Abstract
The in vitro cultures of Bacopa monnieri show poor production of the anti-Alzheimer’s drug, bacoside A. Therefore, suitable bioprocess optimization strategy was developed for callus induction from leaf explants (30 days), followed by callus proliferation (15 days). Central Composite Design was implemented to analyze the effect of pH, photoperiod, naphthalene acetic acid (NAA), and benzylaminopurine (BAP) concentration for maximum biosynthesis of bacoside A using leaf explants as well as callus explants as the inoculum. Using the CCD responses, it was predicted that the best biomass concentration of 4.56 ± 0.53 g/l DW and bacoside A production of 14.04 ± 1.31 mg/g DW can be obtained using 5.4 pH, 18 h/6 h L/D photoperiod regime, and 1.2 mg/l BAP in combination with 0.2 mg/l NAA. The kinetic parameter values for maximum specific growth rate (0.16/day), saturation constant (7.35 g/l), inhibition constant (120 g/l), biomass yield (0.011 g/g), maintenance coefficient (0.02 g/g/day), and growth-associated (0.627 mg/g) and non-growth-associated (1.096 mg/g/day) bacoside A formation constants were determined experimentally in batch cultures using optimized conditions.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02258-6) contains supplementary material, which is available to authorized users.
Keywords: Phytohormones, Modeling, Kinetic parameters, Plant cell suspension culture, Bacoside A
Introduction
Bacopa monnieri (L.) also known as Brahmi is a popular species of the Scrophulariaceae family. This herb is useful in the Indian medicinal system as it has been known to promote regeneration of nerve cells (Sivaramakrishna et al. 2005; Mahato et al. 2000). It also has the potential to cure neurodegenerative diseases such as Alzheimer’s disease (Uabundit et al. 2010) and Parkinson’s disease (Jadiya et al. 2011). Plant-derived drugs and compounds such as artemisinin, etoposide, flavonols, theaflavins, phenolics, saponins, alkaloids, etc. have been extensively used in modern medicine (Ryu et al. 2017; Patra and Srivastava 2018; Gai et al. 2015). The complex biosynthetic pathway makes the heterologous production of these compounds uneconomical and wasteful (Reed and Osbourn 2018). Bacopa monnieri has been included among the threatened species due to its extensive use and demand (Tiwari et al. 1998). Therefore, large-scale cultivation in bioreactors has been the preferred protocol for obtaining large amounts of the native drug at a faster rate in a reproducible manner (Guillon et al. 2006). Statistical experimental design method, Response Surface Methodology (RSM), standardizes all the influential variables collectively (Eilers et al. 1988). Plant cell suspension cultures shows uniform growth kinetics in liquid-phase bioreactors and shake flasks. Therefore, cell suspension culture performed in shake flasks can lead to better industrial scale bioreactor performance for the production of high-value biopharmaceuticals (Zhong and Yuan 2009). The production of plant secondary metabolites has been studied in detail using mathematical modelling-based strategies in Artemisia annua (Patra and Srivastava , 2015), Azadirachta indica (Prakash and Srivastava 2006; Prakash and Srivastava 2008) and Catharanthus roseus (Thakore et al. 2015, 2017). These strategies have been useful for the improvement of the yield of secondary metabolites in large-scale bioreactors.
Keeping in view the previous results, the present study focussed on the improvement of the production of bacoside A in callus cultures of Bacopa monnieri using Central Composite Design for the optimization of phytohormone concentrations, medium pH, and photoperiod regime. Substrate utilization kinetics and inhibition studies were performed using the optimized cultivation conditions and the resulting kinetic parameter values can be used for the effective scale-up of bacoside A production in a bioreactor.
Materials and methods
Inoculum preparation, experimental conditions, and maintenance of callus cultures
Bacopa monnieri cannot be propagated using seeds; therefore, the method for propagation was excision of shoot of plantlets of Bacopa monnieri variety CIM-Jagriti followed by re-plantation in culture tubes containing 20 ml of rooting medium at a temperature of 25 °C. These plantlets were the source of leaf explants for callus induction in MM medium (media names and their respective composition are described in Table S1 in supplementary data) for 15 days under a 14 h/10 h L/D photoperiod regime. The calli obtained were used for the inoculation (inoculum added was 2 g/l DW) of experimental flasks for CCD experiments in RM1 media with pH of 5.8 and 24 h dark photoperiod. Callus cultures were subcultured once in every 20 days in 250 ml Erlenmeyer flasks containing 100 ml MM medium and incubated in complete dark conditions at 25 ± 1 °C. CCD experiments were set in Erlenmeyer flasks containing 30 ml of RM1 or RM2 medium. The final biomass concentration was determined in terms of dry cell weight. Bacoside A content in the biomass was determined using HPLC.
Statistical optimization for callus induction
Central Composite Design was chosen for the optimization of concentration of NAA and BAP in the tissue culture medium for maximum biomass and bacoside A production from leaf explants. RSM not only studies the individual effect of each variable, but also evaluates the pair wise interactive effects of the variables. The callus forms from Bacopa monnieri leaf explants on plant tissue culture medium supplemented with the auxin—NAA and cytokinin—BAP (Leonard et al. 2018).The design of experiments and the analysis of data was performed using the software Design-Expert 5.0.6 (Stat-Ease, Minneapolis). On the basis of initial studies (Leonard et al. 2018), BAP (0.1–2.0 mg/l) and NAA (0.2–1.0 mg/l) were tested at five different levels using a partial factorial CCD-RSM consisting of five central, four axial, and four quadrant points which added to a total of 13 experiments using leaf explants. The responses (biomass and bacoside A concentration) obtained from the RSM experiments performed using variable growth phytohormone concentration can be observed in Table 1. The previous section details experimental conditions for the CCD-RSM experiments. The CCD-RSM experiments were set in 250 ml Erlenmeyer flasks containing RM1 media (30 ml). Each experimental flask had the phytohormones NAA and BAP before sterilization, at concentrations listed in Table 1. At the end of 30 days, the callus biomass obtained from leaf explants was dried at room temperature, and weighed and utilized for bacoside A estimation. The values of the two variables in each of the experiments with their respective responses in terms of biomass and bacoside A concentration have been given in Table 1.
