Abstract
Background
The potential risk of cytokine storm in patients with coronavirus disease 2019 (COVID-19) has been described [1]; we write to share our experience treating a 17-year-old male with haemophagocytic lymphohistiocytosis (HLH) secondary to COVID-19 infection.
Case report
This patient presented with cough, sore throat, anorexia and pyrexia. On examination, he had gross cervical lymphadenopathy and palpable splenomegaly. Nose and throat swab for SARS-CoV-2 was positive and blood tests revealed pancytopaenia with very high ferritin, triglyceride and d-dimer levels. The patient's H-Score [2] was calculated at 220, suggesting probability of HLH of 93–96%. Considering Russell and colleagues' [3] comments about potential harm of corticosteroid use in patients with COVID-19 infection, the patient was commenced on treatment with the selective IL-1 receptor antagonist drug, Anakinra, and a two-day course of intravenous immunoglobulin.
Results
The patient responded rapidly to treatment, becoming apyrexial after 24 h. His lymph nodes and spleen began to normalise after the first 48 h, at which time point the ferritin also started to decrease. He was discharged after 11 days feeling fit and well.
Conclusion
This case certainly illustrates the importance of hyperinflammation syndromes in COVID-19. It also raises the question – is the severe pneumonitis seen in patients with COVID-19 an immunological phenomenon? We know that the viral load of patients with COVID-19 seems to peak in the early stages of illness [4,5]; however, patients deteriorate later in the disease course, at around days 10–14. This patient, who had risk factors for deterioration (male, pancytopaenic), did not develop an oxygen requirement and clinically and biochemically improved rapidly on Anakinra with no adverse events. We might suggest Anakinra to the scientific community as a treatment option in COVID-19 infection.
Keywords: Communicable diseases, Hemophagocytic lymphohistiocytosis, Coronavirus infections
Highlights
-
•
Hyperinflammation syndromes should be considered in coronavirus disease 2019.
-
•
Haemophagocytic lymphohistiocytosis secondary to coronavirus disease 2019
-
•
Avoiding steroids and instead using immunologics in coronavirus disease 2019
-
•
Using selective IL-1 receptor antagonists for treatment in coronavirus disease 2019
Introduction
The potential risk of cytokine storm in patients with coronavirus disease 2019 (COVID-19) has been described [1]; we write to share our experience treating a 17-year-old male with haemophagocytic lymphohistiocytosis (HLH) secondary to COVID-19.
Case
The patient had no past medical history and no regular medications. He was a non-smoker with no alcohol intake and a normal body mass index. He lived with his parents, and there was no family history of haemophagocytic lymphohistiocytosis or other inflammatory disorders.
This patient presented with a six-day history of cough, sore throat, anorexia and pyrexia (recorded at 39.1 °C). On examination, he had gross cervical lymphadenopathy with submandibular nodes more than 10 cm in diameter. There was palpable splenomegaly.
Investigations revealed pancytopaenia, hyponatraemia, hypocalcaemia and elevated alanine aminotransferase, lactate and c-reactive protein. Ferritin was 8197μg/l, triglycerides 5.1 mmol/l, LDH 586 u/l, d-dimer 3758 ng/ml, fibrinogen 1.73 g/l and reticulocytes 13%. Further investigations revealed negative HIV, hepatitis B and C and toxoplasma screening. EBV and CMV IgG were both positive, but IgM and PCR negative. Serum immunoglobulins and electrophoresis were normal. Blood cultures were negative. Admission chest x-ray and seasonal respiratory viral PCR panel (including, but not limited to, influenza A and B, rhinovirus and adenovirus) was negative. SARS-CoV-2 nose and throat swab taken on the day of admission and run using VIASURE by BioTec SARS-CoV-2 with QiaSymphony virus DSP extraction, returned positive on day two of admission with a cycle threshold value of 28.7. Graph 1, Graph 2, Graph 3, Graph 4 show the pattern of change of ferritin, neutrophils, platelets and d-dimer blood parameters during admission.
Graph 1.
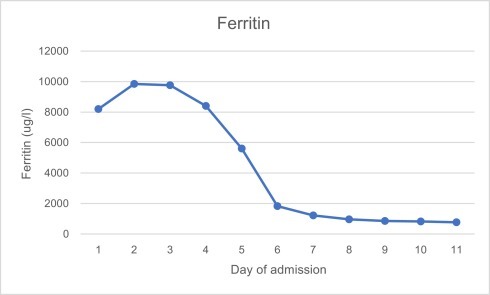
Ferritin values during admission.
Graph 2.
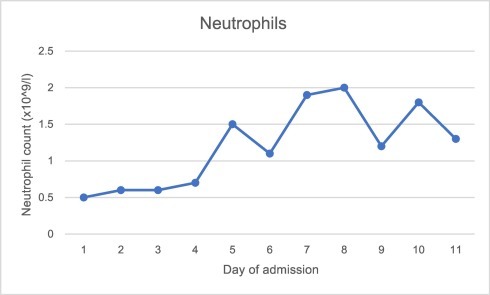
Neutrophil count during admission.
