Abstract
A vast majority of type-2 diabetic patients (~65%) die of cardiovascular complications including heart failure (HF). In diabetic hearts, levels of 4-hydroxy-2-nonenal (4HNE), a reactive aldehyde that is produced upon lipid peroxidation, were increased. We also demonstrated that in diabetic hearts, there is a decrease in the activity of aldehyde dehydrogenase (ALDH) 2, a primary detoxifying enzyme present in cardiac mitochondria. A single point mutation at E487K of ALDH2 in East Asians known as ALDH2 * 2 intrinsically lowers ALDH2 activity. We hypothesize that Empagliflozin (EMP), a sodium-glucose cotransporter (SGLT) 2 inhibitor, can ameliorate diabetic cardiomyopathy by decreasing hyperglycemia-mediated 4HNE protein adducts in ALDH2 * 2 mutant mice which serve as a precision medicine tool as they mimic ALDH2 * 2 carriers. We induced type-2 diabetes in 11–14 month-old male and female ALDH2 * 2 mice through a high-fat diet. Chow-fed ALDH2 * 2 mice served as controls. At the end of 4 months, we treated the diabetic ALDH2 * 2 mice with EMP (3 mg/kg/d) or its vehicle (Veh). After 2 months of EMP treatment, cardiac function was assessed by conscious echocardiography after treadmill exercise stress. EMP improved the cardiac function and running distance and duration significantly compared to Veh-treated ALDH2 * 2 diabetic mice. These beneficial effects can be attributed to the EMP-mediated decrease in cardiac mitochondrial 4HNE adducts and increase in the levels of phospho AKT, AKT, phospho Akt substrate of 160 kDa (pAS160), AS160 and GLUT-4 in the skeletal muscle tissue of the ALDH2*2 mutant diabetic mice, respectively. Finally, our data implicate EMP can ameliorate diabetic cardiomyopathy in diabetic ALDH2 * 2 mutant patients.
Keywords: 4-hydroxy-2-nonenal, Type-2 diabetes, Aldehyde dehydrogenase-2, Empagliflozin, Cardiac function, ALDH2 * 2 mutant mice
1. Introduction
The diabetic patient population is expected to rise to 642 million globally by 2040 from ~425 million in 2015 (Ogurtsova et al., 2017). Many afflicted diabetic patients will incur end-organ damage and poor quality of life (Lichtenauer et al., 2003; Orasanu and Plutzky, 2009). Most importantly, cardiac complications can account for as high as 44% and 68% of deaths in type-1 and type-2 diabetic patients, respectively (Feltbower et al., 2008). Therefore, we strategize to understand the mechanisms behind this cardiomyopathy in order to find and validate unique therapeutic options.
Hyperglycemia-induced oxidative stress is a significant contributor to diabetic cardiac complications such as cardiomyopathy and heart failure (Jay et al., 2006; Mehta et al., 2006). The secondary products of reactive oxygen species namely reactive aldehydes are regarded as significant contributors to organ damage (Semchyshyn, 2014). In diabetes, these aldehyde compounds are not only derived from oxidative stress, but also occur as a result of non-oxidative reactions (Negre-Salvayre et al., 2008). They range from a few carbon lengths to long chains, cyclic aldehydes, and ketones. 4-hydroxy-2-nonenal (4HNE), one such reactive aldehyde, is commonly produced upon lipid peroxidation (Mali and Palaniyandi, 2014). Indeed, diabetic patients have elevated 4HNE in serum (Calabrese et al., 2007). 4HNE forms adducts with macromolecules like protein and DNA, leading to cellular dysfunction and ultimately tissue damage (Humphries et al., 1998; Lashin et al., 2006). We have also found increased 4HNE adducts in the myocardium of diabetic patients (Palaniyandi SS et al., 2010). 4HNE also has been documented to form adducts on mitochondrial complex-2 protein in diabetic heart (Lashin et al., 2006). Therefore, 4HNE is integral in causing cardiotoxicity of the diabetic heart.
4HNE levels are controlled primarily by aldehyde dehydrogenase (ALDH), a family of homo-tetrameric enzymes (Jackson et al., 2011) that detoxify aldehydes into non-toxic acids. ALDH2 is a cardiac mitochondrial enzyme. It has been shown that ALDH2 activation is required to produce cardio protection from ischemic insults (Budas et al., 2009; Chen et al., 2008; Lagranha et al., 2010). ALDH2 is particularly important in the detoxification of aldehydes like 4HNE that accumulate under oxidative stress (carbonyl or aldehydic stress) in diabetic heart (Chen et al., 2008; Vasiliou et al., 2000).
A point mutation (E487K) at the interface of the ALDH2 tetrameric complex decreases catalytic activity to only 1–25% that of WT ALDH2. This mutation is present in ~600 million East Asians (Chen et al., 2008). In a recent study, it was shown that type-2 diabetic patients with ALDH2 * 2 mutation in China exhibited greater cardiac dysfunction compared to diabetic patients without mutation (Wang et al., 2016). This evidence clearly demonstrates that the insufficiency of 4HNE detoxification by ALDH2 * 2 is critical in diabetic cardiac damage. Therefore, we planned to use ALDH2 * 2 knock-in mutant mice with this human mutation which can allow us to investigate the role of ALDH2 in 4HNE-induced mitochondrial and cardiac toxicity.
Due to the magnitude of the East Asian population with ALDH2 * 2 mutation, the prospective burden of diabetic cardiomyopathy is concerning. Thus, we want to use a precision approach to mimic the pathophysiology of the human ALDH2 * 2 patients. ALDH2 * 2 mutant mice which mimic ALDH2 * 2 carriers in East Asia can serve effectively for this precision medicine approach. We postulated that the attenuation of hyperglycemia via EMP would lower 4HNE and thereby ameliorate diabetic cardiomyopathy in ALDH2 * 2 mutant mice despite having low ALDH2 activity.
