Abstract
Intravenous fluid administration should be considered as any other pharmacological prescription. There are three main indications: resuscitation, replacement, and maintenance. Moreover, the impact of fluid administration as drug diluent or to preserve catheter patency, i.e., fluid creep, should also be considered. As for antibiotics, intravenous fluid administration should follow the four Ds: drug, dosing, duration, de-escalation. Among crystalloids, balanced solutions limit acid–base alterations and chloride load and should be preferred, as this likely prevents renal dysfunction. Among colloids, albumin, the only available natural colloid, may have beneficial effects. The last decade has seen growing interest in the potential harms related to fluid overloading. In the perioperative setting, appropriate fluid management that maintains adequate organ perfusion while limiting fluid administration should represent the standard of care. Protocols including a restrictive continuous fluid administration alongside bolus administration to achieve hemodynamic targets have been proposed. A similar approach should be considered also for critically ill patients, in whom increased endothelial permeability makes this strategy more relevant. Active de-escalation protocols may be necessary in a later phase. The R.O.S.E. conceptual model (Resuscitation, Optimization, Stabilization, Evacuation) summarizes accurately a dynamic approach to fluid therapy, maximizing benefits and minimizing harms. Even in specific categories of critically ill patients, i.e., with trauma or burns, fluid therapy should be carefully applied, considering the importance of their specific aims; maintaining peripheral oxygen delivery, while avoiding the consequences of fluid overload.
Keywords: Fluid therapy, Intensive care units, Resuscitation, Maintenance, Water–electrolyte balance, Goal-directed, Crystalloids, Acid base, Sodium, Chloride
Introduction
Intravenous fluids have been in clinical use for over a century, yet the medical and scientific community have only recently begun to appreciate the importance of judicious fluid administration, the necessity to handle them as any other drug we prescribe [1–4], and the considerable side effects with which they may be associated [5, 6].
Three major indications exist for intravenous fluid administration [1, 4, 7–9]: resuscitation, replacement, and maintenance. Resuscitation fluids are used to correct an intravascular volume deficit or acute hypovolemia; replacement solutions are prescribed to correct existing or developing deficits that cannot be compensated by oral intake alone [6]; maintenance solutions are indicated in hemodynamically stable patients that are not able/allowed to drink water in order to cover their daily requirements of water and electrolytes [10, 11]. In addition to these classical indications, the quantitative relevance of fluids administered as drug diluents and to guarantee catheter patency, the so-called fluid creep, has been recently underlined [12, 13].
Although the use of intravenous fluids is one of the most common interventions in medicine, the ideal fluid does not exist. In light of recent evidence, a reappraisal of how intravenous fluids should be used in the perioperative and critical care setting is warranted. Here, we present the executive summary on this area of the International Fluid Academy (https://www.fluidacademy.org).
The four Ds of fluid management
Similarly to antibiotics, the 4 Ds of fluid therapy need to be considered (Table 1) [4].
Table 1.
Analogy between the 4 Ds of antibiotic and fluid therapy Stewardship.