Graph 3.
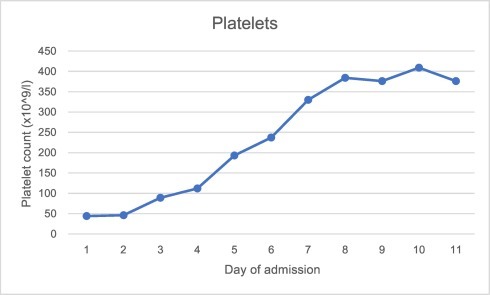
Platelet count during admission.
Graph 4.
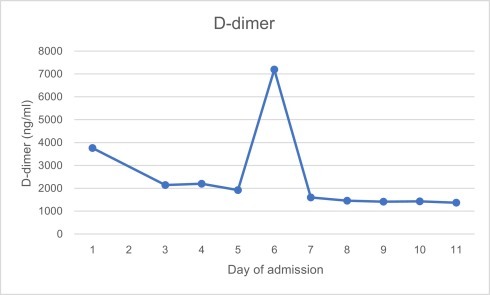
D-dimer values during admission.
On day two of admission there was ongoing pancytopaenia and an increasing ferritin level. Bone marrow aspirate showed reactive marrow with no evidence of malignant infiltration. A bone marrow trephine could not be tolerated. The patient's H-Score [2] was calculated at 220, suggesting a probability of HLH of 93–96%. Considering Russell and colleagues' [3] comments about the potential harm of corticosteroid use in patients with COVID-19 infection, the patient was commenced on treatment with the selective IL-1 receptor antagonist drug, Anakinra (100 mg/day), alongside a two-day course of intravenous immunoglobulin. Bloods were sent to exclude primary HLH, given his young age.
In terms of antimicrobial treatment, oral amoxicillin-clavulanic acid 625 mg three times a day was commenced on admission to provide cover for bacterial infection. On day two, antimicrobial therapy was escalated to intravenous piperacillin-tazobactam 4.5 g three times a day to provide cover for neutropaenic sepsis. After five days of piperacillin-tazobactam, intravenous meropenem 1 g three times a day was started as his liver function tests had not yet stabilised and there was concern that the piperacillin-tazobactam may have been contributing to this. Antibiotics were stopped when a total of seven days had been completed.
The patient responded rapidly to treatment, becoming apyrexial after 24 h of Anakinra. His lymph nodes and spleen began to reduce in size after the first 48 h, at which time point the ferritin also started to decrease. Liver function tests worsened over the first five days, with alanine aminotransferase peaking at 771 u/l, but reduced thereafter. Once his ferritin had fallen to less than 1000 μg/l, on day nine, Anakinra was discontinued. The patient was kept in hospital for a further three days to ensure that his ferritin continued to decrease following treatment. He was discharged on day 11 feeling fit and well, with a ferritin of 766 μg/l and almost normal clotting and liver function tests. Outpatient follow up was arranged to allow for clinical and biochemical review.
Conclusion
This case certainly illustrates the importance of hyperinflammation syndromes in COVID-19. It also raises the question – is the severe pneumonitis seen in patients with COVID-19 an immunological phenomenon? We know that the viral load of patients with COVID-19 seems to peak in the early stages of illness [4,5], however patients deteriorate later in the disease course, at around days 10–14. This patient, who had risk factors for deterioration (male, pancytopaenic), did not develop an oxygen requirement and clinically and biochemically improved rapidly on Anakinra with no adverse events. We might suggest Anakinra to the scientific community as a treatment option in COVID-19 infection, and we understand a clinical trial is indeed in progress [6].
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Kathryn Haigh:Conceptualization, Formal analysis, Investigation, Writing - original draft.Zoe Joanna Syrimi:Investigation.Sharon Irvine:Formal analysis, Writing - review & editing.Tom J. Blanchard:Conceptualization, Formal analysis, Writing - review & editing, Supervision.Muhammad Sajid Pervaiz:Formal analysis, Investigation.Arpad G. Toth:Formal analysis, Investigation.Libuse Ratcliffe:Writing - review & editing.
Footnotes
Declarations of interest: None.
Informed consent: The patient has provided informed consent for us to write this report.
References
- 1.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fardet L., Galicier L., Lambotte O., Marzac C., Aumont C., Chahwan D. Development and validation of the HSCore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613–2620. doi: 10.1002/art.38690. [DOI] [PubMed] [Google Scholar]
- 3.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U.S National Library of medicine; ClinicalTrials.gov. [Internet] https://clinicaltrials.gov/ct2/show/NCT04324021 [updated 2020 April 9, cited 2020 April 25]. Available from:


