Abstract
Even after controlling for stage, comorbidity, age, and insurance status, black women with breast cancer (BC) in the USA have the lowest 5-year survival as compared with all other races for stage-matched disease. One potential cause of this survival difference is the disparity in cancer treatment, evident in many population clinical trials. Specifically, during BC chemotherapy, black women receive less relative dose intensity with more dose reductions and early chemotherapy cessation compared with white women. Symptom incidence, cancer-related distress, and ineffective communication, including the disparity in patient-centeredness of care surrounding patient symptom reporting and clinician assessment, are important factors contributing to racial disparity in dose reduction and early therapy termination. We present an evidence-based overview and an explanatory model for racial disparity in the symptom experience during BC chemotherapy that may lead to a reduction in dose intensity and a subsequent disparity in outcomes. This explanatory model, the Symptom Experience, Management, Outcomes and Adherence according to Race and Social determinants + Genomics Epigenomics and Metabolomics (SEMOARS + GEM), considers essential factors such as social determinants of health, clinician communication, symptoms and symptom management, genomics, epigenomics, and pharmacologic metabolism as contributory factors.
Keywords: Breast cancer, Symptom, Social determinants, Treatment disparity, Chemotherapy, African-American, Dose intensity
Introduction
Breast cancer (BC) incidence is similar among black and white women [1], except for younger black women aged 45 and under, who have higher incidence rates [2]. Yet black women die from BC at a rate 42% higher than white women [1, 3] and are more frequently diagnosed at later disease stages and with aggressive triple-negative (estrogen, progesterone, HER2/neu) tumors [2]. This increase is particularly true in BC, confirmed when a meta-analysis reported a 1.22 odds ratio for a negative effect of African-American ethnicity on BC mortality [4]. These negative outcome differences persist after controlling for disease stage and tumor type, comorbidities, age, and insurance status, which leaves the underlying cause of this disparity unexplained [5, 6]. Receiving ≤ 85% of prescribed BC chemotherapy is associated with poor outcomes [7–9]. Racial disparity in cancer treatment is documented [10] and is a potential source of the racial variance in survival rates [3, 11–17].
Suboptimal adherence to chemotherapy treatment is a multifactorial problem, which involves much more than the patient herself. The International Society for Pharmacoeconomics and Outcomes Research defines medication compliance/adherence as “the degree or extent of conformity (most appropriately a percentage) to the recommendations about day-to-day treatment by the provider with respect to the timing, dosage, and frequency” [18]. Most often, studies investigating cancer treatment adherence focused on oral cancer treatments and have not included factors other than the patient’s role in adherence. The term “adherence” or “compliance” carries some traditionally pejorative connotations, implying that the choice to receive less than full-dose treatment is always patient initiated. In BC treatment, the choices regarding less than full adherence to prescribed BC intravenous chemotherapy are most often initiated by the clinical staff rather than the patient. Treatment decisions such as capping chemotherapy dosing at a body surface area (BSA) of 2.0 [19, 20] instead of treating to full body weight or the clinician’s subjective treatment decisions based on the categorization of certain women as “poor chemotherapy candidates” allowed a differential treatment approach that was potentially racially biased [21]. The standardization of chemotherapy dosing according to BSA without any or minimal cap for overweight and obese patients [22] and the standard use of national treatment guidelines in medical oncology [23] may now more closely regulate the clinician’s discretion during initial treatment prescription, limiting the clinician’s autonomy in prescribing nonstandard therapy or first cycle reduction. Perhaps these changes are reflected in recent studies, including our own, reporting on racial disparity in the initiation of chemotherapy. Slight to no racial disparity was found in the clinician’s prescription or the patient’s initiation of prescribed chemotherapy [24], but racial disparity in receiving full-dose, timely treatment across the chemotherapy continuum was noted [25–31].
The Symptom Experience, Management and Outcomes According to Race and Social determinants (SEMOARS) model was developed to address factors associated with the disparate receipt of chemotherapy. In this model, the exploration of these adjuvant BC chemotherapy receipt variables stresses the importance of the person within a social and environmental context.
The SEMOARS + GEM Model
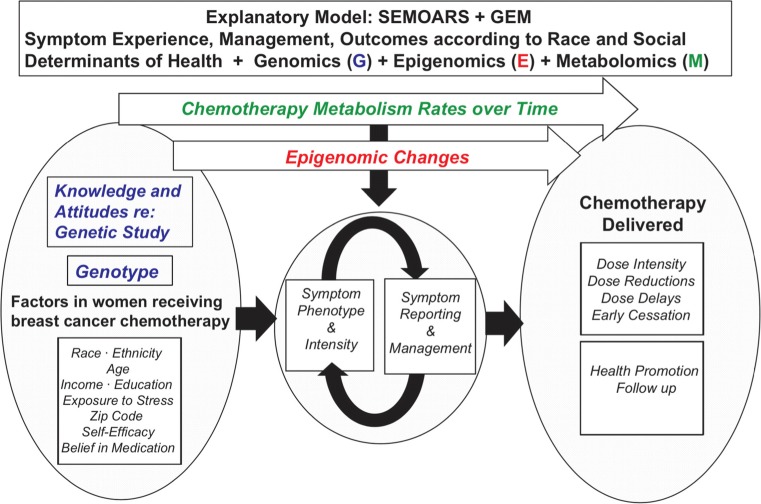
The development of the SEMOARS model, with the addition of Genomics, Epigenomics, and Metabolomics (GEM) (Fig. 1), enables rasearchers to examine the variables contributing to the hypothesized explanatory model [32–41]. The SEMOARS + GEM model identifies crucial factors that contribute to racial disparity in dose reduction and early chemotherapy termination. These factors include symptom phenotype and intensity, symptom reporting and management, and social determinants of health. In addition, the biologic variables of genomics, epigenomics during BC chemotherapy, and chemotherapy metabolism are modeled. The purpose of this paper is to provide a presentation and explanation of this model with relevant science. We will explore each variable in the model (Fig. 1) and provide current supporting evidence (Table 1).
Fig. 1.
The SEMOARS + GEM explanatory model
Table 1.
