Abstract
Recent extensive evidence indicates that air pollution, in addition to causing respiratory and cardiovascular diseases, may also negatively affect the brain and contribute to central nervous system diseases. Air pollution is comprised of ambient particulate matter (PM) of different sizes, gases, organic compounds, and metals. An important contributor to PM is represented by traffic-related air pollution, mostly ascribed to diesel exhaust (DE). Epidemiological and animal studies have shown that exposure to air pollution may be associated with multiple adverse effects on the central nervous system. In addition to a variety of behavioral abnormalities, the most prominent effects caused by air pollution are oxidative stress and neuro-inflammation, which are seen in both humans and animals, and are supported by in vitro studies. Among factors which can affect neurotoxic outcomes, age is considered most relevant. Human and animal studies suggest that air pollution may cause developmental neurotoxicity, and may contribute to the etiology of neurodevelopmental disorders, including autism spectrum disorder. In addition, air pollution exposure has been associated with increased expression of markers of neurodegenerative disease pathologies, such as alpha-synuclein or beta-amyloid, and may thus contribute to the etiopathogenesis of neurodegenerative diseases, particularly Alzheimer’s disease and Parkinson’s disease.
Keywords: Air pollution, Diesel exhaust, Neurotoxicity, Oxidative stress, Neuro-inflammation, Neurodevelopmental disorders, Autism, Neurodegenerative diseases, Alzheimer’s disease
1. Air pollution: sources, composition and major adverse health effects
Full recognition that poor air quality may compromise human health has been achieved only in the twentieth century. The most important episode in this regard is the so-called “London fog” in 1952. A dense smog containing sulfur dioxide and smoke particulate (from coals burning and industrial activities) descended upon London in December 1952, resulting in an unprecedented morbidity and mortality, and a final >10,000 excess deaths by February 1953 (Anderson, Thundiyil, & Stolbach, 2012; Bell & Davis, 2001; Bell, Davis, & Fletcher, 2004). Similar episodes of acute effects of air pollution of similar severity, albeit of lesser consequences, have also been reported in Belgium in 1930 and in Pennsylvania in 1948 (Bell & Davis, 2001). These episodes are believed to have prompted awareness that quality of air is an important determinant of human health, and that it needs to be considered in the overall contributions of environmental factors to potential adverse health effects. In the U.S.A., these considerations culminated in 1970 with the approval of the Clean Air Act whose main goals were the study of air pollution, and the setting of limits on emissions and on specific air pollution components. The National Ambient Air Quality Standards set limits for six primary air pollutants: carbon monoxide (CO), lead, nitrogen dioxide (NO2), ozone (O3), sulfur dioxide (SO2), and particulate matter (PM). The main sources of CO are motor vehicles and industrial boilers, while NO2 derives from motor vehicles, construction equipment, electric utilities and industrial broilers. O3 is formed when nitrogen oxides (NOx) react with volatile organic carbons and oxygen in the presence of heat and light. Its main sources are vehicles, factories and electric utilities. Lead is still released when garbage containing this metal is burned, while SO2 is formed when fuel containing sulfur is burned, mostly in industrial facilities. PM is a complex mixture of acids, organic chemicals, metals and soil or dust particles (Anderson et al., 2012), which can be of natural sources or of man-made sources. The former includes volcanoes, fires, or dust storms, while the latter includes combustion in mechanical and industrial processes, tobacco smoke, and vehicle emissions (Anderson et al., 2012). PM is defined by its aerodynamic equivalent diameter, with particles with the same diameter tending to have similar settling velocity; PM10 is comprised of particles <10 μm in diameter, while PM2.5 represents particles <2.5 μm in diameter. PM with a diameter between 2.5 and 10 is defined as “coarse”, while PM with diameter < 2.5 is defined as “fine”. Of much relevance (particularly related to neurotoxicity) is also ultrafine PM (UFPM or PM0.1, with diameter < 100 nm). It is important to underline the fact that PM10 represents all particles <10 μm in diameter, and thus contains coarse, fine and ultrafine PM fractions. Ambient air pollution is continuously monitored by regulatory agencies in various countries (e.g. the Environmental Protection Agency (EPA) in the U.S.A.). However, only PM2.5 and PM10 are monitored, while PM0.1 is not. It is important to note that the current regulations are “mass-based” and thereby the contribution of UFPM to total PM may be negligible. Given the increasing evidence for UFPM neurotoxicity, including neurodevelopmental and neurodegenerative disorders (Calderon-Garciduenas, Reynoso-Robles, & Gonzalez-Maciel, 2019; Cory-Slechta et al., 2019), future regulation of PM0.1 should consider alternatives to the mass-based approach, such as one based on the number of particles.
Furthermore, the specific composition of PM is not monitored. This latter issue may be relevant, as the assumption that PM2.5 or PM10 from different sources have equivalent biological activity has received little evaluation and may not be true. Indeed, significant variations (up to two orders of magnitude) in relative composition of PM have been found (Forman & Finch, 2018) with largely different biological activities. In the E.U., air quality has been addressed by a series of directives going back to the mid-1980s. Directive 2008/50/EC on ambient air and cleaner air for Europe merges most legislation into a single directive. Air quality standards for the primary air pollutants in the U.S.A. and the E.U. are shown in Table 1.
Table 1.
Air quality standards in the U.S. and in the E.U.
| Pollutant | Averaging period | U.S. | E.U. |
|---|---|---|---|
| Sulfur dioxide | 1 h | 75 ppb | 350 μg/m3 |
| Carbon monoxide | 8 h | 10 mg/m3 | 10 mg/m3 |
| Ozone | 8 h | 0.07 ppm | 120 μg/m3 |
| Nitrogen dioxide | 1 h | 100 ppb | 200 μg/m3 |
| PM2.5 | 1 year | 15 μg/m3 | 25 μg/m3 |
| PM10 | 1 day | 150 μg/m3 | 50 μg/m3 |
| Lead | 1 year | 0.15 μg/m3 | 0.5 μg/m3 |
The Air Quality Index (AQI) is a measure of air pollution, and provides information on levels of air pollutants, and in some cases on their potential adverse effects. In the U.S.A., the AQI is based on the EPA’s five primary pollutants (the six indicated earlier minus lead) and ranges from 0 to 500, with increasing levels of concerns and a different coloring scheme for each level, for easy comprehension by the general population (Table 2). In many urban areas in some countries (e.g. India or China) the AQI could remain above 200 for several days and even exceed it. During the Summer of 2018, the AQI in the city of Seattle, Washington, U.S.A., normally at or below 50, increased for several days to 150–200, because of raging wildfires on the West Coast. Particularly in urban areas, traffic-related air pollution (TRAP) is a major contributor to global air pollution, and diesel exhaust (DE) is one of its most important components (Ghio, Smith, & Madden, 2012). Indeed, as global population increases, anthropogenic pollutants resulting from increased urban density and transportation demands threaten to compromise air quality as never before (Cramer, 2002). In certain megacities such as New Delhi, where use of diesel-powered vehicles predominates, it is estimated that traffic may contribute to up to 72% of air pollution (Goyal, Ghatge, Nema, & Tamhane, 2006). Despite abundant alternative energy sources, diesel remains a fuel of choice for mass transit vehicles, passenger vehicles, heavy machinery, and freight conveyances, resulting in DE being considered as a major contributor to TRAP (Verboven, 2002).
Table 2.
Air Quality Index (AQI) in the U.S.A.
| AQI values | Levels of health concerns | Color |
|---|---|---|
| 0–50 | Good | Green |
| 51–100 | Moderate | Yellow |
| 101–150 | Unhealthy for sensitive groups | Orange |
| 151–200 | Unhealthy | Red |
| 201–300 | Very Unhealthy | Purple |
| 301–500 | Hazardous | Maroon |
DE is a major constituent of ambient PM, particularly PM2.5 and UFPM (USEPA, 2002), and DE exposure is often utilized as a measure of TRAP. DE contains more than forty toxic air pollutants, including nitrogen and sulfur oxides, CO, hydrocarbons, volatile organic compounds (VOCs), metals, organic and elemental carbon, and PM in a variety of sizes, with its makeup varying by engine load conditions and fuel composition (Cooney and Hickey, 2008; Lim, Lim, & Yu, 2009). In recent years, improved diesel engine technology and the use of specialized catalytic converters have changed the composition of DE, resulting in an output lower in concentration of coarse fraction particulates, nitrogen oxides, volatile organic compounds, and carbon monoxide (Su et al., 2008). Additionally, more stringent air quality guidelines in certain localities have reduced allowable sulfur content of diesel fuel, resulting in a lower output of sulfur oxides (Stanislaus, Marafi, & Rana, 2010). However, the content of PM2.5 and of UFPM is estimated to be unchanged, and the inflammatory potential of DE particulates from these low emission engines is increased, due to the higher proportion of fine particulates (Su et al., 2008). It is precisely these fine particulates that are most concerning, as they are capable of penetrating the epithelium of the lungs and the olfactory mucosa, where they can enter into general circulation in the body (Oberdörster et al., 2004).
Inhalation is the main route of exposure to air pollutants. The larger particles (PM10) are filtered out by the nose and upper airways, while PM2.5 are deposited primarily in the lungs, with some ingested after mucociliary transport into the throat (Forman & Finch, 2018). PM2.5 can enter the olfactory epithelium and are transported into the olfactory bulb, further travelling to the olfactory cortex and other brain regions (Ajmani, Suh, & Pinto, 2016). A series of studies by Oberdörster et al. (Oberdörster et al., 2004; Oberdörster & Utell, 2002) has shown clearly that UFPM also enters the brain through the olfactory nerves and distribute to other regions such as the cerebral cortex and the cerebellum. Once PM reaches the lung, it can translocate to the blood. Though lung to blood translocation is not very efficient (Forman & Finch, 2018), some PM can also reach the brain, and certainly other organs, via this pathway. A study by Maher et al. (2016) showed that magnetite nanoparticles abundant in PM pollution were present in brain from individuals from the Mexico City Metropolitan area, even at a young age.
Air pollution is considered “one of the great killers of our age”, as it was responsible in 2015 for 6.4 million deaths worldwide, of which 2.8 million were from household air pollution and 4.2 million from ambient air pollution (Landrigan, 2017). In the same year, air pollution was responsible for 19% of cardiovascular deaths worldwide, 21% of stroke deaths, and 23% of lung cancer deaths (Landrigan, 2017). While household air pollution has been declining since the 1990s, due to the changing of heating and cooking fuels (e.g. from wood or dung to gas or electricity), this has been offset by increases in ambient air pollution with deaths increasing mostly in rapidly industrializing countries.
The first and most studied target for air pollution toxicity are the air-ways. Symptoms such as nose and throat irritation, bronchoconstriction and dyspnea are common in individuals with pre-existing respiratory conditions upon acute exposure to high levels of air pollution as well as chronic exposures. These symptoms are believed to be primarily due to SO2 and NOx (Kampa & Castanas, 2008). PM that penetrates the alveolar epithelium and ozone are responsible for lung inflammation. PM and some metals may induce emphysema and cause lung cancer (Pope & Dockery, 2006). In the case of DE exposure, a potential increase in lung tumors has also been suggested (Benbrahim-Tallaa et al., 2012). In addition to the respiratory system, the cardiovascular system has emerged in the past two decades as an important target for air pollution toxicity (Brook et al., 2010; Brook & Rajagopalan, 2007). Systemic inflammatory changes caused by air pollution affect blood coagulation, atherosclerosis progression, and are associated with consistent increased risk for cardiovascular events (Brook et al., 2010; Brook & Rajagopalan, 2007; Pope & Dockery, 2006). Other systems (e.g. urinary, digestive, etc.) can also be affected by exposure to high levels of air pollution (Kampa & Castanas, 2008), with increasing evidence indicating the Central Nervous System (CNS) as a primary important target.
2. The central nervous system as a target for air pollution
Inhaled UFPM may enter the CNS either by way of the olfactory nerve, or through the tight endothelial junctions that comprise the vascular interface of the blood-brain barrier, which may be rendered more permeable by higher levels of peripheral oxidative stress and inflammation (Lochhead et al., 2010). Increased peripheral inflammation and macrophage activation may also exacerbate existing neuroinflammation, as these conditions can result in increased permeability of the blood-brain barrier to allow both the migration of activated macrophages and the passive diffusion of pathogens and other foreign bodies into the CNS (Elwood, Lim, Naveed, & Galea, 2017; Lochhead et al., 2010).
In recent years evidence from human epidemiological and animal studies supports that air pollution may negatively affect the CNS and contribute to CNS diseases (Calderon-Garciduenas et al., 2002; Block & Calderon-Garciduenas, 2009; Genc, Zadeoglulari, Fuss, & Genc, 2012; Block et al., 2012; Costa et al., 2014; Costa et al., 2017a; Sram, Veleminsky, Veleminsky, & Stejskalova, 2017; Russ, Reis, & van Tongeren, 2019). PM2.5 and UFPM are of particular concern, as these particles can enter systemic circulation and distribute to the brain and other organs (Genc et al., 2012; Oberdörster et al., 2004; Oberdörster & Utell, 2002), as well as gain direct access to the brain through the nasal olfactory mucosa (Garcia, Schroeter, & Kimbell, 2015; Lucchini, Dorman, Elder, & Veronesi, 2012; Oberdörster et al., 2004; Peters et al., 2006). Decreased cognitive function, olfactory dysfunction, auditory deficits, depressive symptoms and other adverse neuropsychological effects have been reported in humans ( Calderon-Garciduenas et al., 2011, 2010; Fonken et al., 2011; Freireet al., 2010; Guxens & Sunyer, 2012; Lee et al., 2019; Petkus et al., 2019; Ranft, Schikowski, Sugiri, Krutmann, & Kramer, 2009). Controlled acute exposure to DE (300 μg/m3) has been shown to induce EEG changes (Crüts et al., 2008). In highly exposed individuals, post-mortem investigations have revealed increased markers of oxidative stress and neuroinflammation (Calderon-Garciduenas et al., 2008; Calderon-Garciduenas et al., 2011; Calderon-Garciduenas et al., 2012; Levesque et al., 2011b).
Animal studies corroborate the human observations (Costa et al.,2014). For example, dogs exposed to heavy air pollution in Mexico City presented evidence of chronic inflammation and neurodegeneration in various brain regions (Calderon-Garciduenas et al., 2002, 2003), and mice exposed to traffic in a highway tunnel had higher levels of pro-inflammatory cytokines in the brain (Bos et al., 2012). Exposure of rats to PM1.0 (16.3 μg/m3, with particle number of 11,257/m3) for 3 to 6 months, caused microglia activation in the hippocampus (Bai et al., 2019). Controlled exposure to DE has been reported to alter motor activity, spatial learning and memory, novel object recognition ability, and emotional behavior, as well as to cause oxidative stress and neuro-inflammation in the CNS (MohanKumar, Campbell, Block, & Veronesi, 2008; Gerlofs-Nijland et al., 2010; Win-Shwe & Fujimaki, 2011; Levesque, Taetzsch, et al., 2011b; Ehsanifar et al., 2019). Changes in the expression of genes related to antioxidant defenses and to inflammation have been reported in the brain of rats exposed to biodiesel fuels for 7 or 28 days (Valand et al., 2018). A three-week exposure to DE (250 μg/m3, 6 h/day, 5 day/week) was found to increase lipid peroxidation and expression of pro-inflammatory cytokines in different mouse brain regions (Dao et al., unpublished data). Even an acute exposure to DE (250 μg/m3 for 6 h) has been shown to cause microglia activation, oxidative stress, neuroinflammation, and to impair adult neurogenesis (Coburn, Cole, Dao, & Costa, 2018; Cole et al., 2016). In most cases the effects were more pronounced in males than in females (Cole et al., 2016; Roqué, Dao, & Costa, 2016; Coburn et al., 2018), a finding that may be related, among others, to sex differences in microglia (Schwartz & Bilbo, 2012), or to the differential expression of antioxidant and anti-inflammatory genes (Giordano et al., 2013; Torres-Rojas & Jones, 2018).
Evidence that air pollution affects the CNS, in addition to its more studied peripheral targets, is undeniable, and supported by dozens of human and animal studies. Of much interest is whether air pollution may be causally associated with neurological or neuropsychiatric disorders, particularly neurodevelopmental and neurodegenerative diseases, and this is discussed in the following sections, together with consideration on possible underlying mechanisms.
3. Developmental neurotoxicity of air pollution and its possible role in neurodevelopmental disorders
Epidemiological and animal studies suggest that young individuals may be particularly susceptible to air pollution-induced neurotoxicity (Calderon-Garciduenas et al., 2008; Calderon-Garciduenas et al., 2011; Calderon-Garciduenas et al., 2012; Freire et al., 2010; Guxens et al., 2014; Guxens & Sunyer, 2012). Studies in Mexico City have revealed elevated levels of neuroinflammatory markers in the brain of children exposed to high air pollution, as well as cognitive deficits (Calderon-Garciduenas et al., 2008; Calderon-Garciduenas et al., 2013, 2011). Newman et al. (2013) reported hyperactivity in 7-year old children associated with early life exposure to traffic-related air pollution. A retrospective cohort study in Catalonia, Spain, also found an association between air pollution (assessed as living <300 m from a motorway) and incidence of attention deficit hyperactivity disorder (ADHD) (Saez, Barcelo, Farrerons, & Lopez-Casasnovas, 2018). In contrast, a large study with eight European population-based birth/child cohorts reported no association between air pollution exposure and ADHD (Forns et al., 2018), and negative findings were also reported in a study in Sweden (Oudin et al., 2019). A recent systematic review concluded that the association between air pollution and ADHD is weak, and that further high-quality studies are needed to clarify this association (Donzelli, Llopis-Gonzalez, Llopis-Morales, Cioni, & Morales-Suarez-Varela, 2020). In six European cohorts, exposure to air pollution during pregnancy was found to be associated with delayed psychomotor development (Guxens et al., 2014). A recent study reported that exposure to traffic-related air pollution is inversely associated with sustained attention in adolescents (Kicinski et al., 2015) and was shown to lower cognitive development in primary school children (Sunyer et al., 2015). The BREATHE project in Catalonia, Spain, found that exposure to TRAP was associated with behavioral problems in children, including cognitive development (Forns et al., 2017, 2016), and that the APOEε4 genotype represented a risk factor for some of them (Alemany et al., 2018). Another recent study in the U.K. reported that children with intellectual disabilities were found to be significantly more likely to live in areas with high levels of DE-PM, NO2 and CO (Emerson, Robertson, Hatton, & Baines, 2018).
Experimental studies support the hypothesis that air pollution may be a developmental neurotoxicant. Ema, Naya, Horimoto, and Kato (2013) indicated that developmental exposure to DE may cause toxicity and neurotoxicity. In utero exposure to high levels of DE (1.0 mg/m3) caused alterations in motor activity, motor coordination and impulsive behavior in male mice (Suzuki et al., 2010; Yokota et al., 2009, 2013). Early postnatal exposure of mice to concentrated ambient PM was reported to cause behavioral changes (enhanced bias towards immediate rewards), as well as long-term impairment of short-term memory and impulsivity-like behavior (Allen et al., 2013; 2014a). Additional studies in mice showed that post-natal administration (PND4–7 and 10–13, for 4 h/day) of DE-PM (~100 μg/m3) caused changes in GFAP expression in various brain regions (Morris-Schaffer et al., 2019), while a lower exposure UFPM (~45 μg/m3) caused male-specific learning and memory dysfunctions (Cory-Slechta, Allen, Conrad, Marvin, & Sobolewski, 2018). Depression-like responses were found in mice exposed prenatally to urban air nanoparticles (Davis et al., 2013). Additional studies have shown that developmental DE exposure of mice alters motor activity, spatial learning and memory, and novel object recognition ability, and causes changes in gene expression, neuroinflammation, and oxidative damage (Hougaard et al., 2008; Hougaard, Saber, Jensen, Vogel, & Wallin, 2009; Tsukue, Watanabe, Kumamoto, Takano, & Takeda, 2009; Win-Shwe, Yamamoto, Fujitani, Hirano, & Fujimaki, 2008; Win-Shwe et al., 2014). In another study, rats were exposed to PM0.2 (340 μg/m3) during gestation and until 25 weeks of age; male rats exhibited activated microglia, impaired hippocampal neurogenesis, and alteration of the blood-brain-barrier, together with behavioral symptoms of depression and deficits in contextual memory (Woodward et al., 2018). In rabbits, Bernal-Melendez et al. (2019) found that prenatal exposure to DE (1 mg/m3) from gestational day 3 to 27, caused changes in olfactory bulb morphology and in olfactory-based behaviors in the offspring, together with alterations in monoaminergic neurotransmission.
