Abstract
Background and Aim:
The soft tick Ornithodoros savignyi is distributed throughout Africa, including Egypt. It primarily attacks camels, cattle, donkeys, and cows; and rarely affects humans. This study evaluated the acaricidal efficacy of ethanolic Curcuma longa extract (Turmeric) on the second nymphs of O. savignyi and then investigated the safety of this herb in rabbits.
Materials and Methods:
The nymphs were immersed in 10, 5, 2.5, 1.25, and 0.625 mg/ml ethanolic C. longa extract. An additional group was immersed in ethanol as a control. On the 1st, 7th, and 15th-day post-treatment, the mortality percentages, LC50, and LC95 were calculated. The ticks exposed to 10mg/ml ethanol C. longa extract were investigated by scanning electron microscopy (SEM). Three male New Zealand White rabbits were orally administered 2ml (two doses) of 10mg/ml ethanolic C. longa extract, and another three rabbits were orally given two doses of 2ml of absolute ethanol as a negative control. Histopathological examination of the kidney and liver hematology and the kidney and liver function was performed. Chemical analysis of the extract was determined by gas chromatography-mass spectrometry (GC-MS).
Results:
The LC50 and LC95 were 1.31 and 15.07, 1.07 and 8.56, and 0.81 and 6.97mg/ml on the 1st, 7th, and 15thday, respectively. SEM revealed that mamillae and spots on the surfaces of the treated ticks were not discriminating except for some clefts on the surfaces. The histological examination, blood profile, and biochemical analyses revealed no significant differences between the treated and untreated rabbits (p>0.05). GC/MS analysis revealed 50 compounds, and curcumene and tumerone were found to be the major constituents of this ethanolic extract.
Conclusion:
The ethanolic C. longa extract produced a strong acaricidal effect on the second nymph of O. savignyi, and it was safe to use in rabbits.
Keywords: histopathology, scanning electron microscopy, soft ticks, tick control, turmeric
Introduction
Ornithodoros savignyi is distributed throughout Africa, including Egypt. It primarily attacks animals (camels, cattle, donkeys, and cows); however, rare cases have been found in humans [1,2]. Bites from these ticks cause severe biting stress, paralysis, and toxicosis [1,3]. O. savignyi is a vector and a causative agent of many diseases such as Borreliaspp.[4,5], Alkhurma hemorrhagic fever virus [6], and Bluetongue virus 8 [7]. Medicinal plants are excellent alternatives to synthetic pesticides as they are eco-friendly, safe for humans and non-target organisms, rapidly biodegradable, and minimally resistant to pests [8,9]. Recent studies have evaluated the effectiveness of crude extracts and essential oils from plants against various tick species [10-12]. Moreover, some commercial acaricides are prepared from plants; and neem and myrrh have exhibited lethal effects against ticks[13,14].
Curcuma longa L. is botanically related to the Zingiberaceae family. Turmeric is a spice obtained from the root of C. longa that has multiple medicinal properties, including antibacterial, antimalarial, antiviral, anti-aging, anticancer, anti-Alzheimer’s disease, antifungal, antioxidant, and anti-inflammatory, as well as efficacy as a snail repellent[8,15-21]. An ethanolic C.longa extract exhibited efficacy against the Toxoplasma gondii tachyzoites in mice [22]. Turmeric possesses well-known insecticidal and repellent properties against insect pests. The ethanolic extracts of three Curcuma species Curcuma aeruginosa, Curcuma aromatica, and Curcuma xanthorrhiza have displayed mosquito repellent activity [23]. Two compounds isolated from the ethanolic C. longa extract ar-turmerone and 8-hydroxyl-ar-turmerone have both exhibited larvicidal effects against the fourth instar larvae of Culex pipiens [24].
However, the effect of C. longa extract on ticks has not previously been studied. Therefore, this research evaluated the acaricidal efficacy of the ethanolic C. longa extract on the soft tick O. savignyi and investigated the safety of this herb in rabbits used as an animal model.
Materials and Methods
Ethical approval
This study was approved to the ethical standards used in this study and the relevant national and institutional guidelines on the care and use of laboratory animals were approved by the Medical and Veterinary Research Ethics Committee (No, 19014) at the National Research Centre in Egypt.
Tick collection
Adult and nymph stage O. savignyi ticks were collected from the grounds of the camel market in Shalateen, Egypt (23° 7′ 54″ N, 35° 35′ 8″ E), during September 2018 (Figure-1). Live ticks were taken to the laboratory and identified morphologically using the taxonomic key of Walker et al. [1]. The second-instar nymphs were used to evaluate the effects of the ethanolic C. longa extract.
Figure-1.

The soft tick Ornithodoros savignyi on the ground of the camel yard: (a) Nymphs, (b) adults.
Preparation of the ethanolic C. longa extract
Dried rhizomes of C. longa were purchased from a local market in Cairo, Egypt. The dried powder was treated with 95% ethanol and evaporated under reduced pressure using a rotatory evaporator. The ethanolic extract was prepared using the dried C. longa rhizomes following the method described by Wang and Waller[25]. Briefly, the powder was macerated several times at room temperature for 1week with 95% ethyl alcohol before filtering using Whatman no.1 filter paper. The solvent was removed under reduced pressure at 40°C using a rotary evaporator until a thick paste was formed. This thick paste was considered a concentrated extract. Extracts were stored at 4°C in a refrigerator until use. The different concentrations of the extract were generated by mixing the appropriate volumes of ethanol.
The toxicity of the ethanolic C. longa extract on ticks
Concentrations of the ethanolic C. longa extract were prepared at 10, 5, 2.5, 1.25, and 0.625mg/ml. Atotal of 360 O. savignyi second nymphs were used during testing; 60 nymphs for each concentration and an additional 60 nymphs as a control group. Each treatment group included three replicates containing 20 nymphs per replicate. The control group contained the same number of ticks, and each was immersed in ethanol as the control treatment. Treatment consisted of immersion of the ticks in the specified extract for 1min before being transferred to the filter paper to absorb the excess extract. Once dry, the nymphs were placed into plastic tubes. These tubes were covered with a piece of cloth secured by a rubber band and incubated at 27°C, 75% relative humidity for 2weeks. The tick mortality was recorded on the 1st, 7th, and 15thdays post-treatment. Images of the dead nymphs (from the treatment groups) and live nymphs (control) were acquired using a stereomicroscope. Several dead nymphs from those exposed to the 10mg/ml extract were preserved in 70% ethanol for imaging with a scanning electron microscope (SEM).