Influence of social determinants of health, symptom experience, genomics and epigenomics on outcomes during breast cancer chemotherapy
| Age | |
|
Griggs et al. [26] Sample N=1403 Black 361 Low-acculturated Hispanic 186 High-acculturated Hispanic 183 Non-Hispanic white 673 |
Multivariable logistic regression o Increased age had lesser odds of receiving chemotherapy: OR 0.91 (95% CI 0.90–0.92) |
|
Inwald et al. [42] Sample N=3463 Bavaria, Germany, no race data reported |
Frequency o Women >70 years old were treated less frequently with chemotherapy + endocrine therapy (6.9%) than 50-69 years old women (28.3%) |
|
Owusu et al. [43] Sample N=689 White 643 Minorities 46 |
Chi-square o Women >75 years old (9%) received less chemotherapy compared with 65 to ≤75 years (28%; p < .0001) |
|
Sandy & Della-Fiorentina [8] Sample N=308 Sydney, Australia, no race data reported |
Multivariable regression with backwards selection o Women age ≥ 65 years old had greater odds of having a dose reduction adjusted OR 8.36; 95% CI 2.40–29.08; p= .001 |
| Income/insurance | |
|
Griggs et al. [26] Sample N=1403 Black 361 Low-acculturated Hispanic 186 High-acculturated Hispanic 183 Non-Hispanic white 673 |
Multivariable logistic regression • Medicaid versus other insurance lesser odds of receiving chemotherapy OR 0.59; 95% CI, 0.37–0.95 |
|
Wells et al. [24] Sample N=99 Black 51 White 48 |
Logistic regression • Medicaid/no insurance versus private/private+Medicare* related to adherence to chemotherapy: β= –2.111; • Adjusted OR 0.121; p= .016 |
| Financial toxicity | |
| Wheeler et al. [44] | Multivariable logistic regression predicted risk for black women compared with white |
| Sample N=2494 |
• Financial barrier adjusted risk difference 13.09 (SE 1.50) p < .001 • Insurance loss adjusted risk difference 3.37 (SE 0.83) p < .001 |
|
Black 49% White 51% | |
| Education and symptoms | |
|
Prigozin et al. [45] Sample N=51 |
Pearson’s r: o Education and total symptom scores were inversely related rs= –0.41; p < .01 |
| Race and adherence | |
|
Griggs et al. [26] Sample N=1403 Black 361 Low-acculturated Hispanic 186 High-acculturated Hispanic 183 Non-Hispanic white 673 |
Multivariable logistic regression receipt of chemotherapy compared with non-Hispanic white women • Black women (ns) OR 0.83 95% CI 0.64–1.08 • Hispanic low acculturated women OR 2.00; 95% CI 1.31–3.04 • Hispanic high acculturated women OR 1.43; 95% CI 1.03–1.98 |
|
Wells et al. [24] Sample N=99 Black 51 White 48 |
Chi-square • No difference in adherence to chemotherapy between black and white patients: χ2= 2.627, p= .10 |
|
K. Smith et al. [46] Sample N=121 Black 21 White 98 |
Relative Risk • Modification of chemotherapy treatment in black versus white women: RR= 1.56; p= .04 • Black women received reduced cumulative doses of adjuvant chemotherapy: RR= 2.49; p= .03 |
|
Fedewa et al. [47] Sample N=107,587 White 69.75% Black 11.52% Hispanic 4.57% Asian 2.84% Other minorities 11.32% |
Multivariate regression results • Greater risk of delay in black women (6.78% versus white 3.59%): 60-day delay RR= 1.36; 95% CI, 1.30–1.41 90-day delay RR= 1.56; 95% CI, 1.44–1.69 • Greater risk of delay in Hispanic women (6.91% versus white 3.59%): 60-day delay RR= 1.31; 95% CI, 1.23–1.39 90-day delay RR= 1.41; 95% CI, 1.26–1.59 |
| Check et al. [48] | Generalized Linear Model Step-wise Regression with cancer-specific physical well-being and 1) race 2) clinical and demographics 3) interpersonal processes of care for black women: |
|
Sample N=4002 (N=2740 for 6-month timepoint) Black 316 White 2672 Hispanic 498 Asian 516 |
• At baseline, interpersonal processes of care domains for compassion (β= 0.40; p= .02), elicited concerns (β= 0.59; p= .0009), and explained results (β= 0.46; p= .002) were positively associated with physical well-being and discrimination due to race was negatively associated (β= –0.58; p= .005) • Black and white women differences in physical well-being widened at 6 months (β= –0.99; p= .02) |
| Newman et al. [4] | Pooled meta-analysis of breast cancer mortality in black compared with white women: |
|
Sample 14 studies N=52,474 Black 10,001 White 42,473 |
• Random effects for mortality OR 1.215; 95% CI 1.13–1.30 • Adjusted for socioeconomic status OR 1.27; 95% CI 1.17–1.38 |
| Symptom/severity | |
|
Simon et al. [49] Sample N=126 Black 27.8% White 65.1% |
Independent sample t test chemotherapy induced peripheral neuropathy (CIPN) black women experienced and reported more CIPN compared with white women: • Sensory scale: 28.6 versus 14.4, p < .002 • Motor scale: 25.0 versus 15.6, p < .012 • Autonomic scale: 24.3 versus 13.4, p < .014 • Reported CIPN: 82.9% versus 67.1% |
|
Yee et al. [32] Sample N=121 Black 100% |
Pearson Correlation • Full dose chemotherapy at midpoint with: o Symptom distress at baseline r= 0.243; p= .007; mid-chemo course r= 0.187, p= .042; and completion r= 0.180, p= .050 o Total number of symptoms at baseline r= –0.225, p= .014 • Full dose chemotherapy at endpoint with: o Total number of symptoms at baseline r= 0.189; p= .039 |
|
Bandos et al. [50] Sample N=1512 |
Multivariable ordinal logistic regression • Women ≥50 were more likely to experience long term peripheral neuropathy OR 1.34; 95% CI 1.10–1.65; p=.005 |
|
Gnerlich et al. [51] Sample N=243,012 |
Cox regression • Younger (<40 years old) were more likely to die with Stage 1 (adjusted HR 1.44; 95% CI 1.27-1.64) or Stage 2 (adjusted HR 1.09; 95% CI 1.03–1.15) than women older than 40 |
|
Gaston-Johansson et al. [52] Sample N=30 Black 100% |
Chi-square • Symptoms increased at midpoint of chemotherapy and then decreased or remained the same at completion. For example, worst pain χ2= 7.81, p= .027 |
|
Schneider et al. [53] Sample N=1779 African descent 213 European descent 1566 |
Logistic regression with Cox hazard ratio • Compared with other races, patients of African descent had increased risk of taxane-induced peripheral neuropathy (TIPN) grade 2-4 HR 2.1; p= 5.6 × 10-16 and grade 3-4 HR 2.6; p= 1.1 × 10-11 |
| Symptoms and race/ethnicity | |
|
Eversley et al. [34] Sample N=116 White 30% Black 30% Latina 25% Other minorities 15% Breast cancer survivors |
Comparing race/ethnicity: o Latina reported more symptoms (μ= 2.5) than black (μ= 1.5) or white (μ= 1.2; p < .01) o Black (91%) and Latina (93%) reported more pain (white 54%; p < .001) o Latina (89%) reported more depressive symptoms compared with black (38%) and white (40%; p < .001) Least Squares Regression for total number of symptoms: o Income β= –0.397 p= .003 o Mastectomy β= 0.340 p= .005 o Chemotherapy β= 0.340 p= .026 o Latina β= 0.340 p= .004 |
|
Miaskowski et al. [54] Sample N=582 Breast, gastrointestinal, gynecological, or lung cancer patients undergoing chemotherapy |
Latent Class Analysis yielded 3 trajectories for symptoms: o “All High” 13.9% of patients o Younger age F= 6.07; p= .002 (low versus moderate and high) o Less education F= 5.00; p= .007 (low versus moderate and high) o Minorities χ2= 8.81; p= .012 (low versus moderate and high) o Lower income KW= 22.81; p < . 0001 (low and moderate versus high) o Breast cancer χ2= 11.17; p= .083 o More comorbidities F= 38.99; p < .0001 (low versus moderate versus high) o Lower reported functional status F= 38.73; p < .0001 (low versus moderate versus high) o “Moderate” 50% of patients o “Low” 36.1% of patients o Fewer females χ2= 24.39; p < .0001 (low versus moderate and high) o More married/partnered χ2= 10.80; p= .005 (low versus high) |
| Comorbidities and cancer | |
|
Leach et al. [55] Sample N=1527 Black 18.1% White 50.5% Other minorities 31.4% |
Prevalence and Linear Regression: o Compared with breast cancer survivors, fewer comorbidities were reported by prostate cancer survivors β= –1.22; p= .0001; as well as colorectal cancer survivors β= –0.62; p= .0243 and ovarian cancer survivors β=–0.55; p= .042 o Compared with white cancer survivors, black cancer survivors reported fewer comorbidities β= –0.89; p= .0112 o Breast cancer survivors reported having experienced more comorbidities (5.8; 95% CI 5.4–6.2) than survivors of other cancers |
| Comorbidity and adherence | |
|
Fedewa et al. [47] Sample N=107,587 Black 11.52% Hispanic 4.57% Asian 2.84% White 69.75% Other minorities 11.32% |
Multivariate regression results Greater risk of delay compared with no comorbidity: • 60-day delay 1 comorbidity RR= 1.09; 95% CI, 1.04–1.14 ≥2 comorbidities RR= 1.32; 95% CI, 1.21–1.45 • 90-day delay 1 comorbidity RR= 1.13; 95% CI, 1.34–1.23 ≥2 comorbidities RR= 1.32; 95% CI, 1.10–1.60 |
| Comorbidity and survival | |
|