Altogether, findings in human and in multiple animal models indicate that exposure to air pollution may damage the developing brain and potentially contribute to neurodevelopmental disorders. A major neurodevelopmental disorder is autism spectrum disorder (ASD), and evidence from human epidemiological and from controlled animal studies suggests that air pollution may be associated with its etiology. The following sections will thus focus on ASD and on the possible contribution of air pollution to this disorder.
3.1. Autism spectrum disorder
Autism is a neurodevelopmental disorder characterized by marked reduction of social and communicative skills, and by the presence of stereotyped behaviors (Levy, Mandell, & Schultz, 2009; Sharma, Gonda, & Tarazi, 2018). The term ASD is usually utilized to include autism and a range of similar disorders. The symptoms of ASD are present before the age of three, and are often accompanied by abnormalities of cognitive functioning, learning, attention, and sensory processing (Levy et al., 2009). The incidence of ASD seems to have increased in the past few decades, and it is now estimated at about 1 in 36 children, up from 1 in 150 children in 2000 (Boyle et al., 2011; Wingate et al., 2012; Sharma et al., 2018; Xuet al., 2018). While this prevalence is similar across ethnic and socioeconomic background, a significant sex difference exists, with a prevalence 4–5 times higher in boys than in girls (Schaafsma & Pfaff, 2014; Sharma et al., 2018; Xu et al., 2018). The economic burden of caring for an individual with ASD and the associated intellectual disability during his or her lifespan has been estimated at $2.4 million (Buescher, Cidav, Knapp, & Mandell, 2014). The etiological basis of ASD is unknown, and susceptibility is attributable to both genetic and environmental factors (Gaugler et al., 2014; Hallmayer et al., 2011; Kalkbrenner, Schmidt, & Penlesky, 2014; Landrigan, 2010; Levy et al., 2009; Lyall, Schmidt, & Hertz-Picciotto, 2014; Rossignol, Genuis, & Frye, 2014). Candidate susceptibility genes for ASD have been identified, but no single anomaly appears to predominate, with the total fraction of ASD attributable to genetic inheritance expected to approach only about 30–50% (Gaugler et al., 2014; Sandin et al., 2014). Studies of ASD concordance in monozygotic and dizygotic twins suggest a moderate genetic heritability and a substantial environmental component (Hallmayer et al., 2011). Thus, ASD likely results from the complex interactions among multiple genes conferring vulnerability and diverse environmental factors (Hertz-Picciotto, Schmidt, & Krakowiak, 2018). Among environmental chemicals and drugs potentially associated with ASD, in addition to air pollution (discussed below), the following have been reported: pesticides (organophosphates, pyrethroids), phthalates, polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), and CNS drugs (valproic acid, antidepressants) (Hertz-Picciotto et al., 2018; Sharma et al., 2018). Of much interest are the findings of an association between maternal infection and ASD (Minakova & Warner, 2018; Patterson, 2011). Studies in various animal species have demonstrated that maternal immune activation (MIA) increases neuroinflammation in the placenta and in the fetal brain, leading to offspring which display ASD-like behaviors (Bauman et al., 2014; Malkova, Yu, Hsiao, Moore, & Patterson, 2012).
Children with ASD present various morphological abnormalities in the brain (Gilbert & Man, 2017; Kulesza, Lukose, & Stevens, 2011; Levy et al., 2009; Stoner et al., 2014; Wegiel et al., 2010), and alterations in certain neurotransmitter systems (e.g. GABA, glutamate, serotonin) (Lam, Aman, & Arnold, 2006; Coghlan et al., 2012; Yang et al. 2014). There is a strong association between decreased reelin, a glycoprotein which modulates neuronal migration in the developing CNS, and ASD (Fatemi et al., 2005; Folsom & Fatemi, 2013). DNA methylation is also altered in the autistic brain, suggesting that epigenetic dysregulation may contribute to ASD (Berko & Greally, 2015; Nardone et al., 2014). Increasing evidence indicates that children with ASD have higher levels of oxidative stress (Frustaci et al., 2012; Napoli, Wong, & Giulivi, 2013; Rose et al., 2012), as well as microglia activation, neuroinflammation and increased systemic inflammation (Chez, Dowling, Patel, Khanna, & Kominsky, 2007; Morgan et al., 2010; Ashwood et al., 2011; El-Ansary & Al-Ayadhi, 2012; Tetreault et al., 2012; Theoharides, Asadi, & Patel, 2013; Suzuki et al., 2013; Depino, 2013).
3.2. Air pollution and autism spectrum disorder
Several epidemiological studies have found associations between exposures to TRAP and ASD (Costa, Cole, et al., 2017a). Two studies in California by Volk, Hertz-Picciotto, Delwiche, Lurmann, and McConnell (2011); Volk, Lurmann, Penfold, Hertz-Picciotto, and McConnell (2013) found that residential proximity to freeways and gestational and early-life exposure to traffic-related air pollution were associated with ASD (OR = 1.86; 95% CI = 1.04–3.45). Similar results were obtained in another epidemiological study in California (Becerra, Wilhelm, Olsen, Cockburn, & Ritz, 2013). Furthermore, another study pulling from the Nurses’ Health Study II, found that perinatal exposure to DE was significantly associated with ASD, particularly in boys (Roberts et al., 2013). A similar boy-specific association between PM2.5 exposure and ASD was reported in a large Southern California pregnancy cohort (Jo et al., 2019). Two studies in Taiwan and in Pennsylvania also reported an increased risk of ASD associated with PM (Jung, Lin, & Hwang, 2013; Talbott et al., 2015). Additionally, a study in two cohorts in North Carolina and California reported an association between PM exposure and ASD, particularly when exposure occurred in the third trimester of pregnancy (Kalkbrenner et al., 2015). The higher susceptibility of third trimester exposure was also evidenced by a study of Raz et al. (2015) in the Nurses’ Health Study II cohort. A population-based cohort study in Vancouver (British Columbia, Canada) reported an association between exposure to NO (10 ppb) and ASD but not for NO2 (10 ppb) or PM2.5 (10 μg/m3) (Pagalan et al., 2019), and an association between O3 (~40 ppb) and PM2.5 (~15 μg/m3) exposure and ASD was found in another study in metropolitan Cincinnati, Ohio (Kaufman et al., 2019). In a Swedish cohort, Oudin et al. (2019) found an association between maternal exposure to NOx (mean 17.7 μg/m3, range 3.5–48.6 μg/m3) and ASD. In Shanghai, China, Chen et al. (2019) found that exposure to PM1 (49 μg/m3), PM2.5 (66 μg/m3), and PM10 (95 μg/m3) during the first three years of life significantly increased the risk of ASD. A recent multisite case-control study reported a significant association between ASD and PM2.5 exposure during the first year of life (OR = 1.3; 95% CI = 1.0–1.6) and between ASD and O3 during the third trimester (OR=1.2; 95% CI 1.1–1.4) (McGuinn et al., 2020). Though these cohort and case-control studies show moderate to strong associations between exposure to air pollution in utero and/or early in life and risk of ASD, some have significant limitations due to the exposure matrix and multiple confounding factors (Fordyce, Leonhard, & Chang, 2018). A recent systematic review and meta-analysis concluded that there is evidence for an association between maternal exposure to PM2.5 and ASD, only weak evidence for NO2 exposure, and little evidence for PM10 or O3 exposure (Chun, Leung, & McDonald, 2020). A recent study in Tehran, Iran, has reported a lack of association between air pollution and ASD (Yousefian et al., 2018); however, only PM10, SO2 and various solvents were measured in air.
Notwithstanding the limitations of human epidemiological studies, animal studies in general agree with the positive human observations (Costa et al., 2014; Costa, Chang, & Cole, 2017b; Costa, Chang, & Cole, 2017b). Prenatal and early life exposure of mice to DE is associated with behaviors like those present in humans with ASD. These include higher levels of motor activity, elevated levels of self-grooming, and increased rearing (Thirtamara Rajamani et al., 2013). Postnatal exposure to concentrated ambient ultrafine particles [on postnatal day (PND) 4–7 and 10–13] caused persistent glial cell activation (both microglia and astrocytes), and ventriculomegaly (lateral ventricular dilation), which occurred preferentially in male mice (Allen et al., 2014b; Allen et al., 2014a). Changes in cytokines and neurotransmitters, which were brain region- and sex-specific were also found in these latter studies (Allen, Liu, Weston, et al., 2014a). These investigators also reported a decreased corpus callosum in both male and female mice, and an increase of glutamate levels, with an excitatory/inhibitory imbalance, all relevant to ASD (Allen et al., 2017). In addition, they also reported male-specific alterations in social novelty preferences (an indication of low sociability) and a reduction in testosterone levels (Sobolewski et al., 2018). Tachibana et al. (2015) found that prenatal exposure to DE disrupts DNA methylation in the brain. Prenatal exposure to UFPM (mean 93 μg/m3; range 33–184 μg/m3) from gestational day (GD) 0.5 to GD16.5 caused a hypermyelination of the cerebellum in male mice only, accompanied by ultrastructural abnormalities and an increase in iron content (Klocke et al., 2018). These effects are also seen in ASD (Fatemi et al., 2012).
Chang, Cole, and Costa (2018) found that perinatal exposure of mice to DE at environmentally relevant concentrations (250–300 μg/m3, from GD 0 to PND 21) caused significant behavioral deficits in the domains of persistent/repetitive behaviors (T-maze and marble burial tests), communication (isolation-induced ultrasonic vocalization and responses to social odors), and social interactions (reciprocal interaction and social novelty tests). Interestingly, the effects of developmental DE exposure were more robust if exposure occurred in both the pre-natal (GD 0 - birth) and post-natal (PND 1–21) periods, in agreement with observations in humans. Indeed, considering the differences in relative rate of brain development between mice and humans, studies indicated that the association between air pollution and ASD is stronger when exposure occurs in the third trimester of pregnancy or early postnatally (Chen et al., 2019; Kalkbrenner et al., 2015; Raz et al., 2015; Rich et al., 2015; Schembari et al., 2015). In a follow-up study, Chang, Daza, Hevner, Costa, and Cole (2019) reported neuroinflammation [increase in interleukin-6 (IL-6)] and reduced expression of reelin in the cerebral cortex of DE-exposed mice, together with subtle alterations in cortical layering of the somatosensory cortex, as also found in ASD (Stoner et al., 2014). In summary, there is suggestive evidence from human epidemiological studies that in-utero and early-life exposure to air pollution and to TRAP is associated with an increased risk of ASD. Controlled animal studies also indicate that developmental exposure to TRAP induces behavioral, biochemical, and morphological effects that are also seen in ASD.
4. Air pollution and neurodegenerative disorders
Aging is associated with a wide variety of clinical and pathological conditions which can be classified generally as neurodegenerative diseases. There is evidence that the aging brain may be particularly susceptible to air pollution-induced neurotoxicity, as epidemiological studies have identified significant behavioral effects, particularly cognitive effects, associated with high air pollution exposure in the elderly (Chen et al., 2015; Power et al., 2011; Ranft et al., 2009; Weuve et al., 2012). Examples of neurodegenerative diseases are Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and Huntington’s disease (HD), with AD and other dementias being the most common, followed by PD (Musgrove, Jewell, & Di Monte, 2015). The main risk factor for neurodegenerative diseases is indeed represented by aging. In the U.S.A., the number of individuals with AD dementia is expected to increase from the current 5 million to about 14 million in 2050 due to the aging of the population (Hebert, Weuve, Scherr, & Evans, 2013). Similarly, in the E.U. it is estimated that by 2060 one third of the population will be aged 65+, and the number of people with various dementias, currently around 10 million, is expected to double (Maresova, Klimova, Novotny, & Kuca, 2016). Most neurodegenerative diseases have complex clinical and pathological pictures, and for most, their etiology is unknown (Musgrove et al., 2015). In some cases, relevant susceptibility genes have been identified, but environmental factors or gene-environment interactions are believed to play a very important role in most cases. The following sections discuss the association of air pollution exposure with AD and PD in more detail, as these neurodegenerative diseases represent the most common ones, and those for which most evidence is available of a potential relationship with air pollution (Costa, 2017).
4.1. Alzheimer’s disease
AD is the main type of dementia present in individuals over 65, accounting for nearly two thirds of all dementia cases (Prince et al., 2013), and is one of the great health care challenges of the 21st century (Scheltens et al., 2016). AD is the prototypical dementia, with disturbances starting in the domain of episodic memory, followed by deficits in language, attention, and executive functions. The number of cases in the world is predicted to reach 115 million by 2050. Other common types of dementia include vascular dementia, whose causes are found in cardiovascular pathology and which presents with clinical signs involving deficits in attention, information processing, and executive function (O’Brien & Thomas, 2015), as well as dementia with Lewy bodies, with clinical signs including hallucinations, Parkinsonism, and altered cognitive status (Walker, Possin, Boeve, & Aarsland, 2015). The brain of AD patients presents diffuse cortical and hippocampal atrophy, and accumulation of abnormally folded amyloid beta (Aβ) and of tau proteins in amyloid plaques and neuronal tangles, which are the neuropathological hallmarks of AD (Khan & Bloom, 2016; Selkoe & Hardy,2016). Amyloid precursor protein (APP) is cleaved by secretases to generate an Aβ polypeptide Aβ42 or Aβ40 with the former being the major form found in amyloid plaques (Pressman & Rabinovici, 2014). Neurofibrillary tangles are composed of hyperphosphorylated tau protein, which cause disassembly of microtubules. Rare mutations in a few genes such as APP, PSEN1 and PSEN2 (presenilins, which provide the catalytic subunit to the secretase which cleaves APP) confer a high risk for AD (Scheltens et al., 2016). However, the major genetic risk factor for AD is apolipoprotein E (APOE); of its three isoforms, APOEε4 predisposes the carrier to AD (Riedel, Thompson, & Brinton, 2016). Indeed, the prevalence of APOEε4 carriers in AD cases is about 60%, while APOEε4 homozygotes are about 10–15%, particularly in Northern Europe (Ward et al., 2012).
4.2. Air pollution and cognitive decline
In the past few years, various epidemiological studies have investigated the possible contribution of air pollution to AD (Cipriani, Danti, & Borin, 2018; Killin, Starr, Shiue, & Russ, 2016; Paul, Haan, Mayeda, & Ritz, 2019; Power, Adar, Yanosky, & Weuve, 2016). Several studies have focused on various assessments of cognitive impairment, as this is an initial important aspect of AD (Clifford, Lang, Chen, Anstey, & Seaton, 2016; Cohen & Gerber, 2017; Peters, Peters, Booth, & Mudway, 2014). Many of these studies have been carried out in the elderly (>65 years), and with few exceptions, have found that increasing levels of air pollution, particularly TRAP, was associated with diminished cognitive abilities (Costa, 2017). For example, Ranft et al. (2009) reported that in women over 65 years old, exposure to PM2.5 and PM10 was associated with increased mild cognitive impairment (MCI). Power et al. (2011) found a strong association between TRAP and decreased cognitive function in older men; additionally, residing near major roadways was associated with poor performance in a series of cognitive tests in a New England population of over 65 years (Wellenius et al., 2012). Higher levels of PM2.5 were associated with faster cognitive decline in the Nurses’ Health Study Cognitive Cohort, which included almost 20,000 women aged 70 to 81 years (Weuve et al., 2012). In two separate studies, Ailshire and Crimmins (2014) and Ailshire and Clarke (2015) reported that PM2.5 was associated with decreased cognitive function in adults 50 years or older. Similar findings were reported for populations in the U.K. (Tonne, Elbaz, Beevers, & Singh-Manoux, 2014), Los Angeles (Gatto et al., 2014), Germany (Tzivian et al., 2016), and China (Zeng, Gu, Purser, Hoenig, & Christakis, 2010). A recent study in older (> 75 years) U.S. women found an increased risk of cognitive decline and of dementia with increasing exposure to PM2.5 (Cacciottolo et al., 2017). A study in a population of Puerto Rican adults (age 45–75 years) in the Greater Boston area, showed an association between long-term exposure to black carbon and nickel (tracers of traffic and oil combustion, respectively), and a decrease in cognitive function (Wurth et al., 2018). Thus, positive association between TRAP and MCI were found in multiple studies in different parts of the world, though two negative studies, both in the U.S. (Loop et al., 2013; Wilker et al., 2016,) found no association with memory impairment. Overall, the evidence to date is sufficiently consistent in indicating that exposure to air pollution is associated with cognitive decline in the elderly. Unfortunately, controlled animal studies in this area are still limited, and epidemiological studies present some limitations (e.g. endpoint measured, different indices of air pollution), as recently underlined (Clifford et al., 2016; Peters et al., 2015).
4.3. Air pollution and Alzheimer’s disease
Several other epidemiological studies have focused on the specific association between air pollution and AD and other dementias, rather than only cognitive impairment (Kilian & Kitazawa, 2018; Shou, Huang, Zhu, Liu, & Hu, Y 7 Wang, H., 2019). In Taiwan, three studies have reported associations between air pollution and AD. Chang et al. (2014) found an increased risk of AD with increasing NO2 and CO. Jung, Lin, and Hwang (2015) reported a significant risk for AD associated with O3 and PM2.5. A third case-control study by Wu et al. (2015) found that high PM10 or O3 exposures were associated with AD and vascular dementia (OR = 4.17 and 2.00, respectively). In Germany, exposure of older women to PM2.5 and to NOx was associated with increased risk of AD (Schikowski et al., 2015), and in Sweden, Oudin et al. (2016) reported that TRAP was a risk factor for AD and vascular dementia, a finding later confirmed in the same population, with estimates of NOx exposure of ~26 μg/m3 (Andersson, Oudin, Sundstrom, Forsberg, & Adolfsson, 2018). In addition, a large population-based cohort study in Canada (involving about 2.2 million individuals aged 55–85 years), found an HR for dementia for people living less than 50 m (164 ft) from a major traffic road of 1.07–1.12, suggesting that 7–11% of dementia cases in patients who live near major roads is attributable to traffic exposure (Chen et al., 2017). PM2.5 and NO2 were positively associated with dementia, but a contribution from other factors, particularly noise, could not be ruled out (Chen et al., 2017). Using a different approach, Zanobetti, Dominici, Wang, and Schwartz (2014) and Kioumourtzoglou et al. (2016) in the U.S. examined the rate of hospitalization for AD and short- or long-term exposure to PM2.5, exposure to which was associated with a significant increase in hospitalization for AD in both studies. Two studies in the E.U. examined occupational exposure to DE and risk of AD and dementia; Helou and Jaecker (2014) found that occupational exposure to DE and to other fuels and certain petroleum-based solvents was associated with an increased risk of AD and of mixed-type dementia among French workers, while Koeman et al. (2015) reported no associations between occupational exposure to DE and solvents and non-vascular dementia in Dutch workers.
In a limited number of cases, it has been possible to examine the brains of individuals exposed to air pollution, and these findings are useful to design and compare with animal studies. In autopsy samples from children, young adults or middle-aged individuals from the highly polluted Mexico City metropolitan area, several studies by Calderon-Garciduenas found increased markers for AD in various brain regions. In an initial study (Calderon-Garciduenas et al., 2004), increased Aβ42 levels were found in frontal cortex and hippocampus of individuals exposed to air pollution, together with increased levels of cyclooxygenase-2 (COX2), an indicator of neuroinflammation which is induced by pro-inflammatory cytokines and is increased in AD. In a follow-up study, similar findings were also reported in olfactory bulb and frontal cortex of young adults from Mexico City; Aβ42 was detected in 58% of young individuals and in 80% of adults (Calderon-Garciduenas et al., 2008). A particularly jarring study in a group of children and young adults (average age = 13 years), found that 51% had Aβ diffuse plaques compared to 0% in controls, and 40% exhibited tau hyperphosphorylation (Calderon-Garciduenas et al., 2012). In agreement with these findings, levels of Aβ42 were significantly decreased in cerebrospinal fluid (CSF) from highly exposed Mexico City children (Calderon-Garciduenas et al., 2016a). This is alarming, as decreases in CSF Aβ42 are considered a very early change in AD, which precede aggregation and deposition of Aβ42 in plaques and may occur decades before the onset of symptoms (Blennow et al., 2015). More recently, Calderon-Garciduenas et al., 2018b) reported higher levels of non-phosphorylated tau (a marker of axonal damage and of AD axonal pathology) in the CSF of 507 children and young adults (age 12.8 ± 6.7) exposed to high air pollution in Mexico City. In the same highly polluted metropolitan Mexico City area, significant increases of hyperphosphorylated tau and of amyloid plaques were found in the olfactory bulb of infants, children, teens and adults under 40 years (Calderon-Garciduenas et al., 2018a). In addition to these studies on early-life exposure and AD, a recent study in older females concluded that late-life exposure to PM2.5 was associated with an accelerated decline in episodic memory, affecting immediate recall and new learning, and was paralleled by an increased incidence of AD pattern similarity, as assessed by MRI (Younan et al., 2020).
The limited biochemical evidence available from post-mortem human brains or from CSF appear to be supported by similar findings deriving from animal studies. High levels of expression of COX2 and of Aβ42 were found in brains of dogs exposed to air pollution in Mexico City (Calderon-Garciduenas et al., 2003). Controlled experimental studies showed that exposure of male rats to DE (311 or 992 μg/m3 for six months) increased levels of Aβ42 and of phosphorylated tau (pS199) in cerebral cortex (Levesque, Taetzsch, et al., 2011b). Mice exposed for a short period (3 h) to nickel nanoparticles (as a model of air pollution) had a significant increase of brain Aβ40 and Aβ42 levels (Kim et al., 2012), while rats exposed to DE nanoparticles (0.3–1.0 mg/L) for three months had higher levels of COX2 and of Aβ42 in various brain regions (Durga, Devasena, & Rajasekar, 2015). In mice exposed to a relatively low level of PM2.5 (66 μg/m3) for 9 months there was an increase in Aβ40, BACE (beta-site APP-cleaving enzyme), and COX2, as well as a decrease of APP, while phosphorylated tau was unchanged (Bhatt, Puig, Gorr, Wold, & Combs, 2015). Similar findings were also reported in vitro in immortalized mouse neurons; exposure to DE particles (50 μg/ml) caused a significant increase in levels of APP and BACE (Milani et al., 2018). Exposure to nanoparticles by inhalation for 15 weeks caused an increase in Aβ42 in brain of mice (Cacciottolo et al., 2017). In the cerebral cortex of male mice exposed for 10 weeks to DE (250 μg/m3, 6 h/day, 5 days/week), Coburn et al. (unpublished) found increases in the levels of Aβ42, phosphorylated tau (pS199) and of Dyrk1a. The latter is the dual-specificity tyrosine phosphorylation-regulated kinase-1A, a protein kinase that phosphorylates APP and tau and plays an important role in AD neuropathology (Arbones, Thomazeau, Nakano-Kobayashi, Hagiwara, & Delabar, 2019; Branca et al., 2017).
As indicated earlier, APOEε4 is the strongest genetic risk factor for AD (Riedel et al., 2016). Hence, the possibility of a gene-environment interaction has been studied by testing the hypothesis that carriers of one or two of the APOEε4 alleles would be more susceptible to the neurotoxic effects of air pollution (Calderon-Garciduenas et al., 2004). Indeed, APOEε4 carriers exposed to air pollution had greater hyperphosphorylated tau and diffuse Aβ plaques and more pronounced olfactory deficits than APOEε3 carriers (Calderon-Garciduenas et al., 2008; Calderon-Garciduenas et al., 2010; Calderon-Garciduenas et al., 2012). The effects of PM2.5, PM10, and NOx on cognitive functions were more pronounced in female carriers of the APOEε4 allele (Schikowski et al., 2015), and female APOEε4 carriers were also more at risk for air pollution-induced hippocampal metabolic alterations and cognitive deficits (Calderon-Garciduenas, Kulesza, Doty, D’Angiulli, & Torres-Jardon, 2015; Calderon-Garciduenas et al., 2016b). Cacciottolo et al. (2017) also found that the risk of cognitive decline and dementia associated with air pollution was greater in homozygotes for the APOEε4 allele (HR = 3.95), compared to those carrying the APOEε3 allele (HR = 1.65). A recent study of a cohort of older adults in northern Manhattan found that long-term exposure to ambient air pollution was associated with a more rapid cognitive decline and that the association was more pronounced in APOε4 carriers (Kulick et al., 2020). For sake of completeness it should also be mentioned that one study did not find differences between APOE genotypes in susceptibility to air pollution-associated dementia (Wu et al., 2015). The study by Cacciottolo et al. (2017) is the only animal study investigating the role of APOE polymorphism in susceptibility to air pollution-induced AD. They found that a 15-week exposure to nanoparticles increased amyloid plaques significantly more in mice carrying the human APOEε4 gene than in mice carrying the human APOEε3 gene. Given the importance of APOEε4 in AD and the availability of transgenic mouse models, further animal testing under controlled exposure conditions is certainly warranted.
4.4. Parkinson’s disease
Air pollution has also been associated with increased risk of PD, though the overall evidence appears to be somewhat less convincing than for AD (Fu, Guo, Cheung, & Yung, 2019; Kasdagli, Katsouyanni, Dimakopoulou, & Samoli, 2019). PD is a neurodegenerative disorder characterized by a progressive degeneration of dopaminergic neurons in the substantia nigra and of nerve terminals in the striatum. Once loss of dopaminergic neurons has reached about 80%, clinical signs appear, which include resting tremor, rigidity, bradykinesia, and gait disturbances (Cubo & Goetz, 2014). PD is primarily a motor system disorder; however, it also presents olfactory dysfunction, cognitive dysfunction, dementia, and depression. Olfactory dysfunction is an important early effect of PD (Doty, 2012; Mesholam, Moberg, Mahr, & Doty, 1998), and damage to the olfactory bulb seems to precede neuropathology in the motor areas (Braak, Ghebremedhin, Rub, Bratzke, & Del Tredici, 2004). Age is the main risk factor for PD, and prevalence is 1% in people above 60 and 3–5% in those over 85 years (Lema-Tomé et al., 2013); in addition, sex is also a risk factor, as incidence of PD is 90% higher in males than in females (Van Den Eeden et al., 2003). Mutations in several genes (PARK1-PARK13) have been associated with some forms of PD, usually of early onset (before the age of 50) and occurring in family clusters. The great majority of PD cases, however, are sporadic, and may be due to environmental factors or to gene-environment interactions. In addition to the dopamine deficiency resulting from degeneration of dopaminergic neurons in the substantia nigra, protein aggregations in the form of Lewy bodies in surviving neurons of adjacent areas are also a hallmark of PD (Beier & Richardson, 2015). Lewy bodies are a dense core inclusion encircled by a halo of radiating fibrils composed of misfolded α-synuclein. A single point mutation in the α-synuclein gene (PARK1/4) is responsible for the first familial form of autosomal dominant PD (Polymeropoulos et al., 1997; Ulusoy & Di Monte, 2013). Levels of α-synuclein are higher in PD brain than in normal aging (Beier & Richardson, 2015; Ulusoy & Di Monte, 2013).
4.5. Air pollution and Parkinson’s disease
A few studies have investigated the possible association between air pollution and PD (see Hu et al., 2019; Kasdagli et al., 2019 for recent reviews and meta-analyses). Kirrane et al. (2015) investigated the incidence of PD among participants in the Agricultural Health Study in two U.S. populations, and found positive associations between PM2.5 and O3 and PD in North Carolina, but not in Iowa, possibly because of lower levels of air pollution in the latter state. In another study, Liu, Young, Chen, Kaufman, and Chen (2016) found evidence of an association between PM10 and PM2.5 and PD in female never smokers (smoking has consistently been found to be a protective factor for PD). A case-control study with 1600 PD patients in Denmark suggested that air pollution from traffic sources was associated with a 9% increased risk of PD for each interquartile range increase of NO2 (2.97 μg/m3), with a higher risk for people living in Copenhagen compared to those living in rural areas (Ritz et al., 2016). In a follow-up study, it was found that in individuals with a functional polymorphism in the gene for the pro-inflammatory cytokine interleukin-1β (IL-1β-rs16944) long-term exposure to NOx increased PD risk more than in the overall population (Lee et al. 2016a). The rs16944 mutation is in the promoter region of IL-1β and causes enhanced transcription, suggesting that individuals with higher levels of IL-1β may be more susceptible to air pollution neurotoxicity. A study by Lee et al., 2016ain Taiwan also found a positive association between air pollution and PD, particularly for NOx and CO, but not for PM10. This latter negative finding agrees with the results of a study by Palacios et al. (2014) in a large (>115,000) cohort of women, part of the Nurses’ Health Study, in which no statistically significant associations were found between PM10 or PM2.5 and PD. A study examining occupational exposures as risk factor for PD found no association between DE exposure and PD mortality in a large Dutch cohort (Brouwer et al., 2015). Finally, a recent study examined the risk of PD when living near major roads in Ontario, Canada, and found no significant association (Chen et al., 2017), while two other studies found that exposure to PM2.5 was associated with a significant increase in hospitalization for PD (Kioumourtzoglou et al., 2016; Zanobetti et al., 2014).
A few studies have examined levels of α-synuclein in post-mortem brains of humans exposed to air pollution (Calderon-Garciduenas et al., 2008; Calderon-Garciduenas et al., 2010; Calderon-Garciduenas et al., 2013). For example, in brains of Mexico City children exposed to high levels of air pollution, particularly in brain regions associated with PD pathology such as olfactory bulb, midbrain, and medulla oblongata, higher levels of α-synuclein were found (Calderon-Garciduenas et al., 2013). These increases in α-synuclein, which were mostly not detected in brains of control children, were paralleled by olfactory dysfunctions in other children living in the same conditions (Calderon-Garciduenas et al., 2010). Olfaction problems have also been reported in individuals exposed to heavy air pollution (Calderon-Garciduenas et al., 2010). Peripheral inflammation (known to be elicited by air pollution) may cause an increase of α-synuclein in the olfactory bulb, and its misfolding and aggregation may spread via the olfactory route, from the olfactory bulb to the midbrain via other olfactory areas and the limbic system (Lema-Tomè et al., 2013). Regarding animal studies, a single controlled study found that exposure of male rats to DE (311 μg/m3 or higher) for six months increased α-synuclein levels in the midbrain (Levesque et al. 2011b). In another study, an increase in α-synuclein levels was found in the cerebral cortex of C57BL6/J mice (male only) exposed to DE (250 μg/m3) for three weeks (Coburn et al. unpublished data). In summary, evidence from epidemiological studies suggesting that air pollution may be associated with PD is still limited and further well-planned longitudinal studies are warranted (Fu et al., 2019; Hu et al., 2019; Palacios, 2017).
5. Possible mechanisms underlying air pollution neurotoxicity
Systemic inflammation is a main mechanism by which air pollution causes pulmonary and cardiovascular toxicity (Fiordelisi et al., 2017), and microglia activation, oxidative stress, and neuroinflammation, all induced by air pollution in the CNS, are believed to be causally associated with air pollution-induced neurotoxicity and to be relevant for neurodevelopmental and neurodegenerative diseases (Costa et al., 2014; Costa, Chang, & Cole, 2017b; Costa et al., 2019). Oxidative and inflammatory responses in the CNS following exposure to air pollution have been found in vivo in animals (e.g. MohanKumar et al., 2008; Levesque et al. 2011b; Cole et al., 2016; Bai et al., 2019), in in vitro systems (e.g. Block et al., 2004; Roqué et al., 2016; Milani et al., 2018; Bai et al., 2019), and to a limited extent in humans (Calderon-Garciduenas et al., 2012; Costa, Chang, & Cole, 2017b; Costa et al., 2019). There is substantial evidence demonstrating an important role played by neuroinflammation in neurodevelopmental disorders, particularly ASD (D’Angiulli, 2019; Davis, 2018; Mottahedin et al., 2017). There is also ample evidence that oxidative stress and neuroinflammation occur in several neurodegenerative diseases and contribute to their etiopathology and progression (Manoharan et al., 2016; Ransohoff,2016). The following sections will highlight selected aspects that may mechanistically link air pollution with ASD, AD and PD by virtue of oxidative stress and neuroinflammation (for additional discussions please see Costa, 2017; Costa et al., 2019).
5.1. Neurodevelopmental disorders
Microglia play an extremely important role in brain development as these cells contribute to neurogenesis, synaptic pruning, myelination and angiogenesis (Davis, 2018). Yet, excessive microglia activation induced by external factors can cause severe inflammation and be associated with adverse effects in the developing nervous system. For example, microglia-generated pro-inflammatory cytokines have been associated with hypomyelination and ventriculomegaly via toxicity to oligodendrocytes (Allen et al., 2017). As said, inflammation plays an important role in neurodevelopmental disorders, particularly in ASD (Brockmeyer & D’Angiulli, 2016; Prata, Santos, Almeida, Coelho, & Barbosa, 2017; Davis, 2018; D’Angiulli, 2019). Maternal infection (i.e. MIA) causes severe neuroinflammation in the fetus’s brain and represents one of the most reliable animal models for ASD (Bilbo, Block, Bolton, Hanamsagar, & Tran, 2018; Patterson, 2011). As discussed below, an increase in pro-inflammatory cytokines may also activate a signaling cascade leading to a decrease in reelin.
5.1.1. Disruption of reelin homeostasis
Secreted in the marginal zone of the developing cerebral cortex, reelin is a signaling glycoprotein known to play an important role in neuronal migration and establishment of neuronal polarity (Folsom & Fatemi, 2013; Förster, 2014; Jossin, 2004; Sekine, Kubo, & Nakajima, 2014). The canonical reelin signaling pathway is activated upon binding of reelin to the VLDL (very low-density lipoprotein) receptor and APOE receptor 2, which triggers tyrosine phosphorylation of the intracellular adaptor protein disabled-1 (Dab1). Phosphorylated Dab1 then activates a kinase cascade involving PI-3 kinase, LIM kinase-1, and several others (Förster, 2014). Such complex networks of signaling pathways mediate the ultimate effects of reelin on neuronal migration and polarity in the developing brain. Several lines of evidence suggest an involvement of reelin in ASD. Reelin expression is significantly decreased in brain from individuals with ASD (Fatemi et al., 2005; Folsom & Fatemi, 2013), and the reelin gene (RELN) is affected in several autistic pedigrees (Lammert & Howell, 2016; Persico et al., 2001; Serajee, Zhong, & Huq, 2006; Wang et al., 2014), as is the methylation pattern at the reelin gene promoter (Lintas, Sacco, & Persico, 2016). Mice lacking the C-terminal region of reelin exhibit behavioral abnormalities related to ASD (Sakai, Shoji, Kohno, Miyakawa, & Hattori, 2016), as does the reeler (rl−/−) mouse (Katsuyama & Terashima, 2009; Reiner, Karzburn, Kshirsagar, & Kaibuchi, 2016). In addition, cortical disorganization has been reported in reelin-deficient mice and in ASD patients, and dysregulation of reelin-driven cortical neuron migration is present in ASD, as evidenced by cortical disorganization (Stoner et al., 2014; Boyle et al., 2011; Reiner et al., 2016). Furthermore, a decrease of reelin protein and mRNA levels are found in the MIA model of ASD (Ghiani et al., 2011; Meyer et al., 2006), and N-acetylcysteine completely prevents lipopolysaccharide (LPS)-induced decreases of reelin (Novais et al., 2013). Chang et al. (2019) found that developmental DE exposure of mice (250–300 μg/m3 from GD0 to PND21), which induces ASD-like behavioral alterations (Chang et al., 2018), causes neuroinflammation (increased IL-6), increases in pSTAT3 and DNA methyltranferase-1 (DNMT1), and a decrease in reelin expression. Structural abnormalities in cerebral cortical layering as observed in ASD (Stoner et al., 2014) were also found (Chang et al., 2019; Cole et al. submitted). Fig. 1 shows a possible mechanism for cortical disruption induced by developmental exposure to DE in mice.
Fig. 1.
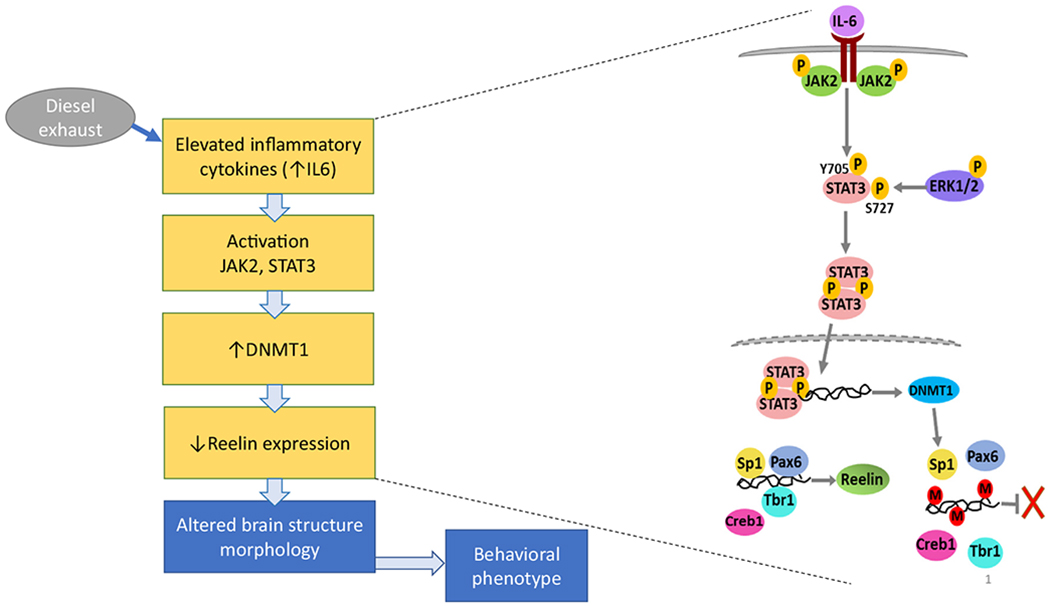
Possible mechanism for developmental DE exposure-induced cortical disruption. Developmental exposure of mice to DE (250 μg/m3, from GD 1 to PND 21) causes neuroinflammation, as evidenced by elevated levels of IL-6. This in turn activates STAT3 which acts as a transcription factor and up-regulates DNMT1 expression, leading to decreased reelin levels. Known for its critical role in guiding the process of neuronal migration during development, decreased expression of reelin during critical expression periods leads to altered cortical structures (Chang et al., 2019), as observed in ASD (Stoner et al., 2014). The same developmental DE exposure causes significant alterations in all three behavioral domains of ASD (communication, repetitive behavior, social interactions) (Chang et al., 2018). Figure reproduced from Chang et al. (2019) with permission.
5.1.2. Excitatory/inhibitory imbalance
An imbalance of excitatory/inhibitory neurotransmission is believed to be relevant in ASD (Uzunova, Pallanti, & Hollander, 2016). The hypothesis of an increased excitation/inhibition ratio was first formulated by Rubenstein and Merzenich (2003) and has influenced research in this area since then. More recently, however, such imbalance, which is found in all animal models of ASD, has been suggested as a compensatory mechanism rather than a causative one in ASD (Antoine, Langberg, Schnepel, & Feldman, 2019). Such imbalance may be due to a reduced GABAergic action or to an increased glutamatergic one, though in individuals with ASD the deficit appears to consist in a reduced GABAergic action (Robertson, Ratai, & Kanwisher, 2016). By inducing neuroinflammation, and specifically by increasing levels of IL-6, air pollution would also increase expression of DNMT1 (Chang et al., 2019). DNMT1 decreases the expression of GAD 67 (glutamic acid decarboxylase 67), and hence inhibitory GABAergic neurotransmission, thereby disrupting the balance of excitation/inhibition, as also found in ASD and in mouse models of ASD (Han et al., 2012). Of interest is that MIA also causes a decrease of GAD67 (Nouel, Burt, Zhang, Harvey, & Boksa, 2012).
5.1.3. Changes in the microbiota
The existence of a vast number of microbes in the body has been known for centuries, but only recently has the microbiota been recognized to play important roles in various diseases including diseases of the CNS (Bell et al., 2019; Dinan & Cryan, 2017; Kim, Jeon, & Chun, 2013; Tillisch, 2014). While microbiota in the nasal cavity, the oral cavity and the lungs exist, the gut microbiota are the most studied in relation to possible dysbiosis and disease (Bell et al., 2019). Earlier indications suggested that in humans, gut microbes outnumbered cells by 10:1; however, more recent studies have questioned this ratio and indicate one much lower (Sender, Fuchs, & Milo, 2016). The microbiota is composed of over 1000 species of bacteria (mostly anaerobic) with over 7000 strains; the most common microorganisms are Bacteroidetes and Firmicutes (Bell et al., 2019). Colonization of the gut begins at birth, and the microbiota change with aging. Gut microbes are essential to digestion and absorption of nutrients and play important role in normal physiology. Dysbiosis of the gastrointestinal tract has been implicated in the development of various disorders, such as GI tract inflammation, but also obesity, type 2 diabetes mellitus, as well as neurodevelopmental and neurodegenerative disorders. Some information on the role of the gut microbiota in CNS disorders has been obtained using germ-free mice or the administration of antibiotics. A gut-brain axis clearly exists by which gut microbiota influence the brain (Bell et al., 2019; Warner, 2019). Such communication can be mediated by multiple pathways such as vagal afferent nerves, immune and HPA axis modulation, and production of active metabolites (Tillisch, 2014). There is evidence that diet, drugs and environmental agents can cause dysbiosis, i.e. change the composition of gut microbiota (Rosenfeld, 2017). Several studies in humans and animals have shown that air pollution can affect the gut microbiota by changing its composition (Alderete et al., 2018; Mutlu et al., 2018; Salim, Kaplan, & Madsen, 2014; Valles & Francino, 2018). Parallel evidence also indicates that dysbiosis of the gut microbiota plays an important role in ASD(Ding, Taur,&Walkup, 2017; Hughes, Rose, & Ashwood, 2018). In genetic and environmental models of ASD (e.g. the BTBR mouse, the valproic acid model, or the MIA model) changes in gut microbiota have been found to parallel the behavioral dysfunctions, and administration of selected deficient bacteria can reverse such impairments (Hsiao et al., 2013; Sgritta et al., 2018).
5.2. Neurodegenerative diseases
There is ample evidence that oxidative and neuroinflammatory processes occur in various neurodegenerative diseases and contribute to their etiopathology (Butterfield & Halliwell, 2019; Heneka, McManus, & Latz, 2019; Manoharan et al., 2016; Ransohoff, 2016). Oxidative stress and neuroinflammation play a cardinal role in AD (Heneka et al., 2015; Huang, Zhang, & Chen, 2016; Moulton & Yang, 2012; Rubio-Perez & Morillas-Ruiz, 2012). Neuro-inflammation can contribute to amyloid toxicity (Minter, Taylor, & Crack, 2016), and ApoEε4 (a strong genetic risk factor for AD) is less protective against neuro-inflammation (Tai et al., 2015). Activation of microglia causes both an increase in oxidative stress and in pro-inflammatory cytokines. Oxidative stress is believed to play a role in PD pathogenesis and has been shown to cause α-synuclein aggregation (Takahashi et al., 2007), and microglia activation and neuroinflammation plays a central role in the pathogenesis of PD (Anderson, Coffey, Berwin, & Havdra, 2018; Hirsch, Vyas, & Hunot, 2012; Lull & Block, 2010; Qian, Flood, & Hong, 2010). An additional aspect that needs to be considered is the contribution of peripheral inflammation to neuroinflammation. Though different from central inflammatory processes (Filiou, Arefin, Moscato, & Graeber, 2014), systemic inflammation affects inflammatory processes in the CNS (Hopkins, 2007; Lema-Tomè et al., 2013; Mumawet al., 2006). As indicated earlier, peripheral inflammation is a cardinal effect of exposure to air pollution (Anderson et al., 2012). Thus, the contribution of peripheral inflammatory processes to adverse CNS effects needs to be further investigated.
More specific hypotheses which may mechanistically link air pollution and neurodegeneration, particularly AD, are also being investigated; these include, for example, changes in miRNAs or in telomere length (Jardim, 2011; Miri et al., 2019). Most have been discussed in detail recently (Costa, 2017), while some of the compelling ones are briefly discussed below. Another very interesting potential mechanism has been recently proposed by Cacciottolo et al. (2020). Exposure of mice to TRAP-PM0.2 (300 μg/m3) or of neuronal cells to the same nanoparticles (1–10 μg/ml) caused an increase in oxidative stress in lipid rafts associated with an increase in Aβ; the latter was inhibited by the antioxidant N-acetyl cysteine, suggesting that oxidative damage may represent an important event in the pro-amyloidogenic effects of air pollution.
5.2.1. Disruption of adult neurogenesis
Findings from post-mortem human brains and from animal studies have shown that adult neurogenesis is impaired in neurodegenerative diseases (Horgusluoglu, Nudelman, Nho, & Saykin, 2017), including AD (Fuster-Matanzo, Llorens-Martin, Hernandez, & Avila, 2013) and PD (Marxreiter, Regensburger, & Winkler, 2013). Alterations in adult neurogenesis differ among different neurodegenerative diseases but are believed to be relevant to their progression. Thus, for example, impairment of adult neurogenesis in the hippocampal region may be associated with decreased cognitive function in AD (Fuster-Matanzo et al., 2013; Horgusluoglu et al., 2017). It has been reported that brain inflammation inhibits basal neurogenesis in the hippocampal subgranular zone, an effect that is prevented by minocycline, an inhibitor of microglia activation (Carpentier & Palmer, 2009; Ekdahl, Claasen, Bonde, Kokaia, & Lindvall, 2003). Evidence of decreased adult neurogenesis following early postnatal inflammation in rodents has also been reported (Dinel et al., 2014; Lajud & Torner, 2015). Given that a main effect of air pollution in the brain is neuro-inflammation, it is conceivable that exposure may result in an impairment of adult neurogenesis. In agreement with this hypothesis, it has been reported that exposure of adult mice to DE (250–300 μg/m3 for 6 h) caused oxidative stress and neuro-inflammation, and decreased adult neurogenesis in mice, particularly in males (Coburn et al., 2018). These findings suggest that air pollution, by virtue of its ability to cause neuro-inflammation, can disrupts the process of adult neurogenesis and may thus contribute to the etiopathology of neurodegenerative diseases.
5.2.2. Alterations of reelin
As for ASD, there is also evidence of an involvement of reelin in AD, and air pollution-induced alterations in reelin expression and/or signaling may indeed play a relevant role in the observed association between exposure and AD. In the adult CNS, reelin is expressed in GABAergic interneurons in the cortex and hippocampus, where it modulates learning and memory processes. Reelin provides functions via proteolytic activity as a serine protease, and via activation of specific receptors which elicit signaling cascades (Bock & May, 2016; Förster, 2014). Brain reelin levels are decreased in natural aging (Stranahan, Haberman, & Gallagher, 2011), in transgenic animal models of AD (Chin et al., 2007; Kocherhans et al., 2010; Mota, Ferreira, & Rego, 2014b; Yu, Tan, Yu, Xie, & Tan, 2016), and in brains of AD patients, particularly in the early stages (Chin et al., 2007; Herring et al., 2012). Reelin mRNA is instead up-regulated in later stages of AD (Botella-Lopez et al., 2010), which may reflect a potential compensatory mechanism or an effect of advanced disease processes (Yu et al., 2016). However, though increased, in these later stages reelin is less effective, as β-amyloid compromises reelin signaling in late AD (Cuchillo-Ibanez et al., 2016). Reelin can indeed inhibit Aβ generation and promote Aβ clearance (Kocherhans et al., 2010; Pujadas et al., 2014), and low levels of reelin are associated with higher tau phosphorylation (Yu et al., 2016). In addition, decreased reelin levels may contribute to the initiation and progression of AD by impairing synaptic functions, cytoskeleton stability, and proper axonal transport (Yu et al., 2016). Reelin is known to play an important role in synaptic plasticity, by stimulating long term potentiation through modulation of NMDA receptors (Weeber et al., 2002), and decreased reelin levels would cause synaptic dysfunction in the hippocampus, leading to memory and cognitive deficits which may precede neuronal loss, as found in AD (Ma & Klann, 2012; Yu et al., 2016). A transcriptomics analysis of the AD brain found that synapse-associated pathways were the most affected, and among these, reelin signaling was one of the few that was significantly altered (Karim et al., 2014). A genome-wide study in elderly, non-demented individuals identified three single nucleotide polymorphisms in the reelin gene (RELN) that significantly correlated with increased tau phosphorylation and concomitant appearance of neurofibrillary tangles (Kramer et al., 2010). In addition, a more recent study found that, among men, genetic polymorphisms of RELN were associated with AD (Feher, Juhasz, Pakaski, Kalman, & Janka, 2015). Overall, decreased reelin levels and signaling appear to be key early events in AD. Hence, experiments aimed at increasing reelin signaling in the CNS to counteract the development of AD have already been underway. For example, in one study, purified recombinant reelin injected into the ventricles increases synaptic function and cognitive abilities of wild-type mice (Rogers et al., 2011). Similarly, exogenous reelin prevented cognitive deficits induced by phencyclidine in mice (Ishii et al., 2015). For interference with reelin homeostasis to represent a mechanism for air pollution in AD, exposure should lead to a decreased expression/signaling of reelin. Neuroinflammation (and specifically an increase in 1L-6) which follows microglia activation, is increased by air pollution (Block & Calderon-Garciduenas, 2009; Cole et al., 2016). As indicated earlier, 1L-6 stimulates theJAK/STAT3 pathway which increases DNMT1 (Garbers, Aparicio-Siegmund, & Rose-John, 2015; Shaun & Thomas, 2012), causing increased methylation of the reelin promoter and a decrease in reelin expression (Noh et al., 2005; Palacios-Garcia et al., 2015). As described in an earlier section, this pathway was activated upon developmental exposure to DE (Chang et al., 2019) (Fig. 4). Another study reported that exposure to the anesthetic sevoflurane increased DNMT1, caused hyper-methylation of RELN, a decrease of reelin mRNA and protein, and associated cognitive impairment (Ju et al., 2016). Interestingly, MIA (elicited by infection during pregnancy), which leads to offspring that display neuroinflammation, has been shown not only to decrease levels of reelin protein and mRNA in brain of offspring (Ghiani et al., 2011; Novais et al., 2013), but also to drive AD-like neuropathology as the animal ages (Krstic et al., 2012). The notion that neuroinflammation (and oxidative stress) may play an important role in modulating reelin expression is also supported by studies showing that N-acetylcysteine completely prevents lipopolysaccharide (LPS)-induced decreases of reelin (Novais et al., 2013). Since impairment of reelin expression and signaling are known to occur in autism and have been found in mice upon developmental exposure to DE (Costa, Cole, et al., 2017a; Chang et al., 2019), the suggestion that ASD and AD may share some genetic and/or etiopathological aspects may be of relevance (Khan et al., 2016), and warrants further investigations.
5.2.3. Alterations in glutamate homeostasis
The correct functioning of glutamatergic synapses is essential for learning and memory, and disruptions of glutamate homeostasis may play a relevant role in neurological/neurodegenerative disorders (Zhang, Li, Feng, & Wu, 2016). Alterations in glutamate receptors, particularly the ionotropic NMDA (N-methyl-d-aspartate) receptors, but also metabotropic receptors, occur in AD, leading to over-activation of the glutamatergic system (Mota, Ferreira, & Rego, 2014b; Lewerenz & Maher, 2015; Rudy, Hunsberger, Weitzner, & Reed, 2015; Ribeiro, Vieira, Pires, Olmo, & Ferguson, 2017). In addition, glutamate transporters, particularly in astrocytes, are altered in AD, leading to dysregulation of the tightly controlled glutamate levels in the extracellular milieu, and contributing to an overstimulation of the glutamatergic system (Murphy-Royal, Dupuis, Groc, & Oliet, 2017; Rudy et al., 2015). Limited evidence from animal and in vitro studies suggests that air pollution may interact with the glutamatergic system. A study by Win-Shwe et al. (2009) reported that exposure of mice to nanoparticle-enriched DE for four weeks caused an increase of the NR1 (now GluN1) subunit of the NMDA receptor in the olfactory bulb. Two other studies reported that exposure of mice to nanoparticles induced a decrease of GluN1 in the hippocampus (Cacciottolo et al., 2017; Morgan et al., 2011). All three studies found that other NMDA receptor subunits were unchanged. The significance of these findings in relationship to air pollution/AD is still unclear. Win-Shwe et al. (2009) also found that DE exposure increased the levels of free glutamate in the olfactory bulb, in agreement with results obtained by various investigators in vitro. Liu et al. (2015) found that PM2.5 caused the release of glutamate from microglia; excess glutamate would then over-activate NMDA receptors on neurons leading to neuronal death. The increase in glutamate appeared to be due to an increase in the activity and release from microglia of glutaminase (which converts glutamine to glutamate) (Liu et al., 2015). In an earlier study, Ye et al. (2013) reported that pro-inflammatory cytokines could increase glutaminase activity and glutamate levels in neurons. Furthermore, Barger, Goodwin, Porter, and Beggs (2007) found that microglia activation (by lipopolysaccharide) caused an increased release of glutamate. They suggested that the initial oxidative stress, particularly lipid peroxidation, would cause glutathione (GSH) depletion, which would be compensated for with an increased activity of the cysteine-glutamate exchanger, leading to increased extracellular glutamate. These findings indicate that neurodegenerative consequences of neuroinflammation may result from the conversion of oxidative stress to excitotoxic stress (Barger et al., 2007). Whatever the exact mechanism (s), it would appear that air pollution may increase extracellular levels of glutamate, and this may indeed contribute to neurotoxicity observed in AD and possible other neurodegenerative diseases.
5.2.4. Changes in the microbiota
In addition to its potential role in neurodevelopmental disorders such as ASD, gut dysbiosis appears also to be involved in neurodegenerative diseases such as AD and PD (Bell et al., 2019; Dinan & Cryan, 2017; Warner, 2019). Changes in the gut microbiome have been found in AD and in experimental models of AD (Jiang, Li, Huang, Liu, & Zhao, 2017; Xu & Wang, 2016). For example, mice overexpressing APP have attenuated microglia activation and decreased Aβ deposition if raised in germ-free-conditions (Harach et al., 2017). Metabolites of gut microbes, such as short chain fatty acids (SCFA, such as acetate, propionate, butyrate), have also been shown to activate microglia and increase neuroinflammation (Warner, 2019). Similar findings have been reported in α-synuclein-overexpressing mice, in which germ-free conditions attenuate motor deficits and brain pathology, suggesting a role played by gut microbiota in PD (Sampson et al., 2016).
6. Conclusions
While, for years, studies on adverse effects of air pollution have been focused first on the respiratory system, then on the cardiovascular system, the past decade has seen a substantial interest in the potential effects of air pollution on the CNS. CNS disorders in the general population are of great concern and appear to be increasing, because of better diagnoses and/or the aging of the population, but also because many may involve environmental factors in their etiopathogenesis. Most studies focusing on air pollution have examined the effects of exposure during development (both pre- and early postnatally) and in the elderly, as these two populations appear to be the most sensitive to air pollution-induced neurotoxicity. Alterations in several neurobehavioral functions have been found in children exposed to high levels of air pollution, and evidence of increased depression and cognitive impairment have been found in adults and in the elderly. Specific CNS disorders have also been shown to be associated with exposure to air pollution, namely ASD as a neurodevelopmental disorder, and AD (in particular) and PD among the neurodegenerative diseases. The great majority of epidemiological studies have revealed positive associations, and the null or negative studies are only a handful. Nevertheless, several limitations remain regarding the human studies. Possibly the most important one relates to the characterization and quantification of the exposure, as often it is based on models considering location of housing, which may provide inaccurate exposure estimations. Indeed, the issue of exposure, in both qualitative and quantitative terms, remains a key element that should guide the design of future epidemiological studies. A better knowledge of exposure would allow for example, an understanding of whether different outcomes in different populations may be related to subtle differences in exposure parameters. In general, PM2.5 appears to be the most significant component of air pollution associated with adverse CNS outcomes, while NOx or O3 have provided more contrasting results. However, as discussed earlier, UFPM (which are comprised in the mass of PM2.5) are believed to be most important PM for neurotoxicity, because of their biological characteristics and their ability to gain access to the CNS. Yet, the number of UFPM is rarely quantified in most studies. Furthermore, elemental contaminants such as iron or sulfur, associated with UFPM, may play important, and not yet recognized roles in determining adverse effects on the CNS (Cory-Slechta et al., 2019). it is not clear whether there are additive and/or synergistic effects among air pollution components.
Confounding factors, from socioeconomic status to smoking or other health conditions, are always important aspects to consider. When studying effects of air pollution in adults and in aging individuals, the relevant time of exposure (i.e. early in life, during adulthood, etc.) also represents a dilemma. For example, when considering the contribution of air pollution to neurodegenerative diseases such as AD, what are the significant “windows of susceptibility” for air pollution exposure? Is it the developmental period (pre- and post-natal), in tune with the Barker hypothesis of early origin of late-life diseases? Similar biochemical/molecular alterations observed in ASD and AD suggest that this area of mechanistic research may provide important new information in this regard, and controlled experimental studies are underway to specifically investigate whether developmental exposures may facilitate late behavioral, biochemical and morphological signs of AD (Cole et al. in progress). Is a prolonged, multidecade exposure necessary or sufficient (even at low levels), as also suggested by some studies (e.g. Kulick et al., 2020) ? Or is a late-life exposure enough to precipitate CNS disease (e.g. Younan et al., 2020)? While difficult to assess in epidemiological studies, these variables could be studied in animals under controlled exposure conditions. Indeed, having established that air pollution is associated with poor brain health, what are needed now are high quality longitudinal cohort studies with large sample size and detailed knowledge of residential history, employment, and mobility (Russ et al., 2019).