SEM of ticks treated with ethanolic C. longa extract
The nymphs that died as a result of the treatment with 10mg/ml ethanol C. longa extract were preserved in 10% ethanol on the 1st-day post-treatment, and several live nymphs from the control group were preserved in 70% ethanol for comparison. The nymphs were prepared for SEM according to themethod described by Abdel-Shafy [26]. Briefly, the nymphs previously preserved in ethanol were cleaned using a water-glycerol-KCl solution, fixed in glutaraldehyde, immersed in a series of graded alcohol, glued by their dorsal and ventral surfaces to the SEM stub, dried with a dryer (Blazer Union, F1-9496 Blazer/Fürstentun Liechtenstein), mounted on SEM stubs, coated with gold using an S15OA Sputter Coater, and examined by SEM.
The safety of the ethanolic C. longa extract in rabbits
Six male New Zealand White rabbits weighing approximately 2.0kg were used for the in vivo evaluation of the ethanolic turmeric extract. Animals were divided randomly into two groups. The test group (n=3) received 2ml of an oral dose of 10mg/ml ethanolic C. longa extract. The second group (n=3) was kept as a negative control where the rabbits were orally administered 2ml of absolute ethanol. All rabbits were monitored for 1week and received the treatment twice during this time with a 3-day interval between the two doses. At the end of the experiment, the rabbits were euthanized by carbon dioxide gas asphyxiation with extra confirmation of death by head dislocation, according to the American Veterinary Medical Association guidelines for the euthanasia of animals [27]. The livers and kidneys were retrieved and fixed in 10% neutral buffered formalin for further pathological examination. Paraffin tissue sections at 4-6 μm thickness were prepared and stained with hematoxylin and eosin for histopathological examination. Histological slides were examined by light microscopy [28]. Blood samples were collected from all rabbits at the end of the experiment. Ahemogram test was conducted according to the method described by Weiss and Wardrop [29]. The activities of aspartate aminotransferase and alanine aminotransferase were determined according to the procedure of Reitman and Frankel [30]. Serum urea and creatinine were determined according to the methods of Patton and Crouch [31], and Houot [32], respectively.
Gas chromatography (GC)/mass spectrometry (MS) analysis
GC (Agilent Technologies 7890A, CA, USA) interfaced with a mass-selective detector (Agilent 7000 Triple Quad, CA, USA) and the Agilent HP-5ms capillary column (30 m×0.25mm ID, 0.25 μm film thickness) were used for analysis. The flow rate was 1mL/min using helium (He) as the carrier. The injector and detector temperatures were 200°C and 250°C, respectively. The acquisition mass range was 50-600. The formulae of the components were identified by comparing the mass spectra and RT information recorded with those of the NIST and WILEY library.
Statistical analysis
A one-way analysis of variance was used for the statistical analysis between the tick mortality percentages using the F and Tukey’s tests at p<0.05. The statistical analysis between the treated and control rabbits for each blood parameter was performed using a Student’s t-test. All statistical analyses were conducted using SPSS Version 14 (IBM Inc., NY, USA).
Results
The toxicity of the ethanolic C. longa extract on the O. savignyi second nymphs
The primary readout for this experiment included the mortality percentages of the O. savignyi nymphs treated with 0.625, 1.25, 2.5, 5, and 10mg/ml ethanolic C. longa extract on the 1st, 7th, and 15thdays post-treatment (Table-1). Statistical analysis revealed significant differences between the mortalities (p<0.001) recorded for all of the concentrations at each time point post-treatment. The highest mortalities were recorded at concentrations of 2.5-10mg/ml during the 2weeks post-treatment. However, the control group exhibited little mortalities (3.3-6.7%). All treatment concentrations and the control group experienced a non-significant increase in mortality throughout the days of post-treatment (p>0.05). The LC50 and LC95 slightly decreased over the days of post-treatment 1.31 and 15.07, 1.07 and 8.56, and 0.81 and 6.97mg/ml on the 1st, 7th, and 15thday, respectively (Table-2). There were changes evident in the O.savignyi nymphs treated with 10mg/ml of the ethanolic C.longa extract compared to that of the controls treated with ethanol. The size and color of the nymphs in the control group remained normal, a dark brown with creamy white legs (Figure-2a and b). In contrast, the treated nymphs were dark red, with swollen bodies, and pink legs (Figure-2c and d).
Table-1.
Mortality percentages (Mean±SE) of O. savignyi second nymphs treated with ethanolic C. longa extract.
| Concentration (mg/ml) | Day post-treatment | ||
|---|---|---|---|
| 1st | 7th | 15th | |
| 10 | 93.3±6.7a | 96.7±3.3ab | 96.7±3.3a |
| 5 | 76.7±8.8ab | 90.0±0.0ab | 93.3±3.3a |
| 2.5 | 73.3±12.0ab | 73.3±12.0abc | 83.3±12.0ab |
| 1.25 | 50.0±5.8bc | 56.7±8.8bc | 63.3±3.3ab |
| 0.625 | 30.0±10.0cd | 36.7±16.7cd | 46.7±14.5b |
| Control | 3.3±3.3d | 3.3±3.3d | 6.7±3.3c |
| Butex | 93.3±6.7a | 100±0.0a | - |
| F value | 17.642 | 16.972 | 17.650 |
| Probability (p) | <0.001 | <0.001 | <0.001 |
Small letters indicate to the significant difference between means using Tukey test at p<0.05. O. savignyi=Ornithodoros savignyi, C. longa=Curcuma longa
Table-2.