Adapted from Malbrain M.L.N.G. et al. [4] with permission
| Description | Antibiotics | Fluids | |
|---|---|---|---|
| Drug | Inappropriate therapy | More organ failure, longer ICU/hospital length of stay, longer duration mechanical ventilation (MV) | Hyperchloremic metabolic acidosis, more acute kidney injury, more need for renal replacement therapy, increased mortality |
| Appropriate therapy | Key factor in empiric AB choice is consideration of patient risk factors (prior AB use, duration of mechanical ventilation, corticosteroids, recent hospitalization, residence in nursing home, etc.) | Key factor in empiric fluid therapy is consideration of patient risk factors (fluid balance, fluid overload, capillary leak, source control, kidney function, organ function). Do not use glucose as a resuscitation fluid | |
| Combination therapy | Possible benefits: broader spectrum, synergy, avoidance of emergency of resistance, less toxicity | Possible benefits: specific fluids for different indications (replacement vs maintenance vs resuscitation), less toxicity | |
| Appropriate timing | Survival decreases with 7% per hour delay. Needs discipline and practical organization | In refractory shock early goal-directed therapy (EGDT) has proven beneficial. The longer the delay, the more microcirculatory hypoperfusion | |
| Dosing | Pharmacokinetics | Depends on distribution volume, clearance (kidney and liver function), albumin level, tissue penetration | Depends on type of fluid: glucose remains 10% intravascular, crystalloids 25%, vs colloids 100% after 1 h, and other factors (distribution volume, osmolality, oncoticity, kidney function) |
| Pharmacodynamics | Reflected by the minimal inhibitory concentration. Reflected by “kill” characteristics, time (T > MIC) vs concentration (Cmax/MIC) dependent | Depends on type of fluid and where you want them to go: intravascular (resuscitation), interstitial vs intracellular (cellular dehydration) | |
| Toxicity | Some ABs are toxic for kidneys, advice on dose adjustment needed. However, not getting infection under control is not helping the kidneys either | Some fluids (HES—starches) are toxic for the kidneys. However, not getting shock under control is not helping the kidneys either | |
| Duration | Appropriate duration | No strong evidence but trend toward shorter duration. Do not use ABs to treat fever, CRP, infiltrates, but use ABs to treat infections | No strong evidence but trend toward shorter duration. Do not use fluids to treat low central venous or mean arterial pressure, urine output, but use fluids to treat hypovolemia |
| Treat to response | Stop ABs when signs and symptoms of active infection resolve. Future role for biomarkers (PCT) | Fluids can be stopped when shock is resolved (normal lactate). Future role for biomarkers (NGAL, cystatin C, citrullin, L-FABP) | |
| De-escalation | Monitoring | Take cultures first and have the guts to change a winning team | After stabilization with early adequate fluid management (normal PPV, normal cardiac output, normal lactate), stop ongoing resuscitation and move to conservative late fluid management and late goal-directed fluid removal (= deresuscitation) |
AB antibiotic, Cmax maximal peak concentration, CRP C reactive protein, EGDT early goal-directed therapy, HES hydroxyl-ethyl starch, L-FABP L-type fatty acid-binding protein, MIC mean inhibitory concentration, MV mechanical ventilation, NGAL neutrophil gelatinase-associated lipocalin, PCT procalcitonin, PPV pulse pressure variation
Drug
Fluids are drugs with indications, contraindications, and side effects. Different indications need different types of fluids, e.g., resuscitation fluids should focus on rapid restoration of circulating volume; replacement fluids must mimic the fluid that has been lost; maintenance fluids must deliver basic electrolytes and glucose for metabolic needs.
Dosing
The dose makes the poison, as stated by Paracelsus. However, timing and administration rate are equally important for fluids [14, 15]. Of note, in contrast to most drugs, there is no standard therapeutic dose for fluids.
Duration
The duration of fluid therapy is crucial and volume must be tapered when shock is resolved. However, while “starting triggers” for fluid resuscitation are quite clear, clinicians are less aware of “stopping triggers” of fluid resuscitation.
De-escalation
The final step in fluid therapy is to withhold/withdraw fluids when they are no longer required, thus reducing the risk of fluid overload and related deleterious effects [16].
Balanced solutions
The basics
Intravenous “balanced” solutions include crystalloids and colloids with minimal effect on the homeostasis of the extracellular compartment, and in particular on acid–base equilibrium and electrolyte concentrations [3]. In addition, the term “balanced” has been recently applied also to fluids with a low chloride content (Cl−). Therefore, there are two main categories of balanced solutions (Table 2): (1) fluids causing a minimal effect on acid–base equilibrium, having an electrolyte content with an in vivo strong ion difference (SID), i.e., the SID after metabolism of the organic anion, close to 24–29 mEq/L; (2) fluids having a normal or sub-normal Cl− content (Cl− ≤ 110 mEq/L).
Table 2.
Electrolyte composition of the main balanced solutions available for intravenous administration.
