Abstract
Purpose
To explore the correlation of tear and conjunctival cytokines and sensory hypersensitivity in mild dry eye (MDE) patients characterized by symptoms outweighing signs (DESOS).
Methods
The subjects comprised 39 patients with MDE characterized by DESOS, 18 patients with common MDE (CMDE), and 15 healthy controls. The patients with DESOS were randomly subdivided into two groups; the C-DESOS group received artificial tears only, and the G-DESOS group received artificial tears and 0.1% fluorometholone eye drops three times a day. Symptoms were assessed using the Ocular Surface Disease Index (OSDI) and the Neuropathic Pain Symptoms Inventory modified for Eye (NPSI-E) questionnaire. Ocular examinations and in vivo confocal microscopy (IVCM) were also employed. Tear and conjunctival cytokines were measured using Multiplex or RT–PCR on Days 0, 7, and 30. The correlation between the expression of cytokines and hypersensitivity status was analyzed.
Results
Compared with the CMDE and control groups, the DESOS groups showed a significant increase in symptom scores and in the ratio of symptoms versus signs. IL-1 β, IL-2, IL-6, and TNF-α in tears and conjunctiva increased in the DESOS groups compared to the CMDE and control groups, indicating a high correlation with hypersensitivity status in the DESOS groups. Glucocorticoid treatment significantly decreased the level of cytokines in tears and conjunctiva in the G-DESOS group and subsequently ameliorated the symptoms.
Conclusions
Tear and conjunctival cytokines, including IL-1 β, IL-2, IL-6, and TNF-α, were correlated with sensory hypersensitivity status in the DESOS groups, suggesting they play an important role in the discordance of symptoms outweighing signs.
Introduction
Dry eye (DE) is a common condition of the ocular surface that varies in severity and that affects millions of people of all ages and demographic backgrounds. It is characterized by unstable tear film that causes a variety of symptoms and/or visual impairment, potentially accompanied by ocular surface damage [1,2]. While the etiology of DE is often unknown, many factors initiate its pathomechanisms, which often overlap and interact. These factors include environmental triggers, medications, refractive surgery, computer use, contact lens use, and low-humidity conditions [3-6] and drive a diverse range of symptoms in parallel with ocular signs, according to the severity of the disease [7]. Upon confirming the diagnosis of DE based on a positive symptom score and one or more positive signs, management and treatment should be guided by the subtype classification and severity. While the majority of DE patients show symptom severity that matches the signs, it is well established that some exhibit conflicting signs and symptoms [8,9]. This discordance between signs and symptoms in DE was mostly shown as two types: symptoms lowering signs (dysesthesia) and symptoms outweighing signs (hypersensitivity). The former commonly appears in DE accompanied by impairment of corneal nerves, such as neurotrophic or diabetic keratopathy [10,11]. However, the latter is usually attributed to the natural variability of clinical tests, disease process, subjective pain thresholds, depression, and lower self-perceived health [12-14], which often frustrates clinicians due to the poor positive signs for the diagnosis of DE and subsequent treatment. Unfortunately, there are few reports on what changes in local molecular levels cause the discordance in DE.
Increasing evidence confirms that DE is associated with ocular surface inflammation and increased levels of inflammatory cytokines (e.g., IL-1, IL-6, IL-8, and TNF-a) in the tear film and conjunctival epithelium in both human and animal models [15-17]. These inflammatory cytokines are correlated with sensory nerve hypersensitivity (apparent pain, itchiness, and irritating symptoms) in psoriasis and atopic dermatitis [18]. In the ophthalmic clinic, it is not uncommon that people with mild signs of DE complain about unendurable itchiness, photophobia, and other irritating symptoms. However, whether the discordance characterized by symptoms outweighing signs in mild DE (MDE) can be attributed to sensory nerve hypersensitivity induced by inflammatory cytokines remains unclear. In this study, we investigated the profiles of the inflammatory cytokines in tears and conjunctiva in patients with MDE characterized by symptoms outweighing signs (DESOS) to explore the potential correlation between cytokines and sensory hypersensitivity in MDE.
Methods
Study subjects
The subjects were recruited from the out-patient clinics at the Shaanxi Institute of Ophthalmology and the Third Affiliated Hospital of Guangzhou Medical University and comprised 39 patients with MDE characterized by DESOS, 18 patients with common MDE (CMDE), and 15 healthy volunteers. The CMDE patients were diagnosed according to the TFOS DEWS II Definition and Classification Report and Dry Eye Definition and Diagnosis: A Consensus Report by the Asia Dry Eye Society and showed Ocular Surface Disease Index (OSDI) scores below 22 [1,19-22]. The DESOS group was characterized by mild DE and OSDI scores over 22. The exclusion criteria were as follows: a history of ocular or refractive surgery, the use of topical or systemic medications affecting DE in the last 3 months, contact lens use, abnormalities of the ocular surface, or any systemic diseases affecting tear secretion. The patients with DESOS were randomly subdivided into two groups; the C-DESOS group received treatment with 1% sodium hyaluronate eye drops 3 to 4 times a day only, and the G-DESOS group received artificial tears with 0.1% fluorometholone eye drops three times a day. In total, four groups were included in this study. The study complied with the tenets of the Declaration of Helsinki and was approved by the Shaanxi Institute of Ophthalmology Institutional Review Board and the Third Affiliated Hospital of Guangzhou Medical University Institutional Review Board. Written informed consent was obtained from all subjects.