One important aspect of the research portfolio on air pollution and brain health is that there is a rather strong convergence of findings in human epidemiological and in experimental animal studies (Costa et al., 2014). This is extremely encouraging, as controlled animal exposure would allow an understanding of the mechanism(s) that may underlie observed behavioral effects. Animal studies would further the understanding of the most significant component of air pollution associated with adverse CNS effects, and this in turn may allow very specific and targeted interventions aimed at prevention. Mechanistic studies are certainly warranted, as they may increase our understanding of CNS effects associated with air pollution and may also lead to potential therapeutic interventions. The recent experimental study by Cacciottolo et al. (2020) is very promising in this regard. Limited in vitro experiments also point to the effects of air pollutants on microglia activation, oxidative stress and neuroinflammation, which appear to be the key responses to air pollution in the CNS. Thus, in vitro approaches may also contribute to our overall understating of air pollution neurotoxicity.
Needless to say, such research efforts must go hand in hand with measures to reduce man-made air pollution. As discussed earlier, TRAP is a major contributor to air pollution, and measures to reduce DE exposures and converting to less-polluting means of transportation are important ways to address this problem. Pollution caused by the processes of rapid industrialization and urbanization is considered highly relevant for air pollution in countries like China, where it has led to some of the worst AQI in the world. Measures to curb pollution have been already implemented in this country, but several challenges remain for the future (Zeng, Cao, Qiao, Seyler, & Tang, 2019). Acknowledgment that there is an urgent need to reduce urban pollution is also recognized in India, where some megacities see levels of PM2.5 several fold higher than the WHO recommended limits for extended periods of time (Patel, 2019).
Acknowledgments
Research by the authors is supported by the grants from NIEHS (R01ES022949, R01ES028273, P30ES07033, T32ES007032, P42ES04696) and NICHD (U54HD08091) and by funds from the University of Washington and the Department of Environmental and Occupational Health Sciences at the University of Washington.
Abbreviations:
- Aβ
Amyloid beta
- AD
Alzheimer’s disease
- ADHD
Attention Deficit Hyperactivity Disorder
- APP
Amyloid Precursor Protein
- AQI
Air Quality Index
- ASD
Autism Spectrum Disorder
- BACE
Beta-site APP-Cleaving Enzyme
- CNS
Central Nervous System
- CO
Carbon Monoxide
- COX
Cyclooxygenase
- CSF
Cerebrospinal Fluid
- DE
Diesel Exhaust
- DNMT
DNA Methyltransferase
- EPA
Environmental Protection Agency
- EU
European Union
- FA
Filtered Air
- GD
Gestational Day
- GFAP
Glial Fibrillary Acidic Protein
- HR
Hazard Ratio
- IL
Interleukin
- MCI
Mild Cognitive Impairment
- MIA
Maternal Immune Activation
- NOx
Nitric Oxides
- NO2
Nitrogen Dioxide
- O3
Ozone
- OR
Odds Ratio
- PD
Parkinson’s Disease
- PM
Particulate Matter
- PND
Postnatal Day
- PPB
Parts per Billion
- PSEN
Presenilin
- RELN
Reelin
- SO2
Sulfur Dioxide
- TRAP
Traffic Related Air Pollution
- VLDL
Very Low-Density Lipoprotein
Footnotes
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
References
- Ailshire JA, & Clarke P (2015). Fine particulate matter air pollution and cognitive function among US older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences 70, 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailshire JA, & Crimmins EM (2014). Fine particulate matter air pollution and cognitive function among older US adults. American Journal of Epidemiology 180, 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajmani GS, Suh HH, & Pinto JM (2016). Effects of ambient air pollution exposure on olfaction: A review. Environmental Health Perspectives 124, 1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete TL, Jones RB, Chen Z, Kim JS, Habre R, Lurman F, et al. (2018). Exposure to traffic-related air pollution and the composition of the gut microbiota in over-weight and obese adolescents. Environmental Research 161, 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemany S, Vilor-Tejedor N, Garcia-Esteban R, Bustamante M, Dadvand P, Esnaola M, et al. (2018). Traffic-related air pollution, APOEe4 status, and neurodevelopmental outcomes among children enrolled in the BREATHE project (Catalonia, Spain). Environmental Health Perspectives 126(8) 087001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JL, Conrad K, Oberdoerster G, Johnston CJ, Sleezer B, & Cory-Slechta DA (2013). Developmental exposure to concentrated ambient particles and preference for immediate reward in mice. Environmental Health Perspectives 121, 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JL, Liu X, Weston D, Prince L, Oberdörster G, Finkelstein JN, et al. (2014a). Developmental exposure to concentrated ambient ultrafine particulate matter air pollution in mice results in persistent and sex dependent behavioral neurotoxicity and glial activation. Toxicological Sciences 140, 160–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JL, Liu X, Pelkowski S, Palmer B, Conrad K, Oberdörster G, et al. (2014b). Early postnatal exposure to ultrafine particulate matter air pollution: Persistent ventriculomegaly, neurochemical disruption, and glial activation preferentially in male mice. Environmental Health Perspectives 122, 939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JL, Oberdoerster G, Morris-Schaffer K,Wong C, Klocke C, Sobolewski M, et al. (2017). Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: Parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology 59, 140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JO, Thundiyil JG, & Stolbach A (2012). Clearing the air: A review of the effects of particulate matter air pollution on human health. Journal of Medical Toxicology 8, 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson FL, Coffey MM, Berwin BL, & Havdra MC (2018). Inflammasomes: An emerging mechanism translating environmental toxicant exposure into neuroinflammation in Parkinson’s disease. Toxicological Sciences 166, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J, Oudin A, Sundström A, Forsberg B, & Adolfsson R (2018). Road traffic noise, air pollution, and risk of dementia – Results from the Betula project. Environmental Research 166, 334–339. [DOI] [PubMed] [Google Scholar]
- Antoine MW, Langberg T, Schnepel P, & Feldman DE (2019). Increased excitation-inhibition ratio stabilizes synapse and circuit excitability in four autism mouse models. Neuron 101, 648–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbones ML, Thomazeau A, Nakano-Kobayashi A, Hagiwara M, & Delabar JM (2019). DYRK1A and cognition, a lifelong relationship. Pharmacology & Therapeutics 194, 199–221. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, & Van de Water J (2011). Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain, Behavior, and Immunity 25, 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai KJ, Chang KJ, Chen CL, Jhan MK, Hsiao TC, Chang TJ, et al. (2019). Microglial activation and inflammation caused by traffic-related particulate matter. Chemico-Biological Interactions 311, 108762. [DOI] [PubMed] [Google Scholar]
- Barger SW, Goodwin ME, Porter MM, & Beggs ML (2007). Glutamate release from activated microglia requires the oxidative burst and lipid peroxidation. Journal of Neurochemistry 101, 1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Iosif AM, Smith SEP, Bregere C, Amaral DG, & Patterson PH (2014). Activation of the maternal immune system during pregnancy alters behavioral development of Rhesus monkey offspring. Biological Psychiatry 75, 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra TA,Wilhelm M, Olsen J, Cockburn M, & Ritz B (2013). Ambient air pollution and autism in Los Angeles County, California. Environmental Health Perspectives 121, 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier EE, & Richardson JR (2015). Parkinson’s disease: Mechanisms, models, and biological plausibility In Aschner M, & Costa LG (Eds.), Environmental factors in neurodevelopmental and neurodegenerative disorders (pp. 267–288). New York: Academic Press/Elsevier. [Google Scholar]
- Bell ML, & Davis DL (2001). Reassessment of the lethal London fog of 1952: Novel indicators of acute and chronic consequences of acute exposure to air pollution. Environmental Health Perspectives 109(Suppl. 3), 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Davis DL, & Fletcher T (2004). A retrospective assessment of mortality from the London smog episode of 1952: The role of influenza and pollution. Environmental Health Perspectives 112, 6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JS, Spencer JI, Yates RL, Yee SA, Jacobs BM, & DeLuca GC (2019). Invited review: From nose to gut- the role of the microbiome in neurological diseases. Neuropathology and Applied Neurobiology 45, 195–215. [DOI] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Baan RA, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, et al. (2012). Carcinogenicity of diesel-engine and gasoline-engine exhaust and some nitroarenes. The Lancet Oncology 13, 663–664. [DOI] [PubMed] [Google Scholar]
- Berko ER, & Greally JM (2015). How might epigenetic dysregulation in early embryonic life contribute to autism spectrum disorder? Epigenomics 7, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal-Melendez E, Lacroix MC, Bouillaud P, Callebert J, Olivier B, Persuy MA, et al. (2019). Repeated gestational exposure to diesel engine exhaust affects the fetal olfactory system and alters olfactory-based behavior in rabbit offspring. Particle and Fibre Toxicology 16, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt DP, Puig KL, Gorr MW,Wold LE, & Combs CK (2015). A pilot study to assess effects of long-term inhalation of airborne particulate matter on early Alzheimer’s-like changes in the mouse brain. PLoS One 10 e0127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Block CL, Bolton JL, Hanamsagar R, & Tran PK (2018). Beyond infection – Maternal immune activation by environmental factors, microglia development, and relevance for autism spectrum disorders. Experimental Neurology 299(Pt. A), 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Dubois B, Fagan AM, Lewczuk P, de Leon MJ, & Hampel H (2015). Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease. Alzheimer’s & Dementia 11, 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, & Calderon-Garciduenas L (2009). Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends in Neurosciences 32, 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML,Wu X, Pei Z, Li G,Wang T, Qin L, et al. (2004). Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: The role of microglia, phagocytosis, and NADPH oxidase. The FASEB Journal 18, 1618–1620. [DOI] [PubMed] [Google Scholar]
- Block ML, Elder A, Auten RL, Bilbo SD, Chen H, Chen JC, et al. (2012). The outdoor pollution and brain health workshop. Neurotoxicology 33, 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock HH, & May P (2016). Canonical and non-canonical reelin signaling. Frontiers in Cellular Neuroscience 10 art. 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos I, DeBoever P, Emmerechts J, Buekers J, Vanoirbeek J, Meeusen R, et al. (2012). Changed gene expression in brains of mice exposed to traffic in a highway tunnel. Inhalation Toxicology 24, 676–686. [DOI] [PubMed] [Google Scholar]
- Botella-Lopez A, Cuchillo-Ibanez I, Cotrufo T, Mok SS, Li QX, Barquero MS, et al. (2010). Beta-amyloid controls altered reelin expression and processing in Alzheimer’s disease. Neurobiology of Disease 37, 682–689. [DOI] [PubMed] [Google Scholar]
- Boyle MP, Bernard A, Thompson CL, Ng L, Boe A, Mortrud M, et al. (2011). Cell-type-specific consequences of reelin deficiency in the mouse neocortex, hippocampus and amygdala. The Journal of Comparative Neurology 519, 2061–2089. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, & Del Tredici K (2004). Stages in the development of Parkinson’s disease pathology. Cell and Tissue Research 318, 121–134. [DOI] [PubMed] [Google Scholar]
- Branca C, Shaw DM, Belfiore R, Gokhale V, Shaw AY, Foley C, et al. (2017). Dyrk1 inhibition improves Alzheimer’s disease-like neuropathology. Aging Cell 16, 1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmeyer S, & D’Angiulli A (2016). How air pollution alters brain development; the role of neuroinflammation. Translational Neuroscience 7, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, & Rajagopalan S (2007). Air pollution and cardiovascular events. New England Journal of Medicine 356, 2104–2105. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope A, Brook JR, Bhatnagar A, Diez-Roux AV, et al. (2010). Particulate matter air pollution and cardiovascular disease. An update to the scientific statement from the American Heart Association. Circulation 121, 2331–2378. [DOI] [PubMed] [Google Scholar]
- Brouwer M, Koeman T, van den Brandt PA, Kromhout H, Schouten LJ, Peters S, et al. (2015). Occupational exposures and Parkinson’s disease mortality in a prospective Dutch cohort. Occupational and Environmental Medicine 72, 448–455. [DOI] [PubMed] [Google Scholar]
- Buescher AV, Cidav Z, Knapp M, &Mandell DS. (2014). Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatrics 168, 721–728. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, & Halliwell B (2019). Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nature Reviews. Neuroscience 20, 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciottolo M,Wang X, Driscoll I,Woodward N, Saffari A, Reyes J, et al. (2017). Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to the amyloidogenesis in experimental models. Translational Psychiatry 7 e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciottolo M, Morgan TE, Saffari AA, Shirmohammadi F, Forman HJ, Sioutas C, et al. (2020). Traffic-related air pollutants (TRAP-PM) promote neuronal amyloidogenesis through oxidative damage to lipid rafts. Free Radical Biology & Medicine 147, 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Azzarelli B, Acuna H, Garcia R, Gambling TM, Osnaya N, et al. (2002). Air pollution and brain damage. Toxicologic Pathology 30, 373–389. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Maronpot RR, Torres-Jardon R, Henriquez-Roldan C, Schoonhoven R, Acuna-Ayala H, et al. (2003). DNA damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicologic Pathology 31, 524–538. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Reed W, Maronpot RR, Henriquez-Roldan C, Delgado-Chavez R, Calderon-Garciduenas A, et al. (2004). Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicologic Pathology 32, 650–658. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, Torres-Jardon R, Nuse B, Herritt L, et al. (2008). Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha -synuclein in children and young adults. Toxicologic Pathology 36, 289–310. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Franco-Lira M, Henriquez-Roldan C, Osnaya N, Gomzalez-Maciel A, Reynoso-Robles R, et al. (2010). Urban air pollution: Influences on olfactory function and pathology in exposed children and young adults. Experimental and Toxicologic Pathology 62, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Engle R, Mora-Tiscareno A, Styner M, Gomez-Garza G, Zhu H, et al. (2011). Exposure to severe urban air pollution influences cognitive outcomes, brain volume and systemic inflammation in clinically healthy children. Brain and Cognition 77, 345–355. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Kavanaugh M, Block M, D’Angiulli A, Delgado-Chavez R, Torres-Jardon R, et al. (2012). Neuroinflammation, hyperphosphorylated tau, diffuse amyloid plaques, and down-regulation of the cellular prion protein in air pollution exposed children and young adults. Journal of Alzheimer’s Disease 28, 93–107. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Cross JV, Franco-Lira M, Aragon-Flores M, Kavanaugh M, Torres-Jardon R, et al. (2013). Brain immune interactions and air pollution: Macrophage inhibitory factor (MIF), prion cellular protein (PrPC), interleukin-6 (IL-6), interleukin 1 receptor antagonist (IL-1Ra), and serum interleukin-2 (IL-2) in cerebrospinal fluid and MIF in serum differentiate urban children exposed to severe vs. low air pollution. Frontiers in Neuroscience 7, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Kulesza RJ, Doty RL, D’Angiulli A, & Torres-Jardon R (2015). Megacities air pollution problems: Mexico City metropolitan area critical issues on the central nervous system pediatric impact. Environmental Research 137, 157–169. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Avila-Ramirez J, Calderon-Garciduenas A, Gonzalez-Heredia T, Acuña-Ayala H, Chao CK, et al. (2016a). Cerebrospinal fluid biomarkers in highly exposed PM2.5 urbanites: The risk of Alzheimer’s and Parkinson’s diseases in young Mexico City residents. Journal of Alzheimer’s Disease 54, 597–613. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Reynoso-Robles R, Vargas-Martinez J, Gomez-Maqueo-Chew A, Perez-Guillé B, Mukherjee PS, et al. (2016b). Prefrontal white matter pathology in air pollution exposed Mexico City young urbanites and their potential impact on neurovascular unit dysfunction and the development of Alzheimer’s disease. Environmental Research 146, 404–417. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Mukherjee PS,Waniek K, Holzer M, Chao CH, Thompson C, et al. (2018a). Non-phosphorylated tau in cerebrospinal fluid is a marker of Alzheimer’s disease continuum in young urbanites exposed to air pollution. Journal of Alzheimer’s Disease 66, 1437–1451. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Gonzalez-Maciel A, Reynoso-Robles R, Kulesza RJ, Mukherjee PS, Torres-Jardon R, et al. (2018b). Alzheimer’s disease and alpha-synuclein pathology in the olfactory bulb of infants, children, teens and adults ≤ 40 years in Metropolitan Mexico City. APOE4 carriers at higher risk of suicide accelerate their olfactory bulb pathology. Environmental Research 166, 348–362. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Reynoso-Robles R, & Gonzalez-Maciel A (2019). Combustion and friction-derived nanoparticles and industrial-sourced nanoparticles: The culprit of Alzheimer and Parkinson’s diseases. Environmental Research 176, 108574. [DOI] [PubMed] [Google Scholar]
- Carpentier PA, & Palmer TD (2009). Immune influence on adult neural stem cell regulation and function. Neuron 64, 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KH, Chang MY, Muo CH,Wu TN, Chen CY, & Kao CH (2014). Increased risk of dementia in patients exposed to nitrogen dioxide and carbon monoxide: A population-based retrospective cohort study. PLoS One 9 e103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Cole TB, & Costa LG (2018). Prenatal and early-life diesel exhaust exposure causes autism-like behavioral changes in mice. Particle and Fibre Toxicology 15, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Daza R, Hevner R, Costa LG, & Cole TB (2019). Prenatal and early-life diesel exhaust exposure disrupts cortical lamina organization: Evidence for a reelin-related pathogenic pathway induced by interleukin-6. Brain, Behavior, and Immunity 78, 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Wang X, Wellenius GA, Serre ML, Driscoll I, Casanova R, et al. (2015). Ambient air pollution and neurotoxicity on brain structure: Evidence from women’s health initiative memory study. Annals of Neurology 78, 466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Kwong JC, Copes R, Tu K, Villeneuve PJ, van Donkelaar A, et al. (2017). Living near major roads and incidence of dementia, Parkinson’s disease, and multiple sclerosis: A population-based cohort study. Lancet 389, 718–726. [DOI] [PubMed] [Google Scholar]
- Chen G, Jin Z, Li S, Jin X, Tong S, Liu S, et al. (2019). Early life exposure to particulate matter air pollution (PM1, PM2.5 and PM10) and autism in Shanghai, China: A case control study. Environment International 121, 1121–1127. [DOI] [PubMed] [Google Scholar]
- Chez MG, Dowling T, Patel PB, Khanna P, & Kominsky M (2007). Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatric Neurology 36, 361–365. [DOI] [PubMed] [Google Scholar]
- Chin J, Massaro CM, Palop JJ, Thwin MT, Yu GQ, Bien-Ly N, et al. (2007). Reelin depletion in the entorhinal cortex of human amyloid precursor protein transgenic mice and humans with Alzheimer’s disease. The Journal of Neuroscience 27, 2727–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun H, Leung C, Wen SW, McDonald J, & Shin HH (2020). Maternal exposure to air pollution and risk of autism in children: A systematic review and meta-analysis. Environmental Pollution 256, 113307. [DOI] [PubMed] [Google Scholar]
- Cipriani G, Danti S, Carlesi C, & Borin G (2018). Danger in the air: Air pollution and cognitive dysfunction. American Journal of Alzheimer’s Diseases Other Dementias 33, 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford A, Lang L, Chen R, Anstey KJ, & Seaton A (2016). Exposure to air pollution and cognitive functioning across the life course-a systematic review. Environmental Research 147, 383–398. [DOI] [PubMed] [Google Scholar]
