Abstract
The objective of the study is to assess the role of cardiopulmonary exercise testing (CPX) variables, including peak oxygen consumption (VO2), which is the most recognized CPX variable, the minute ventilation/carbon dioxide production (VE/VCO2) slope, the oxygen uptake efficiency slope (OUES), and exercise oscillatory ventilation (EOV) in a current meta-analysis investigating the prognostic value of a broader list of CPX-derived variables for major adverse cardiovascular events in patients with HF. A search for relevant CPX articles was performed using standard meta-analysis methods. Of the initial 890 articles found, 30 met our inclusion criteria and were included in the final analysis. The total subject populations included were as follows: peak VO2 (7,319), VE/VCO2 slope (5,044), EOV (1,617), and OUES (584). Peak VO2, the VE/VCO2 slope and EOV were all highly significant prognostic markers (diagnostic odds ratios ≥ 4.10). The OUES also demonstrated promise as a prognostic marker (diagnostic odds ratio = 8.08) but only in a limited number of studies (n = 2). No other independent variables (including age, ejection fraction, and beta-blockade) had a significant effect on the meta-analysis results for peak VO2 and the VE/VCO2 slope. CPX is an important component in the prognostic assessment of patients with HF. The results of this meta-analysis strongly confirm this and support a multivariate approach to the application of CPX in this patient population.
Keywords: Ventilation, Aerobic capacity, Expired gas, Exercise, Heart disease
Introduction
Cardiopulmonary exercise testing (CPX) has been used to gain insights into exertional abnormalities in patients with heart failure (HF) for over a quarter of a century [1, 2]. This premise is supported by numerous investigations demonstrating the ability of key CPX variables to reflect the pathophysiologic processes unique to HF (diminished cardiac output, ventilation-perfusion mismatching, elevated neurohormonal markers, etc.) [3]. As a result of this body of research, CPX has evolved into a highly accurate method for the evaluation of clinical status and prognosis in patients with HF [3]. Thus, modern CPX technology currently enjoys broad clinical acceptance for the assessment of symptomatic patients with HF from the scientific community. Current consensus statements describe the appropriate use, performance, and interpretation of CPX in this chronic disease population [4–7]. Most notably, CPX is a considered a key assessment when heart transplantation or other advanced treatment options are being considered [8, 9]. Despite the current widespread advocacy of CPX in the international literature in both clinical and research laboratories, a true appreciation of the robust body of scientific evidence and available standardized algorithms on its prognostic utility in patients with HF is lacking.
The gap between available scientific evidence and optimally efficacious clinical application may be in part explained by the wide array of CPX-derived variables being presented in a less than cohesive manner. The highly significant prognostic value of these CPX variables has been reported in many studies; however, widely varying outcomes, multivariate modeling methods, and observational time windows have been used [3]. Simply stated, there is a lack of systematic analyses in this area of research. Such investigations are needed to clarify the most effective use of CPX data in patients with HF based on currently available evidence. In this context, the most recent CPX expert consensus statement sponsored by the American Heart Association underscored the need for the continued collection and assessment of prognostic data in large cohorts of HF patients to more clearly elucidate the most effective way to clinically utilize CPX data in this chronic disease population [4].
The wealth of original investigations in this area appropriately lends itself to a meta-analysis, allowing for a comprehensive and scientifically rigorous assessment of the predictive value of CPX in the HF population and adequately assessing the prognostic contribution of multiple metabolic variables. Therefore, we undertook a systematic review and meta-analysis of the available published literature to investigate the prognostic value of a comprehensive list of CPX-derived variables for major adverse cardiovascular (CV) events in patients with HF.
Methods
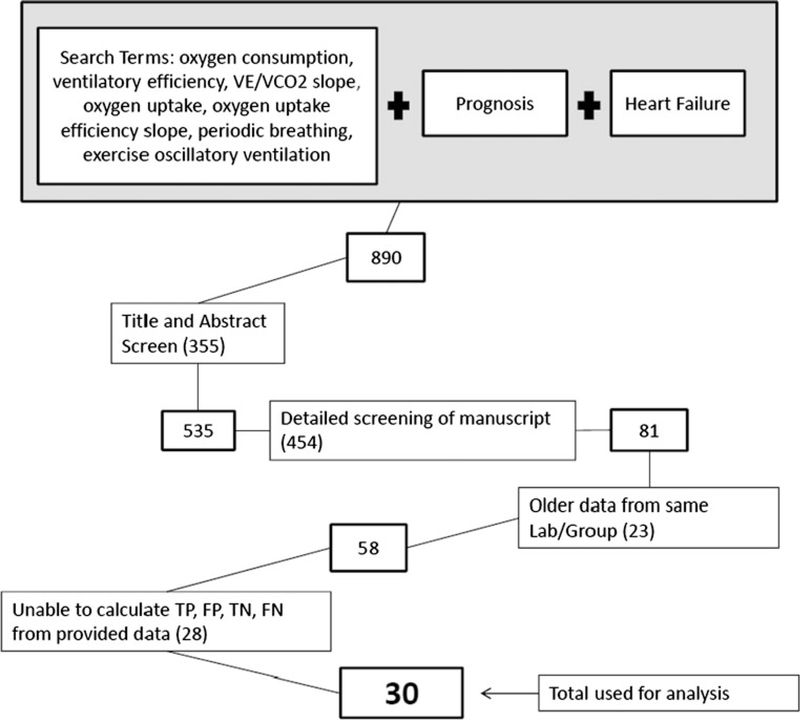
A search for articles was performed using the methods outlined in Fig. 1, with the following search terms used in PubMed in persons with HF: oxygen consumption (VO2), ventilatory efficiency, minute ventilation/carbon dioxide production (VE/VCO2) slope, oxygen uptake, oxygen uptake efficiency slope (OUES), periodic breathing, and exercise oscillatory ventilation (EOV).
Fig. 1.
Manuscript screening process. VE/VCO2, minute ventilation/carbon dioxide production; TP, true positive; FP, false positive; TN, true negative; FN, false negative
Inclusion and exclusion of articles was performed using the four steps outlined in Fig. 1. First, after obtaining the studies from our initial search (n = 890), 2 authors (PC and SP) reviewed the titles and abstracts to determine whether the primary or secondary aim of the study was to determine the prognostic value of the index variable and whether the study included patients with systolic HF only [left ventricular ejection fraction (LVEF) < 50 %] or a confirmed congenital heart defect. Other meta-analyses and review articles were screened out during this title and abstract screen. The remaining 535 studies (including studies that were missing information in the abstract that would determine inclusion/exclusion) were further scrutinized and excluded for: (1) inclusion of patients with chronic obstructive pulmonary disease; (2) evaluation of the index variable as a continuous variable rather than with a dichotomous “cut-off”; and (3) end-points of only hospitalization. Eighty-one studies were evaluated in the third step of the screening process which was performed by one author (PC) and was done to exclude older studies performed by the same group or laboratory (reducing the possibility of analyzing the same data more than once). Finally, one author (PC) extracted the data necessary (i.e., sensitivity/specificity, sample size, number of events, etc.) to determine the number of cases that were true positive (TP), true negative (TN), false positive (FP) and false negative (FN) in each of the remaining 58 studies. There were 28 studies in which the TP, TN, FN and FP could not be determined, which left 30 studies to be analyzed. Though the authors listed at each step of the screening process was primarily responsible for that step, all authors were involved in the final determination of whether an article was included or excluded.
Articles were independently reviewed by 2 authors (SP and LPC) using QUADAS. If needed, it was decided a priori that disagreement between the two reviewing authors would prompt a re-review by each author and review by a third author (MG) who made a final decision regarding study quality if the re-review could not be agreed upon. All reviewers were familiarized with the QUADAS tool and instructions on scoring of studies [10]. Study quality was quantified as a percentage using the 14 QUADAS items, and the number of items receiving an affirmative response with a perfect study accounting for 14/14 items (100 %). Agreement regarding scoring for each QUADAS item was evaluated via weighted kappa statistic. Study quality was included as an independent variable in the meta-regression analysis.