Braithwaite et al. [56] Sample N=1254 Black 416 White 838 |
Logistic regression with Cox hazard ratios • Hypertension increased risk of mortality after adjusting for age and race HR 1.33 95% CI 1.07–1.67 |
|
Klepin et al. [57] Sample N=329 Black 11% White 87% Other minorities 1% Unknown 1% |
Multivariable logistic regression for overall survival • Total number of comorbidities HR 1.18; 95% CI 1.06–1.33; p < .01 |
| Beliefs and adherence | |
|
Gatti et al. [58] Sample N=275 Black 86.2% White 5.1% Other minorities 8.7% |
Multivariable logistic regression on medication adherence in general • Negative beliefs about medication is a predictor of low adherence adjusted OR 2.12; 95% CI 1.3–3.7; p=.006 |
| Spirituality and patient-reported outcomes | |
|
Gaston-Johansson et al. [52] Sample N=30 Black 100% |
Correlation • Negative religious coping correlated with psychological distress r= 0.6; p < .05, anxiety r= 0.51; p < .05, and depression r= 0.65; p < .01 |
| Interpersonal communication and mistrust | |
|
Sutton et al. [59] Sample N=210 Black 100% |
Multiple linear regression • Low rating of chemotherapy communication was associated with greater medical mistrust high school or less p=.02 |
|
Tucker et al. [60] Sample N=298 Black 100% |
Mediation analysis • Trust mediated the role of cultural sensitivity in the domains of provider competence/confidence, provider sensitivity/interpersonal skill, and provider respect/communication with patient satisfaction |
|
Jiang et al. [33] Sample N=101 Black 100% |
Multiple linear regression • Perceived better physician interpersonal communication was positively associated with beliefs in the necessity of chemotherapy β= 0.057; p= .007 |
| Genomics and taxane-induced peripheral neuropathy (TIPN) | |
|
Schneider et al. [61] Sample N=213 Black 100% |
Gene-based case control statistical analysis (SKAT) • SET binding factor 2 (SBF2) was associated with TIPN p= 4.35 × 10−6 |
|
Hertz et al. [62] Sample N = 411 White discovery cohort 209 Black replication cohort 107 |
Log-rank test and Cox proportional hazards • In European-American discovery cohort, CYP2C8*3 genotype increased risk of grade 2+ neuropathy for each allele HR =1.95; 95% CI 1.06–3.58; p = .031 • In African-American replication cohort, no homozygotes were found, but one allele of CYP2C8*3 increase TIPN risk HR = 3.30; 95% CI 1.04–10.45; p = .043 |
|
Baldwin et al. [63] Sample N = 1126 White discovery cohort 855 Black replication cohort 154 White replication cohort 117 |
Ordinal logistic regression o In the white (European) discovery cohort, FGD4 was associated with TIPN HR 1.57; 95% CI 1.30–1.91; p = 2.6 × 10−6 o The white replication cohort was similar HR 1.72; 95% CI 1.06–2.80; p = .013 o The black replication cohort was also associated HR 1.93; 95% CI 1.13–3.28; p = 6.7 × 10−3 |
|
Abraham et al. [64] Sample N = 1303 samples from several trials White 100% |
Unconditional logistic regression and likelihood ratio test o ATP-binding cassette, subfamily B (ABCB1) was associated with decreased odds of TIPN OR 0.47; 95% CI 0.28–0.79; p = .004 o Tubulin Beta 2A Class IIa (TUBB2A) was also associated with increased odds of TIPN OR 1.80; 95% CI 1.20–2.72; p = .005 |
|
Apellaniz-Ruiz et al. [65] Sample N = 146 White 100% |
Cumulative dose analysis and additive model o Ephrin Receptor A5 (EPHA5) was associated with TIPN HR 2.3; 95% CI 1.6–3.9; p = .0074 o Ephrin Receptor A6 (EPHA6) was associated with TIPN HR 1.9; 95% CI 1.2–2.9; p = .0063 o Ephrin Receptor A8 (EPHA8) was associated with TIPN HR 1.9; 95% CI 1.1–3.2; p = .0012 |
|
Boso et al. [66] Sample N = 113 White 100% |
Multivariate logistic regression o Excision repair cross complementation group 1 (ERCC1) was associated with TIPN p = .006 |
| Epigenomics and chemotherapy | |
|
Smith et al. [67.] Sample N = 61 Black 25 White 36 |
Linear regression (MethLAB) o CpG sites with change in methylation after chemotherapy versus no chemotherapy included o cg26077811 β = −.074, p = 3.65 × 10−9 o cg18942579 β = −.161, p = 1.65 × 10−8 o cg12054453 β = −.154, p = 2.75 × 10−8 o cg16936953 β = −.168, p = 3.26 × 10−8 o cg05438378 β = −.089, p = 7.78 × 10−8 o cg25446789 β = −.085, p = 7.84 × 10−8 o cg01409343 β = −.138, p = 9.88 × 10−8 o cg13518625 β = −.051, p = 9.98 × 10−8 |
Where studies categorized race as other or nonwhite (likely grouped due to the sample size), we used the terms minorities or other minorities
ns, not significant; OR, odds ratio; HR, hazard ratio; CI, confidence interval; RR, relative risk; SE, standard error; SD, standard deviation; β, beta; μ, mean; KW, Kruskal-Wallis; χ2 , chi-square; RR, risk ratio; “white” was used in some cases when European ancestry was indicated
*Health insurance variable was a surrogate for income/socioeconomic class
**Only measured number of sessions, not dose. Adherence divided into 100% attendance or less than 100% and was defined by patient factors: missed appointments, cancellations, no shows, etc.; no delays or discontinuations by the provider were noted
Social Determinants of Health
The model begins with the patient (within the context of her social and physical environment) initiating BC chemotherapy. Social determinants of health associated with increased symptom experience and intensity are considered integral and specific to each patient. Race/ethnicity, age, income, education, zip code, allostatic load, comorbidity, and self-efficacy/belief in prescribed medication are social determinants of health that may be associated with increased symptoms resulting in dose reductions, chemotherapy holds, and early therapy cessation [68–74]. The following sections provide a review of evidence of these associations to date.
Race/Ethnicity
Racial and ethnic differences in treatment delivery and symptoms are documented [75–77]. Black women experience more chemotherapy delays compared with white women [47, 78]. Symptoms may be a causative factor. For example, minority women describe more symptom intensity and distress BC treatment [79, 80]. In a recently completed study of 140 black women receiving adjuvant BC chemotherapy, nearly all (99%) black women initiated chemotherapy, and almost 40% received a reduction in dose intensity, early cessation, or delay associated with symptoms and cancer-related distress [32].
Age
Evidence regarding the influence of age on symptom distress is contradictory. Older women are more likely to receive a lower chemotherapy dose intensity, due to fewer prescriptions and more dose reductions than younger women, with a subsequent decrease in overall survival [8, 42, 43]. Most studies adjust for age in their analyses, and when examined as a factor related to symptoms, age produced mixed results. Older age was associated with increased long-term peripheral neuropathy in docetaxel regimens [50] and more overall toxicity, such as chemotherapy-induced bone marrow toxicities [81]. Conversely, younger women experience an increase in symptoms related to cognitive function [82]. Miaskowski et al. examined factors across multiple tumor types associated with increased symptom distress during cancer chemotherapy and found younger age, female sex, low social support, and socioeconomic status to be characteristic of the symptom grouping for greater symptom severity, suggesting multiple factors, including age, are related to symptoms experienced [54].
Income/Education/Zip Code
Indicators of socioeconomic status, including income, education level, and zip code, affect women’s experience of BC chemotherapy and overall treatment. Lower-income women are more likely to report symptoms after treatment [34] as well as a financial burden, and black women report a greater financial burden than white women after controlling for socioeconomic status [44]. Education level, which is often correlated with socioeconomic status, was inversely related to chemotherapy symptoms, with better-educated women reporting a lower symptom burden [45] and being less likely to receive a chemotherapy dose reduction [22]. Additionally, black women with less education were more likely to report perceived discrimination and disparities in their care [59].
Zip code may be used as a surrogate measure of socioeconomic status, such as income, education, and employment, in addition to the geographical region. For example, Griggs et al. found that when compared with the Northeastern region of the USA, patients in the Southern region had greater odds of receiving a reduction in their chemotherapy dose [22]. Financial, educational, and geographic factors influence symptoms and BC treatment intensity.