2. Materials and methods
2.1. Animal Protocol: induction of type-2 diabetes mellitus through high-fat diet in ALDH2 * 2 mice
ALDH2 * 2 knock-in mutant mice (with C57BL/6 background) of either gender were fed a high-fat diet (60% of calories from fat, D12492, Research Diets) N = 12. We employed mice with the age of 11–14 months old to mimic clinical situation where diabetes affects middle aged people. A group of ALDH2 * 2 mice were fed with normal chow (Control, Ctrl N = 6) until sacrifice. After a month, mice with sustained elevated fasting blood glucose levels (> 250 mg/dl) were classified as diabetic mice and selected for further studies. Blood was collected from the tail vein and glucose was then measured with a glucometer. The body weights were also measured and recorded.
2.1.1. ALDH2 * 2 mice were inbred in-house and genotyped by Transnetyx Inc
The animal protocol was approved by the Henry Ford Health System Institutional Animal Care and Use Committee. It adheres to the guiding principles of the care and use of experimental animals in accordance with NIH guidelines. Henry Ford Hospital operates an AAALAC certified animal facility.
2.2. EMP treatment protocol
At the end of 5 months, the diabetic ALDH2 * 2 mice were treated with EMP(3 mg/kg/d) or its vehicle (Veh). We selected the dose of EMP from previous published studies (Benetti et al., 2016; Cheng et al., 2016; Han et al., 2017). After 1 month of treatment, we measured their blood glucose levels and glucose tolerance. After 2 months of treatment, we measured cardiac function by conscious echocardiography as reported briefly below. Additionally, we measured cardiac reserve function by conscious echocardiography after treadmill exercise stress as described in our recent report (Pan et al., 2018) and briefly below. Finally, the mice were killed for biochemical analysis.
2.3. Cardiac function assessment by echocardiography in conscious mice
Left ventricular dimension and function were assessed in conscious mice to avoid the effects of anesthesia. We used an echocardiograph equipped with a 15-MHz linear transducer (Acuson c256) as described previously (Mali et al., 2014; Yang et al., 1999).
2.4. Acute progressive maximal exercise test (exhaustion test)
The control and diabetic ALDH2 * 2 mice were brought to the treadmill room for 2 h in advance so that they could acclimate to the environment. Furthermore, they were allowed to explore and acclimatize to the treadmill for at least 3 min. Next, the mice were subjected to the exhaustion test used in previous studies (Ostler et al., 2014; Petrosino et al., 2016) as described by us (Pan et al., 2018).
2.5. Post-exercise echocardiography
As soon as the mice finished stress treatment, we performed conscious echocardiography on them to record their functional changes as described above.
2.6. Intraperitoneal glucose tolerance test (IPGTT)
The IPGTT was performed in mice from control and diabetic groups as explained elsewhere (Mali et al., 2014). After fasting for 6 h, the mice were injected with 2 g/kg D-glucose. Then, blood glucose levels were measured at 0, 30, 60, 90 and 120 min after D-glucose injection, using a glucometer.
2.7. Western immunoblotting
The Western blot was performed as described earlier (Mali et al., 2016b; Pan et al., 2016). In brief, protein samples from cardiac mitochondria and muscle plasma membrane fractions were separated on SDS-polyacrylamide gels by electrophoresis. The proteins were then transferred to immobilon-P membranes (Millipore, Billerica, MA). Levels of 4HNE-protein adducts in cardiac mitochondrial samples were determined using antibodies of anti-4HNE-Cys/His/Lys rabbit antibody (Millipore) along with anti-aconitase mouse monoclonal antibody (Abcam) was used to detect aconitase, a housekeeping marker, for comparison. In the proteins from plasma membrane fractions of muscle, we measured phospho-AKT using anti-phospho-AKT rabbit monoclonal antibody (Cell Signaling Technology), AKT using AKT rabbit polyclonal antibody (Cell Signaling Technology), phospho-AS160 using anti-phospho-AS160 (Thr642) rabbit polyclonal antibody (Cell Signaling Technology), AS160 using anti-AS160 rabbit polyclonal antibody (Cell Signaling Technology). Anti-Na+K+ATPase rabbit polyclonal antibody (Cell Signaling Technology) and anti- α-sarcomeric actin goat polyclonal antibody (Santacruz Biotech) were used as a loading controls. The bound primary antibodies were added to horseradish peroxidase (HRP)-coupled respective secondary antibodies, and then visualized by chemiluminescence detection reagents.
2.8. Statistical analysis
Data are presented as mean ± standard error of the mean (S.E.M) unless mentioned. We used One-way ANOVA for group comparisons. The difference between control and diabetic groups were analyzed by using the unpaired Student t-test. The difference between the time points from before and after exercise stress testing was analyzed by paired Student t-test.