- Coburn JL, Cole TB, Dao KT, & Costa LG (2018). Acute exposure to diesel exhaust impairs adult neurogenesis in mice: Prominence in males and protective effect of pioglitazone. Archives of Toxicology 92, 1815–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan S, Horder J, Inkster B, Mendez MA, Murphy DG, & Nutt DJ (2012). GABA system dysfunction in autism and related disorders: From synapse to symptoms. Neuroscience and Biobehavioral Reviews 36, 2044–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G, & Gerber Y (2017). Air pollution and successful aging: Recent evidence and new perspectives. Current Environment Health Report 4, 1–11. [DOI] [PubMed] [Google Scholar]
- Cole TB, Coburn J, Dao K, Roque P, Kalia V, Guilarte TR, … Costa LG (2016). Sex and genetic differences in the effects of acute diesel exhaust exposure on inflammation and oxidative stress in mouse brain. Toxicology 374, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Allen JL, Conrad K, Marvin E, & Sobolewski M (2018). Developmental exposure to low level ambient ultrafine particle air pollution and cognitive dysfunction. Neurotoxicology 69, 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Sobolewski M, Marvin E, Conrad K, Merrill A, Anderson T, et al. (2019). The impact of inhaled ambient ultrafine particulate matter on developing brain: Potential importance of elemental contaminants. Toxicologic Pathology 47, 976–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG (2017). Traffic-related air pollution and neurodegenerative diseases: Epidemiological and experimental evidence and potential underlying mechanisms In Aschner M, & Costa LG (Eds.), Advances in Neurotoxicology, Vol. 1, environmental factors in neurodegenerative diseases (pp. 1–46). New York: Elsevier. [Google Scholar]
- Costa LG, Cole TB, Coburn J, Chang YC, Dao K, & Roque P (2014). Neurotoxicants are in the air: convergence of human and in vitro studies on the effects of air pollution on the brain. BioMed Research International. 10.1155/2014/736385 (ID 736385, 8 p) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Coburn J, Chang YC, Dao K, & Roque P (2017a). Neurotoxicity of traffic-related air pollution. Neurotoxicology 59, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Chang YC, & Cole TB (2017b). Developmental neurotoxicity of traffic-related air pollution: Focus on autism. Current Environment Health Report 4, 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Dao K, Chang Y-C , Coburn J, & Garrick J. (2019). Neurotoxicity of air pollution: Role of neuroinflammation In Aschner M, & Costa LG (Eds.), Advances in Neurotoxicology, Vol. 3, role of inflammation in environmental neurotoxicity (pp. 195–221). New York: Elsevier. [Google Scholar]
- Cramer J (2002). Population growth and local air pollution: Methods, models and results. Population and Development Review 28, 22–52. [Google Scholar]
- Crüts B, van Etten L, Tornqvist H, Blomberg A, Sandstrom T, Mills NL, et al. (2008). Exposure to diesel exhaust induces changes in EEG in human volunteers. Particle and Fibre Toxicology 5, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubo E, & Goetz CG (2014). Parkinson’s disease In Aminoff MJ, & Daroff RB (Eds.), The encyclopedia of neurological sciences (2nd ed )3. (pp. 828–832). New York: Elsevier. [Google Scholar]
- Cuchillo-Ibanez I,Mata-Balaguer T, Balmaceda V, Arranz JJ, Nimpf J, & Saez-Valero J (2016). The β-amyloid peptide compromises reelin signaling in Alzheimer’s disease. Scientific Reports 6, 31646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angiulli A (2019). The role of neuroinflammation in developmental neurotoxicity, tackling complexity in children’s exposures and outcomes In Aschner M, & Costa LG (Eds.), Advances in neurotoxicology, Vol. 3, role of inflammation in environmental neurotoxicity (pp. 223–257). New York: Elsevier. [Google Scholar]
- Davis RL (2018). Neurodevelopment: inflammationmatters In Aschner M, & Costa LG (Eds.), Advances in neurotoxicology, Vol. 2, linking environmental exposures to neurodevelopmental disorders (pp. 227–264). New York: Elsevier. [Google Scholar]
- Davis DA, Bortolato M, Godar SC, Sander TK, Iwata N, Pakbin P, et al. (2013). Prenatal exposure to urban air nanoparticles in mice causes altered neuronal differentiation and depression like responses. PLoS One 8 e64128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depino AM (2013). Peripheral and central inflammation in autism spectrum disorders. Molecular and Cellular Neurosciences 53, 69–76. [DOI] [PubMed] [Google Scholar]
- Dinan TG, & Cryan JF (2017). Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. The Journal of Physiology 595, 489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinel AL, Joffre C, Trifilieff P, Aubert A, Foury A, Le Ruyet P, et al. (2014). Inflammation early in life is a vulnerability factor for emotional behavior at adolescence and for lipopolysaccharide-induced spatial memory and neurogenesis alteration at adulthood. Journal of Neuroinflammation 11, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding HT, Taur Y, &Walkup JT. (2017). Gut microbiota and autism: Key concepts and findings. Journal of Autism and Developmental Disorders 47, 480–489. [DOI] [PubMed] [Google Scholar]
- Donzelli G, Llopis-Gonzalez A, Llopis-Morales A, Cioni L, & Morales-Suarez-Varela M (2020). Particulate matter exposure and attention-deficit/hyperactivity disorder in children: A systematic review of epidemiological studies. International Journal of Environmental Research and Public Health 17, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL (2012). Olfaction in Parkinson’s disease and related disorders. Neurobiology of Disease 46, 527–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durga M, Devasena T, & Rajasekar A (2015). Determination of LC50 and sub-chronic neurotoxicity of diesel exhaust nanoparticles. Environmental Toxicology and Pharmacology 40, 615–625. [DOI] [PubMed] [Google Scholar]
- Ehsanifar M, Tameh AA, Farzadkia M, Kalantari RR, Zavareh MS, Nikzaad H, et al. (2019). Exposure to nanoscale diesel exhaust particles: oxidative stress, neuroinflammation, anxiety and depression on adult male mice. Ecotoxicology and Environmental Safety 168, 338–347. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, & Lindvall O (2003). Inflammation is detrimental for neurogenesis in adult brain. Proceedings of the National Academy of Sciences of the United States of America. 100 (pp. 13632–13637). [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ansary A, & Al-Ayadhi L (2012). Neuroinflammation in autism spectrum disorders. Journal of Neuroinflammation 9, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood E, Lim Z, Naveed H, & Galea I (2017). The effect of systemic inflammation on human brain barrier function. Brain, Behavior, and Immunity 62, 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema M, Naya M, Horimoto M, & Kato H (2013). Developmental toxicity of diesel exhaust: A review of studies in experimental animals. Reproductive Toxicology 42, 1–17. [DOI] [PubMed] [Google Scholar]
- Emerson E, Robertson J, Hatton C, & Baines S (2018). Risk of exposure to air pollution among British children with and without intellectual disabilities. Journal of Intellectual Disability Research 63, 161–167. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Snow AV, Stary JM, Araghi-Niknam M, Brooks AI, Pearce DA, et al. (2005). Reelin signaling is impaired in autism. Biological Psychiatry 57, 777–787. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, Chauhan A, et al. (2012). Consensus paper: Pathological role of the cerebellum in autism. Cerebellum 11, 777–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher A, Juhasz A, Pakaski M, Kalman J, & Janka Z (2015). Genetic analysis of the RELN gene: Gender specific association with Alzheimer’s disease. Psychiatry Research 230, 716–718. [DOI] [PubMed] [Google Scholar]
- Filiou MD, Arefin AS, Moscato P, & Graeber MB (2014). Neuroinflammation differs categorically from inflammation: Transcriptomes of Alzheimer’s disease, Parkinson’s disease, schizophrenia and inflammatory diseases compared. Neurogenesis 15, 201–212. [DOI] [PubMed] [Google Scholar]
- Fiordelisi A, Piscitelli P, Trimarco B, Coscioni E, Iaccarino G, & Sorriento D (2017). The mechanisms of air pollution and particulate matter in cardiovascular diseases. Heart Failure Reviews 22, 337–347. [DOI] [PubMed] [Google Scholar]
- Folsom TD, & Fatemi SH (2013). The involvement of reelin in neurodevelopmental disorders. Neuropharmacology 68, 122–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Xu X, Weil ZM, Chen G, Sun Q, Rajagopalan S, et al. (2011). Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Molecular Psychiatry 16, 987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordyce TA, Leonhard MJ, & Chang ET (2018). A critical review of developmental exposure to particulate matter, autism spectrum disorder, and attention deficit hyperactivity disorder. Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances & Environmental Engineering 53, 14–204. [DOI] [PubMed] [Google Scholar]
- Forman HJ, & Finch CE (2018). A critical review of assays for hazardous components of air pollution. Free Radical Biology & Medicine 117, 202–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forns J, Dadvand P, Foraster M, Alvarez-Pedrerol M, Rivas I, Lopez-Vicente M, et al. (2016). Traffic-related air pollution, noise at school, and behavioral problems in Barcelona schoolchildren: A cross-sectional study. Environmental Health Perspectives 124, 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forns J, Dadvand P, Esnaola M, Alvarez-Pedrerol M, Lopez-Vicente M, Garcia-Esteban R, et al. (2017). Longitudinal association between air pollution exposure at school and cognitive development in school children over a period of 3.5 years. Environmental Research 159, 416–421. [DOI] [PubMed] [Google Scholar]
- Forns J, Sunyer J, Garcia-Esteban R, Porta D, Ghassabian A, Giorgis-Allemand L, et al. (2018). Air pollution exposure during pregnancy and symptoms of attention deficit and hyperactivity disorder in children in Europe. Epidemiology 29, 618–626. [DOI] [PubMed] [Google Scholar]
- Förster E (2014). Reelin, neuronal polarity and process orientation of cortical neurons. Neuroscience 269, 102–111. [DOI] [PubMed] [Google Scholar]
- Freire C, Ramos R, Puertas R, Lopez-Espinosa MJ, Julvez J, Aguilera I, et al. (2010). Association of traffic-related air pollution with cognitive development in children. Journal of Epidemiology and Community Health 64, 223–228. [DOI] [PubMed] [Google Scholar]
- Frustaci A, Neri M, Cesario A, Adams JB, Domenici E, Della Bernardina B, et al. (2012). Oxidative stress-related biomarkers in autism: Systematic review and meta-analyses. Free Radical Biology & Medicine 52, 2128–2141. [DOI] [PubMed] [Google Scholar]
- Fu P, Guo X, Cheung FMH, & Yung KKL (2019). The association between PM2.5 exposure and neurological disorders: A systematic review and meta-analysis. Science Total Environment 655, 1240–1248. [DOI] [PubMed] [Google Scholar]
- Fuster-Matanzo A, Llorens-Martin M, Hernandez F, & Avila J (2013). Role of neuroinflammation in adult neurogenesis and Alzheimer disease: Therapeutic approaches. Mediators Inflammation 2013, 260925 10.1155/2013/260925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers C, Aparicio-Siegmund S, & Rose-John S (2015). The IL-6/gp130/STAT3 signaling axis: Recent advances towards specific inhibition. Current Op Immunolgy 34, 75–82. [DOI] [PubMed] [Google Scholar]
- Garcia GJ, Schroeter JD, & Kimbell JS (2015). Olfactory deposition of inhaled nanoparticles in humans. Inhalation Toxicology 27, 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto NM, Henderson VW, Hodis HH, St. John JA, Lurmann F, Chen JC, et al. (2014). Components of air pollution and cognitive function in middle-aged and older adults in Los Angeles. Neurotoxicology 40, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler T, Klei L, Sanders SJ, Bodea CA, Goldberg AP, Lee AB, et al. (2014). Most genetic risk for autism resides in common variation. Nature Genetics 46, 881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc S, Zadeoglulari Z, Fuss SH, & Genc K (2012). The adverse effects of air pollution on the nervous system. Journal of Toxicolgy 2012, 782462 10.1155/2012/782462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlofs-Nijland ME, van Berlo D, Cassee FR, Schins RPF, Wang K, & Campbell A (2010). Effect of prolonged exposure to diesel engine exhaust on proinflammatory markers in different regions of the rat brain. Particle and Fibre Toxicology 7, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani CA, Mattan NS, Nobuta H,Malvar JS, Boles J, Ross MG, et al. (2011). Early effects of lipopolysaccharide-induced inflammation of foetal brain development in rat. ASN Neurology 3(4) art: e00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio AJ, Smith CB, & Madden MC (2012). Diesel exhaust particles and airway inflammation. Curr Op Pulm Medical 18, 144–150. [DOI] [PubMed] [Google Scholar]
- Gilbert J, & Man HY (2017). Fundamental elements in autism: From neurogenesis and neurite growth to synaptic plasticity. Frontiers in Cellular Neuroscience 11, 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano G, Tait L, Furlong CE, Cole TB, Kavanagh TJ, & Costa LG (2013). Gender differences in brain susceptibility to oxidative stress are mediated by levels of paraoxonase-2 (PON2) expression. Free Radical Biology & Medicine 58, 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal SK, Ghatge SV, Nema P, & Tamhane MS (2006). Understanding urban vehicular pollution problem Vis-à-Vis ambient air quality – A case study of a megacity (Delhi, India). Environmental Monitoring and Assessment 119, 557–569. [DOI] [PubMed] [Google Scholar]
- Guxens M, & Sunyer J (2012). A review of epidemiological studies on neuropsychological effects of air pollution. Swiss Medical Weekly 141, w13322. [DOI] [PubMed] [Google Scholar]
- Guxens M, Garcia-Esteban R, Giorgis-Allemand L, Forns J, Badaloni C, Ballester F, et al. (2014). Air pollution during pregnancy and childhood cognitive and psychomotor development. Epidemiology 25, 636–647. [DOI] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. (2011). Genetic heritability and shared environmental factors among twin pairs with autism. Archives of General Psychiatry 68, 1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Tai C,Westenbroek RE, Yu FH, Cheah CS, Potter GB, et al. (2012). Autisticlike behavior in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature 489, 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harach T, Marungruang N, Duthilleul N, Cheatham V, McCoy KD, Frisoni G, et al. (2017). Reduction of Abeta amyloid pathology in APPS1 transgenic mice in the absence of gut microbiota. Scientific Reports 7, 41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE,Weuve J, Scherr PA, & Evans DA (2013). Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80, 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helou R, & Jaecker P (2014). Occupational exposure to mineral turpentine and heavy fuels: A possible risk factor for Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders 4, 160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, et al. (2015). Neuroinflammation in Alzheimer’s disease. Lancet 14, 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, McManus RM, & Latz E (2019). Inflammasome signaling in brain function and neurodegenerative disease. Nature Reviews. Neuroscience 19, 610–621. [DOI] [PubMed] [Google Scholar]
- Herring A, Donath A, Steiner KM, Widera MP, Hamzehian S, Kanakis D, et al. (2012). Reelin depletion is an early phenomenon of Alzheimer’s pathology. Journal of Alzheimer’s Disease 30, 963–979. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Schmidt RJ, & Krakowiak P (2018). Understanding environmental contributions to autism: Causal concepts and the state of science. Autism Research 11, 554–586. [DOI] [PubMed] [Google Scholar]
- Cooney DJ, & Hickey AJ (2008). The generation of diesel exhaust particle aerosols from a bulk source in an aerodynamic size range similar to atmospheric particles. International Journal of Nanomedicine 3, 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Vyas S, & Hunot S (2012). Neuroinflammation in Parkinson’s disease. Parkinsonism & Related Disorders 18(S1), S210–S212. [DOI] [PubMed] [Google Scholar]
- Hopkins SJ (2007). Central nervous system recognition of peripheral inflammation: A neural, hormonal collaboration. Acta Bio-Medica 78(Suppl. 1), 231–247. [PubMed] [Google Scholar]
- Horgusluoglu E, Nudelman K, Nho K, & Saykin AJ (2017). Adult neurogenesis and neurodegenerative diseases: A systems biology perspective. American Journal of Medical Genetics 174, 93–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougaard KS, Jensen KA, Nordly P, Taxvig C, Vogel U, Saber AT, et al. (2008). Effects of prenatal exposure to diesel exhaust particles on postnatal development, behavior, genotoxicity and inflammation in mice. Particle and Fibre Toxicology 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougaard KS, Saber AT, Jensen KA, Vogel U, & Wallin H (2009). Diesel exhaust particles: Effects on neurofunction in female mice. Basic & Clinical Pharmacology & Toxicology 105, 139–143. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. (2013). Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C-Y, Fang Y, Li F-L, Dong B, Hua X-G, Jiang W, et al. (2019). Association between ambient air pollution and Parkinson’s disease: Systematic review and meta-analysis. Environmental Research 168, 448–459. [DOI] [PubMed] [Google Scholar]
- Huang WJ, Zhang X, & Chen WW (2016). Role of oxidative stress in Alzheimer’s disease (review). Biomed. Rep 4, 519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes HK, Rose D, & Ashwood P (2018). The gut microbiota and dysbiosis in autism spectrum disorders. Current Neurology and Neuroscience Reports 18, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Nagai T, Hirota Y, Noda M, Nabeshima T, Yamada K, et al. (2015). Reelin has a preventive effect on phencyclidine-induced cognitive and sensory-motor gating deficits. Neuroscience Research 96, 30–36. [DOI] [PubMed] [Google Scholar]
- Jardim MJ (2011). MicroRNAs: Implications for air pollution research. Mutation Research, Fundamental and Molecular Mechanisms of Mutagenesis 717, 38–45. [DOI] [PubMed] [Google Scholar]
- Jiang C, Li G, Huang P, Liu Z, & Zhao B (2017). The gut microbiota and Alzheimer’s disease. Journal of Alzheimer’s Disease 58, 1–5. [DOI] [PubMed] [Google Scholar]
- Jo H, Eckel SP, Wang X, Chen JC, Cockburn M, Martinez MP, et al. (2019). Sex-specific associations of autism spectrum disorder with residential air pollution exposure in a large Southern California pregnancy cohort. Environmental Pollution 254, 113010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jossin Y (2004). Neuronal migration and the role of reelin during early development of the cerebral cortex. Molecular Neurobiology 30, 225–251. [DOI] [PubMed] [Google Scholar]
- Ju LS, Jia M, Sun J, Sun XR, Zhang H, Ji MH, et al. (2016). Hypermethylation of hippocampal synaptic plasticity-related genes is involved in neonatal sevoflurane exposure-induced cognitive impairments in rats. Neurotoxicity Research 29, 243–255. [DOI] [PubMed] [Google Scholar]
- Jung CR, Lin YT, & Hwang BF (2013). Air pollution and newly diagnostic autism spectrum disorders: A population-based cohort study in Taiwan. PLoS One 8 e75510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CR, Lin YT, & Hwang BF (2015). Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: A population-based cohort study in Taiwan. Journal of Alzheimer’s Disease 44, 573–584. [DOI] [PubMed] [Google Scholar]
- Kalkbrenner AE, Schmidt RJ, & Penlesky AC (2014). Environmental chemical exposures and autism spectrum disorders: A review of epidemiological evidence. Current Problems in Pediatric and Adolescent Health Care 44, 277–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkbrenner AE,Windham GC, Serre ML, Akita Y,Wang X, & Hoffman K (2015). Particulate matter exposure, prenatal and postnatal windows of susceptibility, and autism spectrum disorders. Epidemiology 26, 30–42. [DOI] [PubMed] [Google Scholar]
- Kampa M, & Castanas E (2008). Human health effects of air pollution. Environmental Pollution 151, 362–367. [DOI] [PubMed] [Google Scholar]
- Karim S, Mirza Z, Ansari SA, Rasool M, Igbal Z, Sohrab SS, et al. (2014). Transcriptomics study of neurodegenerative disease: Emphasis on synaptic dysfunction mechanism in Alzheimer’s disease. CNS & Neurological Disorders Drug Targets 13, 1202–1212. [DOI] [PubMed] [Google Scholar]
- Kasdagli MI, Katsouyanni K, Dimakopoulou K, & Samoli E (2019). Air pollution and Parkinson’s disease: A systematic review and meta-analysis up to 2018. International Journal of Hygiene and Environmental Health 222, 402–409. [DOI] [PubMed] [Google Scholar]
- Katsuyama Y, & Terashima T (2009). Developmental anatomy of reeler mutant mouse. Development, Growth & Differentiation 51, 271–286. [DOI] [PubMed] [Google Scholar]
- Kaufman JA,Wright JM, Rice G, Connolly N, Bowers K, & Anixt J (2019). Ambient air and fine particulate matter exposures and autism spectrum disorder in metropolitan Cincinnati, Ohio. Environmental Research 171, 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SS, & Bloom GS (2016). Tau: The center of signaling nexus in Alzheimer’s disease. Frontiers in Neuroscience 10 Art. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Khan SA, Narendra AR, Mushtaq G, Zahran SA, Khan S, et al. (2016). Alzheimer’s disease and autistic spectrum disorder: Is there any association? CNS & Neurological Disorders Drug Targets 15, 390–402. [DOI] [PubMed] [Google Scholar]
- Kicinski M, Vermeir G, Van Larebeke N, Den Hond E, Schoeters G, Bruckers L, et al. (2015). Neurobehavioral performance in adolescents is inversely associated with traffic exposure. Environment International 75, 136–143. [DOI] [PubMed] [Google Scholar]
- Kilian J, & Kitazawa M (2018). The emerging risk of exposure to air pollution on cognitive decline and Alzheimer’s disease-evidence from epidemiological and animal studies. Biomed. J 41, 141–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killin LOJ, Starr JM, Shiue II, & Russ TC (2016). Environmental risk factors for dementia: A systematic review. BMC Geriatrics 16, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Knight EM, Saunders EL, Cuevas AK, Popovech M, Chen LC, et al. (2012). Rapid doubling of Alzheimer’s amyloid-β40 and 42 levels in brain of mice exposed to a nickel nanoparticle model of air pollution. F1000 Research 1, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Jeon YS, & Chun J (2013). Current status and future promise of the human microbiome. Pediatr. Gastroenterol. Hepatol. Nutr 16, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioumourtzoglou MA, Schwartz JD,Weisskopfs MG, Melly SJ, Wang Y, Dominici F, et al. (2016). Long-term PM2.5 exposure and neurological hospital admissions in the northeastern United States. Environ. Health Perspectives 124, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirrane EF, Bowman C, Davis JA, Hoppin JA, Blair A, Chen H, et al. (2015). Associations of ozone and PM2.5 concentrations with Parkinson’s disease among participants in the agricultural health study. Journal of Occupational and Environmental Medicine 57, 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klocke C, Sherina V, Graham UM, Gundersson J, Allen JL, Sobolewski M, et al. (2018). Enhanced cerebellar myelination with concomitant iron elevation and ultrastructural irregularities following prenatal exposures to ambient particulate matter in the mouse. Inhalation Toxicology 30, 381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocherhans S, Madhusudan A, Doehner J, Breu KS, Nitsch RM, Fritschy JM, et al. (2010). Reduced reelin expression accelerates amyloid-β plaque formation and tau pathology in transgenic Alzheimer’s disease mice. The Journal of Neuroscience 30, 9228–9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeman T, Schouten LJ, van den Brandt PA, Slottje P, Huss A, Peters S, et al. (2015). Occupational exposures and risk of dementia-related mortality in the prospective Netherlands cohort study. American Journal of Industrial Medicine 58, 625–635. [DOI] [PubMed] [Google Scholar]
- Kramer PL, Xu H,Woltjer RL,Westaway SK, Clark D, Erten-Lyons D, et al. (2010). Alzheimer’s disease pathology in cognitive healthy elderly: A genome-wide study. Neurobiology of Aging 32, 2113–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krstic D, Madhusudan A, Doehner J, Vogel P, Notter T, Imhof C, et al. (2012). Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. Journal of Neuroinflammation 9, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesza RJ, Lukose R, & Stevens LV (2011). Malformation of the human superior olive in autistic spectrum disorder. Brain Research 1367, 360–371. [DOI] [PubMed] [Google Scholar]
- Kulick ER, Elkind MSV, Boehme AK, Joyce NR, Schupf N, Kaufman JD, et al. (2020). Long-term exposure to ambient air pollution, APO-ε4 status, and cognitive decline in a cohort of older adults in northern Manhattan. Environment International 136, 105440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajud N, & Torner L (2015). Early life stress and hippocampal neurogenesis in the neonate: Sexual dimorphism, long term consequences and possible mediators. Frontiers in Molecular Neuroscience 8, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KS, Aman MG, & Arnold LE (2006). Neurochemical correlates of autistic disorder. A review of the literature. Rev. Dev. Disab 27, 254–289. [DOI] [PubMed] [Google Scholar]
- Lammert DB, & Howell BW (2016). RELN Mutations in autism spectrum disorder. Frontiers in Cellular Neuroscience 10 art. 84 doi:103389/fncel.2016.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ (2010). What causes autism? Exploring the environmental contribution. Curr. Op. Pediatr 22, 219–225. [DOI] [PubMed] [Google Scholar]
- Landrigan P (2017). Air pollution and health. The Lancet Public Health 2, e4–e5. [DOI] [PubMed] [Google Scholar]
- Lee PC, Raaschou-Nielsen O, Lill CM, Bertram L, Sinsheimer JS, Hansen J, et al. (2016a). Gene-environment interactions linking air pollution and inflammation in Parkinson’s disease. Environmental Research 151, 713–720. [DOI] [PubMed] [Google Scholar]
- Lee S, Lee W, Kim D, Kim E, Myung W, Kim SY, et al. (2019). Short-term PM2.5 exposure and emergency hospital admissions for mental disease. Environmental Research 171, 313–320. [DOI] [PubMed] [Google Scholar]
- Lema-Tome’ CM, Tyson T, Rey NL, Grathwohl S, Britschgi M, & Brundin P. (2013). Inflammationa and alpha-synuclein prion-like behavior in Parkinson’s disease – is there a link? Molecular Neurobiology 47, 561–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque S, Taetzsch T, Lull ME, Kodavanti U, Stadler K, Wagner A, et al. (2011b). Diesel exhaust activates and primes microglia: Air pollution, neuroinflammation, and regulation of dopaminergic neurotoxicity. Environmental Health Perspectives 119, 1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy SE, Mandell DS, & Schultz RT (2009). Autism. Lancet 374, 1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz J, & Maher P (2015). Chronic glutamate toxicity in neurodegenerative diseases-what is the evidence? Frontiers in Neuroscience 9 art. 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Lim C, & Yu LE (2009). Composition and size distribution of metals in diesel exhaust particulates. Journal of Environmental Monitoring 11, 1614–1621. [DOI] [PubMed] [Google Scholar]
- Lintas C, Sacco R, & Persico AM (2016). Differential methylation at the RELN gene promoter in temporal cortex from autistic and typically developing post-puberal subjects. Journal of Neurodevelopmental Disorders 8(18), 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Huang Y, Zhang F, Chen Q, Wu B, Rui W, et al. (2015). Macrophages treated with particulate matter PM2.5 induce selective neurotoxicity through glutaminase-mediated glutamate generation. Journal of Neurochemistry 134, 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Young MT, Chen JC, Kaufman JD, & Chen H (2016). Ambient air pollution exposures and risk of Parkinson disease. Environmental Health Perspectives 124, 1759–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead JJ, McCaffrey G, Quigley CE, Finch J, DeMarco KM, Nametz N, et al. (2010). Oxidative stress increases blood-brain barrier permeability and induces alterations in occluding during hypoxia-reoxygenation. Journal of Cerebral Blood Flow and Metabolism 30, 1625–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loop MS, Kent ST, Al-Hamdan MZ, Crosson WL, Estes SM, Ester MG, et al. (2013). Fine particulate matter and incident cognitive impairment in the Reasons for Geographic and Racial Differences in stroke (REGARDS) cohort. PLoS One 8 e75001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini RG, Dorman DC, Elder A, & Veronesi B (2012). Neurological impacts from inhalation of pollutants and the nose-brain connection. Neurotoxicology 33, 838–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lull ME, & Block ML (2010). Microglial activation and chronic neurodegeneration. Neurotherapeutics 7, 354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Schmidt RJ, & Hertz-Picciotto I (2014). Maternal lifestyle and environmental risk factors for autism spectrum disorders. International Journal of Epidemiology 43, 443–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, & Klann E (2012).Amyloid beta: Linking synaptic plasticity failure to memory disruption in Alzheimer’s disease. Journal of Neurochemistry 120(Suppl. 1), 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher BA, Ahmed IAM, Karloukovski V, MacLaren DA, Foulds PG, Allsop D, et al. (2016). Magnetite pollution nanoparticles in the human brain. Proceedings of the National Academy of Sciences of the United States of America 113, 10797–10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova NV, Yu CZ, Hsiao EY, Moore MJ, & Patterson PH (2012). Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain, Behavior, and Immunity 26, 607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoharan S, Guillemein GJ, Abiramasundari RS, Essa MM, Akbar M, & Akbar MD (2016). The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: amini review. Oxidative Medicine and Cellular Longevity 2016, 8590578 10.1155/2016/8590578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresova P, Klimova B, Novotny M, & Kuca K (2016). Alzheimer’s and Parkinson’s diseases: Expected economic impact on Europe-a call for a uniform European strategy. Journal of Alzheimer’s Disease 54, 1123–1133. [DOI] [PubMed] [Google Scholar]
- Marxreiter F, Regensburger M, & Winkler J (2013). Adult neurogenesis in Parkinson’s disease. Cellular and Molecular Life Sciences 70, 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinn LA, Windham GC, Kalkbrenner AE, Bradley C, Di Q, Croen LA, et al. (2020). Early life exposure to air pollution and autism spectrum disorder. Findings from a multisite case-control study. Epidemiology 31, 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam RI, Moberg PJ, Mahr RN, & Doty RL (1998). Olfaction in neurodegenerative disease. A meta-analysis of olfactory functioning in Alzheimer’s and Parkinson’s diseases. Archives of Neurology 55, 84–90. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, et al. (2006). The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. The Journal of Neuroscience 26, 4752–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani C, Corsetto PA, Farina F, Botto L, Lonati E, Massimino L, et al. (2018). Early evidence of stress in immortalized neurons exposed to diesel particles: The role of lipid reshaping behind oxidative stress and inflammation. Toxicology 409, 63–72. [DOI] [PubMed] [Google Scholar]
- Minakova E, & Warner BB (2018). Maternal immune activation, central nervous system development and behavioral phenotypes. Birth Defects Res. 110, 1539–1550. [DOI] [PubMed] [Google Scholar]
- Minter MR, Taylor JM, & Crack PJ (2016). The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease. Journal of Neurochemistry 136, 457–474. [DOI] [PubMed] [Google Scholar]
- Miri M, Nazarzadeh M, Alahabadi A, Ehrampoush MH, Rad A, Lofti MH, et al. (2019). Air pollution and telomere length in adults: A systematic review and meta-analysis of observational studies. Environmental Pollution 244, 636–647. [DOI] [PubMed] [Google Scholar]
- MohanKumar SMJ, Campbell A, Block ML, & Veronesi B (2008). Particulate matter, oxidative stress and neurotoxicity. Neurotoxicology 29, 479–488. [DOI] [PubMed] [Google Scholar]
- Morgan JD, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, et al. (2010). Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biological Psychiatry 68, 368–376. [DOI] [PubMed] [Google Scholar]
- Morgan TE, Davis DA, Iwata N, Tanner JA, Snyder D, Ning Z, et al. (2011). Glutamatergic neurons in rodent models respond to nanoscale particulate urban air pollutants in vivo and in vitro. Environmental Health Perspectives 119, 1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris-Schaffer K, Merrill AK, Wong C, Jew K, Sobolewski M, & Cory-Slechta DA (2019). Limited developmental neurotoxicity from neonatal inhalation exposure to diesel exhaust particles in C57BL/6 mice. Particle and Fibre Toxicology 16, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota SI, Ferreira IL, & Rego AC (2014b). Dysfunctional synapse in Alzheimer’s disease-a focus on NMDA receptors. Neuropharmacology 76(pt. A), 16–26. [DOI] [PubMed] [Google Scholar]
- Mottahedin A, Ardalan M, Chumak T, Riebe I, Ek J, &Mallard C. (2017). Effect of neuroinflammation on synaptic organization and function in the developing brain: Implications for neurodevelopmental and neurodegenerative disorders. Frontiers in Cellular Neuroscience 11, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton PV, & Yang W (2012). Air pollution, oxidative stress, and Alzheimer’s disease. Journal of Environmental and Public Health 2012, 472751 10.1155/2012/472751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumaw CL, Levesque S, McGraw C, Robertson S, Lucas S, Stafflinger JE, et al. (2006). Microglial priming through the lung-brain axis: The role of air pollution-induced circulating factors. The FASEB Journal 30, 1880–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Royal C, Dupuis J, Groc L, & Oliet SH (2017). Astroglial glutamate transporters in the brain: Regulating neurotransmitter homeostasis and synaptic transmission. Journal of Neuroscience Research 95, 2140–2151. [DOI] [PubMed] [Google Scholar]
- Musgrove RE, Jewell SA, & Di Monte DA (2015). Overview of neurodegenerative disorders and susceptibility factors in neurodegenerative processes In Aschner M, & Costa LG (Eds.), Environmental factors in neurodevelopmental and neurodegenerative disorders (pp. 197–210). New York: Academic Press/Elsevier. [Google Scholar]
- Mutlu EA, Comba IY, Cho T, Engen PA, Yazici C, Soberanes S, et al. (2018). Inhalation exposure to particulate matter air pollution alters the composition of the gut microbiome. Environmental Pollution 240, 817–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli E, Wong S, & Giulivi C (2013). Evidence of reactive oxygen species-mediated damage to mitochondrial DNA damage in children with typical autism. Molecular Autism 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone S, Sams D, Reuveni E, Getselter D, Oron O, Karpuj E, et al. (2014). DNA methylation analysis of the autistic brain reveals multiple dysregulated biological pathways. Translational Psychiatry 4 e433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman NC, Ryan P, LeMasters G, Levin L, Bernstein D, Khurana Hershey GK, et al. (2013). Traffic-related air pollution exposure in the first year of life and behavioral scores at 7 years of age. Environmental Health Perspectives 121, 731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh JS, Sharma RP, Veldic M, Salvacion AA, Jia X, Chen Y, et al. (2005). DNA methyltransferase 1 regulates reelin mRNA expression in mouse primary cortical cultures. Proceedings of the National Academy of Sciences of the United States of America 102, 1749–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouel D, Burt M, Zhang Y,Harvey L, & Boksa P (2012). Prenatal exposure to bacterial endotoxin reduces the number of GAD67- and reelin-immunoreactive neurons in the hippocampus of rat offspring. European Neuropsychopharmacology 22, 300–317. [DOI] [PubMed] [Google Scholar]
- Novais ARB, Guiramand J, Cohen-Sola C, Crouzin N, de Jesus Ferreira MC, Vignes M, et al. (2013). N-acetyl-cysteine prevents pyramidal cell disarray and reelin-immunoreactive neuron deficiency in CA3 after prenatal immune challenge in rats. Pediatric Research 73, 750–755. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, & Utell MJ (2002). Ultrafine particles in the urban air: To the respiratory tract –and beyond? Environmental Health Perspectives 110, A440–A441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, et al. (2004). Translocation of inhaled ultrafine particles to the brain. Inhalation Toxicology 16, 437–445. [DOI] [PubMed] [Google Scholar]
- O’Brien JT, & Thomas A (2015). Vascular dementia. Lancet 386, 1698–1706. [DOI] [PubMed] [Google Scholar]
- Oudin A, Forsberg B, Adolfsson AN, Lind N, Modig L, Nordin M, et al. (2016). Traffic-related air pollution and dementia incidence in northern Sweden: A longitudinal study. Environmental Health Perspectives 124, 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudin A, Frondelius K, Haglund N, Kallen K, Forsberg B, Gustafsson P, et al. (2019). Prenatal exposure to air pollution as a potential risk factor for autism and ADHD. Environment International 133, 105149. [DOI] [PubMed] [Google Scholar]
- Pagalan L, Bickford C, Weikum W, Lanphear B, Brauer M, Lanphear N, et al. (2019). Association of prenatal exposure to air pollution with autism spectrum disorder. JAMA Pediatrics 173, 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios N (2017). Air pollution and Parkinson’s disease – Evidence and future directions. Reviews on Environmental Health 32, 303–313. [DOI] [PubMed] [Google Scholar]
- Palacios N, Fitzgerald KC, Hart JE, Weisskopf MG, Schwarzschild MA, Ascherio A, et al. (2014). Particulate matter and risk of Parkinson’s disease in a large prospective study of women. Environmental Health 13, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P (2019). Tackling Delhi’s air pollution problem. ACS Central Science 5, 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH (2011). Maternal infection and immune involvement in autism. Trends in Molecular Medicine 17, 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul KC, Haan M, Mayeda ER, & Ritz BR (2019). Ambient air pollution, noise, and late-life cognitive decline and dementia risk. Annual Review of Public Health 40, 203–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persico AM, D’Agruma L, Maiorano N, Totaro A, Militerni R, Bravaccio C, et al. (2001). Reelin gene alleles and haplotypes as a factor predisposing to autistic disorder. Molecular Psychiatry 6, 150–159. [DOI] [PubMed] [Google Scholar]
- Peters A, Veronesi B, Calderon-Garciduenas L, Gehr P, Chen LC, Geiser M, et al. (2006). Translocation and potential neurological effects of fine and ultrafine particles a critical update. Particle and Fibre Toxicology 3, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R, Peters J, Booth A, & Mudway I (2015). Is air pollution associated with increased risk of cognitive decline? A systematic review. Age and Ageing 44, 755–760. [DOI] [PubMed] [Google Scholar]
- Petkus AJ, Younan D, Wang X, Serre M, Vizuete W, Resnick S, et al. (2019). Particulate air pollutants and trajectories of depressive symptoms in older women. The American Journal of Geriatric Psychiatry 27, 1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. (1997). Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science 276, 2045–2047. [DOI] [PubMed] [Google Scholar]
- Pope CA, & Dockery DW (2006). Health effects of fine particulate air pollution: Lines that connect. Air Waste Manage. Assoc 56, 709–742. [DOI] [PubMed] [Google Scholar]
- Power MC, Weisskopf MG, Alexeeff SE, Coull BA, Spiro A III, & Schwartz J (2011). Traffic-related air pollution and cognitive function in a cohort of older men. Environmental Health Perspectives 119, 682–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power MC, Adar SD, Yanosky JD, & Weuve J (2016). Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: A systematic review of epidemiologic research. Neurotoxicology 56, 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata J, Santos SG, Almeida MI, Coelho R, & Barbosa MA (2017). Bridging autism spectrum disorders and schizophrenia through inflammation and biomarkers preclinical and clinical investigations. Journal of Neuroinflammation 14, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman P, & Rabinovici GD (2014). Alzheimer’s disease In Aminoff MJ, & Daroff RB (Eds.), The encyclopedia of neurological sciences (2nd ed.)3. (pp. 122–127). New York: Elsevier. [Google Scholar]
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, & Ferri CP (2013). The global prevalence of dementia: A systematic review and meta-analysis. Alzheimer’s & Dementia 9, 63–75. [DOI] [PubMed] [Google Scholar]
- Pujadas L, Rossi D, Andres R, Teixeira CM, Serra-Vidal B, Parcerisa A, et al. (2014). Reelin delays amyloid-beta fibril formation and rescues cognitive deficits in a model of Alzheimer’s disease. Nature Communications 5, 3443. [DOI] [PubMed] [Google Scholar]
- Qian L, Flood PM, & Hong JS (2010). Neuroinflammation is a key player in Parkinson’s disease and a prime target for therapy. Journal of Neural Transmission 117, 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranft U, Schikowski T, Sugiri D, Krutmann J, & Kramer U (2009). Long-term exposure to traffic-related particulate matter impairs cognitive function in the elderly. Environmental Research 109, 1004–1011. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM (2016). How neuroinflammation contributes to neurodegeneration. Science 353, 777–783. [DOI] [PubMed] [Google Scholar]