Allostatic Load
Allostatic load is an algorithmic risk factor representing cumulative stress exposure causing persistent, severe psychological and physical symptoms for any illness, specifically cancer. Geronimus et al. used the term “weathering” to characterize the effect of cumulative stress from multiple stressors on US blacks in their residential, occupational, and other environments [83]. Thus, among black and low-income women, there is increasing concern about the impact of a lifetime of accumulated stress on illness outcomes, including BC outcomes [84, 85]. The impact of the full range of childhood and cumulative adult-life stress exposure has not yet been studied in relation to cancer-related symptoms.
Comorbidity
Black women with BC have more comorbid conditions [86] than white women has implications for BC outcomes. For example, hypertension accounted for 30% of racial survival disparity for one BC cohort [56]. An 18% increased risk of death was observed with each additional comorbid condition [57]. Comorbidities may interfere with treatment and are associated with chemotherapy delays [47, 87]. A meta-analysis concluded that patients with comorbidities had lesser odds of receiving chemotherapy and greater odds of toxicity [88]. The precise means by which comorbidities increase symptom incidence and distress and influence chemotherapy intensity is not clear.
Beliefs and Communication
The belief that medication is necessary and efficacious, in addition to the concern over possible harmful effects, can influence whether a patient will carry out a prescribed treatment [58]. Concern may result from mistrust among black patients in a traditionally white health care system or belief among black women that health care providers are not sufficiently culturally sensitive to address specific concerns [59, 60, 89, 90]. Communication is essential to establishing trust in the provider-patient relationship and was negatively correlated with medical mistrust among black women with BC [59]. The communication patterns between clinician and patient, described as the patient centeredness of care (PCC), coded and scored through a 23-item checklist, may be an important explanation for racial differences in communication during BC clinical visits. Rosenzweig’s team described a prospective, comparative pilot study qualitatively coded for PCC during the clinical visit of women undergoing BC chemotherapy and compared by race. Twenty-four clinical visits were recorded in a sample of five black and five white women undergoing BC chemotherapy. Overall for each PCC item, the mean clinician visit scores for black women were higher (worse PCC) than the mean clinician visit scores for white women. Significant differences were found in 27% of the PCC items. The higher scores were evident for three of the four subscales “Invest in the Beginning,” “Elicit the Patient’s Perspective,” and “Demonstrate Empathy” [91].
Symptom Phenotype and Intensity
For all women, once the chemotherapy dosing is calculated and initiated, follow-up doses may be decreased, held, or discontinued if patients exhibit symptoms of toxicity. There is a pattern during chemotherapy that symptoms increase from pre-chemotherapy to mid-therapy but stabilize after chemotherapy treatment midpoint to completion [32, 52], suggesting a symptom tolerance among patients. Associations between the ability to receive ≥ 85% of the prescribed treatment course and symptom distress, severity, and the total number of symptoms at pre-chemotherapy are reported [32]. Minority patients were more likely to belong to the high-symptom group when symptom severity was categorized into low, moderate, and high [54]. Other variables to consider in racial symptom and treatment disparity include baseline genomics and temporal epigenomic changes that may be associated with symptom phenotype and treatment response.
Genomics and Epigenomics of Symptoms and Chemotherapy Metabolomics
Though social determinants of health are factors related to disparity in dosing and completion of chemotherapy, they do not fully account for the disparity in BC symptoms and ability to receive the full dose of chemotherapy in black women compared with white women. Genomic variation may help to explain a portion of these differences. For example, taxane-based chemotherapy used in BC treatment has a highly variable drug response and symptom profile and is metabolized through the cytochrome P450 system. Variations in cytochrome P450 genes CYP3A4, CYP3A5, and CYP2C8, as well as transporter genes ABCB1, ABCB2, and SCLO1B3, would likely result in individual differences in drug metabolism [92]. Genotype variations may result in an increase or decrease in patients’ symptoms, based upon the drug and the gene’s role in metabolism. CYP2C8*3 was associated with grade 2+ neuropathy in European- and African-Americans treated with paclitaxel, but African-Americans with the variant had greater odds of developing taxane-induced peripheral neuropathy, and no homozygotes for the variant were observed [62].
There is considerable variability in absorption, distribution, metabolism, and excretion (ADME) of drugs. These differences can be explained by genetic variation in ADME-related genes [93]. ADME-related genetic variability often differs across populations [94] and helps to explain the link between ancestry and variable chemotherapy drug response [95]. Li et al. observed greater diversity in ADME genes for the African-American population compared with European and African populations [96], predisposing African-Americans to more variable drug response. Gene variations may alter drug metabolism by activating or inactivating a medication, activating or inactivating a drug’s metabolite, affecting the medication’s transport, or affecting the drug’s intended target [97]. Concomitant medications may also facilitate or interfere with drug metabolism.
Genes not directly involved in drug metabolism may affect symptoms experienced among diverse ancestries. For example, taxane-induced peripheral neuropathy (TIPN) is a common symptom with known genomic associations. Variants in the Charcot-Marie-Tooth (CMT) disease gene, SBF2, were predictors of TIPN in black patients [61]. Another CMT gene, FGD4, was associated with paclitaxel-induced peripheral neuropathy in patients of European or African ancestry [63]. ABCB1 (noted as a transporter above), ERCC1, TUBB2A, and EPHA5/6/8 were associated with neuropathy in European samples [64–66]. Most genome-wide databases revealed focus exclusively on European populations, rather than African, Asian, and Latin American, which underscores the gap in genome-wide research and importance of recruitment for all ancestral populations in research [98, 99].
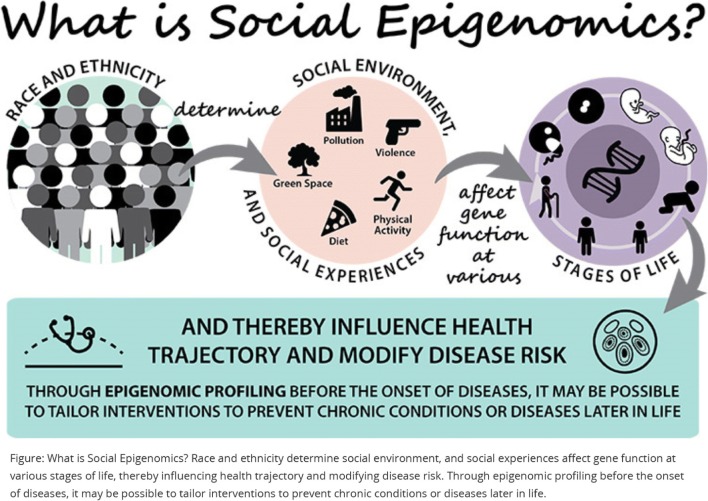
Genomic variation in ancestry and drug metabolism genes are not the only factors associated with the development of toxicities. Epigenetic changes, perhaps those caused by chemotherapy, other cancer treatments, or social determinants of health, may also increase symptoms (Fig. 2). DNA methylation, the addition of a methyl group to the nucleotide cytosine, is one type of epigenetic change which potentially affects gene expression by changing the gene’s activity (increase or decrease). For example, an increase in inflammatory markers was found in patients with BC following a decrease in DNA methylation post-chemotherapy, indicating that DNA methylation mediated a relationship between chemotherapy and inflammatory biomarkers [67]. The study of epigenetic change resulting from chemotherapy and its influence on drug response and symptoms is an emerging field.
Fig. 2.
The role of social determinants and epigenomics in health and disease. Figure used with permission from NIH (https://epi.grants.cancer.gov/epigen.html)
In summary, the SEMOARS + GEM explanatory model for disparity among black women with BC examines psychosocial, clinical, and biological factors impacting treatment delivery, symptoms, and outcomes. Testing each aspect of this model and determining the unique contribution of each component to overall BC treatment disparity is critically important to understanding the most relevant actionable targets for ensuring treatment equity.
Discussion
We presented evidence for the development of the SEMOARS + GEM model of disparity of BC treatment in black women. This comprehensive model, supported by previous research, describes a process that transpires during a BC diagnosis and treatment. Black women bring their life experiences and characteristics, in the form of social determinants of health and genomic profiles, at the time of diagnosis. These factors influence the patient’s symptom experience including symptom phenotype, intensity, reporting to clinicians, and subsequent clinician management. Resultant dose alterations or early cessation may occur. Epigenetic changes and chemotherapy metabolism over time may moderate the symptom experience.
Many of the variables discussed in this paper, for example, education and socioeconomic status and similarly BMI and comorbidities, are typically correlated with one another. Instead of examining race and age as predictors, many studies control for them statistically. Additionally, most study samples do not include a large enough black population to draw inferences, and few focus solely on black women.
A disparity in outcomes for black women with BC has been well-established, but no one factor explains the issue in its entirety. Outcomes for black women are influenced by social determinants of health [100]. Barsevick et al. reported that social determinants such as education, unemployment, marital status, age, comorbidity, and medical mistrust were factors in post-treatment burden for survivors [101]. All of these factors are examined in the SEMOARS + GEM model. Potentially pertinent factors, not examined in depth, are those addressed in the US government’s public health initiative, Healthy People 2020. According to Healthy People 2020, social determinants of health encompass economic stability, education, social and community context, health and health care, and neighborhood and built environment [102]. The SEMOARS + GEM model somewhat superficially measures many of these concepts, though perhaps not as in-depth as prescribed by Healthy People 2020.
Symptom phenotype and intensity experienced by black women are more pronounced than in white women, resulting in an inability of more black women to receive the entire chemotherapy treatment and leading to poorer outcomes. Additionally, Zannas et al. provided evidence of the impact of social determinants of health on epigenetic aging in an African-American cohort, confirming that in addition to genomics, stressors leading to physiologic changes should be further studied [103].
The SEMOARS + GEM model will add in-depth descriptive work to find actionable targets, which will inform further implementation and translation into clinical practice guidelines. Should identification of genomic associations ensue, a precision health care strategy may be routinely implemented into clinical care to identify at-risk patients for early symptom management in order to tailor chemotherapy treatments to the patient. Providing an individualized protocol for all patients with BC, including factors specific to black women, will offer improved symptom management. Biological ancestral differences in chemotherapy metabolism and epigenomics suggest that variations influence symptom and treatment outcome differences.
The SEMOARS + GEM model includes known factors that influence a black woman’s disparate BC treatment experience to inform future interventions to improve the ability to receive complete, effective BC chemotherapy.
Funding Information
Funding supported by the National Institute on Minority Health and Health Disparities (1R01MD012245-01; PI: Rosenzweig) and the Center for Clinical and Translational Science, University of Pittsburgh (Feasibility and Acceptability of Genomics Research Exploring Racial Differences in Metabolism and Toxicity of Breast Cancer Chemotherapy, PI: Rosenzweig). M. McCall is also supported by National Institute of Nursing Research (T32NR009759; PI: Conley), a Doctoral Degree Scholarship in Cancer Nursing, DSCN-19-049-01, from the American Cancer Society.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maura K. McCall, Email: mccallm@pitt.edu
Mary Connolly, Email: mcc55@pitt.edu.
Bethany Nugent, Email: bethany.nugent@pitt.edu.
Yvette P. Conley, Email: yconley@pitt.edu
Catherine M. Bender, Email: cbe100@pitt.edu
Margaret Q. Rosenzweig, Email: mros@pitt.edu
References
- 1.Richardson LC, Henley SJ, Miller JW, Massetti G, Thomas CC. Patterns and trends in age-specific black-white differences in breast cancer incidence and mortality - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2016;65:1093–1098. doi: 10.15585/mmwr.mm6540a1. [DOI] [PubMed] [Google Scholar]
- 2.Shoemaker ML, White MC, M W, Weir HK, Romieu I. Differences in breast cancer incidence among young women aged 20-49 years by stage and tumor characteristics, age, race, and ethnicity, 2004-2013. Breast Cancer Res Treat. 2018;169:595–606. doi: 10.1007/s10549-018-4699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI, Jemal A. Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016;66:290–308. doi: 10.3322/caac.21340. [DOI] [PubMed] [Google Scholar]
- 4.Newman LA, Mason J, Cote D, Vin Y, Carolin K, Bouwman D, Colditz GA. African-American ethnicity, socioeconomic status, and breast cancer survival: a meta-analysis of 14 studies involving over 10,000 African-American and 40,000 white American patients with carcinoma of the breast. Cancer. 2002;94:2844–2854. doi: 10.1002/cncr.10575. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy AM, Yang J, Armstrong K. Increasing disparities in breast cancer mortality from 1979 to 2010 for US black women aged 20 to 49 years. Am J Public Health. 2015;105:S446–S448. doi: 10.2105/AJPH.2014.302297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67(6):439–448. doi: 10.3322/caac.21412. [DOI] [PubMed] [Google Scholar]
- 7.Bonadonna G, Valagussa BS. Dose-response effect of adjuvant chemotherapy in breast cancer. NEJM. 1981;304:10–15. doi: 10.1056/NEJM198101013040103. [DOI] [PubMed] [Google Scholar]
- 8.Sandy J, Della-Fiorentina S. Relative dose intensity in early stage breast cancer chemotherapy: a retrospective analysis of incidence, risk factors and outcomes at a south-west Sydney cancer clinic. Asia-Pacific J Clin Oncol. 2013;9:365–372. doi: 10.1111/ajco.12093. [DOI] [PubMed] [Google Scholar]
- 9.Wildiers H, Reiser M. Relative dose intensity of chemotherapy and its impact on outcomes in patients with early breast cancer or aggressive lymphoma. Crit Rev Oncol Hematol. 2011;77:221–240. doi: 10.1016/j.critrevonc.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Rao D, Debb S, Blitz D, Choi SW, Cella D. Racial/ethnic differences in the health-related quality of life of cancer patients. J Pain Symptom Manag. 2008;36(5):488–496. doi: 10.1016/j.jpainsymman.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams SA, Butler WM, Fulton J, Heiney SP, Williams EM, Delage AF, Khang L, Hebert JR. Racial disparities in breast cancer mortality in a multiethnic cohort in the southeast. Cancer. 2011;118:2693–2699. doi: 10.1002/cncr.26570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bach PB, Schrag D, OW Brawley A, Galaznik SY, Begg CB. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287:2106–2113. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 13.Clegg LX, Li FP, Hankey BF, Chu K, Edwards BK. Cancer survival among us whites and minorities: a SEER (surveillance, epidemiology, and end results) program population-based study. Arch Intern Med. 2002;162:1985–1993. doi: 10.1001/archinte.162.17.1985. [DOI] [PubMed] [Google Scholar]
- 14.Menashe I, Anderson WF, Jatoi I, Rosenberg PS. Underlying causes of the black-white racial disparity in breast cancer mortality: a population-based analysis. J Natl Cancer Inst. 2009;101:993–1000. doi: 10.1093/jnci/djp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris AM, Rhoads KF, Stain SC, Birkmeyer JD. Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg. 2010;211:105–113. doi: 10.1016/j.jamcollsurg.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 16.Penner LA, Eggly S, Griggs JJ, Underwood W, Orom H, Albrecht TL. Life-threatening disparities: the treatment of Black and White cancer patients. J Soc Issues. 2012;68:328–357. doi: 10.1111/j.1540-4560.2012.01751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. JNCI: J Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 18.Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 19.Gurney H. How to calculate the dose of chemotherapy. Br J Cancer. 2002;86:1297–1302. doi: 10.1038/sj/bjc/6600139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurney H. Dose calculation of anticancer drugs: a review of the current practice and introduction of an alternative. J Clin Oncol. 1996;14(9):2590–2611. doi: 10.1200/jco.1996.14.9.2590. [DOI] [PubMed] [Google Scholar]