Table 1.
Uncoded values of the factors (NAA, BAP, pH, and photoperiod) in CCD-RSM studies and their actual and model predicted responses in terms of biomass and bacoside A production and growth index using two different stages of callus induction from leaf and callus proliferation from callus
| S.no | Factor A: BAP (mg/l) | Factor B: NAA (mg/l) | Callus biomass from leaf explants (g/l DW) | Total bacoside A (mg/l) in callus produced from leaf explants | Callus biomass from callus explants (g/l DW) | Total bacoside A (mg/g DW) in callus produced from callus explants | Factor C: pH | Factor D: dark period | Growth index of callus produced by callus explants | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Actual value | Predicted value | Actual value | Predicted value | Actual value | Predicted value | Actual value | Predicted value | Actual value | Predicted value | |||||
| 1 | 0.10 | 1.00 | 2.03 | 1.80 | 19.82 | 16.38 | 2.73 | 2.02 | 11.60 | 14.70 | 5.71 | 18 | 0.63 | 0.83 |
| 2 | 1.05 | 0.60 | 1.37 | 1.30 | 16.01 | 11.16 | 3.77 | 3.77 | 13.03 | 13.64 | 5 | 18 | 0.62 | 1.01 |
| 3 | 1.05 | 0.60 | 1.20 | 1.30 | 6.93 | 11.16 | 3.93 | 3.77 | 13.12 | 13.64 | 5 | 18 | 1.27 | 1.01 |
| 4 | 1.05 | 0.03 | 0.67 | 0.95 | 5.56 | 7.02 | 3.77 | 3.77 | 10.13 | 12.82 | 4.5 | 16 | 0.70 | 0.94 |
| 5 | 0.10 | 0.20 | 1.07 | 0.68 | 7.89 | 5.60 | 5.63 | 4.63 | 16.41 | 14.70 | 5 | 15 | 1.33 | 1.06 |
| 6 | 1.05 | 1.16 | 1.03 | 1.10 | 6.08 | 9.20 | 1.87 | 2.49 | 7.97 | 8.91 | 4.29 | 18 | 0.57 | 0.57 |
| 7 | 0.00 | 0.60 | 1.03 | 1.40 | 9.67 | 12.77 | 2.40 | 3.27 | 14.01 | 14.78 | 5 | 18 | 1.27 | 1.01 |
| 8 | 1.05 | 0.60 | 1.37 | 1.30 | 9.96 | 11.16 | 3.70 | 3.77 | 14.50 | 13.64 | 5 | 21 | 0.65 | 1.13 |
| 9 | 2.00 | 1.00 | 0.53 | 0.57 | 5.55 | 7.02 | 4.50 | 3.86 | 13.01 | 11.07 | 4.5 | 20 | 0.96 | 0.67 |
| 10 | 2.00 | 0.20 | 1.63 | 1.51 | 12.25 | 11.14 | 5.43 | 4.51 | 16.39 | 13.20 | 5 | 18 | 1.27 | 1.01 |
| 11 | 2.39 | 0.60 | 1.13 | 1.12 | 5.99 | 7.43 | 3.73 | 4.49 | 10.79 | 13.66 | 5.5 | 16 | 0.71 | 0.81 |
| 12 | 1.05 | 0.60 | 1.37 | 1.30 | 9.96 | 11.16 | 3.70 | 3.77 | 14.50 | 13.64 | 5 | 18 | 0.63 | 1.01 |
| 13 | 1.05 | 0.60 | 1.37 | 1.30 | 16.01 | 11.16 | 3.77 | 3.77 | 13.03 | 13.64 | 5.5 | 20 | 1.61 | 1.18 |
The 3-D response surfaces, contour plots, and the best combination of NAA and BAP were derived using point prediction tool of the Design-Expert software.
Statistical optimization for callus proliferation
The effect of NAA and BAP concentration on callus proliferation helped in designing a production medium that gives maximum production of biomass as well as bacoside A. Callus explants for the 13 RSM experiments are described in Table 1 in RM1 media. The callus biomass obtained in the 13 experimental flasks after duration of 15 days was subjected to biomass dry cell weight measurement and bacoside A analysis.
ANOVA tables predicted the correlation between experimental and model predicted values. The quadratic mathematical models gave the 3-D contour plots for the prediction of the responses (biomass production and bacoside A) with respect to different phytohormone concentrations. Finally, the ‘point prediction’ tool of Design-Expert software further fine-tuned the optimized concentrations of NAA and BAP.
The optimal NAA and BAP concentrations were predicted for obtaining maximum biomass and bacoside A production. Experiments were set in triplicate to validate the model predicted values. The callus induction stage and the callus proliferation stages, when performed experimentally, validated the model predicted values.
The experiments for the optimization of the medium pH and the photoperiod regime were set using RSM. Table 1 shows the five different levels tested for both the variables. The medium pH was set in the 13 different experimental flasks containing 30 ml of RM2 medium before sterilization. The optimal values predicted by the 3-D plots were also fine-tuned using the ‘point prediction’ tool of software.