- Raz R, Roberts AL, Lyall K, Hart JE, Just AC, Laden F, et al. (2015). Autism spectrum disorder and particulate matter air pollution before, during and after pregnancy: A nested case-control analysis within the nurses’ health study II cohort. Environmental Health Perspectives 123, 264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner O, Karzburn E, Kshirsagar A, & Kaibuchi K (2016). Regulation of neuronal migration, an emerging topic in autism spectrum disorders (ASD). Journal of Neurochemistry 136, 440–456. [DOI] [PubMed] [Google Scholar]
- Ribeiro FM, Vieira LB, Pires RG, Olmo RP, & Ferguson SS (2017). Metabotropic glutamate receptors and neurodegenerative diseases. Pharmacological Research 115, 179–191. [DOI] [PubMed] [Google Scholar]
- Rich DQ, Liu K, Zhang J, Thurstin SW, Stevens TP, Pan Y, et al. (2015). Differences in birthweight associated with the 2008 Beijing Olympics air pollution reduction: Results from a natural experiment. Environmental Health Perspectives 123, 880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel BC, Thompson PM, & Brinton RD (2016). Age, APOE and sex: Triad of risk of Alzheimer’s disease. The Journal of Steroid Biochemistry and Molecular Biology 160, 134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Lee PC, Hansen J, Lassen CF, Ketzel M, Sorensen M, et al. (2016). Traffic related air pollution and Parkinson’s disease in Denmark: A case-control study. Environmental Health Perspectives 124, 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Lyall K, Hart JE, Laden F, Just AC, Bobb JF, et al. (2013). Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses’s health study II participants. Environmental Health Perspectives 121, 978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CE, Ratai EM, & Kanwisher N (2016). Reduced GABAergic action in the autistic brain. Current Biology 26, 80–85. [DOI] [PubMed] [Google Scholar]
- Rogers JT, Rusiana I, Trotter J, Zhao L, Donaldson E, Pak DT, et al. (2011). Reelin supplementation enhances cognitive ability, synaptic plasticity, and dendritic spine density. Learning & Memory 18, 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roqué PJ, Dao K, & Costa LG (2016). Microglia mediate diesel exhaust particle-induced cerebellar neuronal toxicity through neuroinflammatory mechanisms. NeuroToxicology 56, 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S, Melnyk S, Pavliv O, Bai S, Nick TG, Frye RE, et al. (2012). Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Translational Psychiatry 2 e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CR (2017). Gut dysbiosis in animals due to environmental chemical exposures. Frontiers in Cellular and Infection Microbiology 7 Art. 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol DA, Genuis SJ, & Frye RE (2014). Environmental toxicants and autism spectrum disorders: A systematic review. Translational Psychiatry 4 e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JLR, & Merzenich MM (2003). Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes, Brain, and Behavior 2, 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Perez JM, & Morillas-Ruiz JM (2012). A review: inflammatory process in Alzheimer’s disease, role of cytokines. Scientific World Journal 2012, 756357 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy CC, Hunsberger HC,Weitzner DS, & Reed MN (2015). The role of the tripartite glutamatergic synapse in the pathophysiology of Alzheimer’s disease. Aging and Disease 6, 131–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ TC, Reis S, & van Tongeren M (2019). Air pollution and brain health: Defining the research agenda. Curr. Op. Psychiatr 32, 97–104. [DOI] [PubMed] [Google Scholar]
- Saez M, Barcelo MA, Farrerons M, & Lopez-Casasnovas G (2018). The association between exposure to environmental factors and the occurrence of attention-deficit/hyperactivity disorder (ADHD). A population-based retrospective cohort study. Environmental Research 156, 2015–2214. [DOI] [PubMed] [Google Scholar]
- Sakai K, Shoji H, Kohno T, Miyakawa T, & Hattori M (2016). Mice that lack the C-terminal region of reelin exhibit behavioral abnormalities related to neuropsychiatric disorders. SC Reports 6, 28636 10.1038/srep28636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim SY, Kaplan GG, & Madsen KL (2014). Air pollution effects on the gut microbiota. Gut Microbes 5, 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. (2016). Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, & Reichenberg A (2014). The familial risk of autism. JAMA 311, 1770–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaafsma SM, & Pfaff DW (2014). Etiologies underlying sex differences in autism spectrum disorders. Frontiers in Neuroendocrinology 35, 255–271. [DOI] [PubMed] [Google Scholar]
- Scheltens P, Blennow K, Breteler MBB, de Strooper B, Salloway S, & Van der Flier WM (2016). Alzheimer’s disease. Lancet 388, 505–517. [DOI] [PubMed] [Google Scholar]
- Schembari A, de Hoogh K, Pedersen M, Dadvand P, Martinez D, Hoelk G, et al. (2015). Ambient air pollution and newborn size and adiposity at birth: Difference by maternal ethnicity (The Born in Bradford study cohort). Environmental Health Perspectives 123, 1208–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikowski T, Vossoughi M, Vierkötter A, Schulte T, Teichert T, Sugiri D, et al. (2015). Association of air pollution with cognitive functions and its modification by APOE gene variants in elderly women. Environmental Research 142, 10–16. [DOI] [PubMed] [Google Scholar]
- Schwartz JM, & Bilbo SD (2012). Sex, glia, and development: Interactions in health and disease. Hormones and Behavior 62, 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine K, Kubo K, & Nakajima K (2014). How does reelin control neuronal migration and layer formation in the developing mammalian cortex? Neuroscience Research 86, 50–58. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, & Hardy J (2016). The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Molecular Medicine 8, 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender R, Fuchs S, & Milo R (2016). Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 164, 337–340. [DOI] [PubMed] [Google Scholar]
- Serajee FJ, Zhong H, & Huq AHMM (2006). Association of reelin polymorphisms with autism. Genomics 87, 75–83. [DOI] [PubMed] [Google Scholar]
- Sgritta M, Dooling SW, Buffington SA, Momin EN, Francis MB, Britton RA, et al. (2018). Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron 101, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SR, Gonda X, & Tarazi FI (2018). Autism spectrum disorder: Classification, diagnosis and therapy. Pharmacology & Therapeutics 190, 91–104. [DOI] [PubMed] [Google Scholar]
- Shaun N, & Thomas B (2012). The STAT3-DMNT1 connection. JAK-STAT 1(4), 257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou Y, Huang Y, Zhu X, Liu C, & Hu Y7 Wang H (2019). A review of the possible associations between ambient PM2.5 exposures and the development of Alzheimer’s disease. Ecotoxicology and Environmental Safety 174, 344–352. [DOI] [PubMed] [Google Scholar]
- Sobolewski M, Anderson T, Conrad K, Marvin E, Klocke C, Morris-Schaffer K, et al. (2018). Developmental exposures to ultrafine particle air pollution reduces early testosterone levels and adult male social novelty preference: Risk for children’s sex-biased neurobehavioral disorders. Neurotoxicology 68, 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sram RJ, Veleminsky M, Veleminsky M, & Stejskalova J (2017). The impact of air pollution to central nervous system in children and adults. Neuroendocrinology Letters 38, 389–396. [PubMed] [Google Scholar]
- Stanislaus A, Marafi A, & Rana MS (2010). Recent advances in the science and technology of ultra-low sulfur diesel (ULSD) production. Catalysis Today 153, 1–68. [Google Scholar]
- Stoner R, Chow ML, Boyle MP, Sunkin SM, Mouton PR, Roy S, et al. (2014). Patches of disorganization in the neocortex of children with autism. New Engl. J. Med 370, 1209–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Haberman RP, & Gallagher M (2011). Cognitive decline is associated with reduced reelin expression in the entorhinal cortex of aged rats. Cerebral Cortex 21, 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su D, Serafino A, Müller JO, Jentoft R, Schlögl R, & Fiorito S (2008). Cytotoxicity and inflammatory potential of soot particles of low-emission diesel engines. Environmental Science & Technology 42, 1761–1765. [DOI] [PubMed] [Google Scholar]
- Sunyer J, Esnaola M, Alvarez-Pedrerol M, Forns J, Rivas I, Lopez-Vicente M, et al. (2015). Association between traffic-related air pollution in schools and cognitive development in primary school children: A prospective cohort study. PLoS Medicine 12 e1001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Oshio S, Iwata M, Saburi H, Odagiri T, Udagawa T, et al. (2010). In utero exposure to a low concentration of diesel exhaust affects spontaneous locomotor activity and monoaminergic system in male mice. Particle and Fibre Toxicology 7, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Sugihara G, Ouchi Y, Nakamura K, Futatsubashi M, Takebayashi K, et al. (2013). Microglial activation in young adults with autism spectrum disorder. JAMA Psychtr. 70, 49–58. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Takayanagi K, Akimoto A, Ueda K, Shinkai Y, Umezawa M, et al. (2015). Prenatal diesel exhaust exposure disrupts the DNA methylation profile in the brain of mouse offspring. The Journal of Toxicological Sciences 40, 1–11. [DOI] [PubMed] [Google Scholar]
- Tai LM, Ghura S, Koster KP, Liakaite V, Maiensen-Cline M, Kanabar P, et al. (2015). APOE-modulated Aβ-induced neuroinflammation in Alzheimer’s disease: Current landscape, novel data, and future perspective. Journal of Neurochemistry 133, 465–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Ko LW, Kulathingal J, Jiang P, Sevlever D, & Yen SHC (2007). Oxidative stress-induced phosphorylation, degradation and aggregation of α-synuclein are linked to upregulated CK2 and cathepsin D. The European Journal of Neuroscience 26, 863–874. [DOI] [PubMed] [Google Scholar]
- Talbott EO, Arena VC, Rager JR, Clougherty JE, Michanowicz DR, Sharma RK, et al. (2015). Fine particulate matter and the risk of autism spectrum disorders. Environmental Research 140, 414–420. [DOI] [PubMed] [Google Scholar]
- Tetreault NA, Hakeem AY, Jiang S, Williams BA, Allman E, Wold BJ, et al. (2012). Microglia in the cerebral cortex in autism. Journal of Autism and Developmental Disorders 42, 2569–2584. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Asadi S, & Patel AB (2013). Focal brain inflammation and autism. Journal of Neuroinflammation 10, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirtamara Rajamani K, Doherty-Lyons S, Bolden C, Willis D, Hoffman C, Zelikoff J, et al. (2013). Prenatal and early life exposure to high level diesel exhaust particles leads to increased locomotor activity and repetitive behaviors in mice. Autism Research 6, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K (2014). The effects of gut microbiota on CNS function in humans. Gut Microbes 5, 404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonne C, Elbaz A, Beevers S, & Singh-Manoux A (2014). Traffic-related air pollution in relation to cognitive function in older adults. Epidemiology 25, 674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Rojas C, & Jones BC (2018). Sex differences in neurotoxicogenetics. Frontiers in Genetics 9 Art. 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukue N,Watanabe M, Kumamoto T, Takano H, & Takeda K (2009). Perinatal exposure to diesel exhaust affects gene expression in mouse cerebrum. Archives of Toxicology 83, 985–1000. [DOI] [PubMed] [Google Scholar]
- Tzivian L, Dlugaj M,Winkler A, Weinmayr G, Hennig F, Fuks KB, et al. (2016). Long-term air pollution and traffic noise exposures and mild cognitive impairment in older adults: A cross-sectional analysis of the Heinz Nixdorf recall study. Environmental Health Perspectives 124, 1361–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulusoy A, & Di Monte DA (2013). Alpha-synuclein elevation in human neurodegenerative diseases: Experimental, pathogenetic, and therapeutic implications. Molecular Neurobiology 47, 484–494. [DOI] [PubMed] [Google Scholar]
- USEPA (United States Environmental Protection Agency) (2002). Health assessment document for diesel engine exhaust. Washington, DC: National Center for Environmental Assessment, USEPA, 669. [Google Scholar]
- Uzunova G, Pallanti S, & Hollander E (2016). Excitatory/inhibitory imbalance in autism spectrum disorders: Implications for interventions and therapeutics. The World Journal of Biological Psychiatry 17, 174–186. [DOI] [PubMed] [Google Scholar]
- Valand R, Magnusson P, Dziendzikowska K, Gajewska M, Wilczak J, Oczkowski M, et al. (2018). Gene expression changes in rat brain regions after 7- and 28-days inhalation exposure to exhaust emissions from 1st and 2nd generation biodiesel fuels – The FuelHealth project. Inhalation Toxicology 30, 299–312. [DOI] [PubMed] [Google Scholar]
- Valles Y, & Francino MP (2018). Air pollution, early life microbiome, and development. Curr. Environ. Health Rep 5, 512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, et al. (2003). Incidence of Parkinson’s disease: Variation by age, gender, and race/ethnicity. American Journal of Epidemiology 157, 1015–1022. [DOI] [PubMed] [Google Scholar]
- Verboven F (2002). Quality-based price discrimination and tax incidence: Evidence from gasoline and diesel cars. The RAND Journal of Economics 33, 275–297. [Google Scholar]
- Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, & McConnell R (2011). Residential proximity to freeways and autism in the CHARGE study. Environmental Health Perspectives 119, 873–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, & McConnell R (2013). Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry 70, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker Z, Possin KL, Boeve BF, & Aarsland D (2015). Lewy body dementias. Lancet 386, 1683–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Hong Y, Zou L, Zhong R, Zhu B, Shen N, et al. (2014). Reelin gene variants and risk of autism spectrum disorders: An integrated meta-analysis. American Journal of Medical Genetics, 192-200 Pt B 165B. [DOI] [PubMed] [Google Scholar]
- Ward A, Crean S, Mercald CJ, Collins JM, Boyd D, Cook MN, et al. (2012). Prevalence of apolipoprotein E4 genotype and homozygotes (APOE e4/4) among patients diagnosed with Alzheimer’s disease: A systematic review and meta-analysis. Neuroepidemiology 38, 1–17. [DOI] [PubMed] [Google Scholar]
- Warner BB (2019). The contribution of the gut microbiome to neurodevelopmental and neuropsychiatric disorders. Pediatric Research 85, 216–224. [DOI] [PubMed] [Google Scholar]
- Weeber EJ, Beffert U, Jones C, Christian JM, Forster E, Sweatt JD, et al. (2002). Reelin and APoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. The Journal of Biological Chemistry 277, 39944–39952. [DOI] [PubMed] [Google Scholar]
- Wegiel J, Kuchna I, Nowicki K, Imaki H,Wegiel J, Marchi E, et al. (2010). The neuropathology of autism: Defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathologica 119(755-770), 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenius GA, Boyle LD, Coull BA, Milberg WP, Gryparis A, Schwartz J, et al. (2012). Residential proximity to nearest major roadway and cognitive function in community-dwelling seniors: Results from the MOBILIZE Boston study. Journal of the American Geriatrics Society 60, 2075–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, & Grodstein F (2012). Exposure to particulate air pollution and cognitive decline in older women. Archives of Internal Medicine 172, 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilker EH, Mertinez-Ramirez S, Kloog I, Schwarz J, Motofsky E, Koutrakis P, et al. (2016). Fine particulate matter, residential proximity to major roads, and markers of small vessel disease in a memory study population. Journal of Alzheimer’s Disease 53, 1315–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingate M, Mulvihill B, Kirby RS, Pettygrove S, Cunniff C, Meaney F, et al. (2012). Prevalence of autism spectrum disorders-autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR Surveillance Summaries 61, 1–19. [PubMed] [Google Scholar]
- Win-Shwe TT, & Fujimaki H (2011). Nanoparticles and neurotoxicity. International Journal of Molecular Sciences 12, 6267–6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win-Shwe TT, Yamamoto S, Fujitani Y, Hirano S, & Fujimaki H (2008). Spatial learning and memory function-related gene expression in the hippocampus of mouse exposed to nanoparticles-rich diesel exhaust. Neurotoxicology 29, 940–947. [DOI] [PubMed] [Google Scholar]
- Win-Shwe TT, Mitsushima D, Yamamoto S, Fujitani Y, Funabashi T, Hirano S, et al. (2009). Extracellular glutamate level and NMDA receptor subunit expression in mouse olfactory bulb following nanoparticle-rich diesel exhaust exposure. Inhalation Toxicology 21, 828–836. [DOI] [PubMed] [Google Scholar]
- Win-Shwe TT, Fujitani Y, Kyi-Tha-Thu C, Furuyama A, Michikawa T, Tsukahara S, et al. (2014). Effects of diesel engine exhaust origin secondary organic aerosols on novel object recognition ability and maternal behavior in BALB/C mice. International Journal of Environmental Research and Public Health 11, 11286–11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward NC, Haghani A, Johnson RG, Hsu TM, Saffari A, Sioutas C, et al. (2018). Prenatal and early life exposure to air pollution induced hippocampal vascular leakage and impaired neurogenesis in association with behavioral deficits. Translational Psychiatry 8, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YC, Lin YC, Yu HL, Chen JH, Chen TF, Sun Y, et al. (2015). Association between air pollutants and dementia risk in the elderly. Alzheimers Dement 1, 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurth R, Kioumourtzoglou MA, Tucker KL, Griffith J, Manjourides J, & Suh H (2018). Fine particle sources and cognitive function in an older Puerto Rican cohort in greater Boston. Environmental Epidemiolgy 2 e022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, & Wang Q (2016). Towards understanding brain-gut-microbiome connections in Alzheimer’s disease. BMC Systems Biology 10(Suppl. 3), 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Strathearn L, Liu B, O’Brien M, Kopelman TG, Zhu J, … Bao W. (2018). Prevalence and treatment patterns of autism spectrum disorder in the United States, 2016. JAMA Pediatrics 173, 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Huang Y, Zhao L, Li Y, Sun L, Zhou Y, et al. (2013). IL-1β and TNF-α induce neurotoxicity through glutamate production: A potential role for neuronal glutaminase. Journal of Neurochemistry 125, 897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Mizuo K, Moriya N, Oshio S, Sugawara I, & Takeda K (2009). Effect of prenatal exposure to diesel exhaust on dopaminergic system in mice. Neuroscience Letters 449, 38–41. [DOI] [PubMed] [Google Scholar]
- Yokota S, Moriya N, Iwata M, Umezawa M, Oshio S, & Takeda K (2013). Exposure to diesel exhaust during fetal period affects behavior and neurotransmitters in male offspring mice. The Journal of Toxicological Sciences 38, 13–23. [DOI] [PubMed] [Google Scholar]
- Younan D, Petkus AJ, Widaman KF, Wang X, Casanova R, Espeland MA, et al. (2020). Particulate matter and episodic memory decline mediated by early neuroanatomic biomarkers of Alzheimer’s disease. Brain 143, 289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefian F, Mahvi AH, Yunesian M, Hassanvand MS, Kashani H, & Amini H (2018). Long-term exposure to ambient air pollution and autism spectrum disorder in children: A case-control study in Tehran, Iran. Sci. Tot. Environ 643, 1216–1222. [DOI] [PubMed] [Google Scholar]
- Yu NN, Tan MS, Yu JT, Xie AM, & Tan L (2016). The role of reelin signaling in Alzheimer’s disease. Molecular Neurobiology 53, 5692–5700. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Dominici A, Wang Y, & Schwartz JD (2014). A national case-crossover analysis of the short-term effect of PM2.5 on hospitalizations and mortality in subjects with diabetes and neurological disorders. Environmental Health 13, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Gu D, Purser J, Hoenig H, & Christakis N (2010). Associations of environmental factors with elderly health and mortality in China. American Journal of Public Health 100, 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Cao Y, Qiao X, Seyler BC, & Tang Y (2019). Air pollution reduction in China: Recent success but great challenge for the future. Sci. Tot. Environ 663, 329–337. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li P, Feng J, & Wu M (2016). Dysfunction of NMDA receptors in Alzheimer’s disease. Neurological Sciences 37, 1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]