Scores for dry eye symptoms
The DE symptoms of participants were assessed using the OSDI questionnaire (range 0–100) [20] on Days 0, 7, and 30. All participants completed the NPSI-E pain questionnaire to assess the presence, severity, and quality of ocular pain. The NPSI-E is a modified version of the Neuropathic Pain Symptoms Inventory (NPSI) that consists of several scored items that assess neuropathic pain, including burning spontaneous pain, pressing spontaneous pain, paroxysmal pain, evoked pain, and paresthesia/dysesthesia, and its severity. In the current work, the evoked pain section of the NPSI-E targeted aspects of ocular allodynia or hyperalgesia (eye pain caused or increased by (1) wind, (2) light, and (3) heat or cold), according to a previous report [22].
Ocular signs
Ocular signs were investigated using conjunctival lissamine green staining, corneal fluorescein staining, tear film breakup time (BUT), the Schirmer’s test (ST), and the Meibomian gland score and were measured using a slit-lamp microscope and ocular comprehensive analyzer (OCULUS, Germany). All tests were performed bilaterally by the same researcher. As shown in Table 1, all test scores were converted into a common unit system according to previous reports. A composite severity score was calculated for each eye by transforming each sign score to a common unit severity score between 0 and 1, with 0 being no sign of DE and 1 being the severest signs of DE [23]. The discordance between symptoms and signs was highlighted by the ratio of the total OSDI or NPSI-E scores versus the scores of signs.
Table 1. Conversion of dry eye test measurements into a common unit system.
| Test | Severity grade |
||||
|---|---|---|---|---|---|
| 0 | 0.25 | 0.5 | 0.75 | 1 | |
| Schirmer test (mm/5 min) |
35 |
7 |
5 |
2 |
0 |
| Staining cornea (Oxford, 0–5) |
0 |
1 |
2 |
3 |
5 |
| Staining nasal and temporal conjunctiva (Oxford, 0–10) |
0 |
2 |
4 |
6 |
10 |
| Meibomian gland dysfunction score (0–3) |
0 |
0.75 |
1.5 |
2.25 |
3 |
| TBUT (sec) | 10 | 7 | 5 | 3 | 0 |
Tear cytokine assay
After the administration of topical anesthesia consisting of 0.5% proparacaine hydrochloride, a disposable 0.3–0.5mm polyethylene capillary tube was placed in the lower fornix of the conjunctival sac and a tear sample of approximately 5 μl was collected by siphonage on Days 0, 7, and 30. Cytokines in the tear sample, including IL-1 β, IL-2, IL-6, IL-10, IL-17, IFN- γ, and TNF- α, were analyzed using a multiplex cytokine assay kit (Affymetrix, Santa Clara, CA) according to the provided protocol. The multiplexed bead analysis kit makes it possible to measure several cytokines in a single sample, with detection thresholds of 1–2 pg/ml.
Cytokine expression in the conjunctiva
After the administration of topical anesthesia consisting of 0.5% proparacaine hydrochloride, strips of cellulose acetate filter paper (Millipore Corp, Billerica, MA) were placed onto the temporal and superior bulbar conjunctiva adjacent to the corneal limbus, pressed gently with a medical swab for 3 s, and then removed. The impression cytology specimen was put into 350 μl cell-lysis solution containing β -Mercaptoethanol and RLT (1:100). Total RNA was extracted according to the protocol and amplified using RT–PCR (Takara Bio, Inc. Otsu, Japan). The selected primers were shown in the Table 2. Relative mRNA levels of target genes were calculated using the2-△△ct method, as described previously [24].
Table 2. The selected primers in this study.
| Cytokines | Primers |
|---|---|
| IL-2 |
F: CCCAAGAAGGCCACAGAACT |
| |
R: TTGCTGATTAAGTCCCTGGGT |
| IL-10 |
F: GCTGAGAACCAAGACCCAGA |
| |
r-ATTCTTCACCTGCTCCACGG |
| IL-6 |
F: CCAGAGCTGTGCAGATGAGT |
| |
r-AGCTGCGCAGAATGAGATGA |
| IFN-γ |
F: TGGAAAGAGGAGAGTGACAGA |
| |
r-TCTTCCTTGATGGTCTCCACAC |
| TNF-α |
F: AGAGGGAAGAGTTCCCCAGG |
| |
r-CCTCAGCTTGAGGGTTTGCT |
| IL-1β |
F: GCAATGAGGATGACTTGTTCTTTG |
| |
r-CAGAGGTCCAGGTCCTGGAA |
| IL-17 |
F: ACCAATCCCAAAAGGTCC |
| |
r-TGGATGGGGACAGAGTTCAT |
| β -actin |
F: CCTGACTGACTACCTCATGAAG |
| r-GACGTAGCACAGCTTCTCCTTA |
Correlation analysis between variables
The correlation between each of the tear cytokines and the OSDI and NPSI-E symptom scores was analyzed for all subjects. Pearson correlation coefficients and the corresponding p values were calculated.