Data analyses were performed using MetaDiSk 1.4 software (available at www.hrc.es/investigacion/metadisc_en.htm). The Moses-Shapiro-Littenberg model was applied across studies to account for potential use of different thresholds to define positive and negative test results. The potential for greater heterogeneity using different thresholds to define positive and negative test results was also examined via sub-analyses of studies with identical/near identical thresholds. Meta-analysis endpoints consisted of one or more of the following: death, heart transplantation/HT, implantation of a ventricular assist device, and myoplasty. Statistical analyses included calculation of the diagnostic odds ratio (DOR), 95 % CI, sensitivity, specificity, and summary receiver operating characteristic (SROC) curves (both symmetric and asymmetric) to determine the area under the curve (AUC) as well as the Q* between studies (the point on the curve where sensitivity equals specificity) for peak VO2, the VE/VCO2 slope, the OUES, and EOV using a random effects model. Heterogeneity was examined using the Chi-square test of heterogeneity, I2 statistic, the Cochran-Q test, and estimate of between-study variance (tau-squared). The level of significance for statistical analyses was set at 0.05. Sub-study analyses using all of the above statistical tests were also performed by grouping studies with identical or near-identical thresholds for peak VO2 and the VE/VCO2 slope and comparing the analytical results. Peak VO2 sub-study analyses were performed using studies with thresholds <14 ml kg−1 min−1 (N = 4), equal to 14 ml kg−1 min−1 (N = 10), and >14 ml kg−1 min−1 (N = 5). The VE/VCO2 slope sub-study analyses were performed using studies with thresholds ≥34 but <40 (N = 10) and ≥40 (N = 5). Sub-study analyses of peak VO2 and the VE/VCO2 slope were also performed by grouping studies with an identical or near identical mean LVEF and grouping those in which beta-blockade was reported (N = 12) compared with studies not reporting beta-blockade (N = 7). Studies with a mean LVEF ≤ 25 % (N = 6), >25 % but <30 % (N = 7), and >30 % (N = 4) underwent all of the above statistical analyses and results were compared. Meta-analysis results for peak VO2 and the VE/VCO2 slope were also compared by omitting studies demonstrating extreme DOR (>3 times the pooled DOR) in either direction. A comparison of meta-analysis results between peak VO2, the VE/VCO2 slope, and EOV was also performed using studies in which each of these variables were examined and compared. Meta-regression analyses were also performed to examine the effect of potential prognostic independent variables (mean LVEF, New York Heart Association or NYHA classification, age, study sample size, gender, ischemic cardiomyopathy, beta-blockade, and study quality) on the meta-analysis results. Publication bias was examined using funnel plots and the Egger test. The presentation of the results follows current recommendations for meta-analyses [11]. Only symmetric SROC curve analyses are reported since asymmetric SROC curve analyses revealed similar results.
Results
A total of 30 articles were used for this meta-analysis with 19 separate articles examining peak VO2, 15 articles examining the VE/VCO2 slope, 5 articles examining EOV, and 2 articles examining the OUES. Peak VO2 was defined as the highest level of VO2 or averaged level (10–15 or 20–30 s) using breath-by-breath methods in the majority of the articles. The VE/VCO2 slope was calculated using the breath-by-breath VE and VCO2 response throughout exercise and least squares linear regression (y = mx + b, m = slope) in the majority of the articles. Exercise oscillatory ventilation was defined as cyclic fluctuation in ventilation lasting for more than 60 % of the exercise test with an amplitude >15 % of the average amplitude of cyclic fluctuation at rest in the majority of the articles [12]. The OUES was defined as the gradient of the linear relationship of log10 VE to VO2 [VO2 = m (log10VE) + b, where m = slope] using both 50 and 100 % of the exercise data in the two OUES articles. The quality of the included studies was relatively good with the majority of studies being retrospective, having adequate enrollment periods, and meeting QUADAS criteria for diagnostic studies (15). The mean ± SD and median QUADAS quality score for all of the studies included was 72.7 ± 8 % and 71 % (range 57–86 %) with the median QUADAS scores for the peak VO2, the VE/VCO2 slope, EOV, and OUES meta-analyses being 71, 71, 78.6, and 71.3 %, respectively. Agreement between the two reviewers of the studies was high (weighted Kappa statistic for inter-observer agreement varied from 0.74 to 1.0; median = 0.88) and did not require a decision to be made by a third reviewer. Publication bias was not evident based on funnel plot and Egger test analysis results.
Tables 1, 2, 3, 4, 5, and 6 provide the characteristics of the studies and patients included in the analyses of peak VO2, the VE/VCO2 slope, and EOV as well as the OUES, respectively. The total study populations for peak VO2 and the VE/VCO2 slope were 7,319 and 5,044 subjects, respectively. The total study populations for EOV and the OUES were 1,617 and 584 subjects, respectively. The median follow-up duration was 24, 25.3, 45.6, and 72 months for peak VO2, the VE/VCO2 slope, EOV, and the OUES, respectively. The percentage event rate corresponding to death or combined end-points was 25.6, 27.5, 25, and 35.4 % for peak VO2, the VE/VCO2 slope, EOV, and the OUES meta-analyses, respectively. The median age of the population for peak VO2, the VE/VCO2 slope, EOV, and the OUES meta-analyses was 55, 55, 58, and 57.65 years, respectively. The median percentage of male subjects in the meta-analysis for peak VO2, the VE/VCO2 slope, EOV, and the OUES was 74, 78.4, 73, and 85.1 %, respectively. The median percentage of subjects with a diagnosis of ischemic cardiomyopathy for peak VO2, the VE/VCO2 slope, EOV, and the OUES meta-analyses was 39, 45.6, 38, and 55.25 %, respectively. The median NYHA class for the peak VO2, the VE/VCO2 slope, EOV, and the OUES meta-analyses was 2.5, 2.5, 2.2, and 2.15, respectively. The median LVEF for peak VO2, the VE/VCO2 slope, EOV, and the OUES meta-analyses was 26, 28.7, 29, and 32.05 %, respectively. The median threshold values for peak VO2 and the VE/VCO2 slope meta-analyses were 14 ml kg−1 min−1 and 35.6, respectively.
Table 1.
Characteristics of studies included in the meta-analysis of peak VO2
| Author | Years | N | Enrollment | Follow-up (m) | Test mode | Threshold | End point | Event rate (%) | Study quality (%) |
|---|---|---|---|---|---|---|---|---|---|
| Corra et al. | 2006 | 231 | 1995–2001 | 39 | CE | 14.8 | D/CT | 36 | 78.6 |
| Davies et al. | 2006 | 243 | 1992–1996 | 108 | TM | 14.7 | D | 57 | 64 |
| Ferreira et al. | 2010 | 663 | 1999–2006 | 36 | CE & TM | 12–14 | D/CT | 28.9 | 64 |
| Francis et al. | 2000 | 303 | 1992–1996 | 24 | TM | 14 | D | 30 | 64 |
| Goda et al. | 2010 | 681 | 1993–2008 | 32 | TM | 14 | D/CT/VAD | 49.5 | 64 |
| Guimaraes et al. | 2008 | 391 | 1999–2004 | 28 | TM | 16 | CD/CT | 53.7 | 71 |
| Ingle et al. | 2011 | 423 | NR | 12 | TM | 20 | D | 28 | 71 |
| Kruger et al. | 2006 | 83 | 2000–2001 | 14 | CE | 14 | CD/AD | 16.8 | 86 |
| MacGowan et al. | 1997 | 104 | 1991–1993 | 18 | CE & TM | 15 | D | 17 | 71 |
| Mancini et al. | 1991 | 79 | 1986–1989 | 15 | TM | 14 | D | 25 | 78.6 |
| Mezzani et al. | 2003 | 570 | 1996–2000 | 19.6 | CE | 14 | CD/CT | 14 | 71 |
| Myers et al. | 2009 | 847 | 1993–2007 | 36 | CE & TM | 14 | D/CT | 17 | 78.6 |
| Opasich et al. | 1998 | 372 | 1992–1995 | 24 | TM | 14 | CD/CT | 45 | 71 |
| Osada et al. | 1998 | 500 | 1991–1996 | 36 | TM | 14 | D/CT listing | 25.6 | 57 |
| Peterson et al. | 2003 | 540 | 1994–2001 | 36 | TM | 14 | D/CT | 25 | 71 |
| Sarullo et al. | 2010 | 184 | 2006–2007 | 12 | CE | 12.2 | D/C CH | 51 | 78.6 |
| Shakar et al. | 2004 | 127 | 1992–1999 | 31.2 | CE & TM | 14 | D/CT | 19.7 | 71 |
| Sun et al. | 2010 | 508 | NR | 6 | CE & TM | 11 | D/C CH | 25 | 86 |
| Robbins et al. | 1999 | 470 | NR | 18 | TM | 13.9 | D/CT | 15.1 | 64 |
| Median | 391 | 24 | 14 | 25.6 | 71 | ||||
NR not reported, CE cycle ergometer, TM treadmill, D death, CD cardiac death, CT cardiac transplantation, CH cardiac hospitalization, VAD ventricular assist device
Table 2.