LC50 and LC95 values with their confidence limits for O. savignyi second nymphs treated with the ethanolic C. longa extract.
| Day post treatment | LC50 (mg/ml) | LC95 (mg/ml) | Confidence limits | Slope±SE | |||
|---|---|---|---|---|---|---|---|
| LC50 (mg/ml) | LC95 (mg/ml) | ||||||
| Lower | Upper | Lower | Upper | ||||
| 1st | 1.31 | 15.07 | 1.05 | 1.58 | 10.44 | 25.61 | 1.55±0.16 |
| 7th | 1.07 | 8.56 | 0.86 | 1.27 | 6.42 | 12.79 | 1.82±0.17 |
| 15th | 0.81 | 6.97 | 0.62 | 0.99 | 5.22 | 10.55 | 1.76±0.18 |
O. savignyi=Ornithodoros savignyi, C. longa=Curcuma longa
Figure-2.
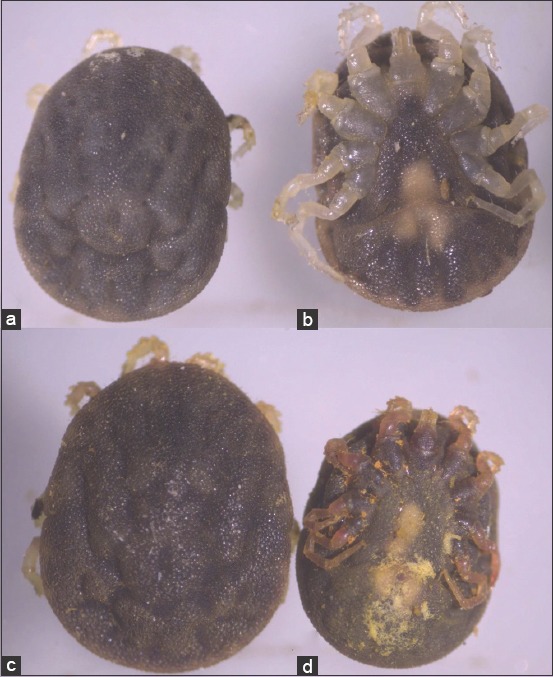
The Ornithodoros savignyi second nymphs treated with 10mg/ml ethanolic Curcuma longa extract: (a) Dorsal view of nymph exposed to the ethanol only as a control, (b) ventral view of nymph exposed to the ethanol only as a control, (c) dorsal view of nymph immersed in the ethanolic C. longa extract, (d) ventral view of nymph immersed in the ethanolic C. longa extract.
SEM analysis of the O. savignyi second nymphs
Untreated nymphs exhibited distinctive vertical and horizontal grooves with spots that were visible inside, as well as mammillae all over the dorsal surface (Figure-3a and b). Amammilla is a broad protuberance with one or two holes at the apex (Figure-3c). Aspot is a small tapering protuberance without any holes on the apex (Figure-3d). However, the dorsal surface of the treated nymphs exhibited shallow vertical and horizontal grooves (Figure-4a), and the mammillae appeared to be adhering to each other (Figure-4b and c). Spots on the treated nymphs were an undiscriminating shape (Figure-4d). The ventral surface of the untreated nymphs exhibited wrinkled integument, mammillae, and spots scattered between mammillae throughout the ventral surface (Figure-5a-d), whereas the ventral surface of a treated nymph showed indistinctive mammillae and spots (Figure-6a-d).
Figure-3.
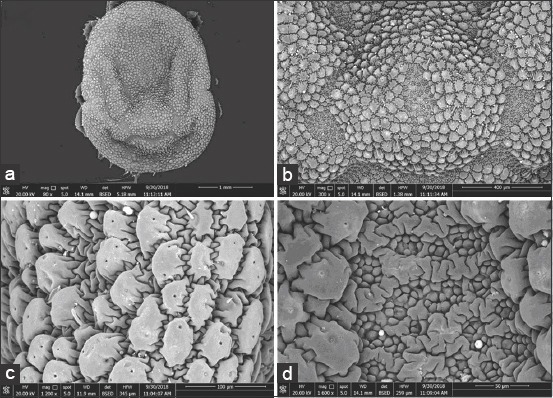
Dorsal view of untreated Ornithodoros savignyi second nymph showing: (a) Depressing areas and well distinctive vertical and horizontal grooves, (b) mamillae and spots together, (c) mamilae with one or two holes on their apex, (d) small spots without any holes on their apex.
Figure-4.

Dorsal view of treated Ornithodoros savignyi second nymph by 10mg/ml ethanolic Curcuma longa extract showing: (a) Slightly depressing areas and shallow vertical and horizontal grooves, (b) mamillae and spots together, (c) mamilae adhere to each other, (d)undiscriminating spots.
Figure-5.
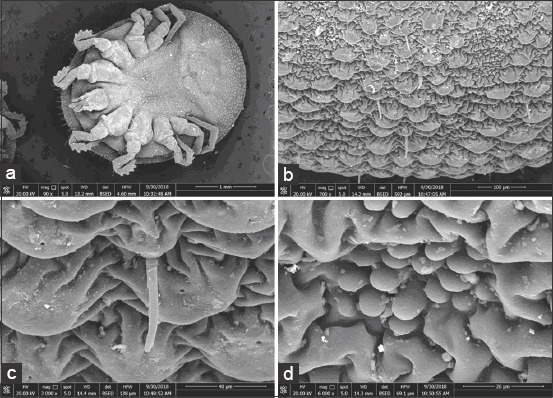
Ventral view of untreated Ornithodoros savignyi second nymph showing: (a) Wrinkle integument, mammillae, and spots, (b) mamillae and spots together, (c) mamillae with one hole on their apex, (d) small spots without any holes on their apex.
Figure-6.
