-day readmission (ρ=−0.22, p=0.0002) (Figure 3).
Figure 2:
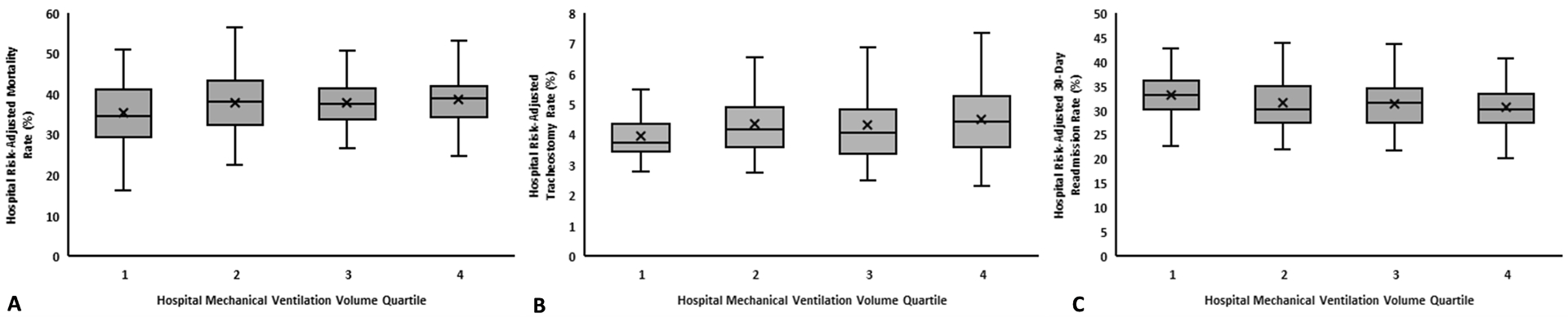
Whisker Plots Demonstrate Linear Trends in Hospital Risk-Adjusted Outcome Rates Across Hospital MV Volume Quartiles. Hospital risk-adjusted outcome rates were calculated using multivariable hierarchical models with the hospital as a random intercept. Hospital risk-adjusted outcome rates were compared across hospital MV volume quartiles using linear regression. Higher hospital MV volume quartiles had higher hospital risk-adjusted rates of the hospital death (p=0.007, Panel A) and tracheostomy (p=0.009, Panel B). Higher hospital MV volume quartiles had lower risk-adjusted rates of 30-day readmission among survivors (p=0.003, Panel C). For all outcomes, pairwise comparisons between quartiles was made with ANOVA testing with Tukey’s adjustment and the only statistically significant pairwise comparison was between Quartile 4 and Quartile 1. Abbreviations: MV – mechanical ventilation.
Figure 3:
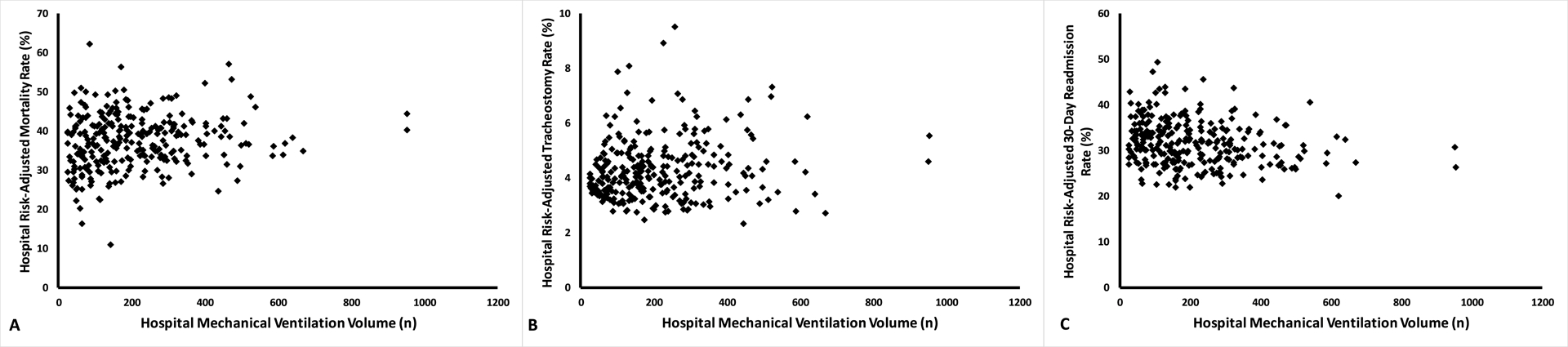
Scatter Plots for Hospital MV Volume and Hospital Risk-Adjusted Outcome Rates Show Weak but Significant Correlation. Hospital risk-adjusted outcome rates were calculated using multivariable hierarchical models with the hospital as a random intercept and Spearman’s correlation test was used to determine nonlinear associations. Higher hospital MV volume was correlated with higher risk-adjusted rates of hospital death (ρ=0.16, p=0.008, Panel A), and tracheostomy (ρ=0.15, p=0.01, Panel B), but lower 30-day readmission (ρ=−0.22, p=0.0002, Panel C). Abbreviations: MV – mechanical ventilation.