3. Results
3.1. High-fat feeding induces type-2 diabetes in ALDH2 * 2 mice and EMP decreases blood glucose levels and glucose intolerance
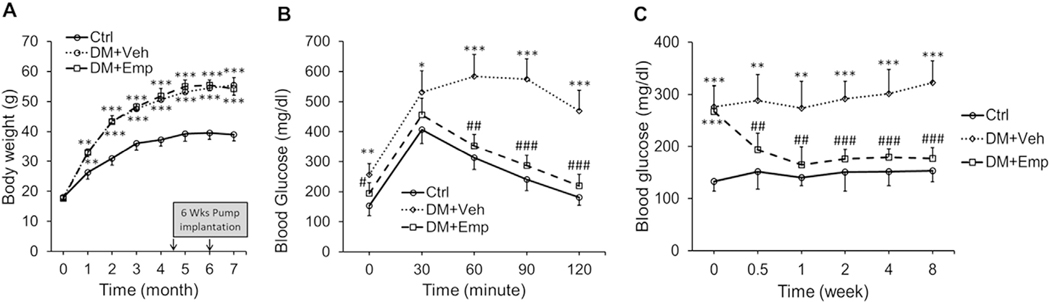
High-fat fed ALDH2 * 2 mice displayed increased body weight (obesity) (Fig. 1A). These mice also exhibited increased blood glucose levels at basal level (hyperglycemia) and upon glucose challenge (Fig. 1B). Therefore, ALDH2 * 2 mice had significantly greater levels of glucose intolerance (insulin resistance) when compared to normal chow-fed ALDH2 * 2 mice (Fig. 1B). This indicates that high-fat fed ALDH2 * 2 mice exhibit type-2 diabetes. EMP treatment has decreased blood glucose levels in the high-fat fed ALDH2 * 2 mice exhibiting type-2 diabetes throughout the treatment period (Fig. 1C) and EMP improved glucose tolerance (Fig. 1B) without decreasing obesity.
Fig. 1. High-fat diet induces obesity, hyperglycemia and glucose intolerance in ALDH2 * 2 mutant mice.
Body weight (A), glucose intolerance (B) and hyperglycemia (C) data were shown from non-diabetic control ALDH2 * 2 mutant mice (Ctrl.), vehicle-treated ALDH2 * 2 mutant diabetic mice (DM + Veh) and EMP-treated ALDH2 * 2 mutant diabetic mice (DM + EMP). Data are presented as mean ± standard error of the mean (S.E.M.). **P < 0.01 and ***P < 0.001 vs Ctrl. ##P < 0.01and ###P < 0.001 vs. DM+Veh.
3.2. EMP improves cardiac function in high-fat fed type-2 diabetic ALDH2 * 2 mice
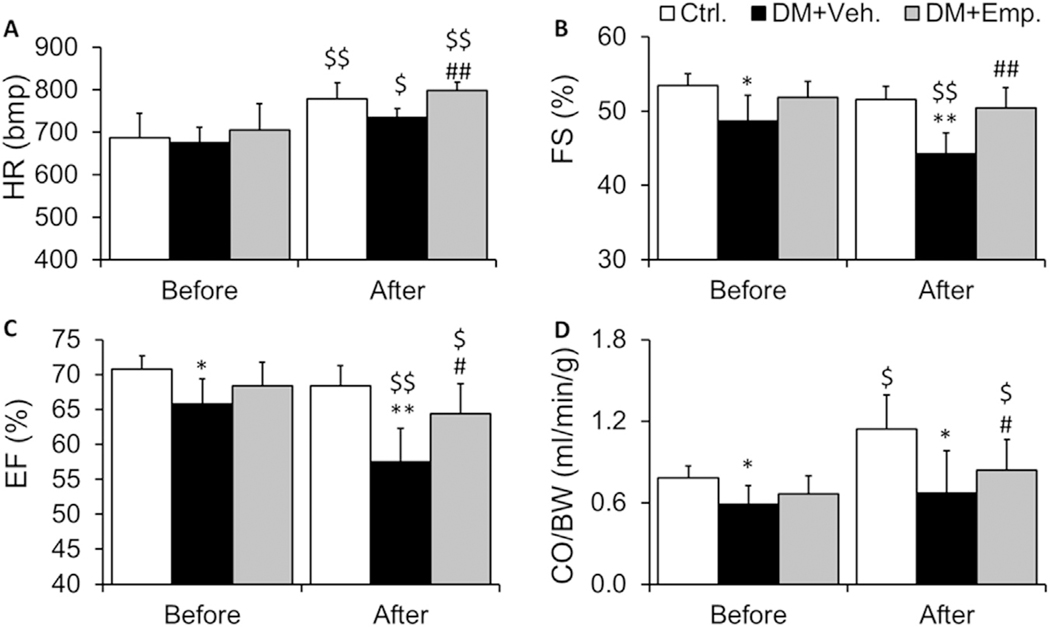
When we performed exercise stress echocardiography after 4 months of diabetes induction, Vehi-treated high-fat diet fed type-2 diabetic ALDH2 * 2 mice exhibited poor cardiac contractile function compared to their non-diabetic control counterparts. EMP improved cardiac functional parameters with the echo-stress test: We found an increase in the heart rate after exercise in both control ALDH2 * 2 mice free of diabetes and ALDH2 * 2 mice with 4 months of type-2 diabetes (Fig. 2A). However, this increase in the heart rate was significantly less in diabetic ALDH2 * 2 mice (Fig. 2A). EMP increased the heart rate after exercise (Fig. 2A). The % fractional shortening (FS) (Fig. 2B) and % ejection fraction (EF) (Fig. 2C) were significantly lower in diabetic ALDH2 * 2 mice after exercise when compared to control ALDH2 * 2 mice. EMP significantly increased %FS and %EF after exercise (Fig. 2B and C). Though there were decreases in % FS and %EF in diabetic ALDH2 * 2 mice relative to control ALDH2 * 2 mice before exercise (at rest) (Fig. 2B and C), but the decreases were much higher after exercise (Fig. 2B and C). A significant decrease in cardiac output (CO) was observed before and after exercise in diabetic ALDH2 * 2 mice compared to their non-diabetic controls (Fig. 2D). There was an increase in CO in non-diabetic control (Fig. 2D). EMP also increased the CO in treated diabetic ALDH2 * 2 mice after exercise stress (Fig. 2D).