In vivo confocal microscopy
In vivo confocal microscopy (IVCM) was used to document the level of corneal sub-basal nerve plexus and the status of antigen-presenting cells (dendritic cells) on Days 0 and 30. The corneal nerve fiber density (CNFD) from IVCM images was quantified using Automatic CCMetrics software V 1.0 (University of Manchester, Manchester, UK), as described earlier [25]. Corneal dendritic cell density (DCD) was analyzed and averaged using five representative IVCM frames. Two blinded observers analyzed the images, and the average of the values was used for statistical analysis.
Statistical analysis
Values were presented as the mean values ± standard deviations. A one-way ANOVA was used to compare the tear and conjunctiva cytokine levels between groups. p<0.05 was considered statistically significant. Person correlation coefficients were calculated for the correlation between the cytokine levels and symptom scores among the groups. Statistical analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, IL) and GraphPad Prism V 6.0 (GraphPad Software, La Jolla, CA).
Results
Demographics of the subjects
The C-DESOS group comprised 19 participants (15 females, 4 males, mean age 47.7±11.2 years) and the G-DESOS group enrolled 20 participants (16 females, 4 males, mean age 45.9±9.7 years). The CMDE group comprised 18 participants (14 females, 4 males, mean age 46.3±8.9 years) and the control group enrolled 15 healthy participants (12 females, 3males, mean age 45.3±9.6 years). There were no statistically significant differences between the groups in terms of age or gender (Table 3).
Table 3. The demographics of all subjects.
| Type | Control | CMDE | C-DESOS | G-DESOS | P value |
|---|---|---|---|---|---|
| Age (mean±SD) |
45.3±9.6 |
46.3±8.9 |
47.7±11.2 |
45.9±9.7 |
0.9046 |
| Male / Female | 3/12 | 4/14 | 4/15 | 4/16 | 0.8424 |
Ocular signs
Compared with the control group, the ST and BUT scores were lower and the conjunctival lissamine green staining, corneal fluorescein staining, and Meibomian gland scores were higher in the CMDE, C-DESOS, and G-DESOS groups (p<0.0001, <0.0001, 0.0014, <0.0001, and 0.0013, respectively). However, there were no statistical differences between the three test groups (Table 4, p=0.4995, 0.1040, 0.7396, 0.1307, and 0.5174, respectively). As shown in the parentheses in Table 4, a composite severity score was calculated for each eye by transforming each sign score to a common unit severity score between 0 and 1, with 0 being no sign of DE and 1 being the severest signs of DE.
Table 4. The ocular sign scores of all subjects before treatment.
| Test | Control | CMDE | C-DESOS | G-DESOS | P value |
|---|---|---|---|---|---|
| Schirmer test |
17.4±9.3 (0.0) |
10.1±2.7 (0.25) |
9.1±4.4 (0.25) |
8.7±3.8 (0.25) |
*<0.0001,**0.4995 |
| BUT |
11.8±4.1 (0.0) |
7.5±1.9 (0.25) |
6.1±2.5 (0.25) |
6.4±1.7 (0.25) |
*<0.0001,**0.1040 |
| Staining conjunctiva |
0.6±0.3 (0.25) |
1.7±1.2 (0.25) |
1.9±1.3 (0.25) |
2.0±1.1 (0.25) |
* 0.0014, **0.7396 |
| Staining cornea |
0.1±0.1 (0.25) |
0.9±0.4 (0.25) |
1.1±0.3 (0.5) |
1.2±0.6 (0.25) |
*<0.0001,**0.1307 |
| Meibomian gland dysfunction score | 0.8±0.2 (0.25) | 1.1±0.2 (0.5) | 1.2±0.4 (0.5) | 1.1±0.3 (0.5) | * 0.0013,**0.5174 |
* refers to statistical P value among the control, CMDE, C-DESOS, and G-DESOS group; ** refers to statistical P value among CMDE, C-DESOS, and G-DESOS group.
Symptom questionnaire score
OSDI scores were higher in the C-DESOS and G-DESOS groups compared with the CMDE and control groups on Days 0 and 7 (both p<0.001). This score decreased in the G-DESOS group after glucocorticoid treatment on Day 30. Corresponding to the OSDI scores, the ratio of the total OSDI scores versus the scores for signs revealed a significant increase in the C-DESOS and G-DESOS groups on Days 0 and 7 compared with the CMDE and control groups (Table 5, p<0.001). There was a significant difference in NPSI-E scores among all groups (p<0.001); this difference was higher in the C-DESOS and G-DESOS groups but not in the CMDE and control groups (Table 6, p<0.001).
Table 5. The OSDI scores of all subjects.
| Time | Scores | Control | CMDED | C-DESSD | G-DESSD | P value |
|---|---|---|---|---|---|---|
| Day 0 |
OSDI |
4.61±1.96 |
14.50±7.12 |
46.60±12.41 |
42.80±21.79 |
*<0.0001,**<0.0001 |
| |
OSDI/Sign |
6.12±2.54 |
9.67±4.75 |
26.63±7.09 |
28.53±14.51 |
*<0.0001,**<0.0001 |
| Day 7 |
OSDI |
5.13±1.20 |
17.70±5.37 |
39.70±19.77 |
34.60±18.88 |
*<0.0001,**0.0004 |
| |
OSDI/Sign |
6.80±1.60 |
11.80±3.58 |
26.47±11.30 |
23.07±12.59 |
*<0.0001,**0.0001 |
| Day 30 |
OSDI |
3.94±1.93 |
16.10±7.74 |
29.80±14.53 |
14.70±10.12 |
*<0.0001,**0.0001 |
| OSDI/Sign | 5.25±2.57 | 10.73±5.16 | 17.03±8.31 | 9.80±6.75 | *<0.0001,**0.0036 |
* refers to statistical P value among the control, CMDE, C-DESOS, and G-DESOS group; ** refers to statistical P value among CMDE, C-DESOS, and G-DESOS group. OSDI/Sign means the ratio of the OSDI scores versus sign scores.
Table 6. The NPSI-E scores of all subjects.
| Test | Control | CMDE | DESOS | P value |
|---|---|---|---|---|
| Total NPSI-E Scores |
2.89±0.44 |
8.74±1.33 |
18.19±6.67 |
*<0.0001,**<0.0001 |
| NPSI-E/sign |
0.10±0.14 |
0.25±0.12 |
1.87±1.02 |
*<0.0001,**<0.0001 |
| Eye pain right now |
0.14±0.17 |
1.14±1.21 |
3.08±2.12 |
*<0.0001,**0.0007 |
| Burning |
0.43±0.61 |
1.34±1.29 |
3.13±1.65 |
*<0.0001,**0.0002 |
| Pressure |
1.12±0.57 |
1.02±1.16 |
2.29±1.37 |
*<0.0001,**0.0016 |
| Pain evoked by wind |
0.18±0.24 |
1.18±1.07 |
2.98±1.68 |
*<0.0001,**0.0001 |
| Pain evoked by light |
0.41±0.46 |
1.54±1.17 |
3.10±1.58 |
*<0.0001,**0.7995 |
| Pain evoked by cold or hot |
0.32±0.38 |
1.23±1.39 |
2.31±1.16 |
*<0.0001,**0.0033 |
| Pins and needles | 0.29±0.53 | 1.29±1.95 | 2.29±1.25 | *<0.0001,**0.0231 |
* refers to statistical P value among the control, CMDE, and DESOS group; ** refers to statistical P value between CMDE and DESOS group.
Cytokine profile in the tears
The results for the cytokine profile in the tears of all subjects were shown in Figure 1. IL-1 β, IL-2, IL-6, IL-17, IFN- γ, and TNF- α measured in this study increased noticeably in the tears of the C-DESOS and G-DESOS groups compared with the CMDE and control groups (p=0.0141). However, the cytokines in the tears of the G-DESOS group all sharply decreased after glucocorticoid treatment on Day 30, but those in the tears of the C-DESOS group did not. There were no significant differences in IL-10 among the groups (p=0.1502).
Figure 1.
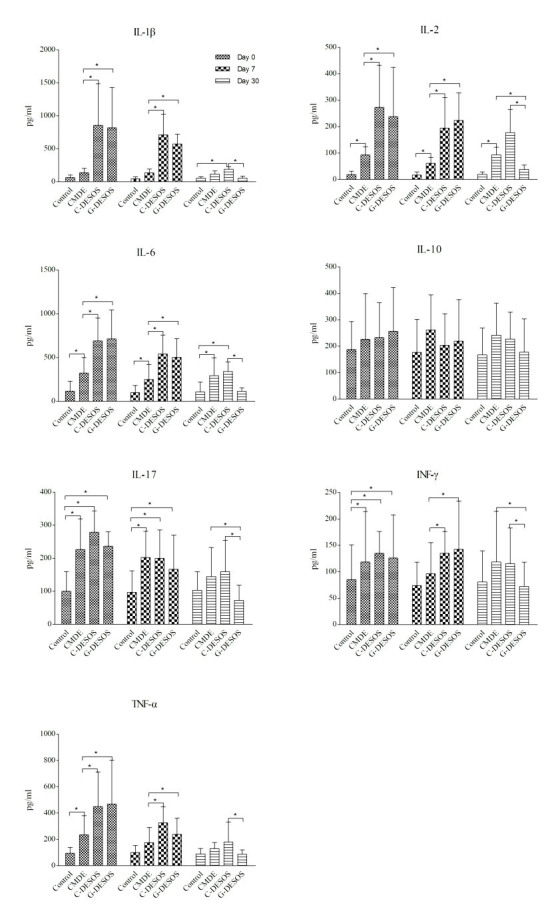
The cytokine profile in the tears of all subjects. IL-1 β, IL-2, IL-6, IL-17, IFN- γ, and TNF- α increased dramatically in the C-DESOS and G-DESOS groups compared to the CMDE and control groups (p=0.0141). However, they all sharply decreased in the G-DESOS group after glucocorticoid treatment on Day 30 but not in the C-DESOS group. There were no significant differences in IL-10 among the groups (p=0.1502).