Adapted from Langer et al. [21] with permission
| Crystalloids | Gelatins | Starches | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lactated Ringer’s | Acetated Ringer’s | Hartmann’s | PlasmaLyte | Sterofundin ISOa | ELO-MEL isoton | Isoplex | Gelaspan | Hextend | Tetraspan | |
| Na+ [mEq/L] | 130 | 132 | 131 | 140 | 145 | 140 | 145 | 151 | 143 | 140 |
| K+ [mEq/L] | 4 | 4 | 5 | 5 | 4 | 5 | 4 | 4 | 3 | 4 |
| Ca2+ [mEq/L] | 3 | 3 | 4 | – | 5 | 5 | – | 2 | 5 | 5 |
| Mg2+ [mEq/L] | – | – | 3 | 3 | 2 | 3 | 1.8 | 2 | 0.9 | 2 |
| Cl− [mEq/L] | 109 | 110 | 111 | 98 | 127 | 108 | 105 | 103 | 124 | 118 |
| Lactate [mEq/L] | 28 | – | 29 | – | – | – | 25 | – | 28 | – |
| Acetate [mEq/L] | – | 29 | – | 27 | 24 | 45 | – | 24 | – | 24 |
| Malate [mEq/L] | – | – | – | – | 5 | – | – | – | – | 5 |
| Gluconate [mEq/L] | – | – | – | 23 | – | – | – | – | – | – |
| Dextrose [g L-1] | – | – | – | – | – | – | – | – | – | – |
| Gelatin [g/L] | – | – | – | – | – | – | 40 | 40 | – | – |
| HES [g/L] | – | – | – | – | – | – | – | – | 60 | 60 |
| Dextran [g/L] | – | – | – | – | – | – | – | – | – | – |
| In-vivo SID [mEq/L] | 28 | 29 | 29 | 50 | 29 | 45 | 45.8 | 56 | 28 | 29b |
| Osmolarity [mOsm/L] | 278 | 277 | 279 | 294 | 309 | 302 | 284 | 284 | 307 | 297 |
In-vivo SID—all organic molecules contained in balanced solutions are strong anions. The resulting calculated SID (in vitro-SID) is equal to 0 mEq/L, due to electrical neutrality. Once infused, the organic molecules are metabolized to CO2 and water; the resulting in vivo-SID corresponds to the amount of organic anions metabolized
aSterofundin-ISO or Ringerfundin
bIn vivo-SID of Tetraspan reported in the Table results from the sum of organic anions; of note, there is a discrepancy as compared to the SID calculated as the difference between inorganic cations and inorganic anions (29 mEq/L vs. 33 mEq/L). No clear explanation has been reported from the seller
According to the quantitative approach to acid–base equilibrium [17, 18], the three variables regulating the pH of biologic fluids independently are (1) partial pressure of carbon dioxide (PCO2); (2) the concentration of non-volatile weak acids (ATOT); (3) the strong ion difference (SID), defined as the difference between the sum of all strong cations and the sum of all strong anions [19]. These principles clearly suggest that intravenous fluids may affect pH due to (i) the specific electrolyte content characterizing the solution, therefore altering the SID of the extracellular compartment and (ii) the dilution effect due to the volume infused, thus reducing the concentration of ATOT [20–22]. Ideally, the fluid able to leave plasma pH unchanged after its administration, at constant PCO2, should balance these variations. Recent studies clearly showed that, in this regard, the ideal balanced solution should have an in vivo SID equal to the baseline concentration of HCO3− [23]. If the SID of the infused fluid is greater than plasma HCO3−, plasma pH will tend toward alkalosis; if the SID of the infused fluid is lower than plasma HCO3−, plasma pH will tend toward acidosis, as it is always the case for NaCl 0.9%, the so-called “normal” saline [24].
As stated above, the definition of “balanced” solution includes also a category of iso- and near-isotonic fluids with a low Cl− content (equal to or lower than 110 mEq/L), as compared to NaCl 0.9%. Nonetheless, the final composition of such a fluid, especially when considering crystalloids, will depend on (1) tonicity; (2) electrical neutrality and (3) SID. Indeed, an isotonic balanced solution leaving unaltered acid–base equilibrium (i.e., with an SID close to 24 mEq/L) will necessarily have a Cl− content > 110 mEq/L (as in Sterofundin-ISO). In contrast, a fluid with an SID of 24 mEq/L and a lower Cl− content will necessarily be slightly hypotonic (as with Lactated Ringer’s). Finally, an isotonic fluid with a low Cl− content will necessarily have a higher SID (as with PlasmaLyte), with a consequent alkalizing effect.
The case for balanced solutions
Balanced and unbalanced (NaCl 0.9%) solutions might have slightly different effects on blood volume expansion, according to the clinical condition. Indeed, different kinetics showing approximately a 10% decrease in plasma volume expansion of balanced solutions as compared to NaCl 0.9% have been described in normovolemic healthy volunteers [25, 26]. On the other hand, in an experimental model of near-fatal hemorrhagic shock, a lower dose of balanced solution was needed, as compared to NaCl 0.9% to restore a target blood pressure [27]. These conflicting results underline the fact that findings about fluid therapy are condition-specific, and that results obtained from septic patients or experimental models should not be extrapolated to all situations.
Despite these controversies, which need further clarification, several definitive differences exist between these two categories of drugs. First, chloride-rich NaCl 0.9% causes a higher dose-dependent degree of acidosis and hyperchloremia, which possibly favors the contraction of vascular smooth muscles [28, 29], potentially leading to a reduced renal perfusion.
When healthy volunteers received 2 L of either saline or Plasma-Lyte over 1 h, saline significantly decreased renal artery blood velocity, decreased renal cortical tissue perfusion, decreased urine output, and increased extravascular fluid accumulation compared with Plasma-Lyte [30]. These findings may support the idea that hyperchloremia may cause increased tubule-glomerular feedback and decreased renal cortical perfusion [31].
Indeed, a large-scale propensity-matched observational analysis of U.S. insurance data showed that the use of PlasmaLyte® versus NaCl 0.9% on the first day of major abdominal surgery led to significantly less renal failure requiring dialysis [32]. In addition to the effect on renal perfusion, NaCl 0.9%, being slightly hypertonic, likely causes an increased incretion of arginine vasopressin. These two effects can conceivably contribute to the slower renal excretion of NaCl 0.9% as compared to balanced solutions [33, 34]. Indeed, more fluid will be retained in the interstitial space, with the consequent propensity to cause more edema [35, 36]. However, it is not merely the renal function that could be deranged by high chloride concentrations; infusion of NaCl 0.9% can cause abdominal discomfort in healthy volunteers [37] and a reduced gastric perfusion in elderly surgical patients [38].
Two important and large randomized controlled trials comparing the use of balanced solutions and normal saline have been published in the last years. The SPLIT study was the first multi-center double-blind randomized controlled trial performed on 2092 patients, comparing balanced and unbalanced fluids in intensive care units. It showed no significant difference in the main outcome, i.e., incidence of acute kidney injury [39]. While providing a high level of evidence, this trial did not give a definitive answer. Indeed, the median volume of study fluid was only 2 L over 90 days. Moreover, both study groups had received a median volume of 1.0–1.2 L of PlasmaLyte within 24 h prior to enrolment, therefore making it plausible that prior administration of PlasmaLyte counterbalanced the effects of low-dose NaCl 0.9%. The SMART-trial was a large study performed in five intensive care units of a single academic center [40]. A total of 15,802 patients were randomized to receive either NaCl 0.9% or a balanced solution (Plasma-Lyte A or Lactated Ringer’s). In both groups, patients received an extremely small amount of fluids: a median of 1 L from admission to day 30 or discharge, whichever came first. Despite the unexpectedly low volume of crystalloids, the authors found a small difference in the primary outcome, i.e., the incidence of major adverse kidney events within 30 days (composite of death, new renal replacement therapy or persistent renal dysfunction) in favor of balance solutions. Looking at the overall outcome, it is important to emphasize that there was no reduction of in-hospital mortality and that neither the incidence of renal replacement therapy (2.5% vs. 2.9%, p = 0.08) nor the incidence of persistent renal dysfunction (6.4% vs. 6.6%, p = 0.60) was statistically significant. A similar study performed by the same authors and published in the same issue of the New England Journal of Medicine, the SALT-ED trial, found a similar difference in the incidence of major adverse kidney events in non-critically ill adults [41].
In summary, we can avoid fluid-induced metabolic acidosis and excessive chloride loading simply using balanced solutions. There is increasing evidence that an excessive chloride administration may have a detrimental effect on renal function, even at low doses. Therefore, the use of balanced solutions, particularly in patients that potentially need a significant amount of intravenous fluids, seems to be a reasonable pragmatic choice [42]. On the contrary, saline may be an intuitive choice for patients with hypovolemic hyponatremia or hypochloremic metabolic alkalosis. In any other settings, the most important reason to choose NaCl 0.9% over balanced solutions is likely economic in nature. Therefore, the patient’s serum chloremia is an important factor to determine the appropriate type of fluids.
Albumin
The basics
Albumin accounts for approximately 50% of the plasma protein content [43] and is the main determinant of plasma oncotic pressure, playing a crucial role in the regulation of microvascular fluid dynamics [44, 45]. Normal plasma concentration of albumin ranges between 35 and 55 g/L, corresponding to approximately 0.54–0.85 mmol/L, and to an in vitro pressure of approximately 9.2 mmHg. In contrast, in vivo colloid-oncotic pressure is lower, since the permeability of the endothelial barrier to albumin is variable, even in healthy subjects. Nonetheless, according to Starling’s law, oncotic pressure is the force counteracting intravascular hydrostatic pressure, therefore acting to reabsorb water and small solutes from the interstitium to the intravascular space. The crucial role of albumin’s oncotic property in the regulation of microcirculatory fluid dynamics also seems to apply to the endothelial glycocalyx layer [46, 47]. This gel-like layer, lining the luminal side of the endothelium, is thought to comprise 20% of the intravascular volume. The current view of the glycocalyx is that it holds many compounds that are mandatory for the functioning of the endothelium and mediates several key physiological processes, such as maintaining the vascular barrier, hemostasis, prevention of cell adhesion to the endothelium and transmission of shear stress [48]. The role of the glycocalyx is however under continuous investigation and its role and function might need to be revised in the future [49]. Of note, shedding of the glycocalyx occurs in the presence of reactive oxygen species, hyperglycemia, cytokines, and endotoxin, and is therefore common in critically ill patients [50]. In the context of fluid homeostasis, loss of barrier function induced by glycocalyx shedding is associated with the formation of edema [51]. Furthermore, fluid therapy itself is known to be potentially deleterious for endothelial function [27], likely because of the resulting oxidative stress. However, the risks probably relate to the specific clinical context. Indeed, while volume loading did not cause glycocalyx shedding in surgical patients and healthy volunteers [52, 53], the amount of glycocalyx shedding was proportional to the volume of fluid given in septic shock patients [54].
The case for albumin
The ALBIOS study, a large Italian randomized controlled trial, gave some suggestions on whether or not albumin administration improves outcomes in severe sepsis and septic shock [55]. Patients with severe sepsis were randomized to receive either 20% albumin and crystalloids or crystalloids alone after initial early goal-directed resuscitation. In patients randomized to albumin treatment, albumin was supplemented for 28 days, to maintain an albumin concentration ≥ 30 g/L. Despite some beneficial physiologic effects (lower heart rates, higher mean arterial pressure, and lower daily net positive fluid balance over the first 7 days), no difference was observed either in mortality at 90 days (41.1% vs. 43.6%) or in overall organ failure scores. However, when analyzing the results according to disease severity, patients with septic shock randomized to albumin supplementation showed a lower risk of death (relative risk 0.87; 95% confidence interval—CI 0.77–0.99) as compared to those just receiving only crystalloids. It is worth mentioning that this trial did not utilize albumin as a resuscitation fluid, but as a drug to correct hypoalbuminemia.
The case against albumin
Colloids should remain in the intravascular space longer than crystalloids, provided that the endothelial barrier is intact, which is often not the case in critically ill patients [56]. Given the recent discussion on the potential adverse effects of artificial colloids, especially of hydroxyethyl starches (HES), a renewed interest in the use of albumin has emerged. However, despite the strong physiologic rationale and significant scientific effort [55, 57], to date, no randomized controlled trial has shown any significant benefit of fluid resuscitation using albumin over other types of fluids, including crystalloids [58]. Some reports have even suggested that albumin administration in the setting of cardiac surgery may be associated with the development of acute kidney injury [59]. As stated previously, one of the largest albumin trials to date, the ALBIOS study, reported a reduction in 90-day mortality in a subgroup of patients with septic shock. However, this result was based on a post-hoc rather than predefined analysis and should, therefore, be interpreted with caution. The results of two ongoing randomized trials, the ALBumin Italian Outcome Septic Shock-BALANCED Trial (ALBIOSS-BALANCED) and the Albumin Replacement Therapy in Septic Shock (ARISS), may provide some answers to the above-mentioned issues.