Fig. 2. Cardiac functional indices.
Heart rate (A), %FS (B), %EF (C) and CO/BW (D) data were shown from non-diabetic control ALDH2 * 2 mutant mice (Ctrl.), vehicle-treated ALDH2 * 2 mutant diabetic mice (DM + Veh) and EMP-treated ALDH2 * 2 mutant diabetic mice (DM + EMP). Data are presented as mean ± standard error of the mean (S.E.M.). *P < 0.05 and **P < 0.01 vs Ctrl. $P < 0.05 and $$P < 0.01 vs. before exercise stress, #P < 0.05, ##P < 0.01 vs. DM + Veh.
3.3. EMP improved the running duration and distance in diabetic ALDH2 * 2 mice
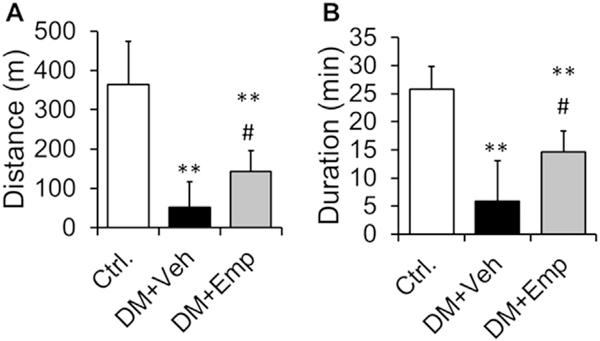
We found that type-2 diabetic ALDH2 * 2 mice (at 4 months after diabetes induction) run for shorter duration and distance until exhaustion, compared to non-diabetic control ALDH2 * 2 mice (Fig. 3A and B). Treatment with EMP improved the running duration and distance in the diabetic ALDH2 * 2 mice (Fig. 3A and B).
Fig. 3. Running distance and duration with treadmill exercise.
Running distance (A) and duration (B) were shown from non-diabetic control ALDH2 * 2 mutant mice (Ctrl.), vehicle-treated ALDH2 * 2 mutant diabetic mice (DM + Veh) and EMP-treated ALDH2 * 2 mutant diabetic mice (DM + EMP). Data are presented as mean ± standard error of the mean (S.E.M.). Data are presented as mean ± standard error of the mean (S.E.M.). **P < 0.001 vs. Ctrl.; #P < 0.05 vs. DM + Veh.
3.4. EMP reduced 4HNE protein adducts in diabetic ALDH2 * 2 mice
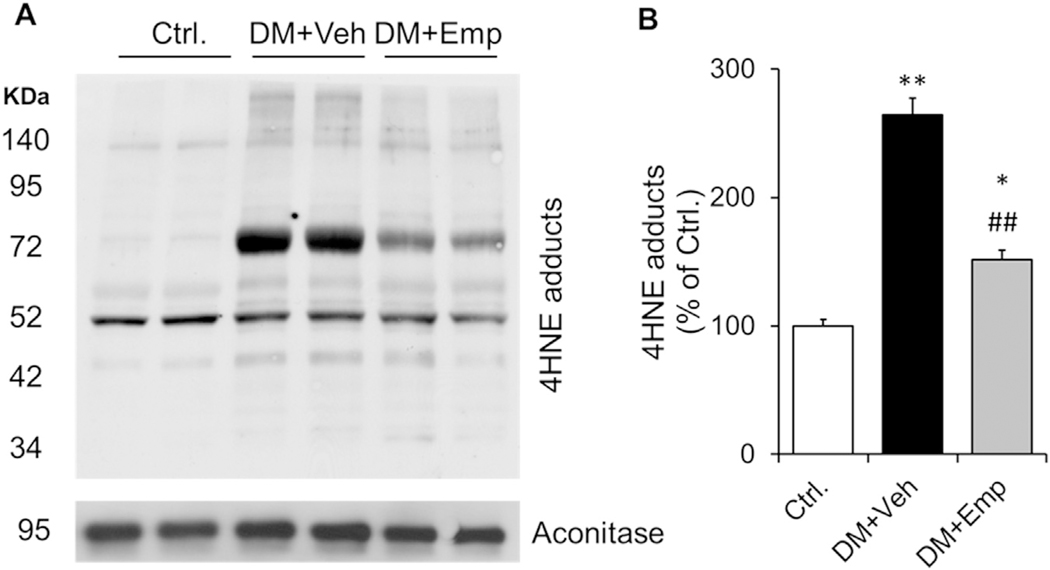
We found that the cardiac mitochondrial levels of 4HNE protein adducts were higher in vehi-treated high-fat diet fed type-2 diabetic ALDH2 * 2 mice compared to the non-diabetic controls (Fig. 4A and B). EMP treatment decreased 4HNE protein adduct levels in the myocardium (Fig. 4A and B).
Fig. 4. Immunoblotting data of 4HNE protein adducts.
Western blot gel images of 4HNE protein adducts in cardiac mitochondrial fraction and the loading control of mitochondrial fraction, aconitase (A) and their densitometric quantification data (B) were shown. Data are presented as mean ± standard error of the mean (S.E.M.). *P < 0.05 and **P < 0.001 vs. Ctrl.; ##P < 0.01 vs. DM + Veh.
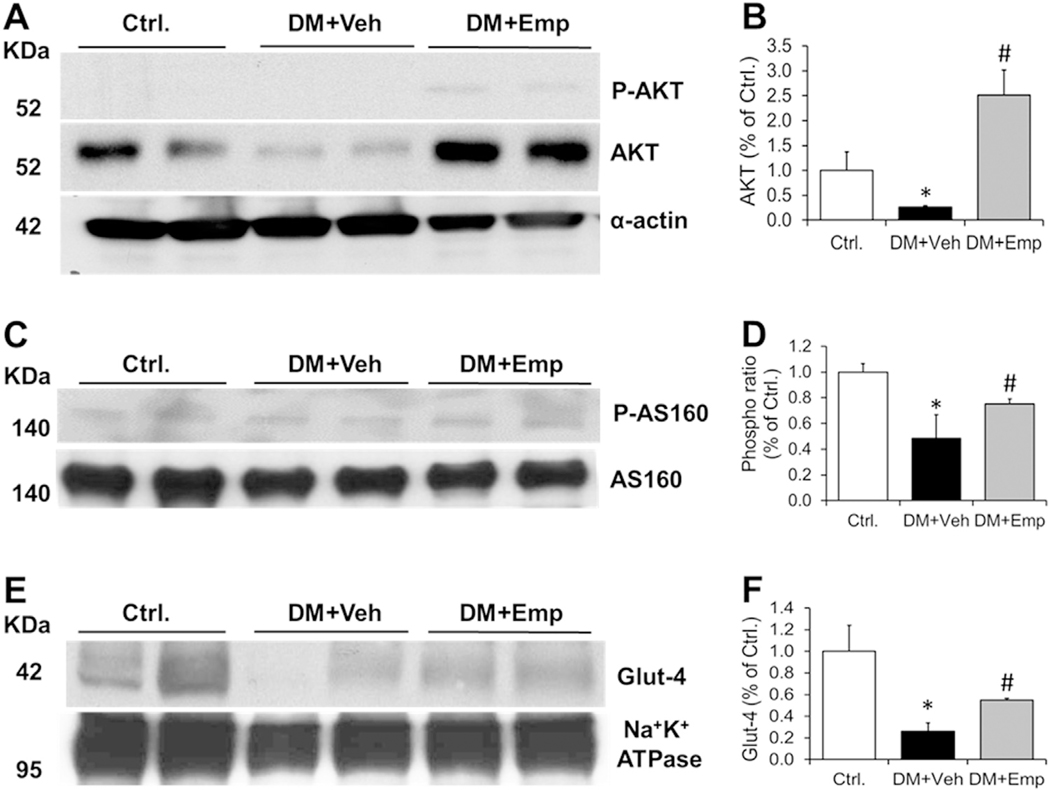
3.5. EMP increased phospho AKT, AKT, phospho AS-160, AS160 and GLUT-4 in diabetic ALDH2 * 2 mice
We found a significant decrease in the muscle plasma membrane levels of AKT, phospho AS160, AS160 and GLUT-4 in vehi-treated high-fat diet fed type-2 diabetic ALDH2 * 2 mice compared to the non-diabetic controls (Fig. 5A–F). We did not see phospho AKT in the control and diabetic samples. However, EMP treatment increased phospho AKT in addition to phospho AS160 and GLUT-4 levels significantly in the muscle plasma membrane compared to veh-treated mice (Fig. 5A–F).
Fig. 5. Levels of phospho AKT and AKT, phospho AS 160 and AS 160 and Glut-4 in plasma membranes of muscle.
Western blot gel images of phospho AKT and AKT (A), phospho AS 160 and AS 160 (C), and Glut-4 (E) in plasma membrane fractions of muscle and their respective densitometric quantification data (B), (D) and (F) were shown. Loading control proteins, α-sarcomeric actin and Na+K+ATPase were shown. Data are presented as mean ± standard error of the mean (S.E.M.). *P < 0.05 vs. Ctrl.; #P < 0.05 vs. DM + Veh.
4. Discussion
EMP ameliorated type-2 diabetes induced hyperglycemia in ALDH2 * 2 mutant diabetic mice. Surprisingly, it also improved glucose tolerance in the aforementioned mice. Two months of EMP treatment improved myocardial contractile function and exercise indices. Furthermore, the treatment also led to a decrease in cardiac mitochondrial 4HNE protein adducts and an increase in phosphorylation of AS 160 in the skeletal muscle.
Increasingly precision medicine and personalized medicine are emerging concepts to treat patients specifically and individually. The ethnicity, weather, diet, life style, socio-economical index, culture and finally genetic polymorphisms play vital role in the severity of patho-physiology of any disease.
Thus, in this study we planned to employ ALDH2 * 2 mutant diabetic mice in order to mimic East Asians with E487K mutation. This ALDH2 * 2 genotype is associated with increased maternal inheritance of diabetes in Japanese (Murata et al., 2004) as well as increased DM and MI in Han-Chinese populations (Xu et al., 2007). DM-induced neuropathy and vasculopathy are also more common in patients carrying ALDH2 * 2 (Suzuki et al., 2004). In 2013, roughly 40% of adults with diabetes were Asians (Rhee, 2015), equating to ~150 million people with high mortality rate. Several studies have indicated that Asians develop diabetes at a younger age compared to their Caucasian counter parts (Rhee, 2015). Additionally, our research using ALDH2 * 2 mutant mice will provide insight into the pathogenesis of diabetic cardiac complications in East Asians with ALDH2 mutation, who represent a growing population in the USA. In a recent paper, we have shown that ALDH2 * 2 diabetic mice exhibit severe heart failure compared wild type C57BL mice (Pan et al., 2018).
Over the last decade, EMP, a SGLT2 inhibitor, is gaining prominence as a drug for T2DM patients with benefits extending beyond its glucose-lowering action (Gallo et al., 2016; Gupte et al., 2017; Ndefo et al., 2015). Specifically, its effect on lowering hospital admissions for heart failure patients and cardiovascular death is very attractive (Zinman et al., 2015). The landmark EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) showed that empagliflozin (EMP), an inhibitor of sodium-glucose cotransporter (SGLT) 2, significantly reduced death from cardio-vascular (CV) causes by 38% and hospitalization for heart failure (HF) by 35% (Zinman et al., 2015). These improvements in primary outcomes occurred within the first 2–3 months of the trial. Consequently, more clinical trials have also confirmed the benefits of EMP (Salsali et al., 2016) as summarized in the report by Gupte et al. (Gupte et al., 2017). Pertinent to our current study, Kaku et al. investigated the potential cardiovascular benefits of EMP treatment in the Asian population within EMPA-REG OUTCOME and found it was beneficial to Asians as well (Kaku et al., 2017). 36% of 366 million individuals affected live in the Western Pacific region, with a significant proportion in East Asia including China, Philippines, Japan and Korea (Rhee, 2015). There are few diabetic animal studies where the beneficial cardiovascular effects of EMP were shown (Gupte et al., 2017; Habibi et al., 2017; Lin et al., 2014). However, no animal studies using ALDH2 * 2 mutant mice that mimic the ALDH2 * 2 mutant patients, Thus, we specifically wanted to address whether EMP improves 1) cardiac function and 2) treadmill running in ALDH2 * 2 mutant diabetic mice.
We started EMP or vehicle treatment in the ALDH2 * 2 mutant diabetic mice at the stage when these diabetic mice start exhibiting cardiac dysfunction i.e. around 4 months. We continued the treatment for another 2 months. At 6 months, we measured their cardiac function before and after treadmill running as an exercise stress test. Additionally, we also evaluated their running performance. We have found in a recent article (Pan et al., 2018) that ALDH2 * 2 mutant diabetic mice exhibit a heart failure of preserved ejection fraction (HFpEF). Since the systolic heart function is preserved in them, this indicates jeopardized myocardial health. We demonstrated exercise stress-echocardiography as a viable approach to enable the detection of cardiac functional deterioration in our type-2 diabetic mice including ALDH2 * 2 mutant mice (Pan et al., 2018). We observed significant improvements in heart rate, %FS, %EF and CO after exercise with EMP treatment. At the signaling level, we measured cardiac mitochondrial 4HNE levels by measuring 4HNE adducts through immunoblotting and found a significant decrease in 4HNE adduct levels. We assume that by lowering the hyperglycemia, EMP decreased reactive oxygen species-mediated 4HNE levels in the cardiac mitochondria of our ALDH2 * 2 mutant diabetic mice. 4HNE modifications of cardiac mitochondrial proteins were shown to be cardio toxic in several experimental studies (Mali and Palaniyandi, 2014) including diabetic cardiomyopathy models (Mali et al., 2016a, 2016b).
When we measured the running performances, we observed EMP treatment improved the running duration and distance in our ALDH2 * 2 mutant diabetic mice. The insulin-mediated AKT signaling is affected in diabetic tissue. The Akt substrate of 160 kDa (AS160), a.k.a. TBC1D4, is an established candidate in Akt/PKB-induced GLUT-4 translocation in skeletal muscle (Bruss et al., 2005; Deshmukh et al., 2006; Sakamoto and Holman, 2008). It was already shown that in type-2 diabetic patients AS160 phosphorylation is decreased (Karlsson et al., 2005). Similarly, we found improved phosphorylation of AKT and AS160 along with increased GLUT-4 in the EMP-treated muscle tissue plasma membrane. We propose that increase in the phosphorylation of AKT, AS160 and translocation of GLUT-4 in our mice by EMP would have improved glucose uptake in muscle and thereby it also might have improved running performance. We did not study how the transcription and translation of this AS 160 is altered by EMP since it is beyond the scope of the current study. The increased glucose tolerance in EMP-treated diabetic mice could also be due to increased AS160 phosphorylation which would improve glucose transport from blood to tissue, thus showing improved insulin sensitivity. In summary, for the first time, our study implicates EMP in the improvement of AKT levels, AS-160 phosphorylation and GLUT-4 translocation in the muscle tissue of ALDH2 * 2 mutant diabetic mice.
Finally, we assert that EMP treatment improves cardiac function and exercise performance by decreasing 4HNE protein adducts in cardiac mitochondria and improving muscle AKT-AS160-GLUT-4 signaling in ALDH2 * 2 mutant diabetic mice.
Acknowledgments
Funding source: this work was partially supported by
1.National Heart, Lung, and Blood Institute: 1R56HL131891-01A1
2.American Heart Association SDG 14SDG20050030 (SSP).
3.Internal Grant from Henry Ford Health System (SSP) A10249
Footnotes
Declaration of conflicting interests
The Author(s) declare(s) that there is no conflict of interest.