Sensitivity Analyses:
The findings were consistent across multiple sensitivity analyses. Inclusion of all patients who received MV, including surgical patients, strengthened the inverse volume-outcome association, especially for the secondary outcome of tracheostomy (Table E9). Adjustments to the definition of the MV cohort, attributing outcomes for patients who were transferred to the originating hospital, choosing the first or last hospitalization for patients with multiple hospitalizations in 2016, and using data from the PDD 2014 to reduce the chances of misclassification bias solely from the use of newer ICD-10 codes showed similar results to our primary analysis (see Online Supplemental Results and Table E10–E18). Based on the e-value, an unmeasured confounder would need to have an aOR=1.65 in order to shift the association between hospital MV volume and hospital mortality to the null. In the instrumental variable analysis, marginal patients admitted to a high vs low MV volume hospital experienced a 10.0% (95% CI 8.2 – 11.8) higher hospital mortality rate. Patients with higher vs lower probability of admission to a high MV volume hospital had higher adjusted odds of death (aOR=1.40, 95% CI 1.05–1.88).
Discussion
We investigated the association of hospital MV volume with patient and hospital outcomes on a population level in a cohort with wide hospital MV volume variation. In contrast to previous studies and our hypothesis, we observed worse patient outcomes at high volume hospitals including death and tracheostomy. Lower risk of readmission at high volume hospital may reflect the fact that marginal patients at high volume hospitals die and, thus, are not eligible for readmission. Our findings were robust to multiple sensitivity analyses including quantification of the effects of unmeasured confounding.
Our findings must be viewed in the context of the confusing history of investigations in the volume-outcome association for MV. Both Needham et al and Kahn et al used data from before 2003 to identify worse outcomes for patients at low MV volume hospitals.11, 14 A follow-up study using Pennsylvania state discharge records from 2004–2006 found similar findings with an instrumental variable analysis and clinical risk-adjustment but not administrative risk-adjustment.13 Cooke et al analyzed the VA database in 2009 and found no volume-outcome association for MV.12 More recently, Ike et al showed improved outcomes specifically for patients with ARDS at high ARDS centers.37 These studies raise questions about the evolution over time of the MV volume-outcome relationship as well as differences across MV subtypes. The variable findings raises the possibility that the volume-outcome relationship for MV, and possibly other critical care processes, may be context specific. Nonetheless, the studies are still cited in arguments for regionalization of critical care services despite the lack of context-specific investigations supporting wide implementation of “high volume centers of excellence”.6, 7, 10, 14
Several factors may help explain our contradictory findings that raise the potential for harm at extremely high volume beyond the simple explanation that investigations of MV volume relationships are context specific. Firstly, our study was the largest to date from a patient and hospital number perspective. Understanding volume-outcome relationships sufficient hospital numbers to create stable comparisons. In Needham et al’s study, the top 2 quintiles had only 6 and 13 hospitals respectively, suggesting significant imbalance across quintiles.14 Kahn et al’s NEJM study compared differences in only 37 hospitals in the APACHE network which were likely not representative of the wide variation in hospital types (e.g. small community vs large academic vs for-profit).11 Cooke et al’s study MV volume was significantly lower than all other published studies with the highest quartile in their study having the same MV incidence as the lowest quartile in Kahn’s study and ours.12 Our findings may differ from prior studies based on the size, diversity, and number of hospitals in our patient sample allowing for more stable estimates across quartiles.