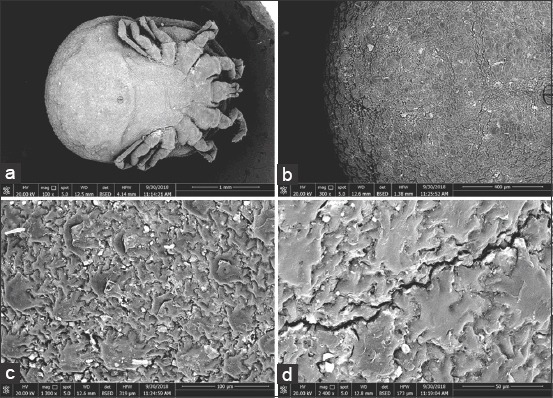
Ventral view of treated Ornithodoros savignyi second nymph by 10mg/ml ethanolic Curcuma longa extract showing: (a) Indistinctive mammillae and spots, (b) diffused mamillae and spots to form flat surface, (c)mamillae merge each other and spots to form flat surface, (d) long cleft on the surface.
The safety of the ethanolic C. longa extract in rabbits
No major abnormalities were seen in the kidneys of either the negative controls or the treated groups of rabbits (Figure-7a and b). The histological examination of the livers from the negative control rabbits showed no pathological abnormalities (Figure-7c). Liver tissue sections of rabbits that ingested ethanolic C. longa extract showed dilation, congestion of the portal, and central veins, areas of vacuolar degeneration, and congestion of the central venules, sinusoids, and portal triad (Figure-7d). The blood profile, kidney function, and liver function were within the normal range in both the control and treated rabbits with non-statistically significant differences (p>0.05) (Table-3).
Figure-7.
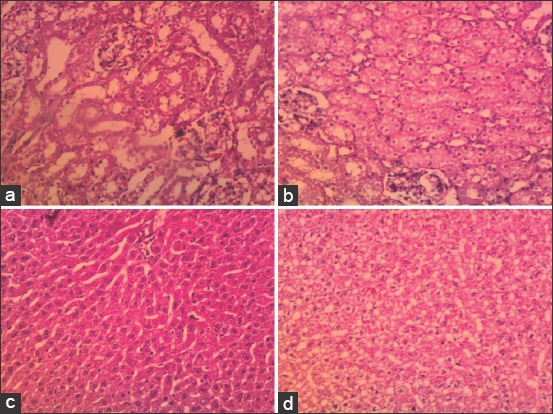
Cross sections in the kidney and liver of the rabbits that orally took 2ml of 10mg/ml ethanolic Curcuma longa extract in the treated group and 2ml of absolute ethanol in the negative control group (Hematoxylin and eosin; ×200): (a) Negative control of the kidney showed the normal architecture. (b) The kidney of the rabbit administered C. longa extract. (c) Negative control of the liver showed normal architecture with few degenerated areas. (d) The liver of the rabbit administered C. longa extract showing moderate reversible liver cell damage in the form of degeneration and congestion.
Table-3.
Blood profile and liver and kidney functions of rabbits ingested 2 times of 10 mg/ml ethanolic C. longa extract.
| Parameter | Treated | Control |
|---|---|---|
| RBCs | 5.52±0.23 | 5.86±0.01 |
| Hb | 12.90±0.17 | 13.00±0.13 |
| HCT | 36.95±0.03 | 37.95±0.38 |
| MCV | 61.55±0.49 | 61.65±0.95 |
| MCH | 21.40±0.46 | 22.05±0.23 |
| MCHC | 34.70±0.40 | 34.20±0.06 |
| Platelets | 452.00±17.90 | 357.50±12.41 |
| WBCs | 6.35±0.20 | 6.40±0.06 |
| Urea | 26.20±0.64 | 20.15±1.01 |
| Creatinine | 1.15±0.03 | 0.95±0.03 |
| ALT | 110.00±9.81 | 95.50±1.44 |
| AST | 58.95±2.34 | 41.50±2.60 |
RBCs=Red blood corpuscular counts, Hb=Hemoglobin, HCT=Hematocrit. MCV=Mean corpuscular volume. MCH=Mean corpuscular hemoglobin. MCHC=Mean corpuscular hemoglobin concentration, WBCs=White blood cell counts, ALT=Alanine aminotransferase. AST=Aspartate aminotransferase, O. savignyi=Ornithodoros savignyi, C. longa=Curcuma longa
Phytochemical analyses of the ethanolic C. longa extract
GC–MS analysis of the ethanol extract of C.longa identified the major chemical constituents based on the peak retention time (Rt) and percentage area. Atotal of 50 compounds were identified, and the most abundant were as follows (the percentage of abundance is stated in brackets) β-curcumene (30.71), tumerone (7.14), germacone (6.08), β-cedrene (4.59), cedr-8-ene (3.87), β-cubebene (3.34), cis-sesquisabinene hydrate (2.51), p-Cymen-2-ol (2.49), β-himachalene (2.26), Cis-p-mentha-2,8-diene-1-ol (2.1), farnesol (2.1), 7,3`-Dimethyoy-3-hydroxyflavone (2.09), and pinane (2.06) (Table-4 and Figure-8).
Table-4.
GC-MS of ethanol extract of C. longa using library.