Characteristics of patients included in the meta-analysis of peak VO2
| Author | Mean age (year) | Gender (% male) | Mean NYHA | Beta-blockade (%) | Ischemic (%) | LVEF (%) |
|---|---|---|---|---|---|---|
| Corra et al. | 57 | 86.6 | 2 | 44 | 56 | 26 |
| Davies et al. | 59 | 87.2 | 2.5 | 31 | 58 | 29 |
| Ferreira et al. | 55 | 84 | 2.5 | 65 | 34 | 26 |
| Francis et al. | 59 | 88.1 | 2.5 | 0 | 58.7 | 25 |
| Goda et al. | 53.6 | 62.7 | 2.82 | 70.5 | 39.7 | 21.6 |
| Guimaraes et al. | 48 | 73.1 | NR | 58.7 | 32.2 | 31.7 |
| Ingle et al. | 63 | 80 | NR | 77 | NR | 36 |
| Kruger et al. | 59 | 74.1 | 2.3 | 86 | 40 | 26 |
| MacGowan et al. | 51 | 74 | NR | 0 | 24 | 29 |
| Mancini et al. | 49 | 86.1 | 2.9 | 0 | 38 | 19.6 |
| Mezzani et al. | 60 | 0 | 2.2 | 0 | NR | 26 |
| Myers et al. | 57 | 71.1 | NR | 66.6 | 39 | 32.1 |
| Opasich et al. | 0 | 0 | NR | 0 | NR | NR |
| Osada et al. | 52 | 75 | NR | 0 | 55 | 24 |
| Peterson et al. | 48.5 | 70.7 | NR | 47 | 32.4 | NR |
| Sarullo et al. | 59.8 | 79.3 | 2.4 | 63 | 52.2 | 35.4 |
| Shakar et al. | 53 | 73 | 2.7 | 100 | 37 | 21 |
| Sun et al. | 64 | 70 | 2.9 | 86 | NR | 26 |
| Robbins et al. | 52 | 71 | NR | 0 | 35 | 20.2 |
| Median | 55 | 74 | 2.5 | 47 | 39 | 26 |
NR not reported, NYHA New York heart association, LVEF left ventricular ejection fraction
Table 3.
Characteristics of studies included in the meta-analysis of the VE/VCO2 slope
| Author | Years | N | Enrollment | Follow-up (m) | Test mode | Threshold | End point | Event rate (%) | Study quality (%) |
|---|---|---|---|---|---|---|---|---|---|
| Chua et al. | 1997 | 166 | 1993–1994 | 25.3 | TM | 34 | D/CT | 23 | 64 |
| Davies et al. | 2006 | 243 | 1992–1996 | 108 | TM | 36.5 | D | 57 | 64 |
| Dimopoulos et al. | 2006 | 478 | 2001–2004 | 20.4 | TM | 38 | D | 5.2 | 78.6 |
| Ferreira et al. | 2010 | 663 | 1999–2006 | 36 | CE & TM | 43 | D/CT | 28.9 | 64 |
| Francis et al. | 2000 | 303 | 1992–1996 | 24 | TM | 40 | D | 30 | 64 |
| Guimaraes et al. | 2008 | 391 | 1999–2004 | 28 | TM | 34 | CD/CT | 53.7 | 71 |
| Ingle et al. | 2011 | 423 | NR | 12 | TM | 34 | D | 28 | 71 |
| Mejhert et al. | 2002 | 67 | 1996–1999 | 30.8 | CE | 45 | D | 20.9 | 86 |
| Myers et al. | 2009 | 847 | 1993–2007 | 36 | CE & TM | 34 | D/CT | 17 | 78.6 |
| Nanas et al. | 2006 | 98 | NR | 20 | TM | 34 | D | 27.5 | 64 |
| Pascual et al. | 2008 | 80 | 2003–2004 | 32.4 | TM | 35 | D/CT/CH | 24 | 78.6 |
| Ponikowski et al. | 2001 | 123 | 1993–1997 | 49 | TM | 34 | D | 28 | 71 |
| Robbins et al. | 1999 | 470 | NR | 18 | TM | 44.7 | D/CT | 15.1 | 64 |
| Sarullo et al. | 2010 | 184 | 2006–2007 | 12 | CE | 35.6 | D/C CH | 51 | 78.6 |
| Sun et al. | 2010 | 508 | NR | 6 | CE & TM | 40 | D/C CH | 25 | 86 |
| Median | 303 | 25.3 | 35.6 | 27.5 | 71 | ||||
NR not reported, CE cycle ergometer, TM treadmill, D death, CD cardiac death, CT cardiac transplantation, CH cardiac hospitalization
Table 4.
Characteristics of patients included in the meta-analysis of the VE/VCO2 slope
| Author | Mean age (year) | Gender (% male) | Mean NYHA | Beta-blockade (%) | Ischemic (%) | LVEF (%) |
|---|---|---|---|---|---|---|
| Chua et al. | 50.8 | 89.6 | 2.5 | 0 | 55.5 | 28.4 |
| Davies et al. | 59 | 87.2 | 2.5 | 31 | 58 | 29 |
| Dimopoulos et al. | 33.2 | 53.2 | 1.7 | 0 | NR | NR |
| Ferreira et al. | 55 | 84 | 2.5 | 65 | 34 | 26 |
| Francis et al. | 59 | 88.1 | 2.5 | 0 | 58.7 | 25 |
| Guimaraes et al. | 48 | 73.1 | NR | 58.7 | 32.2 | 31.7 |
| Ingle et al. | 63 | 80 | NR | 77 | NR | 36 |
| Mejhert et al. | 44 | 66 | NR | 60 | 67 | 36 |
| Myers et al. | 57 | 71.1 | NR | 66.6 | 39 | 32.1 |
| Nanas et al. | 51 | 91.8 | 1.7 | 27 | 35 | 31.2 |
| Pascual et al. | 50 | 77.5 | 2.4 | 100 | 26.3 | 25 |
| Ponikowski et al. | 56 | NR | NR | 0 | 53 | 28 |
| Robbins et al. | 52 | 71 | NR | 0 | 35 | 20.2 |
| Sarullo et al. | 59.8 | 79.3 | 2.4 | 63 | 52.2 | 35.4 |
| Sun et al. | 64 | 70 | 2.9 | 86 | NR | 26 |
| Median | 55 | 78.4 | 2.5 | 58.7 | 45.6 | 28.7 |
NR not reported, NYHA New York heart association, LVEF left ventricular ejection fraction
Table 5.
Characteristics of studies included in the meta-analysis of exercise oscillatory ventilation and the oxygen uptake efficiency slope
| Author | Years | N | Enrollment | Follow-up (m) | Test mode | Threshold | End point | Event rate (%) | Study quality (%) |
|---|---|---|---|---|---|---|---|---|---|
| Studies included in the meta-analysis of exercise oscillatory ventilation | |||||||||
| Arena et al. | 2008 | 154 | 1997–2007 | 36 | TM | 1.1 | CD/CT/VAD | 28 | 78.6 |
| Corra et al. | 2009 | 631 | 1995–2005 | 45.6 | CE | 1.1 | CD | 12.5 | 86 |
| Ingle et al. | 2009 | 240 | NR | 46 | TM | 1.1 | D | 18 | 64 |
| Sun et al. | 2010 | 508 | NR | 6 | CE & TM | 1.2 | D/C CH | 25 | 86 |
| Leite et al. | 2003 | 84 | NR | 49.7 | CE | 1.2 | CD/CT | 30.9 | 71 |
| Median | 240 | 45.6 | 1.1 | 25 | 78.6 | ||||
| Studies included in the meta-analysis of the oxygen uptake efficiency slope | |||||||||
| Davies et al. | 2006 | 243 | 1992–1996 | 108 | TM | 1.47 | D | 57 | 64 |
| Arena et al. | 2007 | 341 | 1993–2006 | 36 | TM | 1.4 | CD/CT/VAD | 13.8 | 78.6 |
| Median | 292 | 72 | 1.435 | 35.4 | 71.3 | ||||
NR not reported, CE cycle ergometer, TM treadmill, D death, CD cardiac death, CT cardiac transplantation, CH cardiac hospitalization, VAD ventricular assist device
Table 6.