A second key consideration for our disparate findings is the influence of secular trends in MV. In the last 2 decades the incidence for MV has doubled, hospital mortality has decreased by one quarter, and MV practices have dramatically changed with shifts towards lower tidal volume and higher PEEP.16–18, 20 As best practices disseminate to smaller non-academic hospitals, outcome differences based on volume may be reduced. Such secular trends may also influence volume-outcome relationships for processes other than MV.
However, the novelty of our findings is the potential for harm at high volume hospitals and secular trends alone would be unlikely to result in such a dramatic change in the association. Therefore, we speculate that a key mechanism of our findings may be hospital strain and increased burnout at high volume hospitals as has been suggested for non-invasive ventilation and ICU/hospital readmission.38–40 Despite the rapid growth in MV in the last 2 decades, similar increases in resources such as nurses, respiratory therapists, physicians and critical care beds have not been observed.41 As such, the shift in volume-outcome relationship may reflect an inflection point at which higher volumes actually lead to worse outcomes as supported by the fact that the primary hospital-level difference in quartiles was observed between the highest and lowest quartile. MV, like non-invasive ventilation and ICU/hospital readmission reduction programs, is a complex multi-disciplinary process of care that may be more susceptible to strain at high volume compared to surgical procedures.
Healthcare provider burnout may also be a key mediator of the proposed strain relationship. Critical care providers (nurses and physicians) have some of the highest rates of burnout syndrome in the healthcare field reaching epidemic proportions based on some studies.42–45 Higher workload has been shown to be a significant contributor to burnout and burnout itself has been shown to be associated with worse patient outcomes.45, 46 It is unclear if higher volume hospitals experience higher levels of burnout. Future studies are needed to assess if burnout may contribute to worse outcomes at high MV volume hospitals.
One limitation of most observational studies is the potential for biased results related to unmeasured confounding such as differences in severity of illness across exposure categories. However, we used multiple statistical techniques to specifically address the issue of risk-adjustment and confounding by severity of illness. We observed that patient characteristics were well balanced across quartiles; Quartile 4 did not appear to be sicker than Quartile 1. Similar to findings from Courtright et al, our statistical risk-adjustment had similar performance characteristics to physiologic risk-adjustment strategies like the APACHE and SOFA scores.47–49
We also used more advanced methods to identify the impact of unmeasured confounding.29 Using the e-value measurement, an unmeasured confounder would have to have an aOR=1.65 to shift the association between hospital volume and death to the null. Given the robustness of our risk-adjustment, we find it unlikely that an individual unmeasured confounder could exert a strong enough effect to negate our observations. Finally, we used an instrumental variable analysis to account for unmeasured confounders. The instrumental variable analysis reveals the association for the marginal patient whose outcome is dependent on the hospital to which they are admitted through a quasi-randomization process for observational data. When the instrumental variable approach was used we observed the same association between higher hospital MV volume and higher odds of death. While each individual analysis might have only yielded a weak or moderate strength association, the combination of all of these approaches does suggest that patients at the highest volume hospitals experience higher odds of death compared to the lowest volume hospitals even in the presence of unmeasured confounders. For hospital volume-outcome relationships, these multiple methods of risk-adjustment and analysis are likely the best estimate of the true association as a prospective randomized trial is neither feasible nor ethical.
Our study had several additional limitations. Use of California data may decrease the generalizability of our findings, but the PDD has a large diverse population of patients with a large number of hospitals needed to obtain an accurate picture of the volume-outcome association. The PDD is also one of the only large databases with DNR status which is a critical variable to consider given its strong association with mortality.50 Use of ICD-10 billing codes is subject to misclassification bias especially given their recent introduction. Differences in coding practices between hospitals may explain the unexpected observation of lower rates of severe sepsis and ARDS at high volume hospitals. However, we found the same volume-outcome associations using 2014 data with ICD-9-CM codes, which have been more extensively validated. We also did not have access to detailed hospital characteristics, which could mediate the volume-outcome relationship. We used administrative data, which, despite the multiple methods of risk-adjustment employed, could still be subject to unmeasured confounding.
