To the Editor,
In heart failure (HF), New York Heart Association (NYHA) classification is widely applied for stratifying disease severity and prognosis [1,2]. The Weber classification differs from the NYHA classification in that it is based on measured peak oxygen consumption (VO2), which has been shown to be more objective and reproducible [3]. Patients in Weber class B are similar to NYHA class II patients in that they are a large, generally stable, and heterogeneous group in whom risk stratification can be relatively complex.
We recently developed a cardiopulmonary exercise test (CPX) score using a summation of readily available responses that improved the prognostic utility of the test [4,5]. We sought to determine whether this score could enhance risk stratification among patients within Weber class B, and compared it to other clinical and CPX responses.
We studied 2635 HF patients who were referred for CPX at 5 centers between 1993 and 2010, followed for up to 3 years. HF diagnosis included ejection fraction (EF) b40% or a history of decompensated HF with normal EF (35% of the sample). A validated CPX score [5] was calculated for each patient based on the summation of abnormal responses as follows: VE/VCO2 slope ≥34 (7 points), heart rate recovery (HRR1) ≤6 beats/min (5 points), OUES ≤1.4 (3 points), PetCO2 b33 mm Hg (3 points) and peak VO2≤14 mL−1 kg−1 min−1 (2 points). The score was divided into quartiles of 0–5, 6–10, 10–15 and N15. All patients completed a written informed consent and institutional review board approval was obtained in each institution. The composite outcome was cardiac-related mortality, heart transplantation or left ventricular assist device (LVAD) implantation.
SPSS version 17.0 (SPSS Inc., Chicago, IL) was used for all analyses. Patients were divided into Weber classes A, B, C and D. Weber B was divided into two groups: Class B1 (CPX summed score b10) or B2 (CPX summed score ≥10). These sub-classes were compared with one another and to the other Weber classes. Weber class A was the reference group. Continuous variables are presented as means ± standard deviation (SD) and categorical variables as proportions. Chi-square and Student t-tests were used for categorical and continuous variables, respectively. ANOVA was used for multiple group comparisons, along with Bonferroni post hoc tests. Kaplan–Meier survival curves were used to compare event-free survival. Multivariable Cox proportional hazards analysis (backward stepwise) was adjusted for age EF, body mass index (BMI) and ischemic etiology.
The population was predominantly male (75%); mean age was 55 ± 14 years; ischemic etiology was present in 30% of the sample and mean values for peak VO2 and EF were 18.1 ± 8.3 mL kg−1 min−1 and 35.4 ± 15.8%, respectively. Overall mortality was 12.2% over 3 years with a median follow-up of 23 ± 12 months (Table 1).
Table 1.
Demographic, clinical, and cardiopulmonary exercise test data and events rates for each Weber Class.
