Abstract
Background and Aim:
The Watish sheep is a strain of desert sheep of smaller size compared to other desert sheep ecotypes, and there is anecdotal evidence that it is endowed with high litter size. The present study was designed for screening for polymorphisms in the known fecundity genes (bone morphogenetic protein receptor type 1B A<G in exon 6, bone morphogenetic protein 15 (BMP15) (FecXB, FecXG, FecXH, and FecXI) in exon2, growth differentiation factor 9 (GDF9) – G1 in exon1 and G8 in exon2 and PRLG<A in intron2) and their association with litter size in Watish.
Materials and Methods:
The study involved 156 Watish ewes of 2-6 years of age, along with data on litter size in the first, second, and third parity from Sinnar state and contiguous Blue Nile State. Genomic DNA was isolated and genotyped using polymerase chain reaction-restriction fragment length polymorphism. Allele and genotype frequencies were calculated by direct counting. Chi-square test for goodness of fit was performed for agreement with Hardy-Weinberg expectations and association testing.
Results:
The results demonstrated that all individuals were non-carriers for the target mutations of FecB, BMP15 (FecXB, FecXH, and FecXI), and GDF9-G8. With regard to the GDF9-G1 gene, the genotypic frequencies were 0.07% (G+) and 0.93% (++), in FecXG gene they were 0.993% (++) and 0.006% (B+), in PRL gene 0.516(++), 0.347(B+), and 0.137(BB). The Chi-square test showed a non-significant association between ewe’s type of birth and the detected mutations genotypes.
Conclusion:
These results preliminarily indicated that GDF9-G1, BMP15 (FecXG), and PRL genes might have had some contribution for improving litter size in Watish Sudanese sheep. However, further studies using larger samples are needed to detect the effects of those mutations on Watish sheep litter size.
Keywords: fecundity, genes, litter size, sheep, Watish
Introduction
Sudan is well endowed with livestock resources. The main sheep type in Sudan is the desert sheep, which encompasses a number of subtypes. The desert sheep ecotype is the most predominant for export and local consumption. Its meat is in high demand for export because of the size of its carcass, and the quality of its mutton and lamb. Desert sheep never had the opportunity to express their real genetic potential in production and reproduction as a result of the nomadic or semi-nomadic systems under which it is raised. Watish sheep, a subtype of desert sheep, are mainly found between latitudes 10° and 11° N along the Blue Nile. They are mainly owned by nomadic and semi-nomadic societies. This breed is of smaller size compared to all other desert sheep subtypes, which means that its ewes will have low maintenance requirements and thereby reduced feed costs. This makes it a possible candidate to be used as a dam breed. In addition, there is anecdotal evidence that the breed has high prolificacy though no systematic study has been carried out on it.
Ovulation rate and litter size are heritably controlled by several genes with minor effects, and sometimes also by single genes with major effects, named fecundity (Fec) genes [1]. The ovulation rate in sheep has been observed to be significantly increased by mutations in a closely linked group of genes. Those genes are bone morphogenetic protein receptor type 1B (BMPR-1B), bone morphogenetic protein 15 (BMP15), and growth differentiation factor 9 (GDF9), which are all part of the ovary-derived transforming growth factor-β (TGFβ) superfamily [2]. These genes have an important effect on ovulation rate and litter size [3]. BMPR-1B, the Booroola gene, also known as activin receptor-like kinase 6 (ALK6) and FecB, was the first major gene identified for influencing prolificacy in sheep [4]. BMPRIB is located at the FecB locus in between the SPP1 and EGF genes [5] in the 6th autosome of sheep. It is inherited as a single autosomal locus with an additive effect for ovulation rate. BMP15 gene, also known as FecX [6], is an X-linked gene (FecX locus) of sheep belonging to the TGFβ family. Ten mutations, labeled FecXG, FecXH, FecXI, FecXL, FecXB, FecXR, FecXGr, FecTT, FecXW, and FecXO, have been detected within the BMP15 with similar phenotypes, i.e. homozygous carrier ewes are sterile [7] and heterozygous carriers show increased ovulation rate [8,9]. GDF9 gene, also known as FecG, is mapped to the 5thautosome of sheep and is expressed in oocytes from the primary stage of follicular development until ovulation [10] playing an important role in folliculogenesis [11]. The gene consists of 2 exons separated by 1126bp intron and encodes a propeptide containing 453 amino acid residues. The active mature peptide is 135 amino acids long. The Prolactin gene is an anterior pituitary hormone having close interaction with gonadotropin, i.e., the elevation in the secretion of prolactin is normally associated with the pronounced reduction in gonadotropin secretion that results in stages of infertility [12].