The significant cost and the availability of equally effective low-cost alternatives do not play in favor of albumin, although a subgroup analysis of the ALBIOS dataset may suggest that albumin infusion is likely cost-effective in patients’ septic shock [60]. Up to date, the theoretical benefits of albumin are not supported by sound clinical evidence, and the case for albumin remains controversial.
Perioperative fluid management
The aim of perioperative fluid therapy, in parallel with the maintenance of the effective circulating blood volume, is to avoid both fluid overload and under-hydration, while maintaining patients’ fluid balance as close as possible to zero. Despite this rationale, it is not unusual for surgical patients to receive 5–10 L of fluid and 600–1000 mmol of sodium, leading to edema and adverse outcomes [61], which is also favored by the marked and mean arterial pressure-dependent reduction of the elimination capacity of crystalloids [62, 63]. On the other hand, overnight fasting and bowel preparation, when traditionally applied, lead to fluid deficits. Apparently, patients develop postoperative complications when fluid retention exceeds 2.5 L [32, 64]. Of course, fluid gain depends not only on the amount of fluid administered, but also on the capacity of the kidney to excrete the excessive fluid and salt [32].
Fluid management before surgery
Fluid therapy is not only meant to compensate intraoperative losses but should also take into account those occurring prior to surgery, induced by poor water intake, bowel preparation, major inflammation associated with a stress response, and possibly, hemorrhage. Dehydration, however, is difficult to detect through clinical methods.
Many studies examined whether a fluid load is capable of reducing hypotension caused by the induction of general/regional anesthesia. However, results regarding a preload strategy have been discouraging [65, 66].
Fluid management during surgery
In response to the ongoing administration of large volumes of crystalloid to patients undergoing major surgery, a ‘fluid restrictive’ strategy has been proposed. For example, Brandstrup et al. demonstrated in a multi-center randomized controlled trial that a more restrictive regimen was associated with better outcomes following colorectal surgery [61]. However, the regimen was restrictive compared with the standard of care that was excessive (e.g., 5 L positive balance due to high crystalloid volumes) [67]. It is therefore conceivable that the group with a better outcome rather benefitted from the avoidance of fluid excess than from fluid restriction. The interpretation of the literature on the topic is hampered by the use of very heterogeneous definitions [68]. What is however clear from observational studies is that both too much and too little fluid are associated with poor outcomes [69–72]. Recently, a large cohort study from 500 U.S. hospitals including adult patients having colon, rectal or primary hip or knee surgery was concluded [72]. A significant association was found between liberal fluid administration on the day of surgery and worse outcomes (increased total costs and length of stay in all patients), as well as increased presence of postoperative ileus, in patients undergoing colorectal surgery. Interestingly, the authors also observed that restrictive fluid utilization (the lowest 25% by volume) was also associated with worse outcomes.
It is common in Enhanced Recovery after Surgery (ERAS) protocols to find the term “intraoperative fluid restriction” [73]. However, alternative terms, such as “zero balance” or the avoidance of salt and water excess, are also available. Protocols advocate the infusion of balanced crystalloid of 1–3 ml/kg/h and to give additional boluses of fluid only to match needs judged by either measured volumes lost during surgery, or the assessment of peripheral perfusion (such as according to the so-called ‘Goal-Directed Fluid Restriction’) [74]. Overall, the literature suggests that algorithm-based perioperative fluid regimens result in improved patient outcomes.
Fluid management after surgery
Fluid management in postoperative patients is a key determinant of their outcomes. While restoring effective volume is critical for these patients, fluid management should not compromise healing processes. Optimal fluid management should thus target efficient central hemodynamics and tissue perfusion while avoiding positive net fluid balance. In theory, colloids offer the advantages over crystalloids of higher plasma expansion capacity and longer plasma half-life. They have the theoretical disadvantage of delaying clotting time and increasing the risk of kidney injury. In randomized trials, the ratio of the cumulative dose of colloids over the cumulative dose of crystalloids ranged roughly from 0.41 to 1 [75]. In patients with overt clinical hypovolemia, colloids were superior to crystalloids in improving cardiac filling pressures and performance [76]. Likewise, in a large multinational randomized trial performed in critically ill patients with acute hypovolemia, colloids reduced vasopressor and ventilator dependency when compared to crystalloids [77]. A recent systematic review of resuscitation with HES in surgical critically ill patients identified 13 randomized trials [78]. However, this review found no statistically significant difference between HES and crystalloids, in terms of mortality (risk ratio 2.97; 95% CI 0.96 to 9.19; I2 = 0%), need for renal replacement therapy (risk ratio 1.11; 95% CI 0.26 to 4.69; I2 = 34%), and major infectious complications (risk ratio 1.19; 95% CI 0.59 to 2.39; I2 = 0%). It is worth mentioning that eligible trials were too small to draw firm conclusions on this issue.