Estimation of kinetic parameters of bacoside A production using batch studies
Batch cultivation of cell suspension culture of Bacopa monnieri was set in shake flasks. The 250 ml Erlenmeyer flasks contained 100 ml of OR4 medium and inoculated with calli grown for 15 days (2 g/l DW). The details of the optimization of OR4 media have been described elsewhere (Leonard et al. 2018). The flasks were incubated under complete dark conditions in an incubator shaker set at 25 °C and the rotational speed was maintained at 100 rpm up to 12 days. One shake flask harvested at a time after every 2 days. The resulting suspension centrifuged at 8000 rpm for 30 min gave plant cell pellet. The cell pellet was dried at room temperature to estimate the dry cell weight and bacoside A concentration. The estimation of residual sucrose, nitrate, and KH2PO4 concentration in the supernatant gave the values of instantaneous substrate concentrations. The values of dry cell weight, bacoside A, residual sucrose, nitrate, and phosphate concentration were utilized for the determination of specific growth, product formation, and substrate utilization rates at every time interval. A mathematical model developed from the values of kinetic parameters.
Substrate inhibition study
The batch kinetic studies indicated that sucrose and phosphate were the limiting nutrients for biomass and bacoside A production. Shake flask studies showed the effect of initial sucrose and KH2PO4 concentration on maximum specific growth. In brief, the sucrose concentration when varied from 20 to 120 g/l and KH2PO4 concentration when varied from 0.18 to 1.0 g/l in OR4 medium showed the effect of substrate inhibition. The flasks inoculated with 2 g/l DW of calli, incubated at 25 °C, and kept at a rotational speed of 100 rpm under complete dark conditions for the experiment. The dry cell weight of this cell suspension culture was determined after each 24 h by harvesting one flask of each concentration at a time during the exponential growth phase of the cultures. The specific growth rate at various substrate concentrations at the corresponding time (t) came from the instantaneous rate of change of biomass concentration (dx/dt) per unit instantaneous biomass concentration (x).
Analytical techniques
The final callus biomass dried at room temperature for the estimation of dry cell weight. HPLC analysis gave the concentration of Bacoside A in the cell pellet and extracellular broth (Deepak et al. 2005). Into the dried and powdered cell pellet, 5 ml of methanol and 1 ml of 1 N HCl were added. The cells suspended in methanol for 1 h were sonicated at 24 kHz for 8 min and stored overnight at 4 °C. The next step for sample preparation was centrifugation at 8000 rpm for 10 min. The bacoside A released in methanol phase and was analyzed using HPLC after filtration using 0.22 µm syringe filters. Analytical separations were carried out by C18 column, using a gradient of acetonitrile (A) and 0.05% orthophosphoric acid (B) at a flow rate of 1.5 ml/min. Column temperature was set at 30 °C. Detection was carried out at 205 nm using a diode array detector (DAD), and the gradient of the mobile phase was changed from 30:70 (A:B) to 40:60 (A:B) during 0–25 min and to 60:40 (A:B) during 25 to 35 min. Pure bacoside A purchased from Sigma (St. Louis, USA) was used as a standard for the estimation of total bacoside A content in the plant cell suspension cultures. All the other reagents were of HPLC grade. The residual sucrose concentration in the cell suspension broth was estimated using di-nitro salicylic acid method (Miller 1959). Nitrate concentration was also determined by spectrophotometry-based method (Cataldo et al. 1975) and the residual phosphate estimation was performed by colorimetric assay using ammonium molybdate and stannous chloride reagents (Murphy and Riley 1962). All the reagents were of analytical grade and purchased from Himedia Pvt. Ltd. (India).
Results
Statistical optimization of callus induction
The calli obtained from the 13 experiments were tested to study the overall biomass and bacoside A productivity. All the 13 experiments using leaf explants were set simultaneously in order to eliminate the effect due to changes in environmental conditions. The responses of the CCD-RSM experiments were analyzed using regression analysis and analysis of variance (ANOVA). Different models (biomass prediction and bacoside A prediction using leaf explants) were generated for the two different responses as follows:
| 1 |
| 2 |
where A = BAP (mg/l), B = NAA (mg/l), Bacoside = Bacoside A concentration (mg/l), and DCW = biomass dry cell weight (g/l).
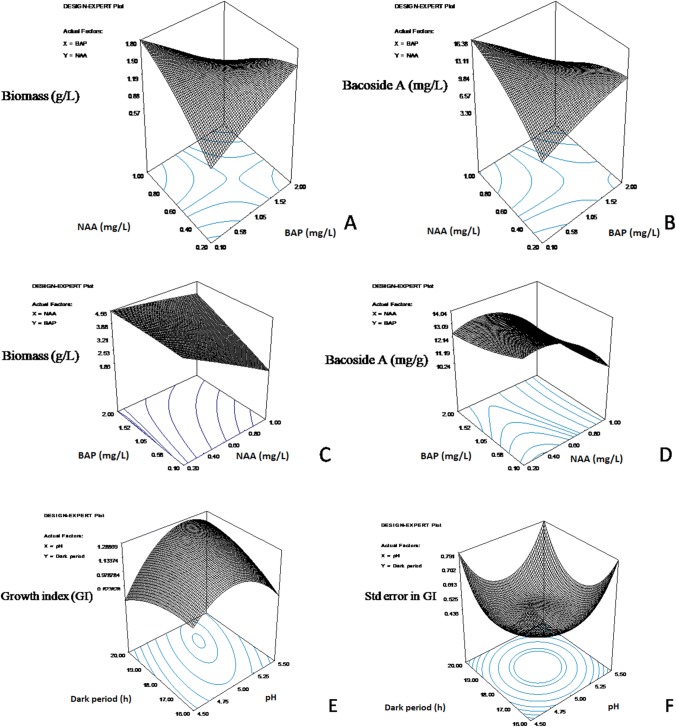
The adequacy of the models was studied using the R2 and (coefficient of determination) values. The CCD-RSM experimental responses, viz., biomass concentration and bacoside A production, were utilized for the development of a quadratic model for the prediction of responses with respect to changes in the values of the factors studied. The statistical validity of the models developed was determined using ANOVA analysis. Model 1 developed for biomass prediction was found to be highly significant (P < 0.0086). Similarly, model 2 developed for bacoside A prediction was not very significant (P < 0.5802) for designing the callus induction medium from leaf explants. However, there was a high degree of correlation between the model predicted and experimental values of biomass and bacoside A production. It was observed from the ANOVA analysis that the adequacy precision of model 1 for biomass prediction was 6.973 and that for model 2 for bacoside A prediction was 4.45. Therefore, the models were highly desirable for the prediction of responses as the adequacy precision value was greater than 4. The R2 value for model 1 was 0.73, and for model 2, it was 0.51. The 3-D response surface plots for the prediction of callus biomass and bacoside A in response to changes in values of factors studied (BAP and NAA concentration) are depicted in Fig. 1. The 3-D contour plots suggested that the optimal biomass concentration was 1.80 g/l and predicted in OR1 medium. The experimental validity of the model showed that the final callus biomass obtained (3.72 ± 0.03 g/l) was much higher than the model predicted values for biomass concentration (1.8 ± 0.20 g/l). This variation in model predicted and experimental values of final biomass in callus developed from leaf explants may be due to each leaf explant being a phenotypically independent entity in the plant.
Fig. 1.
Three-dimensional contour plots representing predicted response for a callus biomass induced from leaf explants in response to changes in NAA and BAP concentration; b bacoside A obtained from callus obtained from leaf explants in response to NAA and BAP concentration; c callus biomass obtained from subculturing callus explants in response to changes in NAA and BAP concentrations; d bacoside A prediction using callus explants obtained from callus explants in response to changes in NAA and BAP concentration; e growth index of callus obtained from callus biomass in response to changes in pH and photoperiod; f standard error in prediction of growth index from callus explants in response to changes in pH and photoperiod
Statistical optimization of callus proliferation
Response surface methodology was utilized for obtaining the quadratic model for the prediction of callus biomass and bacoside A production. Quadratic models were developed for the dry cell weight prediction and bacoside A prediction from the callus explants in response to changes in the concentration of BAP and NAA was as follows:
| 3 |
| 4 |
where DCW = dry cell weight, A = BAP concentration (mg/l), and B = NAA concentration (mg/l).
The statistical validity of the model examined using ANOVA analysis predicted the significance of the quadratic models (Eqs. 3, 4). The R2 value for model 3 (for biomass prediction from callus explants) was 0.58 and the R2 value for model 4 (for the prediction of bacoside A) was 0.47. The adequacy precision, which should be greater than 4, for higher desirability of the model, was found to be 4.57 for model 3 and 3.786 for model 4. The model 3 (P ˂ 0.2014) for biomass was more significant than model 4 (P ˂ 0.3809) for bacoside A which was not statistically significant; however, there was a high degree of correlation between model predicted and experimental values of biomass as well as bacoside A production. This may be due to less value of bacoside A as compared to biomass, which makes the difference in model predicted and experimental values significant. The 3-D contour plots (Fig. 1) showed that the optimal phytohormone concentration for callus proliferation from callus explants was 1.2 mg/l BAP and 0.2 mg/l NAA. OR2 medium was for callus proliferation from callus explants. The correlation between the final biomass obtained (4 g/l) and model predicted value of biomass (4.56 g/l) under optimal phytohormone concentration was 87.77% and 61.1% for bacoside A prediction.
CCD-RSM studied the effect of photoperiod and medium pH on the growth index of callus biomass. Calli (2 g/l DW basis) were inoculated in the experimental flasks. Incubation was in a growth chamber set at 25 °C for variable photoperiod regimes until day 15. Thereafter, the shake flasks were harvested for the estimation of callus dry cell weight (DW). The quadratic equation for the prediction of growth index (GI = (DWfinal − DWinitial)/ (DWinitial)) was as follows:
| 5 |
where GI = growth index, C = pH, and D = dark period in coded terms.
The quadratic mathematical model generated from the 3-D contour plots predicted the GI in response to changes in the photoperiod regime and medium pH. The ANOVA analysis predicted the significance of the results of RSM (P < 0.1007 for growth index). A high degree of correlation was observed between the model predicted values of growth index with the actual values of GI of callus in response to various values of dark periods and pH of the growth medium. The 3-D contour plots obtained using quadratic model (Eq. 5) were utilized to predict the optimal value of pH (pH 5.4) and dark period (18 h) at which maximum GI (1.18 ± 0.68) has been predicted. The 3-D contour plots for GI and standard error in GI are depicted in Fig. 1.