The present study was designed for screening polymorphisms in these four Fec genes (BMPR-1B, BMP15, GDF, and PRL) and to test their association with litter size in the Watish Sudanese Desert sheep.
Materials and Methods
Ethical approval
According to the Animals Use in Research Committee of Khartoum University, this study does not require any special approval.
Sample collection
Venous jugular blood samples from 156, 2 to 6years old Watish ewes along with data on litter size in the first, second, and third parity were collected from Sinnar State and contiguous Blue Nile State. Ewes were divided into two groups, according to their average litter size; the first group consisted of those ewes which had singletons, the second group consisted of those which had multiple births. The collected blood samples were transferred to the laboratory using the cooling chain and stored at −20°C for further analysis.
DNA isolation
Genomic DNA was isolated using Qiagen Commercial kits. The quality and quantity of the extracted DNA was checked by Nanodrop Spectrophotometer and agarose gel electrophoreses. DNA samples were adjusted to a concentration of 100-200ng/μL and exactly 1 μL of the DNA samples were used as templates for polymerase chain reaction (PCR).
Single nucleotide polymorphism detection assays
The BMPR1B gene locus was analyzed, targeting 141bp fragment covering the sequence containing the A<G substitution at 746thposition of the open reading frame (ORF) (rs418841713) in exon 6. Polymorphism evaluation was conducted using PCR amplification, according to the procedure proposed by Wang etal.[13]. Similarly, four polymorphisms in exon 2 in Ovis aries BMP15 (FecXB, FecXG, FecXH, and FecXI) were evaluated. The specific mutations are: Gto T nucleotide change of FecXB at position 1100bp, C<T substitution (rs425019156) at position 718 of ORF in FecXG gene, C<T substitution (rs413916687) at position 871 in FecXH gene, and T<A substitution (rs398521635) at position 896 of the cDNA coding region in FecXI. Polymorphisms evaluation was conducted using PCR amplification according to the procedure proposed by Hanrahan et al. [11] for FecXB and FecXG and Lassoued et al. [6] for FecXH and FecXI.
Two polymorphisms G1 (G860A))rs410123449) at position 260 in the coding region, and G8 (C1184T) of the cDNA at position 395 of the mature protein were detected in GDF9 gene located in exon1 and exon2, respectively, of Watish breed samples which were amplified, according to the procedure proposed by Hanrahan et al. [11].
The polymorphism of Ovis aries Prolactin (PRL) gene was at the G<T substitution (rs424801898) in intron 2, which was amplified according to the procedure proposed by Chu et al. [14] and Chu et al.[15]. Primer sequences, restriction enzymes, expected size, and annealing temperatures are illustrated in Table-1. Each PCR reaction was made in 25 μL volume containing 1× PCR buffer (10 mM Tris-HCl, 50 mM KCl); 1 μLdNTPs (10 mM), and 1.25 μL MgCl2(50 mM). 1 U of Taq polymerase, 1 μL of each primer (10 μM), and 100-200ng/μL DNA, then the volume was completed to 25 μL using d.dH2O. PCR cycles included: Initial denaturation at 94°C for 5min followed by 30cycles of denaturation at 94°C for 30 s, annealing for 30 s (Table-1), extension at 72°C for 30 s, and a last cycle of extension at 72°C for 8min. The PCR product was kept at 4°C until further analysis.
Table-1.
Primer sequences, annealing temperatures, and PCR product sizes of the targeted genes conducted under this study.