| No. | Rt (min) | Name of active compounds | % peak area |
|---|---|---|---|
| 1 | 3.5 | D-2,3-Butane diol | 1.21 |
| 2 | 5.7 | o-cymene | 0.34 |
| 3 | 6.73 | Terpinolene | 0.64 |
| 4 | 7.27 | α-thujone | 0.58 |
| 5 | 7.70 | Cis-sabinol | 0.33 |
| 6 | 8.13 | Ciminaldehyde | 0.5 |
| 7 | 8.68 | Thymol | 0.71 |
| 8 | 8.98 | phenol | 0.57 |
| 9 | 9.19 | Dihydrocurcumene | 1.21 |
| 10 | 9.47 | Hemellitol | 0.38 |
| 11 | 9.57 | Cembrene | 0.42 |
| 12 | 9.71 | α-guaiene | 0.96 |
| 13 | 9.82 | Longipinene | 0.37 |
| 14 | 9.91 | β-bisabolene | 1.75 |
| 15 | 10.04 | γ-gurjunene | 0.54 |
| 16 | 10.28 | β-Curcumene | 1.08 |
| 17 | 10.41 | Cuparene | 0.47 |
| 18 | 10.45 | Cedr-8-ene | 3.87 |
| 19 | 10541 | β-Cedrene | 4.59 |
| 20 | 10.72 | Cis-p-mentha-2,8-diene-1-ol | 2.1 |
| 21 | 10.81 | Cis-p-mentha-6,8-diene-2ol | 0.39 |
| 23 | 10.83 | Cis-Lanceol | 1.05 |
| 24 | 10.87 | Geranyl-α-terpinene | 0.64 |
| 25 | 11.08 | β-Himachalene | 2.26 |
| 26 | 11.19 | Spathulenol | 0.51 |
| 27 | 11.368 | Nopol (terpene) | 1.08 |
| 28 | 11.65 | α-Curcumene | 30.71 |
| 29 | 11.78 | α-bergamotene | 0.55 |
| 30 | 11.81 | Zingiberene | 0.48 |
| 31 | 11.91 | Tumerone | 7.14 |
| 32 | 12.00 | Longiverbenone | 0.66 |
| 33 | 12.102 | Trans-Chrysanthenyl acetate | 1.18 |
| 34 | 12.155 | Caryophyllene oxide | 1.77 |
| 35 | 12.257 | α-terpinene | 0.48 |
| 36 | 12.379 | Germacrone | 6.08 |
| 37 | 12.493 | Elemol | 0.93 |
| 38 | 12.66 | Geranyllinalool | 0.86 |
| 39 | 12.786 | Terpinylformate | 1.51 |
| 40 | 12.969 | Cis-sesquisabinene hydrate | 2.51 |
| 41 | 13.108 | Corymbolone | 1.51 |
| 42 | 13.365 | α-lonone | 0.59 |
| 43 | 13.495 | p-Cymen-2-ol | 2.49 |
| 44 | 13.678 | 7,3`-Dimethyoy-3-hydroxyflavone | 2.09 |
| 45 | 14.738 | Pinane | 2.05 |
| 46 | 14.774 | Farnesol | 2.1 |
| 47 | 14.86 | 5β, 7βH, 10 α-eudesm-11-en-1a-ol | 0.89 |
| 48 | 15.74 | Phytol | 0.38 |
| 49 | 21.456 | Stigmasterol | 0.46 |
| 50 | 22.85 | β-sitosterol | 0.22 |
C. longa=Curcuma longa, GC-MS=Gas chromatography–mass spectrometry
Figure-8.
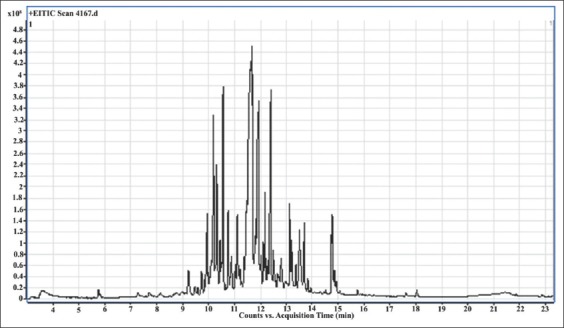
Gas chromatography–mass spectrometry chromatography of the ethanolic Curcuma longa extract.
Discussion
Alcohols are solvents that have demonstrated superior extractive power for almost all-natural substances of low molecular weight, such as alkaloids, saponins, and flavonoids [33]. The ethanolic extracts from the rhizome of C. longa L. possessed a wide variety of biological activities related to the treatment and prevention of various clinical conditions [34]. The C. longa rhizome extract was reportedly a more potent antimicrobial agent than the leaf extract [18], and higher concentrations of curcumin and antioxidant activity were obtained when ethanol was used as the solvent for extraction [17]. Therefore, this study evaluated the effects of the crude ethanolic extract of C. longa L. against the second nymphs of the soft tick O. savignyi, which was found in large numbers on the grounds of a local camel yard in Egypt. The safety of this extract in rabbits was also evaluated. The C.longa L. rhizome was selected for extraction and evaluation against this tick species for three reasons: (1) It is available in the local market in a powder form for a low price, (2) the previous studies found that different C. longa L. derived materials exhibited strong insecticidal activity on various insect pests such mosquitoes, and (3) no previous studies had evaluated the effects of C. longa L. extract on this tick species.
In this study, it was found that the O. savignyi second nymphs were highly susceptible to the ethanolic C. longa extract as 2.5-10mg/ml of the extract caused 73.3-96.7% mortality in the nymphs after 1week of treatment. There were non-statistically significant differences (p>0.05) between the mortality range recorded with the extract and the mortality range of the reference acaricide (93.3 and 100%). The calculated LC50 values of the extract used in this research were 1.31, 1.07, and 0.81mg/ml on the 1st, 7th, and 15thdays, respectively. This indicated that the toxicity of the ethanolic C. longa extract increased over time. Another study[35] had evaluated the effects of C. longa on ticks, using essential oils rather than extract, and their results were similar to the results of the current study[35]. These researchers found that of 11 Brazilian essential oils, C. longa essential oil produced the best results against Rhipicephalus (Boophilus) microplus ticks, with LC50 values of 10.24 and 0.54mg/mL against the females and larvae, respectively[35]. Furthermore, the essential oils of Curcuma spp. demonstrated highly larvicidal activity against Aedes aegypti and Anopheles gambiae with LC50 values of 36.30 and 149ppm, respectively [36,37]. In addition, petroleum ether extract from C. aromatica showed larvicidal activity against Culex quinquefasciatus at an LC50 value of 11.41 [38]. Moreover, two compounds, ar-turmerone and 8-hydroxyl-ar-turmerone isolated from the ethanolic extracts of the C. longa root, exhibited larvicidal activities against the fourth-instar larvae of C. pipiens pallens following 24h of treatment; LC50 values were 138.86 and 257.68ppm, respectively [24].