Another key limitation was our focus on hospital MV volume as the driver for differences in patient outcomes. Non-MV related practices (e.g. time to antibiotics for sepsis, time to catheterization for myocardial infarction, etc.) may heavily influence hospital mortality MV patients. We were unable to explore all of the potential mediators of the volume-outcome relationship we observed. However, this is a limitation in all volume-outcome studies and future studies investigating detailed hospital-level mechanisms of volume-outcome associations are needed.
Conclusions
Our observation of worse hospital outcomes for patients admitted to high MV volume hospitals is contrary to previously published papers. Previous studies have been limited by relatively few hospitals or low MV volume overall. Our findings offer insight from a large, diverse patient cohort with the highest number of hospitals of any previous investigation. We do not believe that our findings suggest that patients should be transferred out of high volume hospitals as some subsets of patients (e.g. ARDS) may derive benefit in higher volume settings. Rather, we argue that the key importance of our findings are threefold. First, previous studies have been limited by low numbers of hospitals or low MV incidence. Strong volume-outcome studies require large numbers of hospitals and wide variation in exposure incidence to generate stable associations. Second, secular trends in practice patterns and outcomes must considered when evaluating or attempting to operationalize volume-outcome relationships described more than a decade ago. Finally, and most importantly, further studies are needed to understand the mechanisms of the volume-outcome relationship for MV as it is one of the most common and complex processes of care for critically ill individuals.
Supplementary Material
Acknowledgements
Guarantor: ABM takes full responsibility for the content of the manuscript, data analysis, and data interpretation.
Sponsors: The sponsors had no role in this study.
Funding: ABM is supported by NIH K12HL137862. AJW is supported by NIH K01HL116768 and R01HL136660. DCE is supported by NIH RHL089897B-08. DM is supported by NIH R01HL136403-01. ISD is supported by NIH. 1R01NR016459-01.
Footnotes
Disclosures: No authors have any disclosures or conflicts of interest for this study.
Work Performed: National Jewish Health
Author Conflict of Interest: No authors have a conflict of interest for this study.
References
- 1.Idrees JJ, Schiltz NK, Johnston DR, et al. Trends, Predictors, and Outcomes of Stroke After Surgical Aortic Valve Replacement in the United States. The Annals of Thoracic Surgery 2015. [DOI] [PubMed] [Google Scholar]
- 2.Fawzy A, Walkey AJ. Association Between Hospital Case Volume of Sepsis, Adherence to Evidence-Based Processes of Care and Patient Outcomes. Critical Care Medicine 2017;45(6):980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walkey AJ, Wiener RS. Hospital case volume and outcomes among patients hospitalized with severe sepsis. American journal of respiratory and critical care medicine 2014;189(5):548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen YL, Wallace DJ, Yordanov Y, et al. The Volume-Outcome Relationship in Critical Care: A Systematic Review and Meta-analysis. Chest 2015;148(1):79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. The New England journal of medicine 1979;301(25):1364–1369. [DOI] [PubMed] [Google Scholar]
- 6.Kahn JM, Branas CC, Schwab CW, et al. Regionalization of medical critical care: what can we learn from the trauma experience? Critical Care Medicine 2008;36(11):3085–3088. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen YL, Kahn JM, Angus DC. Reorganizing adult critical care delivery: the role of regionalization, telemedicine, and community outreach. American journal of respiratory and critical care medicine 2010;181(11):1164–1169. [DOI] [PubMed] [Google Scholar]
- 8.Thompson DR, Clemmer TP, Applefeld JJ, et al. Regionalization of critical care medicine: task force report of the American College of Critical Care Medicine. Critical Care Medicine 1994;22(8):1306–1313. [DOI] [PubMed] [Google Scholar]