Expression profile of cytokines in conjunctival samples
We measured the expression of cytokines in the conjunctival specimens collected using impression cytology with an RT–PCR assay. Consistent with the expression levels of cytokines in tears, IL-1 β, IL-2, IL-6, and TNF-α were significantly increased in the C-DESOS and G-DESOS groups compared with the CMDE and control groups (p=0.0264). These cytokines decreased in the G-DESOS group after glucocorticoid treatment but did not in the C-DESOS group. There was a subtle increase in IL-17 and IFN- γ in the C-DESOS and G-DESOS groups, but there was no significant difference compared with the CMDE and control groups (p=0.0837). There was no significant difference in IL-10 among the groups (Figure 2, p=0.6102).
Figure 2.
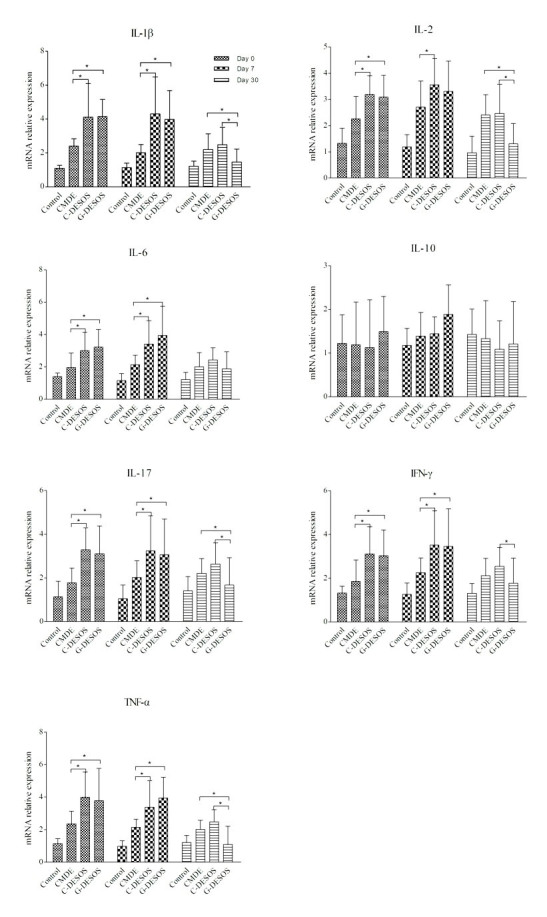
Expression of the cytokines in the conjunctiva. IL-1 β, IL-2, IL-6, IL-17, TNF-α, and IFN- γ increased dramatically in the C-DESOS and G-DESOS groups compared to the CMDE and control groups (p=0.0264). These cytokines decreased significantly in the G-DESOS group after glucocorticoid treatment but not in the C-DESSD group. However, there were no significant differences in IL-10 among the groups (p=0.6102).
Correlations between variables
Figure 3 displayed the correlation, including the correlation coefficient and p value, between each of the cytokines in tears and the symptom scores for all subjects. A high positive correlation was found between IL-1 β, IL-2, IL-6, and TNF- α and the OSDI and NPSI-E scores. IL-17, IFN- γ, and IL-10 showed a very low and nonsignificant correlation with both OSDI and NPSI-E scores.
Figure 3.