Characteristics of patients included in the meta-analysis of exercise oscillatory ventilation and the oxygen uptake efficiency slope
| Author | Mean age (year) | Gender (% male) | Mean NYHA | Beta blockade (%) | Ischemic (%) | LVEF (%) |
|---|---|---|---|---|---|---|
| Studies included in the meta-analysis of exercise oscillatory ventilation | ||||||
| Arena et al. | 49.3 | 60 | 2.2 | 62 | 38 | 29.7 |
| Corra et al. | 58 | 90 | 1.9 | 100 | 69 | 29 |
| Ingle et al. | 59 | 73 | NR | 57 | NR | 34 |
| Sun et al. | 64 | 70 | 2.9 | 86 | NR | 26 |
| Leite et al. | 46 | 77 | NR | 6 | 17.7 | 21.8 |
| Median | 58 | 73 | 2.2 | 62 | 38 | 29 |
| Studies included in the meta-analysis of the oxygen uptake efficiency slope | ||||||
| Davies et al. | 59 | 87.2 | 2.5 | 31 | 58 | 29 |
| Arena et al. | 56.3 | 83 | 1.8 | 61.6 | 52.5 | 35.1 |
| Median | 57.65 | 85.1 | 2.15 | 46.3 | 55.25 | 32.05 |
NR not reported, NYHA New York heart association, LVEF left ventricular ejection fraction
Peak VO2
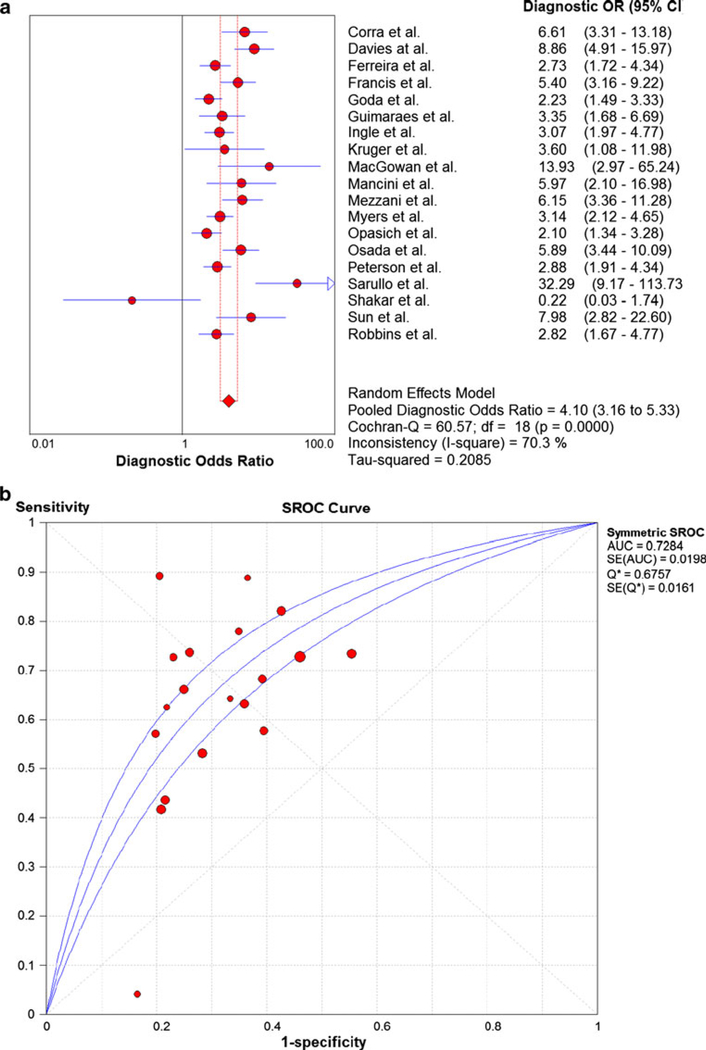
The meta-analysis results for peak VO2 are shown in Fig. 2a. The pooled DOR was 4.10 (3.16–5.33) with a high level of heterogeneity (Cochran-Q statistic and I2 values of 60.57 and 70.3 %, respectively). The sensitivity and specificity of peak VO2 in predicting study endpoints was 0.64 (0.61–0.66) and 0.67 (0.65–0.68), respectively. The SROC AUC for peak VO2 was 0.7284 as shown in Fig. 2b. Removal of studies with extreme DOR (Sarullo et al., Shakar et al., and MacGowan et al.) yielded a pooled DOR of 3.83 and less heterogeneity (Cochran-Q statistic and I2 values of 38.81 and 61.3 %, respectively). Sub-study analyses of several different threshold levels revealed mostly less heterogeneity with varying pooled DOR (Table 7). The peak VO2 threshold demonstrating the highest pooled DOR (5.58) and level of heterogeneity (I2 = 81.8 %) was <14 ml kg−1 min−1. The peak VO2 threshold demonstrating the lowest level of heterogeneity (I2 = 66.1 %) was a peak VO2 >14 ml kg−1 min−1 which was associated with a pooled DOR of 5.34. The pooled DOR and I2 associated with a peak VO2 threshold = 14 ml kg−1 min−1 was 3.43 and 67.5 %, respectively. Sub-study analyses of different threshold levels revealed varying levels of sensitivity and specificity (0.68–0.71). Sub-study analyses of peak VO2 and LVEF revealed that studies in which the mean LVEF was>25 and <30 % (N = 7) yielded the highest DOR and lowest level of heterogeneity (5.79 and 57.2 %, respectively) (Table 7). Sub-study analyses of peak VO2 and beta-blockade found that studies not reporting beta-blockade (N = 7) yielded a greater DOR and lower level of heterogeneity than studies reporting of beta-blockade (N = 12) (4.48 vs. 3.90 and 66.7 vs. 73.4 %, respectively).
Fig. 2.
a Summary statistics for meta-analysis of peak VO2. b Summary receiver operating characteristic curve results for 19 studies of peak VO2. VO2, oxygen consumption
Table 7.
Results of sub-study meta-analyses
| # Studies | DOR (95 % CI) | I-squared | Cochran-Q/p value | Tau-squared | |
|---|---|---|---|---|---|
| Sub-study | |||||
| Peak VO2 threshold | |||||
| Peak VO2 < 14 ml kg−1 min−1 | 4 | 5.58 (2.39–13.02) | 81.8 | 16.47/0.0009 | 0.569 |
| Peak VO2 = 14 ml kg−1 min−1 | 10 | 3.43 (2.48–4.74) | 67.5 | 27.73/0.001 | 0.162 |
| Peak VO2 > 14 ml kg−1 min−1 | 5 | 5.34 (3.15–9.04) | 66.1 | 11.80/0.02 | 0.222 |
| VE/VCO2 slope threshold | |||||
| VE/VCO2 = 34 | 6 | 4.53 (3.13–6.54) | 43.5 | 8.85/0.12 | 0.085 |
| VE/VCO2 > 34 < 40 | 10 | 6.22 (4.10–9.43) | 63.9 | 24.95/0.003 | 0.244 |
| VE/VCO2 > 40 | 5 | 4.83 (3.68–6.35) | 0 | 1.26/0.86 | 0 |
| Peak VO2 with and without beta-blockade | |||||
| Peak VO2 without beta-blockade | 7 | 4.48 (2.95–6.80) | 66.7 | 17.99/0.006 | 0.195 |
| Peak VO2 with beta-blockade | 12 | 3.90 (2.76–5.52) | 73.4 | 41.37/0.0000 | 0.239 |
| VE/VCO2 slope with and without beta-blockade | |||||
| VE/VCO2 without beta-blockade | 5 | 5.25 (3.82–7.23) | 0 | 3.72/0.44 | 0 |
| VE/VCO2 with beta-blockade | 10 | 5.44 (3.79–7.79) | 59.6 | 22.26/0.008 | 0.169 |
| Peak VO2 and left ventricular ejection fraction | |||||
| Peak VO2 with LVEF < 25 % | 6 | 3.41 (2.02–5.75) | 74.6 | 19.66/0.001 | 0.281 |
| Peak VO2 with LVEF > 25 % < 30 % | 7 | 5.79 (3.73–8.99) | 57.2 | 14.03/0.03 | 0.183 |
| Peak VO2 with LVEF > 30 % | 4 | 4.41 (2.39–8.15) | 76.4 | 12.71/0.005 | 0.274 |
| VE/VCO2 slope and ejection fraction | |||||
| VE/VCO2 with LVEF < 30 % | 8 | 5.37 (4.24–6.81) | 0 | 5.34/0.62 | 0 |
| VE/VCO2 with LVEF > 30 % | 6 | 5.59 (3.16–9.87) | 73.7 | 19.00/0.002 | 0.328 |
The VE/VCO2 slope
The meta-analysis results for the VE/VCO2 slope are shown in Fig. 3a. The pooled DOR was 5.40 (4.17–6.99) with a modest level of heterogeneity (Cochran-Q statistic and I2 values of 26.22 and 46.6 %, respectively). The sensitivity and specificity of the VE/VCO2 slope in predicting study endpoints was 0.66 (0.63–0.69) and 0.72 (0.71–0.73), respectively. The SROC AUC for the VE/VCO2 slope was 0.77 as shown in Fig. 3b. Removal of studies with extreme DOR (Pascual et al. and Sarullo et al.) yielded a pooled DOR of 4.87 and less heterogeneity (Cochran-Q statistic and I2 values of 11.07 and 36.7 %, respectively). Sub-study analyses of several different threshold levels revealed varying heterogeneity and pooled DOR (Table 7). The VE/VCO2 slope threshold demonstrating the highest pooled DOR (12.46) and level of heterogeneity (I2 = 66.2 %) was a VE/VCO2 slope >34 and <40. However, this analysis was limited to four studies. The VE/VCO2 slope threshold demonstrating the lowest level of heterogeneity (I2 = 0.0 %) was ≥40, which was associated with a pooled DOR of 4.83 (N = 5 studies). The pooled DOR and I2 for the 10 studies in which the VE/VCO2 slope was ≥34 and <40 were 6.22 and 63.9 %, respectively. Sub-study analyses of different threshold levels revealed varying levels of sensitivity and specificity (0.66–0.74). Sub-study analyses of the VE/VCO2 slope and LVEF revealed that studies in which the mean LVEF was <30 % (N = 8) yielded a similar pooled DOR to the meta-analysis results that included all studies (5.37 vs. 5.40), but without heterogeneity (I2 = 0.0 %) (Table 7). Sub-study analyses of the VE/VCO2 slope and beta-blockade found that studies not reporting beta-blockade (N = 5) yielded a similar DOR to the DOR that included all studies (5.25 vs. 5.40), but without heterogeneity (I2 = 0.0 %) (Table 7).