- 9.Singh JM, MacDonald RD. Pro/con debate: do the benefits of regionalized critical care delivery outweigh the risks of interfacility patient transport? Crit Care 2009;13(4):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn JM, Linde-Zwirble WT, Wunsch H, et al. Potential value of regionalized intensive care for mechanically ventilated medical patients. Am J Respir Crit Care Med 2008;177(3):285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahn JM, Goss CH, Heagerty PJ, et al. Hospital volume and the outcomes of mechanical ventilation. The New England journal of medicine 2006;355(1):41–50. [DOI] [PubMed] [Google Scholar]
- 12.Cooke CR, Kennedy EH, Wiitala WL, et al. Despite variation in volume, Veterans Affairs hospitals show consistent outcomes among patients with non-postoperative mechanical ventilation. Critical Care Medicine 2012;40(9):2569–2575. [DOI] [PubMed] [Google Scholar]
- 13.Kahn JM, Ten Have TR, Iwashyna TJ. The relationship between hospital volume and mortality in mechanical ventilation: an instrumental variable analysis. Health services research 2009;44(3):862–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Needham DM, Bronskill SE, Rothwell DM, et al. Hospital volume and mortality for mechanical ventilation of medical and surgical patients: a population-based analysis using administrative data. Crit Care Med 2006;34(9):2349–2354. [DOI] [PubMed] [Google Scholar]
- 15.Mehta AB, Syeda SN, Bajpayee L, et al. Trends in Tracheostomy for Mechanically Ventilated Patients in the United States, 1993–2012. American journal of respiratory and critical care medicine 2015;192(4):446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta AB, Syeda SN, Wiener RS, et al. Epidemiological trends in invasive mechanical ventilation in the United States: A population-based study. Journal of critical care 2015;30(6):1217–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esteban A, Ferguson ND, Meade MO, et al. Evolution of mechanical ventilation in response to clinical research. American journal of respiratory and critical care medicine 2008;177(2):170–177. [DOI] [PubMed] [Google Scholar]
- 18.Duan EH, Adhikari NKJ, D’Aragon F, et al. Management of Acute Respiratory Distress Syndrome and Refractory Hypoxemia. A Multicenter Observational Study. Ann Am Thorac Soc 2017;14(12):1818–1826. [DOI] [PubMed] [Google Scholar]
- 19.Silveira MJ, Wiitala W, Piette J. Advance directive completion by elderly Americans: a decade of change. Journal of the American Geriatrics Society 2014;62(4):706–710. [DOI] [PubMed] [Google Scholar]
- 20.Esteban A, Frutos-Vivar F, Muriel A, et al. Evolution of Mortality over Time in Patients Receiving Mechanical Ventilation. Am J Respir Crit Car Med 2013;188(2):220. [DOI] [PubMed] [Google Scholar]
- 21.California Office of Statewide Health Planning and Development: Patient Discharge Data. California Office of Statewide Health Planning and Development. Available at: https://www.oshpd.ca.gov/HID/Patient-Discharge-Data.html. Last updated: May 26, 2017. Last accessed: May 29, 2017.
- 22.Goldman LE, Chu PW, Prothro C, et al. Accuracy of condition present on admission, do not resuscitate, and e-codes in California Patient Discharge Data: prepared for the Office of Statewide Health Planning and Development , Healthcare Outcomes Center. Office of Statewide Health Planning and Development. Available at: https://www.oshpd.ca.gov/documents/HID/PDDValidation/PDD_Validation_Study.pdf. Last updated: Spring 2011. Last accessed: March 3, 2016.
- 23.Beta Procedure Classes for ICD-10 PCS. Healthcase Cost and Utilization Project. Available at: https://www.hcup-us.ahrq.gov/toolssoftware/procedureicd10/procedure_icd10.jsp. Last updated: September 22, 2017. Last accessed: October 25, 2017.
- 24.Beta Elixhauser Comorbidity Software for ICD-10-CM. Healthcare Cost and Utilization Project. Available at: https://www.hcup-us.ahrq.gov/toolssoftware/comorbidityicd10/comorbidity_icd10.jsp. Last updated: October 16, 2017. Last accessed: October 25, 2017.