References
- Benetti E, Mastrocola R, Vitarelli G, Cutrin JC, Nigro D, Chiazza F, Mayoux E, Collino M, Fantozzi R, 2016. Empagliflozin protects against diet-induced NLRP-3 inflammasome activation and lipid accumulation. J. Pharmacol. Exp. Ther. 359, 45–53. [DOI] [PubMed] [Google Scholar]
- Bruss MD, Arias EB, Lienhard GE, Cartee GD, 2005. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes 54, 41–50. [DOI] [PubMed] [Google Scholar]
- Budas GR, Disatnik MH, Mochly-Rosen D, 2009. Aldehyde dehydrogenase 2 in cardiac protection: a new therapeutic target? Trends Cardiovasc. Med. 19, 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Mancuso C, Sapienza M, Puleo E, Calafato S, Cornelius C, Finocchiaro M, Mangiameli A, Di Mauro M, Stella AM, Castellino P, 2007. Oxidative stress and cellular stress response in diabetic nephropathy. Cell Stress Chaperon. 12, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D, 2008. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science 321, 1493–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng ST, Chen L, Li SY, Mayoux E, Leung PS, 2016. The effects of empagliflozin, an SGLT2 inhibitor, on pancreatic beta-cell mass and glucose homeostasis in type 1 diabetes. PLoS One 11, e0147391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh A, Coffey VG, Zhong Z, Chibalin AV, Hawley JA, Zierath JR, 2006. Exercise-induced phosphorylation of the novel Akt substrates AS160 and filamin A in human skeletal muscle. Diabetes 55, 1776–1782. [DOI] [PubMed] [Google Scholar]
- Feltbower RG, Bodansky HJ, Patterson CC, Parslow RC, Stephenson CR, Reynolds C, McKinney PA, 2008. Acute complications and drug misuse are important causes of death for children and young adults with type 1 diabetes: results from the Yorkshire Register of diabetes in children and young adults. Diabetes Care 31, 922–926. [DOI] [PubMed] [Google Scholar]
- Gallo LA, Ward MS, Fotheringham AK, Zhuang A, Borg DJ, Flemming NB, Harvie BM, Kinneally TL, Yeh SM, McCarthy DA, Koepsell H, Vallon V, Pollock C, Panchapakesan U, Forbes JM, 2016. Once daily administration of the SGLT2 inhibitor, empagliflozin, attenuates markers of renal fibrosis without improving albuminuria in diabetic db/db mice. Sci. Rep. 6, 26428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte M, Umbarkar P, Lal H, 2017. Mechanistic insights of empagliflozin-mediated cardiac benefits: nearing the starting line: editorial to: “empagliflozin improves left ventricular diastolic dysfunction in a genetic model of type 2 diabetes” by N. Hammoudi et al. Cardiovasc. Drugs Ther. 31, 229–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi J, Aroor AR, Sowers JR, Jia G, Hayden MR, Garro M, Barron B, Mayoux E, Rector RS, Whaley-Connell A, DeMarco VG, 2017. Sodium glucose transporter 2 (SGLT2) inhibition with empagliflozin improves cardiac diastolic function in a female rodent model of diabetes. Cardiovasc. Diabetol. 16, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JH, Oh TJ, Lee G, Maeng HJ, Lee DH, Kim KM, Choi SH, Jang HC, Lee HS, Park KS, Kim YB, Lim S, 2017. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE (−/−) mice fed a western diet. Diabetologia 60, 364–376. [DOI] [PubMed] [Google Scholar]
- Humphries KM, Yoo Y, Szweda LI, 1998. Inhibition of NADH-linked mitochondrial respiration by 4-hydroxy-2-nonenal. Biochemistry 37, 552–557. [DOI] [PubMed] [Google Scholar]
- Jackson B, Brocker C, Thompson DC, Black W, Vasiliou K, Nebert DW, Vasiliou V, 2011. Update on the aldehyde dehydrogenase gene (ALDH) superfamily. Human. Genom. 5, 283–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay D, Hitomi H, Griendling KK, 2006. Oxidative stress and diabetic cardiovascular complications. Free Radic. Biol. Med. 40, 183–192. [DOI] [PubMed] [Google Scholar]
- Kaku K, Lee J, Mattheus M, Kaspers S, George J, Woerle HJ, Investigators, E.-R.O., 2017. Empagliflozin and cardiovascular outcomes in asian patients with type 2 diabetes and established cardiovascular disease- results from EMPA-REG OUTCOME ((R)). Circ. J. 81, 227–234. [DOI] [PubMed] [Google Scholar]
- Karlsson HK, Zierath JR, Kane S, Krook A, Lienhard GE, Wallberg-Henriksson H, 2005. Insulin-stimulated phosphorylation of the Akt substrate AS160 is impaired in skeletal muscle of type 2 diabetic subjects. Diabetes 54, 1692–1697. [DOI] [PubMed] [Google Scholar]
- Lagranha CJ, Deschamps A, Aponte A, Steenbergen C, Murphy E, 2010. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ. Res. 106, 1681–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashin OM, Szweda PA, Szweda LI, Romani AM, 2006. Decreased complex II respiration and HNE-modified SDH subunit in diabetic heart. Free Radic. Biol. Med. 40, 886–896. [DOI] [PubMed] [Google Scholar]
- Lichtenauer UD, Seissler J, Scherbaum WA, 2003. Diabetic complications. Micro and macroangiopathic end-organ damage. Internist 44 (840–846), 848–852. [DOI] [PubMed] [Google Scholar]
- Lin B, Koibuchi N, Hasegawa Y, Sueta D, Toyama K, Uekawa K, Ma M, Nakagawa T, Kusaka H, Kim-Mitsuyama S, 2014. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc. Diabetol. 13, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali VR, Deshpande M, Pan G, Thandavarayan RA, Palaniyandi SS, 2016a. Impaired ALDH2 activity decreases the mitochondrial respiration in H9C2 cardiomyocytes. Cell Signal. 28, 1–6. [DOI] [PubMed] [Google Scholar]