| Gene | (Primers 5’®3’) | Annealing temperature | Restriction enzyme | Product size |
|---|---|---|---|---|
| BMPR-IB | F:GTCGCTATGGGGAAGTTTGGATG | 62°C | AvaII | 140 bp |
| FecB | R:CAAGATGTTTTCATGCCTCATCAACACGGTC | |||
| BMP15 | F:GCCTTCCTGTGTCCCTTATAAGTATGTTCCCCTTA | 62°C | DdeI | 153 bp |
| FecXB | R:TTCTTGGGAAACCTGAGCTAGC | |||
| BMP15 | F:CACTGTCTTCTTGTTACTGTATTTCAATGAGAC | 66°C | Hinf1 | 141 bp |
| FecXG | F:GATGCAATACTGCCTGCTTG | |||
| BMP15 | F:TATTTCAATGACACTCAGAG | 59°C | SpeI | 240 bp |
| FecXH | R:GAGCAATGATCCAGTGATCCCA | |||
| BMP15 | F:GAAAGTAACCAGTGTTCCCTCCACCCTTTTCT | 63°C | XbaI | 150 bp |
| FecXI | R:CATGATTGGGAGAATTGAGACC | |||
| GDF9-G1 | F- GAAGACTGGTATGGGGAAATG | 58°C | HhaI | 462 bp |
| R- CCAATCTGCTCCTACACACCT | ||||
| GDF9-G8 | F-CTTTAGTCAGCTGAAGTGGGACAAC | 62°C | DdeI | 139 bp |
| R-ATGGATGATGTTCTGCACCATGGTG | ||||
| TGAACCTGA | ||||
| PRL | F-ACCTCTCCTCGGAAATGTTCA | 56°C | HaeIII | 1211 bp |
| R-GGGACACTGAAGGACCAGAA |
The products of PCR were digested in a total of 10 μL reaction containing (10×) buffer 1 μL, 0.2 μL restriction enzyme, 5 μL PCR products, and 3.8 μL distilled water at a constant temperature of 37°C overnight in restriction fragment length polymorphism (RFLP) reaction. After 2% electrophoresis, the gel PCR products were visualized by a gel documentation system.
Statistical analysis
Gene and genotypes frequencies were calculated by direct counting. AChi-square test for goodness-of-fit was performed to verify if genotype frequencies agreed with Hardy–Weinberg equilibrium expectations and for association testing.
Results
Genotyping of BMPR-1B gene
The missense mutation in BMPR1B gene causing substitution of A<G that results in a nonsynonymous substitution of glutamine with an arginine (Q249R) was not present in the studied populations. The digestion of the 140bp (PCR product) with AvaII (AG/CT) endonuclease enzyme resulted in one type of restriction pattern, which was assigned as homozygous wild type genotype (++) 140bp that produced one fragment (Figure-1). The allele and genotype frequencies of the BMPR-1B gene are shown in Table-2.
Figure-1.
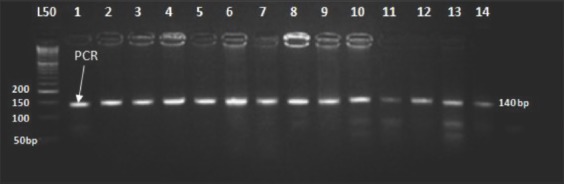
Agarose gel electrophoresis (4%) displaying AvaII1restriction site on an amplified portion of Watish BMPR1B gene in exon 6. Lane 1: Polymerase chain reaction product. Lane 2-14: Restriction enzyme representing BB genotype (140bp). Lane L: 50bp DNA ladder.
Table-2.
Allele and genotype frequencies of the ovine BMPR-IB, BMP15 (FecXB, FecXG, FecXH, and FecXI), GDF9 (G1-G8), and PRL genes in experimental population.
| Gene | Genotype frequency | Allele frequency | |||
|---|---|---|---|---|---|
| BMPR-IB | BB | D+ | ++ | B | + |
| 0.00 | 0.00 | 1 | 0.00 | 1 | |
| FecXB | BB | B+ | ++ | B | + |
| 0.00 | 0.00 | 1` | 0.00 | 1 | |
| FecXG | GG | G+ | ++ | G | + |
| 0.00 | 0.006 | 0.993 | 0.003 | 0.997 | |
| FecXH | CC | TC | TT | C | T |
| 0.00 | 0.00 | 1 | 0.00 | 1 | |
| FecXI | AA | TA | TT | A | T |
| 0.00 | 0.00 | 1 | 0.00 | 1 | |
| GDF9-G1 | GG | G+ | ++ | G | + |
| 0.00 | 0.07 | 0.93 | 0.036 | 0.964 | |
| GDF9-G8 | GG | G+ | ++ | G | + |
| 0.00 | 0.00 | 1 | 0.00 | 1 | |
| PRL | BB | B+ | ++ | B | + |
| 0.137 | 0.347 | 0.516 | 0.31 | 0.69 | |
Genotyping of BMP15 gene
The BMP15 (FecXB) locus was analyzed, targeting a 153bp fragment covering the sequence containing the missense mutation 1100 G <T(S99I) in exon 2 causing a non-synonymous substitution of serine with isoleucine. The PCR reaction was carried out with the restriction endonuclease enzyme DdeI (C/TTAG). It resulted in one type of restriction pattern that produced two fragments in all animals under study and was assigned as homozygous genotype (wild type) (122-31bp). There was a complete absence of both the homozygous genotype (mutant type) (153bp) and the heterozygous genotype (153-122 and 31bp) (Figure-2).