Changes to the morphological features of the ticks were observed on treatment with the ethanolic C. longa extract, cuticles became darker, and the ticks’ legs changed from a creamy white color to a pinkish hue. This suggested that the extract could penetrate the tick’s cuticle, causing damage to the guts, which spread throughout the tick’s body, including the legs. Furthermore, the SEM images revealed additional changes that occurred on the surfaces of the ticks that were exposed to the ethanolic C. longa extract. The mammillae of the treated ticks appeared to overlap, the spots on the surface were different, and there were many crevices on the surfaces of treated ticks. These changes might be due to swelling of the tick’s body. The ethanolic C. longa extract might cause inhibition of the acetylcholinesterase activity as it contained high levels of polyphenols [39]. Consistent with the effects seen in the present study, Chaaban et al. [40] observed that C. longa essential oil caused damage to the tick cuticles, midgut, and brain, and produced a fattening of the body of the third-instar Lucilia cuprina (Diptera: Calliphoridae) larvae.
Two doses of 2ml (10mg/ml ethanolic) C.longa extract were administered to rabbits with 3days between doses to assess the safety of the extract in a mammalian system. Biochemical blood parameters were measured for assessment. These parameters were related to health status and considered good indicators of the physiological, pathological, and nutritional status of the animals [41]. The results confirmed the safety of the extract in rabbits. There were no significant changes observed in the kidney function tests or the histological analysis in rabbits that received the extract compared to the control group. Furthermore, there were no significant changes in the blood profile or liver functions between the treated and control rabbits. The results agreed with the findings in the literature [42], in which the authors found no adverse effects on the erythrocytes, leukocytes, or the concentrations of blood constituents. However, slight changes were observed in the livers of the C. longa extract-treated rabbits compared with the control group. Mesa et al.[43] found that oral administration of the C.longa extract reduced susceptibility to the oxidation of erythrocytes and liver microsome membranes. Turmeric’s hepatoprotective effect was primarily a result of the antioxidant properties and the ability to reduce the formation of the pro-inflammatory cytokines. Moreover, curcumin administration significantly decreased liver injury [43].
In the present study, the GC–MS analyses of the ethanolic C. longa extract revealed 50 compounds, of which the 13 most abundant comprised the majority of the sample. Many of these compounds were major components of essential oils. The natural phytochemicals of C. longa have been associated with anticancer, anti-inflammatory, neuroprotective, anti-Alzheimer’s, and antioxidant activities. Furthermore, the volatile oil of turmeric has been widely used in cosmetics or health products and possesses antimicrobial, antifungal, and anti-arthritic properties. The natural phytochemicals of C. longa are also considered to affect a variety of biological activities [44,45]. The two most abundant compounds of the ethanolic C. longa extract were curcumene and tumerone, which might be responsible for the acaricidal activity against the second nymphs of O. savignyi in the present study. Furthermore, some studies have identified that other compounds isolated from the ethanolic extracts of C. longa root have exhibited insecticidal activity against some insects such as mosquitoes. Liu etal.[24] isolated ar-turmerone and 8-hydroxyl-ar-turmerone from the C. longa root ethanolic extracts and identified larvicidal activities against the fourth-instar larvae of C. pipiens. Furthermore, Ali et al. [46] discovered that the ethanolic extract of C.longa was comprised 15.4% of ar-turmerone, 6.6% of bisdesmethoxycurcumin, 6.1% of desmethoxycurcumin, and 22.6% of curcumin.
Conclusion
The ethanolic curcumene and tumerone were the most abundant constituents identified in the extract. The ethanolic C. longa extract elicited a strong acaricidal effect on the second nymphs of the soft tick O. savignyi, while no adverse effects were found in the rabbits. Therefore, this extract could be used in pest management applications against ticks found on animals.
Authors’ Contributions
All authors shared in the study plan. SA, HSMG performed the bioassay of the ethanolic C. longa extract on the ticks. SA, HSMG, and AMA participated in the administration of the ethanolic C. longa extract in the rabbits. SA and DES photograph the ticks by light microscope and scanning electron microscope and analyzed the data. AMA analyzed the blood and performed the cross-sections of the rabbit organs. HAAA and RAM participated in the chemical analyses of the ethanolic C. longa extract. SA, AMA, HAAA, RAM, ADA, and MYA shared in writing the manuscript. All authors revised and approved the final manuscript.
Acknowledgments
This study is a part of project No.11020303, which was financially supported by the National Research Centre, Giza, Egypt.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Walker A.R, Bouattour A, Camicas J.L, Estrada-Pena A, Horak I.G, Latif A.A, Pegram R.G, Preston P.M. Ticks of Domestic Animals in Africa:A Guide to Identification of Species. Edinburgh: Bioscience Reports; 2003. [Google Scholar]