Estimation of kinetic parameters of bacoside A production in cell suspension culture of Bacopa monnieri
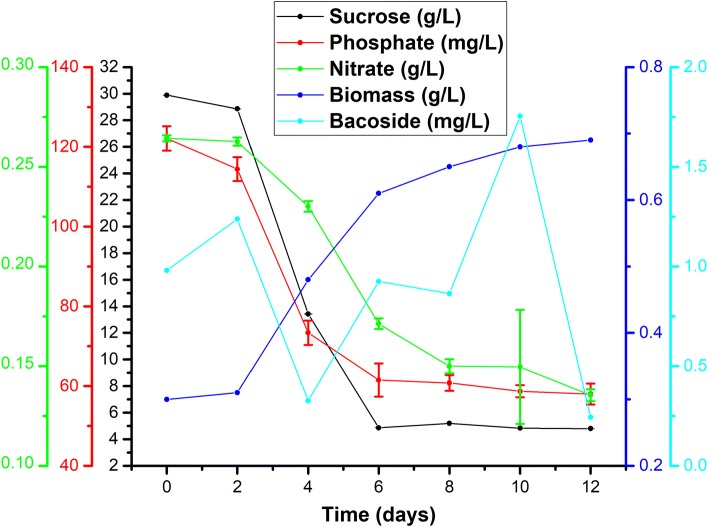
The time course of biomass production, bacoside A concentration, residual sucrose, nitrate, and KH2PO4 concentration is depicted in Fig. 2. There was no significant change in the biomass concentration during the initial 2 days. The bacoside A concentration reached 1.24 mg/l in the cell suspension on day 2 and then again dropped to 0.33 mg/l on day 4. This abrupt increase in bacoside A concentration on day 2 followed by a sudden drop could be due to exposure to high concentrations of nutrient medium. During the lag phase, formation of uniform cell suspension culture takes place. The callus pieces were less friable and this led to gradual disintegration of callus accompanied by the loss of some bacoside A in the medium by day 4. It was observed that the bacoside A concentration increased exponentially from day 4 (0.33 mg/l) up to day 10 (1.75 mg/l). The biomass growth rate decreases and stationary phase sets in by day 6. The kinetic studies showed that the maximum biomass (0.69 g/l DW) was obtained on day 12, whereas the maximum bacoside A production (1.75 mg/l) was obtained on day 10 of cultivation during the stationary phase. The amount of residual sucrose at the end of cultivation on day 12 was 4.79 g/l, residual phosphate concentration was 59 mg/l, and residual nitrate concentration was 0.14 g/l.
Fig. 2.
Kinetic profiles of biomass production, bacoside A production, sucrose concentration, nitrate concentration, and KH2PO4 concentration. The error bars show standard deviation values for three parallel experiments
Substrate inhibition studies
The values of kinetic parameters indicated that sucrose and KH2PO4 were the major limiting nutrients for biomass and bacoside A production. Therefore, substrate inhibition study showed whether excess concentration of these nutrients in the growth culture medium inhibits the biomass growth. The experimental results predicted that after a certain point of increase in the initial concentration of sucrose or KH2PO4 in the medium, there was a decrease in maximum specific growth rate of Bacopa monnieri cell suspension. This decrease in biomass growth was observed after the initial sucrose concentration in the medium reached to 60 g/l. The maximum specific growth rate of 0.15 d−1 was achieved at an initial sucrose concentration of 60 g/l. Similarly, it was observed that increasing the initial KH2PO4 concentration in the OR4 medium led to decrease in the maximum specific growth rate from 0.12/day at 0.2 g/l KH2PO4 to 0.02/day at 1.0 g/l KH2PO4 concentration. The substrate concentration at which the specific growth rate of cell suspension culture became zero was 120 g/l (Inhibition constant for sucrose) of sucrose and 1.25 g/l of KH2PO4 (inhibition constant for phosphate). Nitrate concentration remained non-limiting even after biomass growth becomes limiting and, therefore, was not considered as a limiting substrate.
The values of kinetic parameters for bacoside A production are summarized in Table S3. The batch kinetic and inhibitory data for biomass, bacoside A production, sucrose, and phosphate inhibition were utilized for the development of an unstructured mathematical model based on Monod’s model (Monod 1958).
The model assumptions were:
Sucrose and KH2PO4 were the main limiting nutrients for biomass and bacoside A production, while rest of the components remained in excess till the end of cultivation.
The physical conditions such as pH, temperature, ionic strength, etc. remained constant throughout the cultivation.
The following mathematical model was proposed:
| 6 |
| 7 |
| 8 |
| 9 |
where µ = specific growth rate, S1 = sucrose concentration, S2 = phosphate concentration, = specific sucrose consumption rate, = specific phosphate consumption rate, and qp = specific bacoside A production rate.
Discussion
Bacoside A has wide applications in the treatment of cognitive ailments such as memory disorders and Alzheimer’s disease (Sivaramakrishna et al. 2005; Rastogi et al. 1994; Ramasamy et al. 2015; Russo and Borrelli 2005). Leaves serve as ideal explants for the induction of calli. It has been seen that 70–90% of callus induction have been reported using leaf explants of Bacopa monnieri (Majumdar et al. 2011; Vijayakumar et al. 2010; Hegazi et al. 2017; Rahman et al. 2002). The other explants reported for callus induction are nodal explants and stem explants (Zote et al. 2018; Ranjan et al. 2018).
CCD-RSM experiments predicted that the highest amount of callus biomass (2.03 g/l DW) from leaf explants was obtained in OR1 medium. These studies indicated that a higher auxin (here NAA) to cytokinin (here BAP) ratio leads to high biomass production and high bacoside A production. Therefore, this study suggests that bacoside A production was mainly a non-growth-associated process. It has been reported in the literature that synthetic and natural auxins such as IBA/IAA/NAA (at concentrations greater than 0.5 mg/l) cause callus formation and slight rooting from leaf explants because of auxin cross-talk with other endogenous phytohormones (Deepthi and Satheeshkumar 2017). Small amount of BAP in the growth media can stop rooting from calli (Leonard et al. 2018). The experimental verification of the model predicted values of biomass and bacoside A in OR1 media showed that high amount of biomass (3.72 g/l) and bacoside A (11.31 mg/g) were obtained using the statistically optimized conditions.
Similarly, in the RSM study performed for optimized callus proliferation, it was predicted that the highest amount of biomass (5.63 g/l DW) and bacoside A (8.58 mg/g) were obtained in OR2 medium (MS solid medium + 0.2 mg/l NAA + 1.2 mg/l BAP with pH of 5.8 and photoperiod of 24 h dark). Therefore, it can be concluded that low auxin-to-cytokinin ratio favors the proliferation of callus explants and bacoside A production thereof. Production of bacoside A is a two-stage process in which high auxin-to-cytokinin ratio is required for leaf to callus induction and a second phase of low auxin-to-cytokinin ratio is required for the proliferation of calli. If the callus induction MM medium (MS solid medium + 0.5 mg/l NAA + 0.1 mg/l BAP with pH of 5.8 and photoperiod of 24 h dark) was utilized for callus proliferation, it led to browning of the callus. A similar study reported two-phase culture for large-scale production of azadirachtin-related limonoids, whereby different concentrations of phosphorus and nitrogen salts led to growth phase and production phase of the secondary metabolites (Raval et al. 2003). Our study showed that the callus biomass obtained in optimized OR1 medium resulted in 29.37% higher production of biomass as compared to that of control cultures grown in MM medium.
The value of optimal pH for the callus biomass proliferation was 5.4 and the optimum photoperiod was found to be 18 h dark and 6 h light using the CCD-RSM experiments. This study showed that the exposure to high intensity light for longer duration promoted unwanted shoot induction in Bacopa monnieri callus cultures. Similarly, as the MS medium (pH 5.8) was autoclaved, its pH rose to 6.1 ± 0.1; therefore, it can be inferred that setting a lower initial value of pH (pH 5.4) before autoclaving produces optimal callus proliferation. In another study (Bansal et al. 2017), the production of callus biomass and bacoside A accumulation in Bacopa monnieri-derived cell suspension culture were enhanced by optimization of factors, such as inoculum density, concentrations of potassium dihydrogen phosphate, potassium nitrate, and glucose, and showed approximately 2- and 1.7-fold increase, respectively. However, their study reported less overall biomass production.
The kinetic studies in batch culture indicated that the stationary phase sets in at the 6th day. The appearance of stationary phase could be due to changes in the medium pH due to cell biomass development or due to the accumulation of harmful phenolic compounds in response to stress during shake flask cultivation. A longer exponential phase for growth can be obtained in different plant cell bioreactors under controlled pH, temperature, dissolved oxygen, and light conditions (Prakash and Srivastava 2008; Patra and Srivastava 2016). The maximum specific growth rate value of the cell suspension culture was found to be 0.16/day and the Ks value was found to be 7.35 g/l that indicated fast consumption of substrate due to rapid growth of the Bacopa monnieri cell suspension culture. The yield of biomass per unit sucrose consumed was found to be 0.01 g/g and the bacoside A productivity was found to be 0.18 g/(l day). The value of growth-associated product formation constant was estimated to be 0.627 mg/g, whereas the value of non-growth-associated product formation constant was found to be 1.096 mg/(g day). As the value of non-growth-associated product formation constant is more than the growth-associated product formation constant, therefore, the production of the secondary metabolite, bacoside A, is mainly non-growth-associated process. The material balance of substrate utilization, biomass, and bacoside A utilized for the development of a mathematical model as described in the literature (Monod 1958; Panda et al. 2018). To the best of our knowledge, there have been no reports of batch kinetic studies for the production of bacoside A in cell suspension cultures. Some reports have demonstrated the production of lower amount of bacoside A in shoot cultures using a small-scale Growtek bioreactor (Sharma et al. 2015) and callus cultures (Bansal et al. 2017).
The growth inhibition of cell suspension culture of Bacopa monnieri at 120 g/l sucrose or 1.25 g/l of KH2PO4 used for predicting the substrate inhibition terms in the batch model as discussed in literature (Kaur et al. 2012). Sucrose acts as carbon source as well as osmolite in the growth medium. Excess substrate in the medium can lead to non-competitive inhibition of biochemical enzymes. Inorganic phosphate acts as part of co-enzyme of many biochemical reactions. It is also directly involved in the ATP generation process during cellular respiration. Therefore, an increase in inorganic phosphate may be affecting cellular respiration enzymes (Shuler and Kargi 1992). The present study can be utilized for the optimal large-scale production of bacoside A using bioreactor. This technology, in turn, can curb the exploitation of the indigenous herb for the successful commercialization of bacoside A as an anti-Alzheimer’s disease drug.
Conclusion
The optimum phytohormone concentration of 1.00 mg/l NAA and 0.1 mg/l BAP in OR1 media resulted in maximum biomass (3.72 ± 0.03 g/l DW) and bacoside A (11.31 ± 2.6 mg/g DW) production from leaf explants during callus induction of Bacopa monnieri. The phytohormone concentration of 0.2 mg/l NAA and 1.2 mg/l BAP in OR2 media led to maximum biomass (3.92 ± 0.002 g/l DW) and bacoside A (8.58 ± 2.7 mg/g DW) production during callus proliferation in B. monnieri. The experimental validation of model predicted data was performed and the experimental values showed 83.67% correlation for biomass and 61.11% correlation with the model predicted data for bacoside A production. The optimal values were 5.4 pH and photoperiod of 18 h dark and 6 h light. The batch kinetic parameters for the cell suspension culture of Bacopa monnieri were: µm = 0.16/day, = 7.35 g/l, = 120 g/l, = 0.011 g/g, = 0.02 g/g/day, K1 = 0.627 mg/g, K2 = 1.096 mg/g/day. The growth of cell suspension culture was complete at a concentration of 120 g/l of sucrose or 1.25 g/l of KH2PO4 in the cell culture medium. The batch kinetic parameters based on the development of a mathematical model is useful for the optimal production of secondary metabolites in large-scale bioreactors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors obtained the elite plant material from CIMAP Lucknow. The financial support was provided by SERB (File number: ECR/2017/001113) (Govt. of India) to one of the authors for pursuing PhD (Bishwanath Seth).MHRD (India) provided the fellowship for pursuing M.Tech. to two of the authors (Krishna Kalyani Sahoo and K.R. Aravind). The authors are also thankful to the Life Science Department of NIT Rourkela for providing the HPLC facility which was funded by DST (FIST), India [File number: SR/FST/LSI-025/2014].
Author contributions
The kinetic studies, and spectrophotometric and HPLC analysis have been standardized and performed by Bishwanath Seth. Krishna Kalyani Sahoo performed the RSM experiments for phytohormones, its experimental validation, and substrate inhibition studies. K. R. Aravind performed RSM experiments for pH and photoperiod optimization. B. B. Sahu and V. R. Singh were the collaborators for this work. Nivedita Patra has been the thesis supervisor for Bishwanath Seth, Krishna Kalyani Sahoo, and Aravind in this research work.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Bansal M, Sudhakara Reddy M, Kumar A. Optimization of cell growth and bacoside-A production in suspension cultures of Bacopa monnieri (L.) Wettst. using response surface methodology. In Vitro Cell Dev Biol Plant. 2017;53:527–537. doi: 10.1007/s11627-017-9847-0. [DOI] [Google Scholar]
- Cataldo DA, Maroon M, Schrader LE, Youngs VL. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Plant Anal. 1975;6:71–80. doi: 10.1080/00103627509366547. [DOI] [Google Scholar]
- Deepak M, Sangli GK, Arun PC, Amit A. Quantitative determination of the major saponin mixture bacoside A in Bacopa monnieri by HPLC. Phytochem Anal. 2005;16:24–29. doi: 10.1002/pca.805. [DOI] [PubMed] [Google Scholar]
- Deepthi S, Satheeshkumar K. Effects of major nutrients, growth regulators and inoculum size on enhanced growth and camptothecin production in adventitious root cultures of Ophiorrhiza mungos L. Biochem Eng J. 2017;117:198–209. doi: 10.1016/j.bej.2016.10.016. [DOI] [Google Scholar]
- Eilers RJ, Sullivan JG, Skirvin RM. Analyzing the effects of exogeneous polyamines and growth regulators on plating efficiency of sweet potato protoplasts using a central composite test design. Plant Cell Rep. 1988;7:216–219. doi: 10.1007/BF00269328. [DOI] [PubMed] [Google Scholar]
- Gai QY, Jiao J, Luo M, Wang W, Ma W, Zu YG, Fu YJ. Establishment of high-productive Isatis tinctoria L. hairy root cultures: a promising approach for efficient production of bioactive alkaloids. Biochem Eng J. 2015;95:37–47. doi: 10.1016/j.bej.2014.12.006. [DOI] [Google Scholar]
- Guillon S, Tremouillaux-Guiller J, Pati PK, Rideau M, Gantet P. Harnessing the potential of hairy roots: dawn of a new era. Trends Biotechnol. 2006;24:403–409. doi: 10.1016/j.tibtech.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Hegazi G, Taha H, Monem Mohamed Sharaf A, Ramzy ES. Enhancing in vitro production of bacoside A from Bacopa monnieriusing precursor and elicitors feeding. J Basic Appl Sci Res. 2017;7:27–35. [Google Scholar]
- Jadiya P, Khan A, Sammi SR, Kaur S, Mir SS, Nazir A. Anti-Parkinsonian effects of Bacopa monnieri: insights from transgenic and pharmacological Caenorhabditis elegans models of Parkinson's disease. Biochem Biophys Res Commun. 2011;413:605–610. doi: 10.1016/j.bbrc.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Kaur G, Srivastava AK, Chand S. Mathematical modelling approach for concentration and productivity enhancement of 1,3-propanediol using Clostridium diolis. Biochem Eng J. 2012;68:34–41. doi: 10.1016/j.bej.2012.07.004. [DOI] [Google Scholar]
- Leonard J, Seth B, Sahu BB, Singh VR, Patra N. Statistical optimization for enhanced bacoside A production in plant cell cultures of Bacopa monnieri. Plant Cell Tissue Organ Cult. 2018;133:203–214. doi: 10.1007/s11240-017-1373-6. [DOI] [Google Scholar]
- Mahato SB, Garai S, Chakravarty AK. Bacopasaponins E and F: two jujubogenin bisdesmosides from Bacopa monniera. Phytochemistry. 2000;53:711–714. doi: 10.1016/S0031-9422(99)00384-2. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Garai S, Jha S. Genetic transformation of Bacopa monnieri by wild type strains of Agrobacterium rhizogenes stimulates production of bacopa saponins in transformed calli and plants. Plant Cell Rep. 2011;30:941–954. doi: 10.1007/s00299-011-1035-9. [DOI] [PubMed] [Google Scholar]
- Monod J (1958) Recherches sur la croissance des cultures bactériennes. Hermann
- Murphy J, Riley JP. A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta. 1962;27:31–36. doi: 10.1016/S0003-2670(00)88444-5. [DOI] [Google Scholar]
- Panda I, Balabantaray S, Sahoo SK, Patra N. Mathematical model of growth and polyhydroxybutyrate production using microbial fermentation of Bacillus subtilis. Chem Eng Commun. 2018;205:249–256. doi: 10.1080/00986445.2017.1384923. [DOI] [Google Scholar]
- Patra N, Srivastava AK. Use of model-based nutrient feeding for improved production of artemisinin by hairy roots of Artemisia Annua in a modified stirred tank bioreactor. Appl Biochem Biotechnol. 2015;177:373–388. doi: 10.1007/s12010-015-1750-8. [DOI] [PubMed] [Google Scholar]
- Patra N, Srivastava AK. Artemisinin production by plant hairy root cultures in gas- and liquid-phase bioreactors. Plant Cell Rep. 2016;35:143–153. doi: 10.1007/s00299-015-1875-9. [DOI] [PubMed] [Google Scholar]