Figure-2.

Agarose gel electrophoresis (4%) displaying DdeI1 restriction digest on an amplified portion of Watish BMP15-FecXB gene in exon 2. Lane: 1 polymerase chain reaction product. Lane: 2-14 representing ++ genotype (122-31bp). Lane L: 50 bpDNA ladder.
The PCR product of FecXG was about 141bp, the non-sense mutation C<T which causes the substitution of glutamine with a stop codon (Q239*) resulting in premature termination of translation was detected by digestion with Hinf1I (G/ACT) endonuclease enzyme, generating two types of restriction patterns (112bp + 29bp) and (141bp + 112bp + 29bp) representing ++ and G+ genotypes, respectively. The wild type (++) was the more frequent type in the population, but none of the animals carried the homozygous mutant genotypeGG (141bp), which was not sensitive to the enzyme at the site of cutting (Figure-3).
Figure-3.

Agarose gel electrophoresis (4%) displaying Hinf I/digest an amplified portion of exon2 of the Watish BMP15-FecXG gene. Lane 1: Polymerase chain reaction product. Lanes 2-5, 7-15: Represent ++. Lane 6: Represent G+. Lane L: 50bp DNA Ladder.
The point mutation polymorphism C<T in the FecXH gene causes the substitution of glutamine with a stop codon. The PCR products (240bp) evaluation was carried out with SpeI (A/CTAGT) endonuclease enzyme. The uncut pattern (240bp) resulted in one type of restriction fragment pattern, which was assigned as wild type genotype ++ in all animals under study (Figure-4).
Figure-4.

Agarose gel electrophoresis (4%) displaying SpeI/digest on an amplified product of Watish BMP15-FecXH in exon2. Lane 1: Polymerase chain reaction product. Lanes 2-15: Represent ++ 240bp. Lane L: 100bp DNA Ladder.
For detection of the FecXI allele, 150bp of the PCR products covering the missense mutation polymorphism T<A was digested with the endonuclease restriction enzyme XbaI (T/CTAGA). This mutation results in nonsynonymous substitution of Valine with aspartic acid at amino acid position 308. Wild-type individuals for FecXI showed 150bp undigested fragments in all animals under study (Figure-5).
Figure-5.

Agarose gel electrophoresis (4%) displaying XbaI digest in an amplified product of Watish BMP15-FecXI gene in exon 2. Lane 1: PCR product. Lanes 2-14: Represent ++ 150bp. Lane L: 50bp DNA Ladder.
Genotyping of GDF9 gene
The PCR product is about 462bp, located in exon 1 of the GDF9-G1, which included the transition mutation that changes adenine into guanine (G860A) at position 260 in the coding region. The mutation causes the substitution of amino acid arginine with histidine at residue 87. The product was digested with the endonuclease restriction enzyme Hha1 (GCG↑C). Digestion of the PCR product from wild-typeFecG(++) animals resulted in cleavage of the 462-bp product (at two internal HhaI sites) into fragments of 52, 156, and 254bp. The DNA fragments containing the A nucleotide gave only two fragments of 52 and 410bp (GG). Animals heterozygous (G+) for the mutation had fragments of all four sizes (52, 156, 254, and 410bp) (Figure-6).
Figure-6.

Agarose gel electrophoresis (4%) displaying HhaI digest on an amplified exon1of Watish FecG-G1 gene. Lanes 2-7, 9-16: Represent++. Lanes 1, 8: (G+) lane L: 50bp DNA ladder.
The transition mutation C<T in the GDF9-G8 causes the substitution of serine to phenylalanine at position 395 of the mature protein (Ser395Phe) in exon 2. Detection of the mutation in the 139bp PCR product in exon 2 of the GDF9-G8 that includes the mutation was carried out by digestion with the restriction endonuclease enzyme DdeI (C/TNAG). The digestion resulted in one type of restriction fragment pattern, which was assigned as wild type genotype ++: (108-31bp) and produced one fragment as displayed by the NEBcutter program in all animals under study. There was the complete absence of both homozygous (BB) genotype (139bp) and the heterozygous (B+) genotype (139-108 and 31bp) in all animals under study (Figure-7).