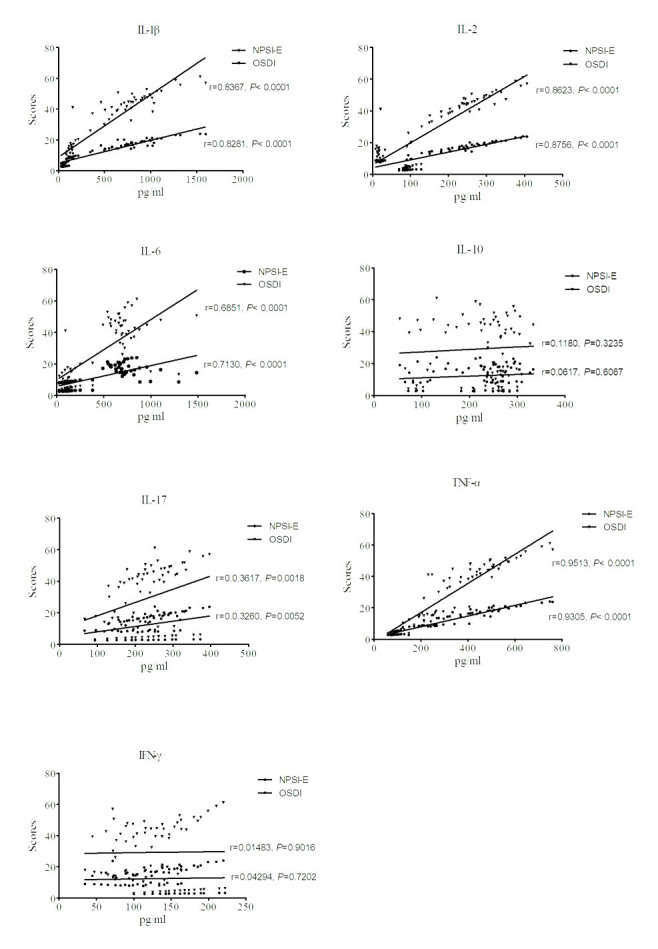
The correlation between each of the cytokines in tears and the symptom scores among all subjects. A high positive correlation was found between IL-1 β, IL-2, IL-6, and TNF- α and OSDI scores. A similar correlation was also found between these cytokines and NPSI-E scores. IL-17, IFN- γ, and IL-10 had very low and nonsignificant correlations with both OSDI and NPSI-E scores.
In vivo confocal microscope
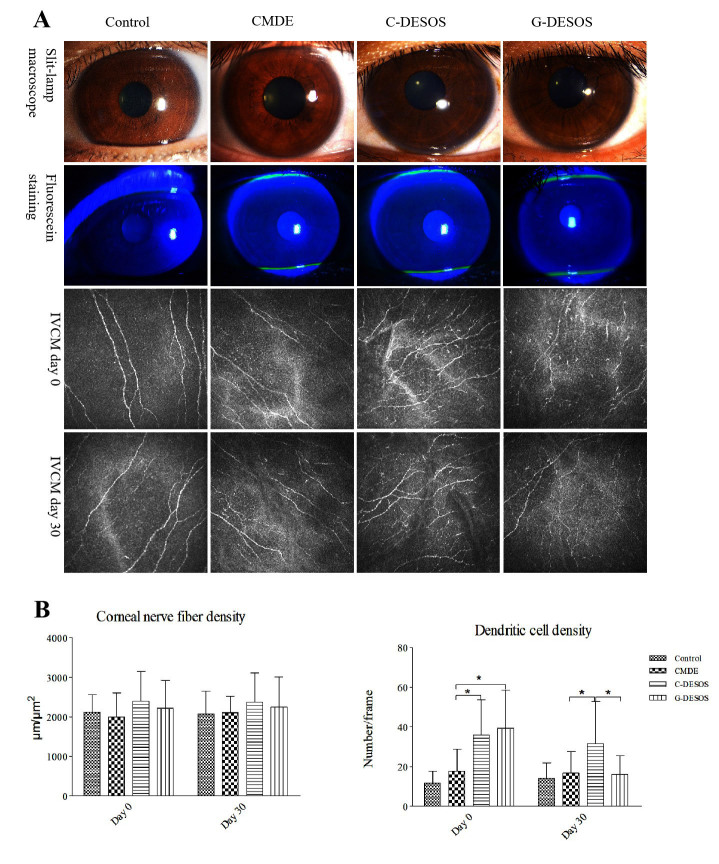
As shown in Figure 4A,B, there was no significant difference in CNFD among the groups. In addition, there were no statistical changes in CNFD in the G-DESOS group on Days 0 and 30 (p=0.1926). There were more dendritic cells clustered in the corneal epithelial layer in the C-DESOS and G-DESOS groups than in the CMDE and control groups (p=0.0052). After glucocorticoid treatment, the number of these dendritic cells significantly decreased (p<0.001).
Figure 4.
Photo of the slit-lamp microscope and IVCM. A and B: Photo of the slit-lamp microscope and IVCM. There was no significant difference in CNFD among the groups (p=0.2116) or in the G-DESOS group on Days 0 and 30 (p=0.1926). More dendritic cells clustered in the corneal epithelial layer in the C-DESOS and G-DESOS groups compared to the CMDE and control groups (p=0.0052). After glucocorticoid treatment, the number of dendritic cells decreased significantly (p<0.001).