Fig. 3.
a Summary statistics for meta-analysis of the VE/VCO2 slope. b Summary receiver operating characteristic curve results for 15 studies of the VE/VCO2 slope. VE/VCO2, minute ventilation/carbon dioxide production
EOV
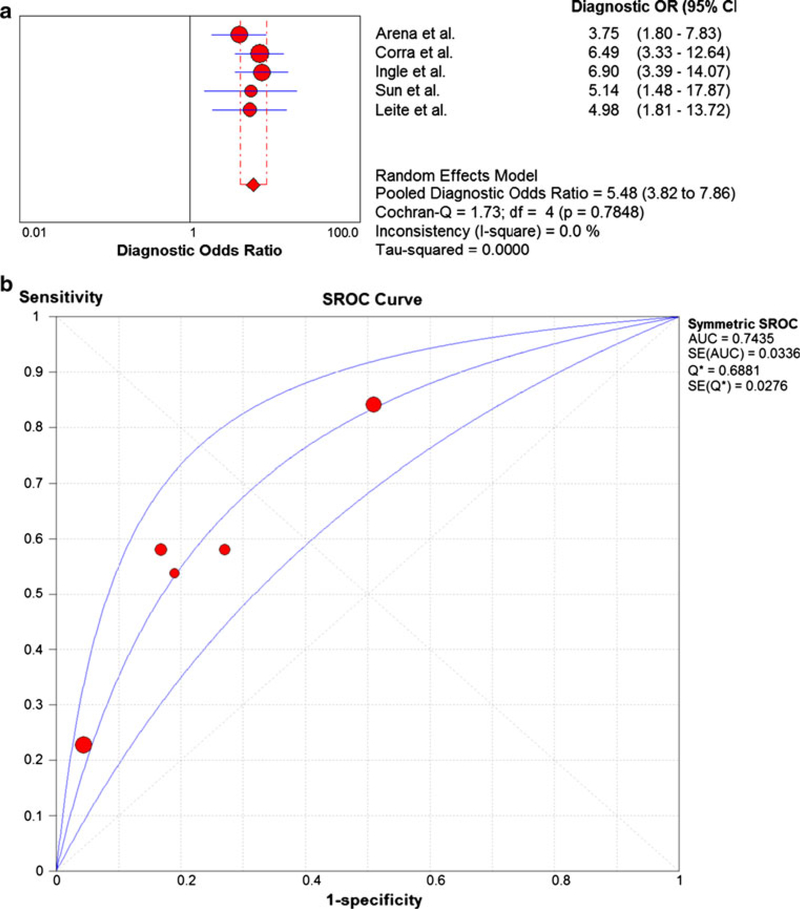
The meta-analysis results for EOV are shown in Fig. 4a. The pooled DOR was 5.48 (3.82–7.86) with no heterogeneity (Cochran-Q statistic and I2 values of 1.73 and 0.0 %, respectively). The sensitivity and specificity of EOV in predicting study endpoints was 0.47 (0.40–0.54) and 0.75 (0.73–0.78), respectively. The SROC AUC for the VE/VCO2 slope was 0.74 as shown in Fig. 4b.
Fig. 4.
a Summary statistics for meta-analysis of exercise oscillatory ventilation. b Summary receiver operating characteristic curve results for 5 studies of exercise oscillatory ventilation
OUES
The meta-analysis results for OUES are shown in Fig. 5. The pooled DOR was 8.08 (4.19–15.58) with a modest level of heterogeneity (Cochran-Q statistic and I2 values of 1.99 and 49.7 %, respectively). The sensitivity and specificity of the OUES in predicting study endpoints was 0.72 (0.65–0.78) and 0.70 (0.65–0.75), respectively. Because of limited studies, no OUES sub-study analysis was performed and the SROC AUC for the OUES could not be calculated.
Fig. 5.
Summary statistics for meta-analysis of oxygen uptake efficiency slope
Meta-regression analyses
The results of meta-regression analyses are shown in Table 8. None of the potential prognostic independent variables had a significant effect on the meta-analysis results for peak VO2 and the VE/VCO2 slope. A near significant effect of beta-blockade and study thresholds on the meta-analysis results of peak VO2 was observed. The coefficient and SE of the effect of beta-blockade were −0.012 and 0.006, respectively (p = 0.06), and the coefficient and SE of the effect of study thresholds were 0.308 and 0.144, respectively (p = 0.06). Univariate meta-regression could not be performed on the OUES and EOV due to limited studies and model saturation, respectively.
Table 8.