- 21.Griggs JJ, Mangu PB, Temin S, Lyman GH, Anderson H, Balaban EP, Dignam JJ, et al. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Oncol Pract. 2012;8:e59–e61. doi: 10.1200/JOP.2012.000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griggs JJ, Culakova E, Sorbero MES, Van Ryn M, Poniewierski MS, Wolff DA, Crawford J, Dale DC, Lyman GH. Effect of patient socioeconomic status and body mass index on the quality of breast cancer adjuvant chemotherapy. J Clin Oncol. 2007;25:277–284. doi: 10.1200/JCO.2006.08.3063. [DOI] [PubMed] [Google Scholar]
- 23.Smith TJ, Hillner BE. Ensuring quality cancer care by the use of clinical practice guidelines and critical pathways. J Clin Oncol. 2001;19(11):2886–2897. doi: 10.1200/jco.2001.19.11.2886. [DOI] [PubMed] [Google Scholar]
- 24.Wells JS, Strickland OL, Dalton JA, Freeman S. Adherence to intravenous chemotherapy in African American and white women with early-stage breast cancer. Cancer Nurs. 2015;38(2):89–98. doi: 10.1097/NCC.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griggs JJ, Culakova E, Sorbero ME, Poniewierski MS, Wolff DA, Crawford J, Dale DC, Lyman GH. Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J Clin Oncol. 2007;25(18):2522–2527. doi: 10.1200/JCO.2006.10.2749. [DOI] [PubMed] [Google Scholar]
- 26.Griggs JJ, Hawley ST, Graff JJ, Hamilton AS, Jagsi R, Janz NK, Mujahid MS, et al. Factors associated with receipt of breast cancer adjuvant chemotherapy in a diverse population-based sample. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2012;30:3058–3064. doi: 10.1200/JCO.2012.41.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hershman D, Weinberg M, Rosner Z, Alexis K, Tiersten A, Grann VR, Troxel A, Neugut AI. Ethnic neutropenia and treatment delay in African American women undergoing chemotherapy for early-stage breast cancer. J Natl Cancer Inst. 2003;95:1545–1548. doi: 10.1093/jnci/djg073. [DOI] [PubMed] [Google Scholar]
- 28.Wu XC, Lund MJ, Kimmick GG, Richardson LC, Sabatino SA, Chen VW, Fleming ST, Morris CR, Huang B, Trentham-Dietz A, Lipscomb J. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol. 2012;30(2):142–150. doi: 10.1200/jco.2011.36.8399. [DOI] [PubMed] [Google Scholar]
- 29.Mandelblatt JS, Sheppard VB, Neugut AI. Black-white differences in breast cancer outcomes among older Medicare beneficiaries: does systemic treatment matter? JAMA. 2013;310(4):376–377. doi: 10.1001/jama.2013.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jemal A, Robbins AS, Lin CC, Flanders WD, DeSantis CE, Ward EM, Freedman RA. Factors that contributed to black-white disparities in survival among nonelderly women with breast cancer between 2004 and 2013. J Clin Oncol. 2018;36:14–24. doi: 10.1200/JCO.2017.73.7932. [DOI] [PubMed] [Google Scholar]
- 31.Silber JH, Rosenbaum PR, Clark AS, Giantonio BJ, Ross RN, Teng Y, Wang M, Niknam BA, Ludwig JM, Wang W, Even-Shoshan O, Fox KR. Characteristics associated with differences in survival among black and white women with breast cancer. Jama. 2013;310(4):389–397. doi: 10.1001/jama.2013.8272. [DOI] [PubMed] [Google Scholar]
- 32.Yee MK, Sereika SM, Bender CM, Brufsky AM, Connolly MC, Rosenzweig MQ. Symptom incidence, distress, cancer-related distress, and adherence to chemotherapy among African American women with breast cancer. Cancer. 2017;123:2061–2069. doi: 10.1002/cncr.30575. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Y, Sereika S, Bender C, Brufsky A, Rosenzweig M. Beliefs in chemotherapy and knowledge of cancer and treatment among African American women with newly diagnosed breast cancer. Oncol Nurs Forum. 2016;43:180–189. doi: 10.1188/16.ONF.180-189. [DOI] [PubMed] [Google Scholar]
- 34.Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Post-treatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32:250–256. doi: 10.1188/05.ONF.250-256. [DOI] [PubMed] [Google Scholar]
- 35.Haggstrom DA, Quale C, Smith-Bindman R. Differences in the quality of breast cancer care among vulnerable populations. Cancer. 2005;104:2347–2358. doi: 10.1002/cncr.21443. [DOI] [PubMed] [Google Scholar]
- 36.Richardson LC, Wang W, Hartzema AG, Wagner S. The role of health-related quality of life in early discontinuation of chemotherapy for breast cancer. Breast J. 2007;13:581–587. doi: 10.1111/j.1524-4741.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 37.Rosenzweig M, Connolly M. Total dose of chemotherapy received in cohort of African American women with breast cancer. Oncol Nurs Forum. 2016;43:133. doi: 10.1188/16.ONF.180-189. [DOI] [PubMed] [Google Scholar]
- 38.Rosenzweig M, Brufsky A, Rastogi P, Puhalla S, Simon J, Underwood S. The attitudes, communication, treatment, and support intervention to reduce breast cancer treatment disparity. Oncol Nurs Forum. 2011;38:85–89. doi: 10.1188/11.onf.85-89. [DOI] [PubMed] [Google Scholar]
- 39.Rosenzweig MQ, Wiehagen T, Brufsky A, Arnold R. Challenges of illness in metastatic breast cancer: a low-income African American perspective. Palliat Support Care. 2009;7:143–152. doi: 10.1017/S1478951509000194. [DOI] [PubMed] [Google Scholar]
- 40.von Friederichs-Fitzwater MM, Denyse RT. The unmet needs of African American women with breast cancer. Adv Breast Cancer Res. 2012;2012:1–6. doi: 10.4236/abcr.2012.11001. [DOI] [Google Scholar]
- 41.Yoon J, Malin JL, Tisnado DM, Tao ML, Adams JL, Timmer MJ, Ganz PA, Kahn KL. Symptom management after breast cancer treatment: is it influenced by patient characteristics? Breast Cancer Res Treat. 2008;108:69–77. doi: 10.1007/s10549-007-9580-1. [DOI] [PubMed] [Google Scholar]
- 42.Inwald E, Ortmann O, Koller M, Zeman F, Hofstädter F, Evert M, Brockhoff G, Klinkhammer-Schalke M. Screening-relevant age threshold of 70 years and older is a stronger determinant for the choice of adjuvant treatment in breast cancer patients than tumor biology. Breast Cancer Res Treat. 2017;163:119–130. doi: 10.1007/s10549-017-4151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Owusu C, Lash TL, Silliman RA. Effect of undertreatment on the disparity in age-related breast cancer-specific survival among older women. Breast Cancer Res Treat. 2007;102:227–236. doi: 10.1007/s10549-006-9321-x. [DOI] [PubMed] [Google Scholar]
- 44.Wheeler SB, Spencer JC, Pinheiro LC, Carey LA, Olshan AF, Reeder-Hayes KE. Financial impact of breast cancer in black versus white women. J Clin Oncol. 2018;36:1695–1701. doi: 10.1200/JCO.2017.77.6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prigozin A, Uziely B, Musgrave CF. The relationship between symptom severity and symptom interference, education, age, marital status, and type of chemotherapy treatment in Israeli women with early-stage breast cancer. Oncol Nurs Forum. 2010;37:E411–E418. doi: 10.1188/10.ONF.E411-E418. [DOI] [PubMed] [Google Scholar]
- 46.Smith K, Wray L, Klein-Cabral M, Schuchter L, Fox K, Glick J, DeMichele A. Ethnic disparities in adjuvant chemotherapy for breast cancer are not caused by excess toxicity in black patients. Clin Breast Cancer. 2005;6(3):260–266. doi: 10.3816/CBC.2005.n.029. [DOI] [PubMed] [Google Scholar]
- 47.Fedewa SA, Ward EM, Stewart AK, Edge SB. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: a national cohort study 2004-2006. J Clin Oncol. 2010;28:4135–4141. doi: 10.1200/JCO.2009.27.2427. [DOI] [PubMed] [Google Scholar]