Discussion
Subjective symptoms coupled with objective signs were considered to the classification of DE and guide its management and treatment [1,7,18]. Despite its widespread prevalence, DE remains quite difficult to manage because of the apparent lack of the identified pathophysiological cytokines involved in the discordance between commonly used clinical tests and patient-reported symptoms [26,27]. In this study, we found that the expression of cytokines, especially IL-1 β, IL-2, IL-6, and TNF-α, in tears and conjunctiva significantly increased in the DESOS groups compared with the CMDE group, which showed a linear positive correlation with the higher OSDI and NPSI-E scores. Cytokines such as IL-1 β, IL-6, and TNF- α bind the correspondent nociceptor on the ending of a sensory nerve, directly igniting the action potential of the sensory nerve and indirectly inducing some neuropeptides to release, such as substance P and calcitonin gene-related peptide (CGRP). These neuropeptides positively activate the nociceptor and have been implicated in sensory hypersensitivity [28,29]. Anti-inflammatory treatment with glucocorticoid decreased the expression of these cytokines and in turn improved the symptoms of DESOS patients. This supports our above-mentioned hypothesis that cytokines were correlated with the sensory hypersensitivity of the DESOS patients. However, this incidental observation warrants further investigation, particularly regarding whether glucocorticoid agents can reduce the progression of DESOS.
The question we ask here is whether the elevated cytokines affected trigeminal nerve anatomy and function and thus caused sensory hypersensitivity in the DESOS group. The IVCM results did not show any significant differences in CNFD between the DESOS groups, suggesting the functional hypersensitivity of the trigeminal nerve rather than anatomic or morphologic changes was responsible for the discordance of symptoms outweighing signs in the DESOS groups. An increasing number of dendritic cells appeared in the corneas of participants in the DESOS groups, but the number decreased after the glucocorticoid treatment. Dendritic cells adjacent to the corneal sensory fibers might relay the signal stimuli of cytokines and might be involved in the cytokine-associated hypersensitivity of the trigeminal nerve in the DESOS groups [25].
The reason for the lack of corresponding ocular signs in the DESOS groups remains unclear. A previous study hypothesized that the phenomenon of symptoms outweighing signs perhaps occurred in the preclinical phase of DE [19]. However, it must be clarified whether DESOS was only a characteristic stage in the progression of DE or a new special disease. In conclusion, cytokines such as IL-1 β, IL-2, IL-6, and TNF- α showed a positive correlation with the OSDI and NPSI-E scores, which suggests they were involved in the sensory hypersensitivity of the DESOS participants.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (NO. 81870631). The authors are also grateful to the subjects for their enthusiastic participation. Contributors: Contribution of each author is listed as the following. Shuangyong Wang: substantial contribution to the conception or design of the work. Ying Tian and Bei Li: the acquisition, analysis or interpretation of data and drafting the work or revising it. Ethics declarations: The study was approved by the Shaanxi Institute of Ophthalmology Institutional Review Board and the Third Affiliated Hospital of Guangzhou Medical University Institutional Review Board.
References
- 1.Tsubota K, Yokoi N, Shimazaki J, Watanabe H, Dogru M, Yamada M, Kinoshita S, Kim HM, Tchah HW, Hyon JY. New Perspectives on Dry Eye Definition and Diagnosis: A Consensus Report by the Asia Dry Eye Society. Ocul Surf. 2017;15:65–76. doi: 10.1016/j.jtos.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Benítez-Del-Castillo J, Labetoulle M, Baudouin C, Rolando M, Akova YA, Aragona P, Geerling G, Merayo-Lloves J, Messmer EM, Boboridis K. Visual acuity and quality of life in dry eye disease: Proceedings of the OCEAN group meeting. Ocul Surf. 2016;15:169–78. doi: 10.1016/j.jtos.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Clayton JA. Dry Eye. N Engl J Med. 2018;379:e19. doi: 10.1056/NEJMc1808906. [DOI] [PubMed] [Google Scholar]
- 4.van Setten G, Labetoulle M, Baudouin C, Rolando M. Evidence of seasonality and effects of psychrometry in dry eye disease. Acta Ophthalmol. 2016;94:499–506. doi: 10.1111/aos.12985. [DOI] [PubMed] [Google Scholar]
- 5.Tong L, Zhao Y, Lee R. Corneal refractive surgery-related dry eye: risk factors and management. Expert Rev Ophthalmol. 2014;8:561–75. [Google Scholar]
- 6.Nichols JJ, Sinnott LT. Tear Film, Contact Lens, and Patient-Related Factors Associated with Contact Lens–Related Dry Eye. Invest Ophthalmol Vis Sci. 2006;47:1319–28. doi: 10.1167/iovs.05-1392. [DOI] [PubMed] [Google Scholar]