Y, years; n, number of subjects; BMI, body mass index; kg, kilogram; m2, square meters; ACE, angiotensin conversion enzyme; NYHA, New York Heart Association; RER, respiratory exchange ratio; VE, ventilation; VCO2, carbon dioxide production; OUES, oxygen uptake efficiency slope; HHR1, heart rate recovery at first minute; PetCO2, end-tidal carbon dioxide partial pressure; CPX, cardiopulmonary exercise test.
| All (n 2625) | Weber A (n 811) | Weber B1 (n 459) | Weber B2 (n 103) | Weber C (n 990) | Weber D (n 262) | |
|---|---|---|---|---|---|---|
| Age, y | 55 ± 14 | 51 ± 14 | 57 ± 14* | 60 ± 14* | 57 ± 13* | 59 ± 13* |
| Male sex, %(n) | 75% (1981) | 82% (665) | 77% (355) | 84% (87) | 69% (688)* | 69% (180)* |
| BMI, kg/m2 | 28.5 ± 6 | 27 ± 5.3 | 29 ± 5.7*‡† | 27 ± 4.5£†‡ | 29 ± 6.0* | 30 ± 7.5* |
| Medications %(n) | ||||||
| Beta-blocker | 61% (1611) | 53% (387) | 66% (284) | 74% (73)* | 72% (678)* | 73% (185)* |
| ACE inhibitor | 56% (1469) | 44% (360) | 61% (281)* | 63% (65)* | 60% (598)* | 63% (165)* |
| Diuretic | 84% (2210) | 31% (216) | 56% (213)* | 56% (49)* | 71% (592)* | 78% (174)* |
| Ejection fraction, % | 35.4 ± 15.8 | 44 ± 15.4 | 35 ± 14.7*†‡ | 30 ± 15.9* | 31 ± 14.2*£ | 29 ± 14.3* |
| NYHA class | 2.4 ± 0.8 | 1.8 ± 0.8 | 2.1 0.8†‡ | 2.3 ± 0.7*‡† | 2.5 ± 0.7*‡ | 3.0 ± 0.6* |
| Peak VO2, ml−1 kg−1 min−1 | 18.1 ± 8.3 | 27.7 ± 7.9 | 17.8 ± 1.2*†‡ | 17.5 ± 1.1*†‡ | 13.1 ± 1.6*‡ | 8.0 ± 1.3* |
| Peak RER | 1.10 ± 0.14 | 1.11 ± 0.12 | 1.11 ± 0.13 | 1.06 ± 0.11*£ | 1.10 ± 0.14 | 1.08 ± 0.17* |
| VE/VCO2 slope | 33.3 ± 9.2 | 28 ± 5.2 | 29 ± 4.9*‡† | 39 ± 7.5*£†‡ | 36 ± 8.7*£‡ | 44 ± 13* |
| OUES | 2.03 ± 0.88 | 2.60 ± 0.88 | 1.98 ± 0.61*†‡ | 1.71 ± 0.43*‡ | 1.50 ± 0.49* | 1.07 ± 0.44* |
| HRR1, beats | 19 ± 13 | 26 ± 14 | 21 ± 12* | 16 ± 11*£ | 16 ± 11*£ | 13 ± 13*£ |
| Resting PetCO2, mm Hg | 33.6 ± 4.6 | 34 ± 4.4 | 35 ± 3.7*†‡ | 29 ± 3.9*£†‡ | 33 ± 4.7*‡ | 32 ± 5.0* |
| CPX summed score | 5.6 ± 4.4 | 3.7 ± 3.1 | 2.8 ± 2.5*†‡ | 11 ± 1.7*£‡† | 6.8 ± 4.6*‡ | 9.4 ± 3.8* |
| Combined Events, % (n) | 12.2% (321) | 2.8% (23) | 6.1% (28)* | 12.1% (13)*£‡ | 16.5% (163)*£‡ | 35.9% (94)*£ |
p < 0.05 versus Weber D.
p < 0.05 versus Weber A
p < 0.05 versus Weber B1.
p < 0.05 versus Weber C.
Table 1 shows comparisons between Weber classes with classes B1 and B2 separately. Compared to Weber class B1, patients in class B2 had a lower BMI (27.0 ± 4.5 versus 29.0 ± 5.7 kg/m2, p b 0.05), lower peak respiratory quotient (RER—1.06 ± 0.11 versus 1.11 ± 0.13, p < 0.05), higher VE/VCO2 slope (39.0 ± 7.5 versus 29.0 ± 4.9, p b 0.01), lower HRR1 (16 ± 11 versus 21 ± 12 beats, p b 0.05), lower resting PetCO2 (29.0 ± 3.9 versus 35.0 ± 3.7 mm Hg, p < 0.01) and a higher CPX score (11.0 ± 1.7 versus 2.8 ± 2.5, p < 0.01). Mean peak VO2 values in groups B1 and B2 were similar (17.5 ± 1.1 and 17.8 ± 1.2 mL kg−1 min−1, respectively; p = 0.99). The overall event rate in group B2 was nearly twice that in group B1 (12.1% versus 6.1%, p < 0.01).
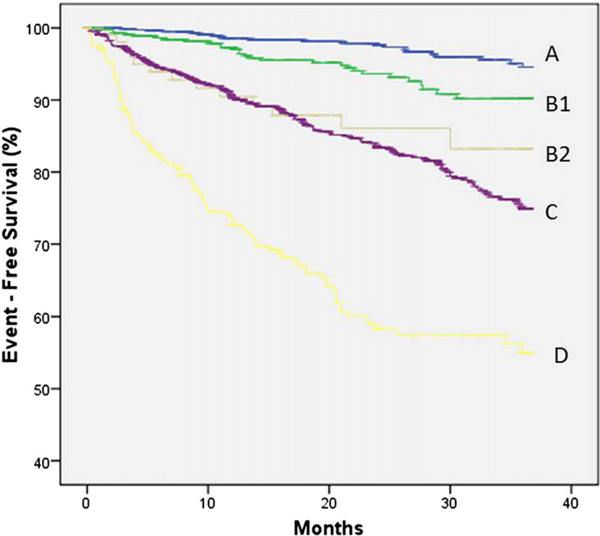
Fig. 1 shows event free survival curves, with Weber class B divided into B1 and B2. Class B2 patients had a lower event-free survival than class B1 (p < 0.01) and an event-free survival comparable to those in class C (p = 0.69).
Fig. 1.
Kaplan–Meier curves illustrating cumulative event-free survival for Weber Classes, including Classes B1 and B2 (p < 0.01 for trend;B2 compared to B1; p = 0.02, B2 compared to C; p = 0.69).
In multivariable Cox hazards analysis, compared to class A, class B1 had a similar event rate (HR 1.65 95% CI 0.88–3.08, p = 0.11). In contrast, patients in class B2 had a nearly 3-fold higher risk (HR 2.64, 95% CI 1.38–5.05, p b 0.01). Also compared to Weber A, other significant predictors of risk included Weber class C (HR 3.35, 95% CI 2.36– 4.76, p < 0.01), Weber class D (HR 8.77, 95% CI 5.99–12.82, p b 0.01), ischemic etiology (HR 1.27, 95% CI 1.00–1.60, p = 0.04), EF (HR 1.03, 95% CI 1.02–1.04, p < 0.01—for each decrease of 1%) and BMI (HR 1.03, 95% CI 1.01–1.05, p b 0.01—for each 1 kg/m2 decrease).
These results suggest that improved estimation of risk is achieved among patients in Weber class B when applying a CPX score. These patients frequently present a management quandary because they fall into neither what is considered high risk (peak VO2< 14 mL kg−1 min−1) or low risk (peak VO2> 20 mL kg−1 min−1) categories. Our salient finding was that patients in Weber Class B with an abnormal CPX score had a markedly higher (≈3-fold) risk for an adverse event than Class B patients with a normal score. Patients in Class B2 had an event rate that was in fact comparable to patients in Weber Class C. These findings highlight the importance of a multivariable approach to estimating risk based on CPX responses [6–10], and also suggest the utility of Weber classes B1 and B2 given the variation in risk within this group despite similar values for peak VO2.
In conclusion, the application of a CPX composite score in HF patients within Weber Class B more precisely stratified patients into high and low risk groups, even though subjects had similar values for peak VO2. For subjects with HF and intermediate values for peak VO2, these findings have the potential to improve risk stratification and thereby facilitate more appropriate therapeutic decisions.
Footnotes
Declaration of conflicting interests
None declared
References
- [1].Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;2013:128: •••–•••. [DOI] [PubMed] [Google Scholar]
- [2].The Criteria Committee of the New York Heart Association: Diseases of the Heart and Blood Vessels Nomenclature and Criteria for Diagnosis. 6th ed Boston, Little, Brown and Co; 1964. [Google Scholar]
- [3].Weber KT, Kinasewitz GT, Janicki JS, Fishman AP. Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure. Circulation 1982;65: 1213–23. [DOI] [PubMed] [Google Scholar]
- [4].Myers J, Arena R, Dewey F, Bensimhon D, Abella J, Hsu L, et al. A cardiopulmonary exercise testing score for predicting outcomes in patients with heart failure. Am Heart J 2008;156:1177–83. [DOI] [PubMed] [Google Scholar]
- [5].Myers J, Oliveira R, Dewey F, Arena R, Guazzi M, Chase P, et al. Validation of a cardiopulmonary exercise test score in heart failure. Circ Heart Fail 2013;6:11–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Myers J, Gullestad L, Vagelos R, Do D, Bellin D, Ross H, et al. Cardiopulmonary exercise testing and prognosis in severe heart failure: 14 mL/kg/min revisited. Am Heart J 2000;139:78–84. [DOI] [PubMed] [Google Scholar]
- [7].Guazzi M, Myers J, Abella J, Peberdy MA, Bensimhon D, Chase P, et al. The added prognostic value of ventilatory efficiency to the Weber classification system in patients with heart failure. Int J Cardiol 2008;129:86–92. [DOI] [PubMed] [Google Scholar]
- [8].Arena R, Myers J, Aslam SS, Varughese EB, Peberdy MA. Peak VO2 and VE/VCO2 slope in patients with heart failure: a prognostic comparison. Am Heart J 2004;147: 354–60. [DOI] [PubMed] [Google Scholar]
- [9].Baba R, Nagashima M, Goto M, Nagano Y, Yokota M, Tauchi N, et al. Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J Am Coll Cardiol 1996;28:1567–72. [DOI] [PubMed] [Google Scholar]
- [10].Ritt LE, Oliveira RB, Myers J, Arena R, Peberdy MA, Bensimhon D, et al. Patients with heart failure in the “intermediate range” of peak oxygen uptake: additive value of heart rate recovery and the minute ventilation/carbon dioxide output slope in predicting mortality. J Cardiopulm Rehabil Prev May-Jun 2012;32(3):141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]