- Mali VR, Ning R, Chen J, Yang XP, Xu J, Palaniyandi SS, 2014. Impairment of aldehyde dehydrogenase-2 by 4-hydroxy-2-nonenal adduct formation and cardio-myocyte hypertrophy in mice fed a high-fat diet and injected with low-dose streptozotocin. Exp. Biol. Med. (Maywood) 239, 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali VR, Palaniyandi SS, 2014. Regulation and therapeutic strategies of 4-hydroxy-2-nonenal metabolism in heart disease. Free Radic. Res 48, 251–263. [DOI] [PubMed] [Google Scholar]
- Mali VR, Pan G, Deshpande M, Thandavarayan RA, Xu J, Yang XP, Palaniyandi SS, 2016b. Cardiac mitochondrial respiratory dysfunction and tissue damage in chronic hyperglycemia correlate with reduced aldehyde dehydrogenase-2 activity. PLoS One 11, e0163158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta JL, Rasouli N, Sinha AK, Molavi B, 2006. Oxidative stress in diabetes: a mechanistic overview of its effects on atherogenesis and myocardial dysfunction. Int. J. Biochem. Cell Biol. 38, 794–803. [DOI] [PubMed] [Google Scholar]
- Murata C, Taniyama M, Kuriyama S, Muramatsu T, Atsumi Y, Matsuoka K, Suzuki Y, 2004. Meta-analysis of three diabetes population studies: association of inactive ALDH2 genotype with maternal inheritance of diabetes. Diabetes Res. Clin. Pract. 66 (Suppl 1), S145–S147. [DOI] [PubMed] [Google Scholar]
- Ndefo UA, Anidiobi NO, Basheer E, Eaton AT, 2015. Empagliflozin (Jardiance): a novel SGLT2 inhibitor for the treatment of type-2 diabetes. P T 40, 364–368. [PMC free article] [PubMed] [Google Scholar]
- Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R, 2008. Advanced lipid peroxidation end products in oxidative damage to proteins: potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharmacol. 153, 6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE, 2017. IDF diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 128, 40–50. [DOI] [PubMed] [Google Scholar]
- Orasanu G, Plutzky J, 2009. The pathologic continuum of diabetic vascular disease. J. Am. Coll. Cardiol. 53, S35–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostler JE, Maurya SK, Dials J, Roof SR, Devor ST, Ziolo MT, Periasamy M, 2014. Effects of insulin resistance on skeletal muscle growth and exercise capacity in type 2 diabetic mouse models. Am. J. Physiol. Endocrinol. Metab. 306, E592–E605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyandi SS DM-H, Sun L, Vishnumangalam JJ, Xia X, Pavlovic A, Bhalla V, Ashley E, Mochly-Rosen D, 2010. Aldehyde dehydrogenase activator attenuates diabetic cardiomyopathy; a role in improving the quality of resident cardiac stem cells? EB Meet. 24 (Supplement 572), 573. [Google Scholar]
- Pan G, Deshpande M, Thandavarayan RA, Palaniyandi SS, 2016. ALDH2 inhibition potentiates high glucose stress-induced injury in cultured cardiomyocytes. J. Diabetes Res. 2016, 1390861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G, M S, Kar A, Gardinier J, Thandavarayan RA, Palaniyandi SS, 2018. Type-2 diabetic aldehyde dehydrogenase 2 mutant mice (ALDH 22) exhibiting heart failure with preserved ejection fraction phenotype can be determined by exercise stress echocardiography. PLos One E 13, e0195796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosino JM, Heiss VJ, Maurya SK, Kalyanasundaram A, Periasamy M, LaFountain RA, Wilson JM, Simonetti OP, Ziouzenkova O, 2016. Graded maximal exercise testing to assess mouse cardio-metabolic phenotypes. PLoS One 11, e0148010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee EJ, 2015. Diabetes in Asians. Endocrinol. Metab. 30, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Holman GD, 2008. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am. J. Physiol. Endocrinol. Metab. 295, E29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salsali A, Kim G, Woerle HJ, Broedl UC, Hantel S, 2016. Cardiovascular safety of empagliflozin in patients with type 2 diabetes: a meta-analysis of data from randomized placebo-controlled trials. Diabetes Obes. Metab. 18, 1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semchyshyn HM, 2014. Reactive carbonyl species in vivo: generation and dual biological effects. Sci. World J. 2014, 417842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Taniyama M, Muramatsu T, Higuchi S, Ohta S, Atsumi Y, Matsuoka K, 2004. ALDH2/ADH2 polymorphism associated with vasculopathy and neuropathy in type 2 diabetes. Alcohol Clin. Exp. Res. 28, 111S–116S. [DOI] [PubMed] [Google Scholar]
- Vasiliou V, Pappa A, Petersen DR, 2000. Role of aldehyde dehydrogenases in endogenous and xenobiotic metabolism. Chem. Biol. Interact. 129, 1–19. [DOI] [PubMed] [Google Scholar]
- Wang C, Fan F, Cao Q, Shen C, Zhu H, Wang P, Zhao X, Sun X, Dong Z, Ma X, Liu X, Han S, Wu C, Zou Y, Hu K, Ge J, Sun A, 2016. Mitochondrial aldehyde dehydrogenase 2 deficiency aggravates energy metabolism disturbance and diastolic dysfunction in diabetic mice. J. Mol. Med. 94, 1229–1240. [DOI] [PubMed] [Google Scholar]
- Xu F, Chen YG, Geng YJ, Zhang H, Jiang CX, Sun Y, Li RJ, Sagar MB, Xue L, Zhang Y, 2007. The polymorphism in acetaldehyde dehydrogenase 2 gene, causing a substitution of Glu > Lys(504), is not associated with coronary atherosclerosis severity in Han Chinese. Tohoku J. Exp. Med. 213, 215–220. [DOI] [PubMed] [Google Scholar]
- Yang XP, Liu YH, Rhaleb NE, Kurihara N, Kim HE, Carretero OA, 1999. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am. J. Physiol. 277, H1967–1974. [DOI] [PubMed] [Google Scholar]
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, Investigators, E.-R.O., 2015. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373, 2117–2128. [DOI] [PubMed] [Google Scholar]