Figure-7.

Agarose gel electrophoresis (4%) displaying DdeI/digest on an amplified exon 2 of Watish FecG-G8. Lanes 1, 8: Polymerase chain reaction product (139bp). Lane 2-7, 9-15: Represent ++(108-31) bp. Lane L: 100bp DNA Ladder.
Genotyping of PRL gene
The digestion of the PCR product 1211bp located in intron 2, which includes the mutation causing the substitution of G<T, with endonuclease restriction enzyme HaeIII (GG/CC) resulted in three types of restriction patterns: Homozygous genotypes which were assigned as wild type (++) (541, 372, 151, and 147bp), a mutant type (BB) (688, 372, and 151bp), and heterozygous genotype (B+) (688, 541, 372, 151, and 147bp) (Figure-8).
Figure-8.

Agarose gel electrophoresis (2%) of the digested product of PRL gene at intron 2 by HaeIII restriction enzyme. Lane L: DNA marker 100bp, Lane 1, 3, 5, and 6 heterozygous genotype. Lane 2 and 7 mutant type. Lane 4: Wild type genotype.
Allele and genotype frequencies calculated for all SNPs of genes under study are given in Table-2. The allele and genotype frequencies were calculated separately for ewes with single lambs, and those with more than one lamb (Table-3), and the frequencies were not significantly different in BMPR-IB, BMP15 (FecXB, FecXH, and FecXI), and GDF9-G8 genes between the two groups of ewes (Table-3). However, there were differences between the two groups in the frequencies of FecXG and GDF9-G1 and PRL genes, but the differences were not statistically significant. The calculated Chi-square values were 0.219 and 3.39 for the three genes, respectively. There were no significant associations between birth type and the genotypes of these mutations (p>0.05).
Table-3.
Allele and genotype frequencies of single and multiple litter ewes of the ovine BMPR-IB, BMP15 (FecXB, FecXG,FecXH, and FecXI), GDF9 (G1-G8), and PRL genes in the experimental population.
| Gene | Single lamb ewes | Multiple litter ewes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genotype frequency | Allele frequency | Genotype frequency | Allele frequency | |||||||
| BMPR-IB | BB | D+ | ++ | B | + | BB | D+ | ++ | B | + |
| 0.00 | 0.00 | 1 | 0.00 | 1 | 0.00 | 0.00 | 1 | 0.00 | 1 | |
| FecXB | BB | B+ | ++ | B | + | BB | B+ | ++ | B | + |
| 0.00 | 0.00 | 1 | 0.00 | 1 | 0.00 | 0.00 | 1 | 0.00 | 1 | |
| FecXG | GG | G+ | ++ | G | + | GG | G+ | ++ | G | + |
| 0.00 | 0.00 | 1 | 0.00 | 1 | 0.00 | 0.008 | 0.992 | 0.004 | 0.996 | |
| FecXH | CC | TC | TT | C | T | CC | TC | TT | C | T |
| 0.00 | 0.00 | 1 | 0.00 | 1 | 0.00 | 0.00 | 1 | 0.00 | 1 | |
| FecXI | AA | TA | TT | A | T | AA | TA | TT | A | T |
| 0.00 | 0.00 | 1 | 0.00 | 1 | 0.00 | 0.00 | 1 | 0.00 | 1 | |
| GDF9-G1 | GG | G+ | ++ | G | + | GG | G+ | ++ | G | + |
| 0.00 | 0.14 | 0.86 | 0.07 | 0.93 | 0.00 | 0.05 | 0.95 | 0.03 | 0.97 | |
| GDF9-G8 | GG | G+ | ++ | G | + | GG | G+ | ++ | G | + |
| 0.00 | 0.00 | 1 | 0.00 | 1 | 0.00 | 0.00 | 1 | 0.00 | 1 | |
| PRL | BB | B+ | ++ | B | + | BB | B+ | ++ | B | + |
| 0.121 | 0.364 | 0.515 | 0.30 | 0.70 | 0.145 | 0.339 | 0.516 | 0.31 | 0.69 | |
Discussion
The Watish ecotype is an important genetic resource of Sudan. It is also different from other desert ecotypes in being of smaller size, of relatively high prolificacy and has good meat quality. This makes it a good candidate for use as a dam breed in a more specialized production system. Such a line may be used in crossbreeding with males from large ecotypes such as the Hamari and Kabashi.