- 2.Horak I.G, Jordaan A.J, Nel P.J, van Heerden J, Heyne H, van Dalen E.M. Distribution of endemic and introduced tick species in free state Province, South Africa. J. S. Afr. Vet. Assoc. 2015;86(1):1–9. doi: 10.4102/jsava.v86i1.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mans B.J, Steinmann C.M.L, Venter J.D, Louw A.I, Neitz A.W.H. Pathogenic mechanisms of sand tampan toxicoses induced by the tick Ornithodoros savignyi. Toxicon. 2002;40(7):1007–1016. doi: 10.1016/s0041-0101(02)00098-3. [DOI] [PubMed] [Google Scholar]
- 4.Adham F.K, El-Samie-Abd E.M, Gabre R.M, El Hussein H. Detection of tick blood parasites in Egypt using PCR assay II-Borrelia burgdorferi sensu lato. J. Egypt. Soc. Parasitol. 2010;40(3):553–564. [PubMed] [Google Scholar]
- 5.Hassan M.I, Gabr H.S.M, Abdel-Shafy S, Hammad K.M, Mokhtar M.M. Molecular detection of Borrelia sp. In Ornithodoros savignyi and Rhipicephalus annulatus by FlaB gene and Babesia bigemina in R. annulatus by 18S rRNA gene. J. Egypt. Soc. Parasitol. 2017;47(2):403–414. [Google Scholar]
- 6.Charrel R.N, Fagbo S, Moureau G, Alqahtani M.H, Temmam S, De Lamballerie X. Alkhurma hemorrhagic fever virus in Ornithodoros savignyi ticks. Emerg. Infect. Dis. 2007;13(1):153–155. doi: 10.3201/eid1301.061094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouwknegt C, van Rijn P.A, Schipper J.J.M, Hölzel D, Boonstra J, Nijhof A.M, van Rooij E.M.A, Jongejan F. Potential role of ticks as vectors of bluetongue virus. Exp. Appl. Acarol. 2010;52(2):183–192. doi: 10.1007/s10493-010-9359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latip S.N.H, Nawi F.W.M, Mansur S.H.P. Snails:Biodiversity, Biology and Behavioral Insights. Hauppauge, New York: Nova Science Publishers Inc; 2017. Potential of selected indigenous plant extracts as botanical pesticides for controlling golden apple snail Pomacea canaliculata; pp. 95–116. [Google Scholar]
- 9.Walia S, Saha S, Tripathi V, Sharma, K.K Phytochemical biopesticides:Some recent developments. Phytochem. Rev. 2017;16(5):989–1007. [Google Scholar]
- 10.Abdel-Shafy S, Soliman M.M.M. Toxicity of some essential oils on eggs, larvae and females of Boophilus annulatus(Acari Ixodidae) infesting cattle in Egypt. Acarologia. 2004;44(1-2):23–30. [Google Scholar]
- 11.Abdel-Shafy S, Soliman M.M.M, Habeeb S.M. In vitro acaricidal effect of some crude extracts and essential oils of wild plants against certain tick species. Acarologia. 2007;47(1-2):31–42. [Google Scholar]
- 12.Habeeb S.M, Abdel-Shafy S, Youssef A.A. Light, scanning electron microscopy and SDS-PAGE studies on the effect of the essential oil Citrus sinensis var. balady on the embryonic development of camel tick Hyalomma dromedarii(Koch, 1818) (Acari Ixodidae) Pak. J. Biol. Sci. 2007;10(8):1151–1160. doi: 10.3923/pjbs.2007.1151.1160. [DOI] [PubMed] [Google Scholar]
- 13.Abdel-Shafy S, Zayed A.A. In vitro acaricidal effect of plant extract of neem seed oil (Azadirachta indica) on egg, immature, and adult stages of Hyalomma anatolicum excavatum(Ixodoidea Ixodidae) Vet. Parasitol. 2002;106(1):89–96. doi: 10.1016/s0304-4017(02)00023-7. [DOI] [PubMed] [Google Scholar]
- 14.Massoud A.M, Kutkat M.A, Abdel-Shafy S, El-Khateeb R.M. Acaricidal efficacy of Myrrh (Commiphora molmol) on the fowl tick Argas persicus(Acari Argasidae) J. Egypt. Soc. Parasitol. 2005;35(2):667–686. [PubMed] [Google Scholar]
- 15.Álvarez N.M, Ortíz A.A, Martínez O.C. In vitro antibacterial activity of Curcuma longa(Zingiberaceae) against nosocomial bacteria in Montería, Colombia. Rev. Biol. Trop. 2016;64(3):1201–1208. [PubMed] [Google Scholar]
- 16.Hefnawy H.T, El-Shourbagy G.A, Ramadan M.F. Phenolic extracts of carrot, grape leaf and turmeric powder:antioxidant potential and application in biscuits. J. Food Meas. Charact. 2016;10(3):576–583. [Google Scholar]
- 17.Martinez-Correa H.A, Paula J.T, Kayano A.C.A, Queiroga C.L, Magalhães P.M, Costa F.T.M, Cabral F.A. Composition and antimalarial activity of extracts of Curcuma longa L. Obtained by a combination of extraction processes using supercritical CO2ethanol and water as solvents. J. Supercrit. Fluids. 2017;119(Jan):122–129. [Google Scholar]
- 18.Singh N, Gupta S, Rathore V. Comparative antimicrobial study of ethanolic extract of leaf and rhizome of Curcuma longa Linn. Pharmacogn. J. 2017;9(2):208–212. [Google Scholar]
- 19.Sornpet B, Potha T, Tragoolpua Y, Pringproa K. Antiviral activity of five Asian medicinal plant crude extracts against highly pathogenic H5N1 avian influenza virus. Asian Pac. J. Trop. Med. 2017;10(9):871–876. doi: 10.1016/j.apjtm.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Wang L, Pan L, Jia X.G, Yan L, Jiang Y.Y. Antifungal activity of 122 kinds of Uighur medicines in vitro. Acad. J. Second Mil. Med. Univ. 2017;38(5):554–562. [Google Scholar]
- 21.Lu P.S, Inbaraj B.S, Chen B.H. Determination of oral bioavailability of curcuminoid dispersions and nanoemulsions prepared from Curcuma longa Linnaeus. J. Sci. Food Agric. 2018;98(1):51–63. doi: 10.1002/jsfa.8437. [DOI] [PubMed] [Google Scholar]
- 22.Al-Zanbagi N.A, Zelai N.T. Two methods for attenuating Toxoplasma gondii tachyzoites RH strain by using ethanol extract of Curcuma longa. J. Egypt. Soc. Parasitol. 2008;38(3):965–976. [PubMed] [Google Scholar]
- 23.Pitasawat B, Choochote W, Tuetun B, Tippawangkosol P, Kanjanapothi D, Jilpakdi A, Riyong D. Repellency of aromatic turmeric Curcuma aromatica under laboratory and field conditions. J. Vector. Ecol. 2003;28(2):234–240. [PubMed] [Google Scholar]
- 24.Liu J, Zhang M, Fu W.J, Hu J.F, Dai G.H. Efficacy of bioactive compounds from Curcuma longa L. against mosquito larvae. J. Appl. Entomol. 2018;142(8):792–799. [Google Scholar]