- 25.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical care 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 26.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Medical care 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 27.Rubin EB, Buehler AE, Halpern SD. States Worse Than Death Among Hospitalized Patients With Serious Illnesses. JAMA internal medicine 2016;176(10):1557–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathur MB, Ding P, VanderWeele TJ. Website and R package for computing E-Values. Epidemiology (Cambridge, Mass) 2018;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med 2017;167(4):268–274. [DOI] [PubMed] [Google Scholar]
- 30.Gershon AS, Jafarzadeh SR, Wilson KC, et al. Clinical Knowledge from Observational Studies: Everything You Wanted to Know but Were Afraid to Ask. Am J Respir Crit Care Med 2018. [DOI] [PubMed] [Google Scholar]
- 31.Valley TS, Sjoding MW, Ryan AM, et al. Association of Intensive Care Unit Admission With Mortality Among Older Patients With Pneumonia. Jama 2015;314(12):1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rassen JA, Brookhart MA, Glynn RJ, et al. Instrumental variables I: instrumental variables exploit natural variation in nonexperimental data to estimate causal relationships. Journal of clinical epidemiology 2009;62(12):1226–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rassen JA, Brookhart MA, Glynn RJ, et al. Instrumental variables II: instrumental variable application-in 25 variations, the physician prescribing preference generally was strong and reduced covariate imbalance. J Clin Epidemiol 2009;62(12):1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris KM, Remler DK. Who is the marginal patient? Understanding instrumental variables estimates of treatment effects. Health Serv Res 1998;33(5 Pt 1):1337–1360. [PMC free article] [PubMed] [Google Scholar]
- 35.Angrist JD, Pischke JS. Mostly Harmless Econometrics: An Empiricist’s Companion. Princeton, NJ: Princeton University Press; 2008. [Google Scholar]
- 36.Newhouse JP, McClellan M. Econometrics in outcomes research: the use of instrumental variables. Annual review of public health 1998;19:17–34. [DOI] [PubMed] [Google Scholar]
- 37.Ike JD, Kempker JA, Kramer MR, et al. The Association Between Acute Respiratory Distress Syndrome Hospital Case Volume and Mortality in a U.S. Cohort, 2002–2011. Crit Care Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehta AB, Douglas IS, Walkey AJ. Hospital Non-Invasive Ventilation Case-Volume and Outcomes for Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Annals of the American Thoracic Society 2016;13(10):1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner J, Gabler NB, Ratcliffe SJ, et al. Outcomes among patients discharged from busy intensive care units. Annals of Internal Medicine 2013;159(7):447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horwitz LI, Lin Z, Herrin J, et al. Association of hospital volume with readmission rates: a retrospective cross-sectional study. BMJ (Clinical research ed) 2015;350:h447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.HCUPnet. Healthcare Cost and Utilization Project. Available at: http://hcupnet.ahrq.gov/. Last updated: January 1, 2017. Last accessed: October 25, 2017.
- 42.Moss M, Good VS, Gozal D, et al. A Critical Care Societies Collaborative Statement: Burnout Syndrome in Critical Care Health-care Professionals. A Call for Action. Am J Respir Crit Care Med 2016;194(1):106–113. [DOI] [PubMed] [Google Scholar]
- 43.van Mol MM, Kompanje EJ, Benoit DD, et al. The Prevalence of Compassion Fatigue and Burnout among Healthcare Professionals in Intensive Care Units: A Systematic Review. PLoS One 2015;10(8):e0136955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Epp K Burnout in critical care nurses: a literature review. Dynamics (Pembroke, Ont) 2012;23(4):25–31. [PubMed] [Google Scholar]
- 45.Embriaco N, Azoulay E, Barrau K, et al. High level of burnout in intensivists: prevalence and associated factors. Am J Respir Crit Care Med 2007;175(7):686–692. [DOI] [PubMed] [Google Scholar]
- 46.Dewa CS, Loong D, Bonato S, et al. The relationship between physician burnout and quality of healthcare in terms of safety and acceptability: a systematic review. BMJ open 2017. 7(6):e015141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zimmerman JE, Kramer AA, McNair DS, et al. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Critical Care Medicine 2006;34(5):1297–1310. [DOI] [PubMed] [Google Scholar]
- 48.Minne L, Abu-Hanna A, de Jonge E. Evaluation of SOFA-based models for predicting mortality in the ICU: A systematic review. Crit Care 2008;12(6):R161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Courtright KR, Halpern SD, Bayes B, et al. Adaptation of the Acute Organ Failure Score for Use in a Medicare Population. Crit Care Med 2017;45(11):1863–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bradford MA, Lindenauer PK, Wiener RS, et al. Do-not-resuscitate status and observational comparative effectiveness research in patients with septic shock. Critical Care Medicine 2014;42(9):2042–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