SNPs sites in BMPR-IB, BMP15, GDF9, and PRL genes, which could affect litter size, were tested. The results showed that the Watish samples were homozygous for the 746 A allele (monomorphic for the glutamine variant) in the A>G SNP (Q249A, glutamine to an arginine). This indicates the absence of the Booroola mutation (FecB) in all studied animals and probably that it is not present in the Watish population at large. Similar results were found in other studies previously reported by Al-Barzinji and Othman [16], Nejhad and Ahmadi [17], and Abouheif et al. [18] in different sheep breeds in the Middle East.
Four previously reported mutations in exon 2 of the sheep BMP-15 gene have been identified: FecXB (Belclare) (G<T), FecXG (Gallway) (C<T), FecXH (Hanna) (C<T), and FecXI (Inverdale) (T<A) were considered as candidate polymorphisms for the present study. No mutations in the FecXB, FecXH, and FecXI genes were found in Watish samples. Consequently, based on the evidence from this study, these mutations cannot be considered a cause of the anecdotal high prolificacy of Watish sheep. This suggests that the molecular mechanism affecting the multiparous performance in Watish sheep may be different from those of Inverdale, Hanna, and Belclare sheep. These results are in agreement with the results from Chios sheep [19], Hu sheep, and Chinese Merino [20].
The nonsense mutation in FecXG (C<T) at the position 718 leads to a premature stop codon at amino acid 239 of the unprocessed protein. The PCR product digested with HinfI was 141bp. The digestion demonstrated the existence of two patterns: The homozygous wild type ++ genotype (f=0.993) and a low frequency of heterozygous G+ genotype (0.006). The reason for the low frequency of the mutant allele is probably the fact that most Watish owners either slaughtered or sold infertile or barren ewes by the end of the 1styear of age. Similarly, Chu et al. [15] reported the absence of GG mutant genotype in Small-Tailed Han sheep. Ewes heterozygous for any one of these BMP-15 mutations have experienced increased ovulation rates, whereas homozygous ewes were sterile due to a failure of normal ovarian follicular development [6,11].
The results of PCR-RFLP also indicated that Watish ecotype showed the existence of the targeted SNP of the GDF9-G1detected using HhaI digestion enzyme. The reaction produced two types of bands of 52, 156, and 254bp indicating homozygous wild type animals (++), four bands of 52, 156, 254, and 410bp for the heterozygous genotype (G+). The frequency of the wild type allele was 0.964, while the frequency of the mutant (G) allele was 0.036. The homozygous genotype (GG), which should produce 52 and 410bp bands as reported by Moradband et al. [21] in Baluchi breed and Hanrahan et al. [11] in Belclare and Cambridge breeds, did not appear in our Watish samples. Furthermore, Liandris et al. [22] did not find the mutant genotype in Karagouniki sheep.
The C/T mutation at 1184bp of GDF9–G8 leads to the change of Serine at amino acid 77 into Phenylalanine. All animals showed the same banding pattern of 139 pb in agreement with previously published results [23].
The PCR-RFLP amplification of the Prolactin gene (1211bp) showed that all three genotypes were present in our population. The allelic frequencies were 0.31 for the mutant B allele and 0.69 for the wild type allele (+), and the genotypic frequencies were 0.137 for BB, 0.347 for B+, and 0.516 for ++. These results are also congruent with reports in Awassi sheep in which the allelic frequencies were 0.757 for A allele and 0.243 for B allele, and the genotypic frequencies were 0.646 for AA, 0.221 for AB, and 0.131 for BB[24]. The results showed that prolificacy seemed to be affected by Prolactin gene variants. In Romanov sheep breed, Jawasreh et al. [25] reported the homozygous individuals (MM genotype) of GDF9 gene to produce 0.792 more lambs born per lambing than that of heterozygous NM genotype.
Conclusion
No mutations in the FecXB, FecXH, and FecXI genes were found in Watish samples. Consequently, based on the evidence from this study, these mutations cannot be considered a cause of the anecdotal high prolificacy of Watish sheep. The Prolactin, GDF9 (G1) and FecXG genes, showed variation but no association with litter size was detected. However, further studies using larger samples are needed to confirm or reject these results.