- Patra N, Srivastava AK. Mass production of artemisinin using hairy root cultivation of Artemisia annua in bioreactor. In: Pavlov A, Bley T, editors. Bioprocessing of plant in vitro systems. Cham: Springer International Publishing; 2018. pp. 343–359. [Google Scholar]
- Prakash G, Srivastava AK. Modeling of azadirachtin production by Azadirachta indica and its use for feed forward optimization studies. Biochem Eng J. 2006;29:62–68. doi: 10.1016/j.bej.2005.02.027. [DOI] [Google Scholar]
- Prakash G, Srivastava AK. Statistical elicitor optimization studies for the enhancement of azadirachtin production in bioreactor Azadirachta indica cell cultivation. Biochem Eng J. 2008;40:218–226. doi: 10.1016/j.bej.2007.12.017. [DOI] [Google Scholar]
- Rahman LU, Verma PC, Singh D, Gupta MM, Banerjee S. Bacoside production by suspension cultures of Bacopa monnieri (L.) Pennell. Biotechnol Lett. 2002;24:1427–1429. doi: 10.1023/A:1019815018436. [DOI] [Google Scholar]
- Ramasamy S, Chin SP, Sukumaran SD, Buckle MJC, Kiew LV, Chung LY. In silico and in vitro analysis of bacoside A aglycones and its derivatives as the constituents responsible for the cognitive effects of Bacopa monnieri. PLoS One. 2015;10:e0126565. doi: 10.1371/journal.pone.0126565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan R, Kumar S, Singh AK. An efficient in vitro propagation protocol of local germplasm of Bacopa monnieri (L.) found in Bihar: a plant with wide variety of medicinal properties. J Pharmacogn Phytochem. 2018;7:1803–1807. [Google Scholar]
- Rastogi S, Pal R, Kulshreshtha DK. Bacoside A3—a triterpenoid saponin from Bacopa monniera. Phytochemistry. 1994;36:133–137. doi: 10.1016/S0031-9422(00)97026-2. [DOI] [PubMed] [Google Scholar]
- Raval KN, Hellwig S, Prakash G, Ramos-Plasencia A, Srivastava A, Buchs J. Necessity of a two-stage process for the production of azadirachtin-related limonoids in suspension cultures of Azadirachta indica. J Biosci Bioeng. 2003;96:16–22. doi: 10.1016/S1389-1723(03)90091-0. [DOI] [PubMed] [Google Scholar]
- Reed J, Osbourn A. Engineering terpenoid production through transient expression in Nicotiana benthamiana. Plant Cell Rep. 2018;37:1431–1441. doi: 10.1007/s00299-018-2296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A, Borrelli F. Bacopa monniera, a reputed nootropic plant: an overview. Phytomedicine. 2005;12:305–317. doi: 10.1016/j.phymed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Ryu HW, Yuk HJ, An JH, Kim DY, Song HH, Oh SR. Comparison of secondary metabolite changes in Camellia sinensis leaves depending on the growth stage. Food Control. 2017;73:916–921. doi: 10.1016/j.foodcont.2016.10.017. [DOI] [Google Scholar]
- Sharma M, Gupta R, Khajuria RK, Mallubhotla S, Ahuja A. Bacoside biosynthesis during in vitro shoot multiplication in Bacopa monnieri L. Wettst. grown in Growtek and air lift bioreactor. Indian J Biotechnol. 2015;14:547–551. [Google Scholar]
- Shuler ML, Kargı F. Bioprocess engineering: basic concepts. USA: Prentice Hall; 1992. [Google Scholar]
- Sivaramakrishna C, Rao CV, Trimurtulu G, Vanisree M, Subbaraju GV. Triterpenoid glycosides from Bacopa monnieri. Phytochemistry. 2005;66:2719–2728. doi: 10.1016/j.phytochem.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Thakore D, Srivastava AK, Sinha AK. Model based fed batch cultivation and elicitation for the overproduction of ajmalicine from hairy roots of Catharanthus roseus. Biochem Eng J. 2015;97:73–80. doi: 10.1016/j.bej.2015.02.005. [DOI] [Google Scholar]
- Thakore D, Srivastava AK, Sinha AK. Mass production of ajmalicine by bioreactor cultivation of hairy roots of Catharanthus roseus. Biochem Eng J. 2017;119:84–91. doi: 10.1016/j.bej.2016.12.010. [DOI] [Google Scholar]
- Tiwari V, Singh BD, Tiwari KN. Shoot regeneration and somatic embryogenesis from different explants of Brahmi [Bacopa monniera (L.) Wettst.] Plant Cell Rep. 1998;17:538–543. doi: 10.1007/s002990050438. [DOI] [PubMed] [Google Scholar]
- Uabundit N, Wattanathorn J, Mucimapura S, Ingkaninan K. Cognitive enhancement and neuroprotective effects of Bacopa monnieri in Alzheimer's disease model. J Ethnopharmacol. 2010;127:26–31. doi: 10.1016/j.jep.2009.09.056. [DOI] [PubMed] [Google Scholar]
- Vijayakumar M, Vijayakumar R, Stephen R. In vitro propagation of Bacopa monnieri L.—a multipurpose medicinal plant. Indian J Sci Technol. 2010;3:781–786. [Google Scholar]
- Zhong C, Yuan YJ. Responses of Taxus cuspidata to hydrodynamics in bubble column bioreactors with different sparging nozzle sizes. Biochem Eng J. 2009;45:100–106. doi: 10.1016/j.bej.2009.03.001. [DOI] [Google Scholar]
- Zote RK, Pati YK, Londhe SS, Thakur VV, Choudhari NB. In vitro regeneration of Bacopa monnieri (L.) from leaf and stem explants. Int J Chem Stud. 2018;6:1577–1580. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.