Univariate meta-regression results for peak VO2 and VE/VCO2 slope
| Variables | Peak VO2 p value | VE/VCO2 slope p value |
|---|---|---|
| Mean LVEF (%) | .79 | .64 |
| Mean NYHA (I-IV) | .53 | .75 |
| Mean age (years) | .50 | .72 |
| Male (%) | .38 | .71 |
| Ischemic cardiomyopathy (%) | .84 | .71 |
| Beta-blockers (%) | .06 | .65 |
| Study sample size | .40 | .21 |
| Study quality | .42 | .97 |
| Threshold | .06 | .20 |
Univariate meta-regression could not be performed on OUES and EOB due to limited studies and model saturation, respectively VO2 oxygen consumption, VE/VCO2 minute ventilation/carbon dioxide production, LVEF left ventricular ejection fraction, NYHA New York Heart Association
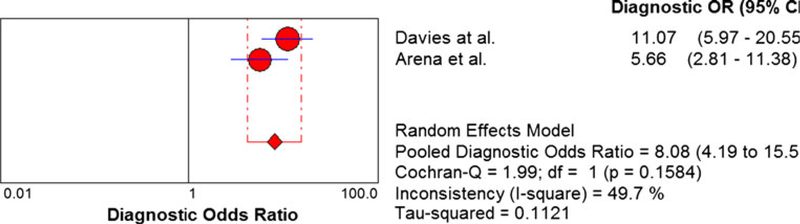
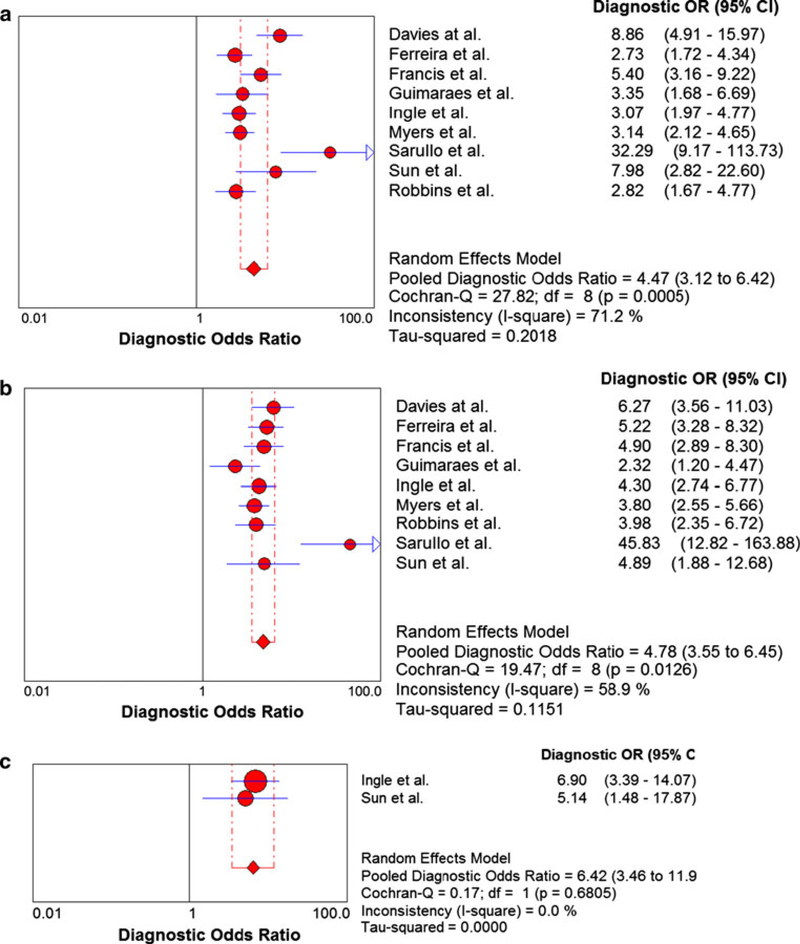
Comparison of peak VO2, the VE/VCO2 slope, and EOV
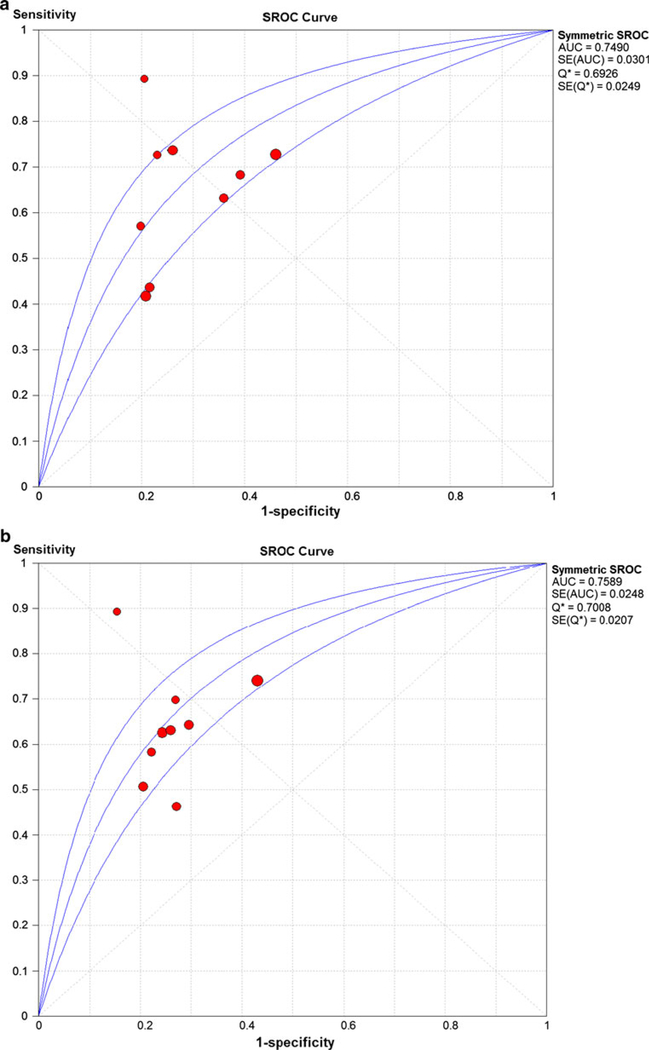
The meta-analysis results of the 9 studies used to compare peak VO2 and the VE/VCO2 slope are shown in Fig. 6. Two of these 9 studies examined EOV and the meta-analysis results for these two studies are shown in Fig. 6c. The EOV meta-analysis results revealed a pooled DOR of 6.42 and no heterogeneity (Cochran-Q statistic and I2 values of 0.17 and 0.0 %, respectively). The peak VO2 meta-analysis results revealed a pooled DOR of 4.47 and high level of heterogeneity (Cochran-Q statistic and I2 values of 27.82 and 71.2 %, respectively) (Fig. 6a). The SROC AUC for peak VO2 was 0.7490 (Fig. 7a). The VE/VCO2 slope meta-analysis results revealed a pooled DOR of 4.78 and modest level of heterogeneity (Cochran-Q statistic and I2 values of 19.47 and 58.9 %, respectively) (Fig. 6b). The SROC AUC for the VE/VCO2 slope was 0.76 (Fig. 7b).
Fig. 6.
Summary statistics comparing peak VO2, the VE/VCO2 slope, and exercise oscillatory ventilation. a Summary statistics for meta-analysis of peak VO2. VO2, oxygen consumption. b Summary statistics for meta-analysis of the VE/VCO2 slope. VE/VCO2, minute ventilation/carbon dioxide production. c Summary statistics for meta-analysis of exercise oscillatory ventilation
Fig. 7.
Summary receiver operating characteristic curve results comparing peak oxygen consumption and the VE/VCO2 slope for 9 studies. a Summary receiver operating characteristic curve results of peak VO2. VO2, oxygen consumption. b Summary receiver operating characteristic curve results of the VE/VCO2 slope. VE/VCO2, minute ventilation/carbon dioxide production
Discussion
To our knowledge, this is the largest CPX meta-analysis of prognosis in patients with HF which has examined the largest number of CPX variables and presents results that strongly support the role of CPX in patients with HF. The key findings from this meta-analysis include the robust prognostic value of peak VO2, the VE/VCO2 slope, the OUES, and EOV in patients with HF. Although each of these variables demonstrated significant prognostic power, EOV appears to be a very important measure to include in CPX since the meta-analysis results observed no heterogeneity and a relatively large DOR (5.48) from the 5 studies meeting our inclusion criteria which comprised 1,617 patients. Further examination of EOV in patients with HF is warranted. Further examination of the prognostic value of the OUES in patients with HF is also needed.
The clinical value of CPX in patients with HF is clearly established in the literature. The groundbreaking work by Mancini et al. in 1991 [13], demonstrating the prognostic value of peak VO2, paved the way for CPX to be supported as an integral component in the assessment of heart transplant candidacy. Numerous investigations since 1991 have confirmed the prognostic value of peak VO2 [14–16], which are used to support the continued recommendation that aerobic capacity be used as a listing criterion for HT [8, 9, 17]. However, there is a growing wealth of literature demonstrating that other CPX variables, including the VE/VCO2 slope [18, 19], EOV [12, 20] and the OUES [21], are also strong prognostic markers in patients with HF. Numerous investigations demonstrate that these indices of ventilatory inefficiency prognostically outperform peak VO2 and are readily obtained from standard CPX testing [3]. It is now broadly recognized that a multivariate CPX approach in patients with HF provides optimal estimates of prognosis [4]. Therefore, the current meta-analysis on the value of CPX in patients with HF is timely and novel in that the prognostic strength of four variables was examined in a rigorous manner.
Recently, a meta-analysis by Poggio et al. [22] reported on the diagnostic accuracy and prognostic value of CPX. While this systematic analysis was an important step forward, additional issues remain in this area of research. For example, while it is increasingly recognized that a host of CPX variables provide clinically valuable information in HF, this prior analysis was limited to only 2 CPX variables, peak VO2 and the VE/VCO2 slope [22]. Moreover, the meta-analysis by Poggio et al. [22] evaluated 2,171 and 2,628 subjects for peak VO2 and the VE/VCO2 slope, respectively. Our meta-analysis totaled 5,044 subjects for the VE/VCO2 slope, 7,319 for peak VO2, 1,617 for EOV, and 584 for the OUES. Therefore, both the number of subjects and number of CPX variables assessed in this meta-analysis were greater compared with the only other meta-analysis currently published in this area.