Authors’ Contributions
MAA supervised, designed, and coordinated the study. SEIM collected samples, executed genotyping methods, analyzed genotyping data, and wrote significant parts of the manuscript. RMA wrote significant parts of the manuscript and coordinated the study. KIZJ contributed to data analysis, and contributed to the genotyping process reading and revising the manuscript. LMAM analyzed the data and coordinated the study. MAMS, DMA, and AWA contributed to laboratory work. MTO contributed in critical review and correction of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors gratefully acknowledge the funding of this research provided by the Ministry of Animal Resources, Sudan. The technical support of the Department of Genetics and Animal Breeding, Faculty of Animal Production, University of Khartoum and the Animal Production Research Centre, Ministry of Animal Resources is also acknowledged. Special thanks are extended to the staff of the Central Laboratory, Ministry of Higher Education and Scientific Research, Khartoum, Sudan and we are grateful to the staff of the Animal Biotechnology Laboratory, Department of Animal Production, Jordan University of Science and Technology.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Abdoli R, Mirhoseini S.Z, Hossein-Zadeh N.G, Zamani P. Screening for causative mutations of major prolificacy genes in Iranian fat-tailed sheep. Int. J. Fertil. Steril. 2018;12(1):51–55. doi: 10.22074/ijfs.2018.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monsivais D, Matzuk M.M, Pangas S.A. The TGF-βfamily in the reproductive tract. Cold Spring Harb. Perspect. Biol. 2017;9(10):a022251. doi: 10.1101/cshperspect.a022251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal S, Aggarwal J, Dubey P.K, Mishra B.P, Ghalsasi P, Nimbkar C, Joshi B.K, Kataria R.S. Expression analysis of genes associated with prolificacy in FecB carrier and non-carrier Indian sheep. Anim. Biotechnol. 2017;28(3):220–227. doi: 10.1080/10495398.2016.1262869. [DOI] [PubMed] [Google Scholar]
- 4.Tang J, Hu W, Di R, Liu Q, Wang X, Zhang X, Zhang J, Chu M. Expression analysis of the prolific candidate genes, BMPR1B, BMP15, and GDF9 in small tail Han ewes with three fecundity (FecB Gene) genotypes. Animals. 2018;8(10):166. doi: 10.3390/ani8100166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montgomery G.W, Lord E.A, Penty J.M, Dodds K.G, Broad T.E, Cambridge L, Sunden S.L, Stone R.T, Crawford A.M. The booroola fecundity (FecB) gene maps to sheep chromosome 6. Genomics. 1994;22(1):148–153. doi: 10.1006/geno.1994.1355. [DOI] [PubMed] [Google Scholar]
- 6.Lassoued N, Benkhlil Z, Woloszyn F, Rejeb A, Aouina M, Rekik M, Bedhiaf-Romdhani S. FecXBara Novel BMP15 mutation responsible for prolificacy and female sterility in Tunisian Barbarin sheep. BMC Genet. 2017;18(1):43. doi: 10.1186/s12863-017-0510-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scaramuzzi R, Baird D.T, Campbell B.K, Driancourt M.A, Dupont J, Fortune J.E, Gilchrist R.B, Martin G.B, McNatty K.P, McNeilly A.S, Monget P, Monniaux D, Viñoles C, Webb R. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod. Fertil. Dev. 2011;23(3):444–467. doi: 10.1071/RD09161. [DOI] [PubMed] [Google Scholar]
- 8.Davis G.H, Balakrishnan L, Ross I.K, Wilson T, Galloway S.M, Lumsden B.M, Hanrahan J.P, Mullen M, Moa X.Z, Wang G.L, Zhao Z.S, Zeng Y.Q, Robinson J.J, Mavrogenis A.P, Papachristoforou C, Peter C, Baumung R, Cardyn P, Boujenane I, Cockett N.E, Eythorsdottir E, Arranz J.J, Notter D.R. Investigation of the booroola (FecB) and inverdale (FecXI) mutations in 21 prolific breeds and strains of sheep sampled in 13 countries. Anim. Reprod. Sci. 2006;92:8–96. doi: 10.1016/j.anireprosci.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Monteagudo L.V, Ponz R, Tejedor M.T, Lavi˜na A, Sierra I. A 17bp deletion in the bone morphogenetic protein 15 (BMP15) gene is associated to increased prolificacy in the Rasa Aragonesa sheep breed. Anim. Reprod. Sci. 2009;110:139–146. doi: 10.1016/j.anireprosci.