- 48.Check DK, Chawla N, Kwan ML, Pinheiro L, Roh JM, Ergas IJ, Stewart AL, Kolevska T, Ambrosone C, Kushi LH. Understanding racial/ethnic differences in breast cancer-related physical well-being: the role of patient–provider interactions. Breast Cancer Res Treat. 2018;170:593–603. doi: 10.1007/s10549-018-4776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simon NB, Danso MA, Alberico TA, Basch E, Bennett AV. The prevalence and pattern of chemotherapy-induced peripheral neuropathy among women with breast cancer receiving care in a large community oncology practice. Qual Life Res. 2017;26:2763–2772. doi: 10.1007/s11136-017-1635-0. [DOI] [PubMed] [Google Scholar]
- 50.Bandos H, Melnikow J, Rivera DR, Swain SM, Sturtz K, Fehrenbacher L, Wade JL, 3rd, et al. Long-term peripheral neuropathy in breast cancer patients treated with adjuvant chemotherapy: NRG Oncology/NSABP B-30. J Natl Cancer Inst. 2018;110:1. doi: 10.1093/jnci/djx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gnerlich JL, Deshpande AD, Jeffe DB, Sweet A, White N, Margenthaler JA. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg. 2009;208:341–347. doi: 10.1016/j.jamcollsurg.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaston-Johansson F, Watkins CC, Kanu IK, Whitehouse E, Sarenmalm EK, Brovall M, Kozachik SL. The effects of symptoms on quality of life during chemotherapy in African-American women with breast cancer. J Natl Black Nurses Assoc : JNBNA. 2015;26:7–16. [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider BP, Li L, Radovich M, Shen F, Miller KD, Flockhart DA, Jiang G, Vance G, Gardner L, Vatta M, Bai S, Lai D, Koller D, Zhao F, O’Neill A, Smith ML, Railey E, White C, Partridge A, Sparano J, Davidson NE, Foroud T, Sledge GW. Genome-wide association studies for taxane-induced peripheral neuropathy in ECOG-5103 and ECOG-1199. Clin Cancer Res. 2015;21:5082–5091. doi: 10.1158/1078-0432.Ccr-15-0586.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miaskowski C, Cooper BA, Melisko M, Chen LM, Mastick J, West C, Paul SM, Dunn LB, Schmidt BL, Hammer M, Cartwright F, Wright F, Langford DJ, Lee K, Aouizerat BE. Disease and treatment characteristics do not predict symptom occurrence profiles in oncology outpatients receiving chemotherapy. Cancer. 2014;120:2371–2378. doi: 10.1002/cncr.28699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leach CR, Weaver KE, Aziz NM, Alfano CM, Bellizzi KM, Kent EE, Forsythe LP, Rowland JH. The complex health profile of long-term cancer survivors: prevalence and predictors of comorbid conditions. J Cancer Surviv. 2015;9(2):239–251. doi: 10.1007/s11764-014-0403-1. [DOI] [PubMed] [Google Scholar]
- 56.Braithwaite D, Tammemagi CM, Moore DH, Ozanne EM, Hiatt RA, Belkora J, West DW, Satariano WA, Liebman M, Esserman L. Hypertension is an independent predictor of survival disparity between African-American and white breast cancer patients. Int J Cancer. 2009;124:1213–1219. doi: 10.1002/ijc.24054. [DOI] [PubMed] [Google Scholar]
- 57.Klepin HD, Pitcher BN, Ballman KV, Kornblith AB, Hurria A, Winer EP, Hudis C, Cohen HJ, Muss HB, Kimmick GG. Comorbidity, chemotherapy toxicity, and outcomes among older women receiving adjuvant chemotherapy for breast cancer on a clinical trial: CALGB 49907 and CALGB 361004 (Alliance) J Oncol Pract. 2014;10:e285–e292. doi: 10.1200/JOP.2014.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gatti ME, Jacobson KL, Gazmararian JA, Schmotzer B, Kripalani S. Relationships between beliefs about medications and adherence. Am J Health Syst Pharm. 2009;66:657–664. doi: 10.2146/ajhp080064. [DOI] [PubMed] [Google Scholar]
- 59.Sutton AL, He J, Edmonds MC, Sheppard VB. Medical mistrust in black breast cancer patients: acknowledging the roles of the trustor and the trustee. J Cancer Educ. 2018;34:1–8. doi: 10.1007/s13187-018-1347-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tucker CM, Moradi B, Wall W, Nghiem K. Roles of perceived provider cultural sensitivity and health care justice in African American/Black patients’ satisfaction with provider. J Clin Psychol Med Settings. 2014;21:282–290. doi: 10.1007/s10880-014-9397-0. [DOI] [PubMed] [Google Scholar]
- 61.Schneider BP, Lai D, Shen F, Jiang G, Radovich M, Li L, Gardner L, Miller KD, O’Neill A, Sparano JA, Xue G, Foroud T, Sledge GW., Jr Charcot-Marie-Tooth gene, SBF2, associated with taxane-induced peripheral neuropathy in African Americans. Oncotarget. 2016;7:82244–82253. doi: 10.18632/oncotarget.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hertz DL, Roy S, Motsinger-Reif AA, Drobish A, Clark LS, McLeod HL, Carey LA, Dees EC. CYP2C8*3 increases risk of neuropathy in breast cancer patients treated with paclitaxel. Ann Oncol. 2013;24:1472–1478. doi: 10.1093/annonc/mdt018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baldwin RM, Owzar K, Zembutsu H, Chhibber A, Kubo M, Jiang C, Watson D, Eclov RJ, Mefford J, McLeod HL, Friedman PN, Hudis CA, Winer EP, Jorgenson EM, Witte JS, Shulman LN, Nakamura Y, Ratain MJ, Kroetz DL. A genome-wide association study identifies novel loci for paclitaxel-induced sensory peripheral neuropathy in CALGB 40101. Clin Cancer Res. 2012;18:5099–5109. doi: 10.1158/1078-0432.CCR-12-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abraham JE, Guo Q, Dorling L, Tyrer J, Ingle S, Hardy R, Vallier AL, Hiller L, Burns R, Jones L, Bowden SJ, Dunn JA, Poole CJ, Caldas C, Pharoah PPD, Earl HM. Replication of genetic polymorphisms reported to be associated with taxane-related sensory neuropathy in patients with early breast cancer treated with paclitaxel. Clin Cancer Res. 2014;20:2466–2475. doi: 10.1158/1078-0432.CCR-13-3232. [DOI] [PubMed] [Google Scholar]
- 65.Apellaniz-Ruiz M, Sanchez-Barroso L, Gutierrez-Gutierrez G, Sereno M, Garcia-Donas J, Avall-Lundqvist E, Green H, Brosen K, Bergmann TK, Rodriguez-Antona C. Replication of genetic polymorphisms reported to be associated with taxane-related sensory neuropathy in patients with early breast cancer treated with paclitaxel--letter.erratum appears in. Clin Cancer Res. 2015;21(18):4244. doi: 10.1158/1078-0432.CCR-15-1693. [DOI] [PubMed] [Google Scholar]
- 66.Boso V, M J Herrero A, Santaballa L, Palomar JE, Megias H, de la Cueva L, Rojas, et al. SNPs and taxane toxicity in breast cancer patients. Pharmacogenomics. 2014;15:1845–1858. doi: 10.2217/pgs.14.127. [DOI] [PubMed] [Google Scholar]
- 67.Smith AK, Conneely KN, Pace TWW, Mister D, Felger JC, Kilaru V, Akel MJ, Vertino PM, Miller AH, Torres MA. Epigenetic changes associated with inflammation in breast cancer patients treated with chemotherapy. Brain Behav Immun. 2014;38:227–236. doi: 10.1016/j.bbi.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bigby JA, Holmes MD. Disparities across the breast cancer continuum. Cancer Causes Control. 2005;16(1):35–44. doi: 10.1007/s10552-004-1263-1. [DOI] [PubMed] [Google Scholar]
- 69.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. JNCI: J Natl Cancer Inst. 2002;94(7):490–496. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 70.Bustami RT, Shulkin DB, O’Donnell N, Whitman ED. Variations in time to receiving first surgical treatment for breast cancer as a function of racial/ethnic background: a cohort study. JRSM open. 2014;5(7):2042533313515863–2042533313515863. doi: 10.1177/2042533313515863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Daly B, Olopade OI. A perfect storm: how tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015;65:221–238. doi: 10.3322/caac.21271. [DOI] [PubMed] [Google Scholar]
- 72.Lund MJ, Brawley OP, Ward KC, Young JL, Gabram SS, Eley JW. Parity and disparity in first course treatment of invasive breast cancer. Breast Cancer Res Treat. 2008;109(3):545–557. doi: 10.1007/s10549-007-9675-8. [DOI] [PubMed] [Google Scholar]