Our findings are generally consistent with those of Poggio et al. [22] except that the previous group found no heterogeneity in their meta-analysis of the VE/VCO2 slope while the current study observed moderate heterogeneity (I2 of 46.6 %). Despite the meta-analysis results of the VE/VCO2 slope in the current study revealing moderate heterogeneity (I2 = 46.6 %), the Cochran-Q statistic was relatively low (26.22) although statistically significant (p = .02). These findings suggest that the VE/VCO2 slope results may be a bit more robust than implied by the I2 statistic. Our DOR for the analysis of the VE/VCO2 slope was 5.40 compared with 5.02 in the study by Poggio et al. Also, Poggio et al. found a DOR of 4.07 in their meta-analysis of peak VO2 while the current analysis found a DOR of 4.10, but with greater heterogeneity in our analysis (I2 of 70.3 vs. 51.1 %). It appears that our inclusion of more subjects with different thresholds produced more heterogeneity. Regardless, the results from both these meta-analyses: (1) confirm the robust prognostic value of both peak VO2 and the VE/VCO2 slope and (2) support the widely held view that the VE/VCO2 slope possesses greater prognostic strength in comparison with peak VO2. Even so, there is ample evidence justifying the combined analysis of peak VO2 and the VE/VCO2 slope when determining prognosis in patients with HF since these responses provide independent and complementary information [3]. For example, systolic HF patients with a combined peak VO2 <10 ml kg−1 min−1 and a VE/VCO2 slope >40 are considered to be at very high risk of major adverse events [4].
The OUES was proposed as an alternate approach to assessing ventilatory efficiency by Baba et al. [23] in 1996. While this variable has been available for approximately 15 years, assessment of its prognostic value in HF is relatively new. Currently there are a limited number of studies assessing the prognostic value of the OUES (only two studies met inclusion criteria for the current meta-analysis). Accordingly, the Cochrane-Q statistic was comparatively low and was not significant in our analysis (Q-stat = 1.99; p = .158) despite an I2 statistic of 49.7 %. However, given the high DOR (8.08) for the OUES, further study of this index is warranted to determine its prognostic value relative to other CPX responses.
Perhaps one of the most compelling findings of the current analysis was the prognostic strength of EOV. This unique ventilatory pattern demonstrated no heterogeneity and a relatively large DOR (5.48) from the meta-analysis results of the 5 studies (comprising 1,617 patients) meeting our inclusion criteria). To put these results into perspective, Poggio et al. [22] included 6 articles in their meta-analysis of peak VO2, which comprised approximately 500 more subjects compared with the EOV analysis in the current investigation. A challenge in clinically utilizing EOV for prognostic purposes in patients with HF is the fact that it is not automatically calculated by current software packages operating ventilatory expired gas units. EOV is most commonly defined as an oscillatory pattern at rest that persists for ≥60 % of the EST at an amplitude of ≥15 % of the average resting value [12]. This criteria has to be manually determined by a health professional with CPX expertise. The results of the current meta-analysis clearly demonstrate the strong prognostic value of determining EOV, warranting continued efforts to standardize and automate assessment of the ventilatory pattern in patients with HF. Such efforts would certainly help to improve clinical acceptance and utilization of EOV.
While peak VO2 has remained the most commonly assessed variable in clinical practice, predominantly in a univariate manner, the results of this meta-analysis as well as the majority of other recent publications support expanding the list of CPX variables assessed for prognostic purposes. Our findings indicate a multivariate prognostic approach should be used with the consideration of peak VO2, the VE/VCO2 slope and the presence or absence of EOV. Patients who demonstrate a higher number of abnormal responses (i.e., low peak VO2, high VE/VCO2 slope and EOV) are likely to have a progressively higher risk of adverse CV events. Given the strong prognostic value of these CPX variables observed in the current meta-analysis, these variables should be used to guide clinical management strategies (pharmacologic, lifestyle, surgical) with the hope of reducing the risk of future adverse CV events [4, 24]. As additional evidence on the prognostic utility of CPX becomes available, inclusion of the OUES may be added to peak VO2, the VE/VCO2 slope and EOV as a core variable. Future investigations are needed to insure the expanding list of CPX variables provide independent prognostic value when assessed in a multivariate fashion. Educating clinicians responsible for ordering and interpreting CPX in patients with HF on the expanding list of variables that provide clinically valuable information should be considered a priority that would greatly enhance the quality of information gained from this exercise assessment.
As with any investigation, there are several notable limitations. First, CPX-HF investigations included in this meta-analysis have consistently assessed cohorts that were primarily male. Thus, while there is initial evidence to indicate CPX is equally prognostic in female patients with HF [25], additional research is needed. Heart failure management strategies continue to evolve and the possibility that some of the studies included in the current meta-analysis may be less applicable to current therapeutic approaches. However, a number of more current investigations were used in this meta-analysis and the potential for this bias was examined via the sub-study analysis of beta-blockade with the results being relatively similar or better using more current literature with more current medical therapy. Also, over 75 % of the studies used in our meta-analysis received an affirmative score for the QUADAS question examining “current clinical data and practice when the study results were reported”. The use of different threshold levels for peak VO2 and the VE/VCO2 slope as well as the presence of markedly different patient characteristics in the studies used for this meta-analysis could be possible limitations to this study, but we accounted for such differences by performing sub-study analyses which consistently demonstrated the prognostic value of these variables. Recently published results from the HF-ACTION study indicate variables more readily obtainable from exercise testing, specifically exercise test duration, was a very robust prognostic marker in their cohort, stronger than both peak VO2 and the VE/VCO2 slope [26]. While there were some similarities in subject characteristics between subjects from studies included in the current meta-analysis and subjects from HF-ACTION (i.e., age, sex distribution, ejection fraction, NYHA class distribution), others differed. For example, subjects enrolled in the HF-ACTION trial were well screened for participation in the study while subjects included in this meta-analysis were largely referred to CPX for refinement of clinical decision making, specifically decisions regarding transplant listing/device implantation. Moreover, approximately half of the patients included in the HF-ACTION analysis received an exercise intervention following CPX while subjects from studies included in the current meta-analysis did not. Lastly, a substantially higher percentage of patients in HF-ACTION were prescribed a beta-blocking agent compared with the studies included in the current meta-analysis (95 vs. 46 %), indicating a greater percentage of the patients included in HF-ACTION were managed under current standards of care, at least from the perspective of beta-blockade. Given the discordance in findings from this meta-analysis and the recently published prognostic investigation from HF-ACTION [26], the choice of optimal prognostic variables from exercise testing in the current clinical setting continues to require further investigation. Perhaps prognostic weighting of CPX variables is dependent upon the circumstances of testing; that is, performance of CPX in a broader HF population who may or may not receive and exercise intervention versus CPX in a more narrow HF population, clinically screened and being considered for transplantation or device implantation. However, we can say with great confidence that exercise testing, either performed in standard fashion or with the addition of ventilatory expired gas analysis, provides substantial prognostic insight and should continue to be a standard clinical assessment in this patient population. Also, other patient characteristics such as depression and renal function were not included as covariates in our meta-regression analysis and are limitations to this meta-analysis and an area in need of further investigation. Lastly, although not necessarily a limitation of our meta-analysis, the median age of the patients in all of our analyses was <60 years of age (median range of 55–58 years) which highlights the need to more thoroughly examine the role of CPX in older persons with HF.
In conclusion, CPX is clearly an important component in the clinical assessment of patients with HF. The results of this meta-analysis strongly confirm this view and support a multivariate approach to CPX assessment. Specifically, the combined quantification of aerobic capacity (peak VO2) and ventilatory efficiency (the VE/VCO2 slope and EOV) appear to provide a more comprehensive insight into path-ophysiology, disease severity, and prognosis.
Acknowledgments
Partial support provided by Cosmed, Rome, Italy.
Abbreviations
- AUC
Area under curve
- CPX
Cardiopulmonary exercise testing
- CV
Cardiovascular
- DOR
Diagnostic odds ratio
- EOV
Exercise oscillatory ventilation
- EST
Exercise stress testing
- FN
False negative
- FP
False positive
- HF
Heart failure
- HT
Heart transplantation
- LVEF
Left ventricular ejection fraction
- NYHA
New York heart association
- OUES
Oxygen uptake efficiency slope
- SROC
Summary receiver operating characteristic
- TN
True negative
- TP
True positive
- VE/VCO2
Minute ventilation/carbon dioxide production
- VO2
Oxygen consumption
Footnotes
Conflict of interest None.