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 10.El Fiky Z.A, Hassan G.M, Nassar M.I. Genetic polymorphism of growth differentiation factor 9 (GDF9) gene related to fecundity in two Egyptian sheep breeds. J. Assist. Reprod. Genet. 2017;34(12):1683–1690. doi: 10.1007/s10815-017-1007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanrahan J.P, Gregan S.M, Mulsant P, Mullen M, Davis G.H, Powell R, Galloway S.M. Mutations in the genes for oocyte-derived growth factorsGDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries) Biol. Reprod. 2004;70(4):900–909. doi: 10.1095/biolreprod.103.023093. [DOI] [PubMed] [Google Scholar]
- 12.Saleem M, Martin H, Coates P. Prolactin biology and laboratory measurement:An update on physiology and current analytical issues. Clin. Biochem. Rev. 2018;39(1):3–16. [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Liu S, Li F, Pan X, Li C, Zhang X, Ma Y, La Y, Xi R, Li T. Polymorphisms of the ovine BMPR-IB, BMP-15 and FSHR and their associations with litter size in two Chinese indigenous sheep breeds. Int. J. Mol. Sci. 2015;16(5):11385–11397. doi: 10.3390/ijms160511385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu M.X, Mu Y.L, Fang L, Ye S.C, Sun S.H. Polymorphism of prolactin gene and its relationship with prolificacy of small tail Han sheep. J. Agric. Biotechnol. 2007;18(1):65–73. doi: 10.1080/10495390601090950. [DOI] [PubMed] [Google Scholar]
- 15.Chu M.X, Wang X.C, Jin M, Di R, Chen H.Q, Zhu G.Q, Fang L, Mal Y.H, li K. DNA polymorphism of 5'flanking region of prolactin gene and its association with litter size in sheep. J. Anim. Breed. Genet. 2008;126(1):931–2668. doi: 10.1111/j.1439-0388.2008.00763.x. [DOI] [PubMed] [Google Scholar]
- 16.Al-Barzinji Y.M.S, Othman G.U. Genetic polymorphism in FecB gene in Iraqi sheep breeds. IOSR-JAVS. 2013;2(4):46–48. [Google Scholar]
- 17.Nejhad R.K, Ahmadi A.K. Genetic polymorphism in GDF9 and FecB in Dalagh sheep breed of Iran. JAVA. 2012;11(6):766–768. [Google Scholar]
- 18.Abouheif M.A, Al-Owaimer A.N, Shafey T.M, AlShaikh M.A. Polymorphism of booroola FecB gene in prolific individual from Najdi and Naeimi sheep breed of Saudi Arabia. J. Anim. Vet. Adv. 2011;10(10):1262–1264. [Google Scholar]
- 19.Dinçel D, Ardıçl S, Şaml H, Balc F. Genotype frequency of FecXB (Belclare) mutation of BMP15 gene in Chios (Sakiz) sheep. Uludag Univ. J. Fac. Vet. Med. 2018;37(2):87–91. [Google Scholar]
- 20.Guan F, Liu S.R, Shi G.Q, Yang L.G. Polymorphism of FecB gene in nine sheep breeds or strains and its effects on the little size, lamb growth and development. Anim. Reprod. Sci. 2007;99(1):44–52. doi: 10.1016/j.anireprosci.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 21.Moradband F, Rahimi G, Gholizadeh M. Association of polymorphisms in fecundity genes of GDF9, BMP15 and BMP15-1B with litter size in Iranian Baluchi sheep. Asian Aust. J. Anim. Sci. 2011;24(9):1179–1183. [Google Scholar]
- 22.Yu A.K, Getmantseva L.V, Shirockova N.V, Klimenko A, Yu B.S. Polymorphism of the GDF9 gene in Russian sheep breeds. J. Cytol. Histol. 2015;6(1):305. [Google Scholar]
- 23.Ghaffari M, Nejati-Javaremi A, Rahimi-Mianji G. Lack of polymorphism in the oocyte derived growth factor (GDF9) gene in the Shal breed of sheep. S. Afr. J. Anim. Sci. 2009;39(4):355–360. [Google Scholar]
- 24.Jawasreh K.I.Z, Awawdeh F.T, Al-Qaisy A. Association between GDF9, FecB and Prolactin Gene Polymorphisms and Prolificacy of Awassi Sheep. Canada: Proceedings, 10th World Congress of Genetics Applied to Livestock Production; 2014. [Google Scholar]
- 25.Jawasreh K.I.Z, Jadallah R, Al-Massad M.M. Association between the prolificacy of Romanov sheep breed and fecundity gene, growth differentiation factor 9 gene and prolactin gene genotypes. Malays. Appl. Biol. 2017;46(2):41–48. [Google Scholar]