- 25.Wang L, Waller C.L. Recent advances in extraction of neutraceuticals from plants. Trends Food Sci. Technol. 2006;17(6):300–312. [Google Scholar]
- 26.Abdel-Shafy S. Scanning electron microscopy and comparative morphology of Hyalomma anatolicum excavatum H. dromedarii and H. marginatum marginatum(Acari Ixodidae) based on nymphs. Acarologia. 2008;48(1-2):111–126. [Google Scholar]
- 27.Underwood W, Anthony R, Cartner S, Corey D, Grandin T, Greenacre C, Gwaltney-Brant S, McCrackin M.A, Meyer R, Miller D. AVMA Guidelines for the Euthanasia of Animals:2013 edition. Schaumburg, IL: American Veterinary Medical Association; 2013. [Google Scholar]
- 28.Bancroft J.D, Layton C. The hematoxylins and eosin. In: Suvarna S.K, Layton C, Bancroft J.D, editors. Bancroft's Theory and Practice of Histological Techniques. 7th ed. Oxford: Churchill Livingstone Elsevier; 2013. pp. 173–186. [Google Scholar]
- 29.Weiss D.J, Wardrop K.J. Schalm's Veterinary Hematology. 6th ed. Ames, Iowa, USA: Blackwell Publishing Ltd; 2010. [Google Scholar]
- 30.Reitman S, Frankel S. A colorimetric method for determination of oxaloacetic transaminase and serum glutamic pyruvic transaminase. Am. J. Clin. Pathol. 1957;28(1):56–60. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 31.Patton C.J, Crouch S.R. Spectrophotometric and kinetics investigation of the Berthelot reaction for the determination of ammonia. Anal. Chem. 1977;49(3):464–469. [Google Scholar]
- 32.Houot O. In: Interpretation of Clinical Laboratory Tests. Siest G, Henny J, Schiele F, Young D.S, editors. California, USA: Biochemical Publications, Foster City; 1985. pp. 220–234. [Google Scholar]
- 33.Alamgir A.N.M. Herbal drugs:Their collection, preservation, and preparation;evaluation, quality control, and standardization of herbal drugs. Prog. Drug Res. 2017;73:453–495. [Google Scholar]
- 34.Xia X, Pan Y, Zhang W.Y, Cheng G, Kong L.D. Ethanolic extracts from Curcuma longa attenuates behavioral, immune, and neuroendocrine alterations in a rat chronic mild stress model. Biol. Pharm. Bull. 2006;29(5):938–944. doi: 10.1248/bpb.29.938. [DOI] [PubMed] [Google Scholar]
- 35.Chagas A.C.S, Oliveira M.C.S, Giglioti R, Santana R.C.M, Bizzo H.R, Gama P.E, Chaves F.C.M. Efficacy of 11 Brazilian essential oils on lethality of the cattle tick Rhipicephalus (Boophilus) microplus. Ticks Tick Borne Dis. 2016;7(3):427–432. doi: 10.1016/j.ttbdis.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Choochote W, Chaiyasit D, Kanjanapothi D, Rattanachanpichai E, Jitpakdi A, Tuetun B, Pitasawat B. Chemical composition and anti-mosquito potential of rhizome extract and volatile oil derived from Curcuma aromatica against Aedes aegypti(Diptera Culicidae) J. Vector Ecol. 2005;30(2):302–309. [PubMed] [Google Scholar]
- 37.Kemabonta K.A, Adediran O.I, Ajelara K.O. The insecticidal efficacy of the extracts of Piper nigrum(Black Pepper) and Curcuma longa(Turmeric) in the control of Anopheles gambiae Giles (Dip Culicidae) Jordan J. Biol. Sci. 2018;11(2):195–200. [Google Scholar]
- 38.Madhu S.K, Vijayan V.A. Evaluation of the larvicidal efficacy of extracts from three plants and their synergistic action with propoxur against larvae of the filarial vector Culex quinquefasciatus(Say) Toxicol. Environ. Chem. 2010;92(1):115–126. [Google Scholar]
- 39.Jung Y.S, Park S.J, Park J.H, Jhee K.H, Lee I.S, Yang S.A. Effects of ethanol extracts from Zingiber officinale Rosc Curcuma longa L, and Curcuma aromatica Salisb. On acetylcholinesterase and antioxidant activities as well as GABA contents. J. Korean Soc. Food Sci. Nutr. 2012;41(10):1395–1401. [Google Scholar]
- 40.Chaaban A, Richardi V.S, Carrer A.R, Brum J.S, Cipriano R.R, Martins C.E.N, Silva M.A.N, Deschamps C, Molento M.B. Insecticide activity of Curcuma longa(leaves) essential oil and its major compound α-phellandrene against Lucilia cuprina larvae (Diptera Calliphoridae):Histological and ultrastructural biomarkers assessment. Pestic. Biochem. Physiol. 2019;1530(Jan):17–27. doi: 10.1016/j.pestbp.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Ozaki M, Fuchinoue S, Teraoka S, Ota K. The in vivo cytoprotection of ascorbic acid against ischemia/reoxygenation injury of rat liver. Arch. Biochem. Biophys. 1995;318(2):439–445. doi: 10.1006/abbi.1995.1252. [DOI] [PubMed] [Google Scholar]
- 42.Sambaiah K, Ratankumar S, Kamanna V.S, Satyanarayana M.N, Rao M.V.L. Influence of turmeric and curcumin on growth, blood constituents and serum enzymes in rats. J. Food Sci. Technol. 1982;19(5):187–190. [Google Scholar]
- 43.Mesa M.D, Aguilera C.M, Ramírez-Tortosa C.L, Ramírez-Tortosa M.C, Quiles J.L, Baró L, Martínez de Victoria E, Gil A. Oral administration of a turmeric extract inhibits erythrocyte and liver microsome membrane oxidation in rabbits fed with an atherogenic diet. Nutrition. 2003;19(9):800–804. doi: 10.1016/s0899-9007(03)00093-5. [DOI] [PubMed] [Google Scholar]
- 44.Dewanto V, Wu X, Adom K, Liu R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002;50(10):3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- 45.Naczk M, Shahidi F. Extraction and analysis of phenolics in food. J. Chromatogr. A. 2004;1054(1-2):95–111. [PubMed] [Google Scholar]
- 46.Ali A, Wang Y.H, Khan I.A. Larvicidal and biting deterrent activity of essential oils of Curcuma longa ar-turmerone, and curcuminoids against Aedes aegypti and Anopheles quadrimaculatus(Culicidae Diptera) J. Med. Entomol. 2015;52(5):979–986. doi: 10.1093/jme/tjv072. [DOI] [PubMed] [Google Scholar]