Contributor Information
Lawrence P. Cahalin, Department of Physical Therapy, Leonard M. Miller School of Medicine, University of Miami, 5915 Ponce de Leon Blvd., 5th Floor, Coral Gables, FL 33146-2435, USA
Paul Chase, Lebauer Cardiovascular Research Foundation, Greensboro, NC, USA.
Ross Arena, Physical Therapy Program, Department of Orthopaedics and Department of Internal Medicine, Division of Cardiology, University of New Mexico, Albuquerque, NM, USA.
Jonathan Myers, Division of Cardiology, VA Palo Alto Healthcare System, Palo Alto, CA, USA.
Daniel Bensimhon, Lebauer Cardiovascular Research Foundation, Greensboro, NC, USA.
Mary Ann Peberdy, Department of Internal Medicine, Virginia Commonwealth University, Richmond, VA, USA.
Euan Ashley, Cardiovascular Medicine, Stanford University, Palo Alto, CA, USA.
Erin West, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA, USA.
Daniel E. Forman, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA, USA
Sherry Pinkstaff, Physical Therapy Program, Brooks College of Health, University of North Florida, Jacksonville, FL, USA.
Carl J. Lavie, Department of Cardiovascular Diseases, John Ochsner Heart and Vascular Institute, Ochsner Clinical School, The University of Queensland School of Medicine, New Orleans, LA, USA Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, LA, USA.
Marco Guazzi, Cardiology, I.R.C.C.S. Policlinico San Donato, University of Milano, San Donato Milanese, Italy.
References
- 1.Weber KT, Kinasewitz GT, Janicki JS, Fishman AP (1982) Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure. Circulation 65:1213–1223 [DOI] [PubMed] [Google Scholar]
- 2.Wilson JR, Ferraro N, Weber KT (1983) Respiratory gas analysis during exercise as a noninvasive measure of lactate concentration in chronic congestive heart failure. Am J Cardiol 51:1639–1643 [DOI] [PubMed] [Google Scholar]
- 3.Arena R, Myers J, Guazzi M (2008) The clinical and research applications of aerobic capacity and ventilatory efficiency in heart failure: an evidence-based review. Heart Fail Rev 13:245–269 [DOI] [PubMed] [Google Scholar]
- 4.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV (2010) Clinician’s guide to cardiopulmonary exercise testing in adults. A scientific statement from the American heart association. Circulation 122:191–225 [DOI] [PubMed] [Google Scholar]
- 5.Piepoli MF, Corra U, Agostoni PG, Belardinelli R, Cohen-Solal A, Hambrecht R, Vanhees L (2006) Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction: recommendations for performance and interpretation Part II: how to perform cardiopulmonary exercise testing in chronic heart failure. Eur J Cardiovasc Prev Rehabil 13: 300–311 [DOI] [PubMed] [Google Scholar]
- 6.Piepoli MF, Corra U, Agostoni PG, Belardinelli R, Cohen-Solal A, Hambrecht R, Vanhees L (2006) Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction: recommendations for performance and interpretation. Part I: definition of cardiopulmonary exercise testing parameters for appropriate use in chronic heart failure. Eur J Cardiovasc Prev Rehabil 13:150–164 [DOI] [PubMed] [Google Scholar]
- 7.Piepoli MF, Corra U, Agostoni PG, Belardinelli R, Cohen-Solal A, Hambrecht R, Vanhees L (2006) Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction: recommendations for performance and interpretation Part III: interpretation of cardiopulmonary exercise testing in chronic heart failure and future applications. Eur J Cardiovasc Prev Rehabil 13:485–494 [DOI] [PubMed] [Google Scholar]
- 8.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW (2009) 2009 Focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in Collaboration With the International Society for Heart and Lung Transplantation. Circulation 119: 1977–2016 [DOI] [PubMed] [Google Scholar]
- 9.Gibbons RJ, Balady GJ, Timothy BJ, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O’Reilly MG, Winters WL, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC (2002) ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol 40:1531–1540 [DOI] [PubMed] [Google Scholar]
- 10.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J (2003) The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB, for the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Group (2000) Meta-analysis of observational studies in epidemiology. JAMA 283:2008–2012 [DOI] [PubMed] [Google Scholar]
- 12.Corra U, Giordano A, Bosimini E, Mezzani A, Piepoli M, Coats AJ, Giannuzzi P (2002) Oscillatory ventilation during exercise in patients with chronic heart failure: clinical correlates and prognostic implications. Chest 121:1572–1580 [DOI] [PubMed] [Google Scholar]
- 13.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH Jr, Wilson JR (1991) Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 83:778–786 [DOI] [PubMed] [Google Scholar]
- 14.Myers J, Gullestad L, Vagelos R, Do D, Bellin D, Ross H, Fowler MB (2000) Cardiopulmonary exercise testing and prognosis in severe heart failure: 14 mL/kg/min revisited. Am Heart J 139: 78–84 [DOI] [PubMed] [Google Scholar]
- 15.Parikh MN, Lund LH, Goda A, Mancini D (2009) Usefulness of peak exercise oxygen consumption and the heart failure survival score to predict survival in patients >65 years of age with heart failure. Am J Cardiol 103:998–1002 [DOI] [PubMed] [Google Scholar]
- 16.O’Neill JO, Young JB, Pothier CE, Lauer MS (2005) Peak oxygen consumption as a predictor of death in patients with heart failure receiving {beta}-blockers. Circulation 111:2313–2318 [DOI] [PubMed] [Google Scholar]
- 17.Gibbons RJ, Balady GJ, Beasley JW, Bricker JT, Duvernoy WF, Froelicher VF, Mark DB, Marwick TH, McCallister BD, Thompson PD Jr, Winters WL, Yanowitz FG, Ritchie JL, Gibbons RJ, Cheitlin MD, Eagle KA, Gardner TJ, Garson A Jr, Lewis RP, O’Rourke RA, Ryan TJ (1997) ACC/AHA guidelines for exercise testing. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). J Am Coll Cardiol 30:260–311 [DOI] [PubMed] [Google Scholar]
- 18.Arena R, Myers J, Abella J, Peberdy MA, Bensimhon D, Chase P, Guazzi M (2007) Development of a ventilatory classification system in patients with heart failure. Circulation 115:2410–2417 [DOI] [PubMed] [Google Scholar]
- 19.Francis DP, Shamim W, Davies LC, Piepoli MF, Ponikowski P, Anker SD, Coats AJ (2000) Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2)slope and peak VO(2). Eur Heart J 21:154–161 [DOI] [PubMed] [Google Scholar]
- 20.Guazzi M, Arena R, Ascione A, Piepoli M, Guazzi MD (2007) Exercise oscillatory breathing and increased ventilation to carbon dioxide production slope in heart failure: an unfavorable combination with high prognostic value. Am Heart J 153:859–867 [DOI] [PubMed] [Google Scholar]
- 21.Davies LC, Wensel R, Georgiadou P, Cicoira M, Coats AJ, Piepoli MF, Francis DP (2006) Enhanced prognostic value from cardiopulmonary exercise testing in chronic heart failure by non-linear analysis: oxygen uptake efficiency slope. Eur Heart J 27: 684–690 [DOI] [PubMed] [Google Scholar]
- 22.Poggio R, Arazi HC, Giorgi M, Miriuka SG (2010) Prediction of severe cardiovascular events by VE/Vco2 slope versus peak Vo2 in systolic heart failure: a meta-analysis of the published literature. Am Heart J 160:1004–1014 [DOI] [PubMed] [Google Scholar]
- 23.Baba R, Nagashima M, Goto M, Nagano Y, Yokota M, Tauchi N, Nishibata K (1996) Oxygen intake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relationship between oxygen consumption and minute ventilation during incremental exercise. Nagoya J Med Sci 59:55–62 [PubMed] [Google Scholar]
- 24.Arena R, Myers J, Guazzi M (2011) Cardiopulmonary exercise testing is a core assessment for patients with heart failure. Congest Heart Failure 17:115–119 [DOI] [PubMed] [Google Scholar]
- 25.Guazzi M, Arena R, Myers J (2006) Comparison of the prognostic value of cardiopulmonary exercise testing between male and female patients with heart failure. Int J Cardiol 113:395–400 [DOI] [PubMed] [Google Scholar]
- 26.O’Connor CM, Whellan DJ, Wojdyla D, Leifer E, Clare RM, Ellis SJ, Fine LJ, Fleg JL, Zannad F, Keteyian SJ, Kitzman DW, Kraus WE, Rendall D, Pinã IL, Cooper LS, Fiuzat M, Lee KL (2012) Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction. Clin Perspect Circ Heart Fail 5:63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]