- 7.Craig JP, Nelson JD, Azar DT, Belmonte C, Bron AJ, Chauhan SK, Paiva CSD, Gomes JAP, Hammitt KM, Jones L. TFOS DEWS II Report Executive Summary. Ocul Surf. 2017;15:802–12. doi: 10.1016/j.jtos.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett JD, Keith MS, Sudharshan L, Snedecor SJ. Associations between signs and symptoms of dry eye disease: a systematic review. Clin Ophthalmol. 2015;9:1719–30. doi: 10.2147/OPTH.S89700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004;23:762–70. doi: 10.1097/01.ico.0000133997.07144.9e. [DOI] [PubMed] [Google Scholar]
- 10.Zou X, Lu L, Xu Y, Zhu J, He J, Zhang B, Zou H. Prevalence and clinical characteristics of dry eye disease in community-based type 2 diabetic patients: the Beixinjing eye study. BMC Ophthalmol. 2018;18:117. doi: 10.1186/s12886-018-0781-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Zhao L, Deng S, Sun X, Wang N. Dry Eye Syndrome in Patients with Diabetes Mellitus: Prevalence, Etiology, and Clinical Characteristics. J Ophthalmol. 2016;2016:8201053. doi: 10.1155/2016/8201053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bron AJ, Tomlinson A, Foulks GN, Pepose JS, Baudouin C, Geerling G, Nichols KK, Lemp MA. Rethinking dry eye disease: a perspective on clinical implications. Ocul Surf. 2014;12(Suppl):S1–31. doi: 10.1016/j.jtos.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Shtein RM, Harper DE, Pallazola V, Harte SE, Hussain M, Sugar A, Williams DA, Clauw DJ. Discordant Dry Eye Disease (An American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc 2016; 114:T4. [PMC free article] [PubMed] [Google Scholar]
- 14.Kato K, Sullivan PF, Evengard B, Pedersen NL. A population-based twin study of functional somatic syndromes. Psychol Med. 2009;39:497–505. doi: 10.1017/S0033291708003784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of Inflammatory Cytokines in the Tears of Dry Eye Patients. Cornea. 2009;28:1023–7. doi: 10.1097/ICO.0b013e3181a16578. [DOI] [PubMed] [Google Scholar]
- 16.Zhu L, Shen J, Zhang C, Park CY, Kohanim S, Yew M, Parker JS, Chuck RS. Inflammatory cytokine expression on the ocular surface in the Botulium toxin B induced murine dry eye model. Mol Vis. 2009;15:250–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Mrugacz MG, Ostrowska L, Bryl A, Szulc A, Zelazowska-Rutkowska B, Mrugacz G. Pro-inflammatory cytokines associated with clinical severity of dry eye disease of patients with depression. ADV MED SCI-POLAND. 2017;62:338–44. doi: 10.1016/j.advms.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Sauer SK, Reeh PW. Inflammation and hypersensitivity in the context of the sensory functions of axonal membranes: what are the molecular mechanisms? Dig Dis. 2009;27(Suppl 1):11–5. doi: 10.1159/000268116. [DOI] [PubMed] [Google Scholar]
- 19.Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, Liu Z, Nelson JD, Nichols JJ, Tsubota K. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15:276–83. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–21. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 21.Asiedu K, Kyei S, Mensah SN, Ocansey S, Abu LS, Kyere EA. Ocular Surface Disease Index (OSDI) Versus the Standard Patient Evaluation of Eye Dryness (SPEED): A Study of a Nonclinical Sample. Cornea. 2016;35:175–80. doi: 10.1097/ICO.0000000000000712. [DOI] [PubMed] [Google Scholar]
- 22.Farhangi M, Feuer W, Galor A, Bouhassira D, Levitt RC, Sarantopoulos CD, Felix ER. Modification of the Neuropathic Pain Symptom Inventory for use in eye pain (NPSI-Eye). Pain. 2019;160:1541–50. doi: 10.1097/j.pain.0000000000001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vehof J, Sillevis Smitt-Kamminga N, Nibourg SA, Hammond CJ. Predictors of Discordance between Symptoms and Signs in Dry Eye Disease. Ophthalmology. 2017;124:280–6. doi: 10.1016/j.ophtha.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Hamzaoui A, Maalmi H, Berraies A, Abid H, Ammar J, Hamzaoui K. Transcriptional characteristics of CD4 T cells in young asthmatic children: RORC and FOXP3 axis. J Inflamm Res. 2011;4:139–46. doi: 10.2147/JIR.S25314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shetty R, Sethu S, Deshmukh R, Deshpande K, Ghosh A, Agrawal A, Shroff R. Corneal Dendritic Cell Density Is Associated with Subbasal Nerve Plexus Features, Ocular Surface Disease Index, and Serum Vitamin D in Evaporative Dry Eye Disease. BioMed Res Int. 2016;2016:1–10. doi: 10.1155/2016/4369750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan BD, Crews LA, Messmer EM, Foulks GN, Nichols KK, Baenninger P, Geerling G, Figueiredo F, Lemp MA. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: clinical implications. Acta Ophthalmol. 2014;92:161–6. doi: 10.1111/aos.12012. [DOI] [PubMed] [Google Scholar]
- 27.Hua R, Yao K, Hu Y, Chen L. Discrepancy between subjectively reported symptoms and objectively measured clinical findings in dry eye: a population based analysis. BMJ Open. 2014;4:e5296. doi: 10.1136/bmjopen-2014-005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chavan SS, Ma P, Chiu IM. Neuro-immune interactions in inflammation and host defense: Implications for transplantation. Am J Transplant. 2018;18:556–63. doi: 10.1111/ajt.14515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reardon C, Murray K, Lomax AE. Neuroimmune Communication in Health and Disease. Physiol Rev. 2018;98:2287–316. doi: 10.1152/physrev.00035.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]