- 73.Popescu I, Schrag D, Ang A, Wong M. Racial/ethnic and socioeconomic differences in colorectal and breast cancer treatment quality: the role of physician-level variations in care. Med Care. 2016;54(8):780–788. doi: 10.1097/mlr.0000000000000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wheeler SB, Reeder-Hayes KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18(9):986–993. doi: 10.1634/theoncologist.2013-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gwyn K, Bondy ML, Cohen DS, Lund MJ, Liff JM, Flagg EW, Brinton LA, Eley JW, Coates RJ. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004;100(8):1595–1604. doi: 10.1002/cncr.20169. [DOI] [PubMed] [Google Scholar]
- 76.Chen L, Li CI. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomark Prev. 2015;24(11):1666–1672. doi: 10.1158/1055-9965.Epi-15-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bickell NA, Wang JJ, Oluwole S, Schrag D, Godfrey H, Hiotis K, Mendez J, Guth AA. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357–1362. doi: 10.1200/jco.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 78.Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH. Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncology. 2016;2:322–329. doi: 10.1001/jamaoncol.2015.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McFarland DC, Shaffer KM, Tiersten A, Holland J. Prevalence of physical problems detected by the distress thermometer and problem list in patients with breast cancer. Psychooncology. 2018;27(5):1394–1403. doi: 10.1002/pon.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miaskowski C, Cooper B, Paul SM, West C, Langford D, Levine JD, Abrams G, Hamolsky D, Dunn L, Dodd M, Neuhaus J, Baggott C, Dhruva A, Schmidt B, Cataldo J, Merriman J, Aouizerat BE. Identification of patient subgroups and risk factors for persistent breast pain following breast cancer surgery. J Pain : official journal of the American Pain Society. 2012;13(12):1172–1187. doi: 10.1016/j.jpain.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nurgalieva Z, Liu C-C, Du XL. Chemotherapy use and risk of bone marrow suppression in a large population-based cohort of older women with breast and ovarian cancer. Med Oncol. 2011;28:716–725. doi: 10.1007/s12032-010-9512-5. [DOI] [PubMed] [Google Scholar]
- 82.Janelsins MC, Heckler CE, Peppone LJ, Kamen C, Mustian KM, Mohile SG, Magnuson A, Kleckner IR, Guido JJ, Young KL, Conlin AK, Weiselberg LR, Mitchell JW, Ambrosone CA, Ahles TA, Morrow GR. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: an analysis from a nationwide, multicenter, prospective longitudinal study. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2017;35(5):506–514. doi: 10.1200/JCO.2016.68.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96(5):826–833. doi: 10.2105/ajph.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, Cruz TD. Do US black women experience stress-related accelerated biological aging?: a novel theory and first population-based test of black-white differences in telomere length. Hum Nat. 2010;21(1):19–38. doi: 10.1007/s12110-010-9078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pearson JA, Geronimus AT. A practical guide to biological primary data collection in an impoverished urban setting: illuminating structural and social influences on population health inequity. London: SAGE Research Methods Cases; 2018. [Google Scholar]
- 86.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294(14):1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 87.Gallups SF, Connolly MC, Bender CM, Rosenzweig MQ. Predictors of adherence and treatment delays among African American women recommended to receive breast cancer chemotherapy. Womens Health Issues. 2018;28(6):553–558. doi: 10.1016/j.whi.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 88.Edwards MJ, Campbell ID, Lawrenson RA, Kuper-Hommel MJ. Influence of comorbidity on chemotherapy use for early breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2017;165(1):17–39. doi: 10.1007/s10549-017-4295-4. [DOI] [PubMed] [Google Scholar]
- 89.Gaston-Johansson F, Haisfield-Wolfe ME, Reddick B, Goldstein N, Lawal TA. The relationships among coping strategies, religious coping, and spirituality in African American women with breast cancer receiving chemotherapy. Oncol Nurs Forum. 2013;40:120–131. doi: 10.1188/13.ONF.120-131. [DOI] [PubMed] [Google Scholar]
- 90.Han W, Lee S. Racial/ethnic variation in health care satisfaction: the role of acculturation. Soc Work Health Care. 2016;55:694–710. doi: 10.1080/00981389.2016.1191580. [DOI] [PubMed] [Google Scholar]
- 91.Robertson-Jones TA, Tissue MM, Connolly M, Gallups SF, Bender CM, Rosenzweig MQ. Exploring racial differences in patient centeredness of care (PCC) during breast cancer (BC) chemotherapy clinical visits. J Racial Ethn Health Disparities. 2018;6:94–100. doi: 10.1007/s40615-018-0503-0. [DOI] [PubMed] [Google Scholar]
- 92.Jabir RS, Naidu R, Annuar MABA, Ho GF, Munisamy M, Stanslas J. Pharmacogenetics of taxanes: impact of gene polymorphisms of drug transporters on pharmacokinetics and toxicity. Pharmacogenomics. 2012;13:1979–1988. doi: 10.2217/pgs.12.165. [DOI] [PubMed] [Google Scholar]
- 93.Arbitrio Mariamena, Martino Maria Teresa Di, Scionti Francesca, Barbieri Vito, Pensabene Licia, Tagliaferri Pierosandro. Pharmacogenomic Profiling of ADME Gene Variants: Current Challenges and Validation Perspectives. High-Throughput. 2018;7(4):40. doi: 10.3390/ht7040040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hovelson DH, Xue Z, Zawistowski M, Ehm MG, Harris EC, Stocker SL, Gross AS, Jang IJ, Ieiri I, Lee JE, Cardon LR, Chissoe SL, Abecasis G, Nelson MR. Characterization of ADME gene variation in 21 populations by exome sequencing. Pharmacogenet Genomics. 2017;27(3):89–100. doi: 10.1097/fpc.0000000000000260.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jack J, Havener TM, McLeod HL, Motsinger-Reif AA, Foster M. Evaluating the role of admixture in cancer therapy via in vitro drug response and multivariate genome-wide associations. Pharmacogenomics. 2015;16:1451–1463. doi: 10.2217/PGS.15.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li J, Lao X, Zhang C, Tian L, Lu D, Xu S. Increased genetic diversity of ADME genes in African Americans compared with their putative ancestral source populations and implications for pharmacogenomics. BMC Genet. 2014;15:52. doi: 10.1186/1471-2156-15-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lynch T, Price A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician. 2007;76:391–396. [PubMed] [Google Scholar]
- 98.Landry LG, Ali N, Williams DR, Rehm HL, Bonham VL. Lack of diversity in genomic databases is a barrier to translating precision medicine research into practice. Health Aff (Millwood) 2018;37:780–785. doi: 10.1377/hlthaff.2017.1595. [DOI] [PubMed] [Google Scholar]
- 99.Morales J, Welter D, Emily H, Bowler MC, Harris LW, McMahon AC, Hall P, et al. A standardized framework for representation of ancestry data in genomics studies, with application to the NHGRI-EBI GWAS Catalog. Genome Biol. 2018;19:1–10. doi: 10.1186/s13059-018-1396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roseland ME, Schwartz K, Ruterbusch JJ, Lamerato L, Krajenta R, Booza J, Simon MS. Influence of clinical, societal, and treatment variables on racial differences in ER−/PR− breast cancer survival. Breast Cancer Res Treat. 2017;165:163–168. doi: 10.1007/s10549-017-4300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barsevick AM, Leader A, Bradley PK, Avery T, Dean LT, DiCarlo M, Hegarty SE. Post-treatment problems of African American breast cancer survivors. Support Care Cancer. 2016;24(12):4979–4986. doi: 10.1007/s00520-016-3359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Healthy People 2020. U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health. Accessed 3/19/2019.
- 103.Zannas AS, Arloth J, Carrillo-Roa T, Iurato S, Roh S, Ressler KJ, Nemeroff CB, et al. Lifetime stress accelerates epigenetic aging in an urban, African American cohort: relevance of glucocorticoid signaling. Genome Biol. 2015;16:266. doi: 10.1186/s13059-015-0828-5.. [DOI] [PMC free article] [PubMed] [Google Scholar]