It should also be stated that there are opposing views regarding the use of starches [79]. For example, several criticisms regarding the CHEST trial have been put forward which still require to be addressed [80, 81]. Furthermore, it can be stated that in the CHEST trial starches were administered to patients that were not hypovolemic. On the other hand, the CRISTAL trial (where 70% of the colloid group received HES) concluded that significantly less volume was required to achieve hemodynamic stability for HES vs. NaCl in the initial phase of fluid resuscitation in severe sepsis patients without any difference for adverse events in both groups [77]. Taking these opposing views into consideration, the ongoing debate about the use of starches in hypovolemic critically ill patients still requires more data.
Among patients undergoing major abdominal surgery, the recent results of the FLASH trial, showed no significant difference in a composite outcome of death or major postoperative complications within 14 days after surgery [82].
Pending the results of ongoing trials, there are currently insufficient data to ban the use of colloids in the surgical intensive care unit.
Many patients undergoing surgery are not able to ingest food or fluids for some time following surgery and will require maintenance fluids. Recently, a debate emerged on the tonicity of these solutions: although guidelines traditionally recommended the use of hypotonic maintenance fluids, in pediatric literature, these were shown to be associated with an increased incidence of symptomatic hyponatremia [83, 84]. The recent randomized controlled TOPMAST trial in adults undergoing major thoracic surgery found this problem to be mild in these patients. Isotonic maintenance fluids, on the other hand, were associated with a considerably larger positive cumulative fluid balance (estimated at 1.4L more positive under fluids containing 154 compared to 54 mmol/L of sodium) [85].
Fluid overload
The problem with fluid overload in the perioperative setting
A certain degree of hypervolemia is necessary to maintain organ perfusion during anesthesia and surgery. However, fluid given after the induction of anesthesia mainly increases “unstressed” blood volume, because vasodilatation occurs as a consequence of anesthesia. At this point, additional fluid administration is needed to optimize stroke volume, i.e., to add to the “stressed” intravascular volume [86]. Many clinicians still consider this “wet” approach as the gold standard for intraoperative fluid therapy [87], although intravascular volume expansion certainly bears some dangers. Myocardial work and cardiac pressures increase when infused fluids have exceeded the degree of anesthesia-induced vasodilatation. Moreover, fluid overload reduces the colloid osmotic pressure that, together with raised cardiac pressures, might promote pulmonary edema [88]. These issues are of particular relevance in patients with poor cardiovascular status. Finally, hypervolemia may be responsible for another important effect: the release of atrial natriuretic peptides (ANPs) to the circulation caused by the stretching of atrial myocardial fibers [68, 89]. Indeed, in response to a rapid infusion of crystalloids, ANP levels increase 2- to 3-fold [90–92], therefore reducing strain on the circulation by promoting natriuresis and capillary leakage of albumin.
The problem with fluid overload in the Intensive Care Unit
Fluid administration is one of the cornerstones of hemodynamic resuscitation in critically ill patients. How much fluid to give has been the subject of lively debate over the years. Too much fluid can have harmful consequences on multiple organ systems, e.g., worsening gas exchange, renal function and wound healing. Fluid overload is particularly likely to arise in conditions when capillary permeability is altered due to an inflammatory response, such as during sepsis. A positive fluid balance has been associated with worse outcomes in several studies in various groups of intensive care unit (ICU) patients [16, 93–95]. In patients with septic shock, fluid administration and positive fluid balance were independently associated with increased mortality rates [93, 96]. Similarly, in patients admitted to the ICU after major surgery, fluid balance was an independent risk factor for death [95]. Indeed, a multi-modal restrictive fluid strategy aiming for negative fluid balance (PAL-treatment) in patients with acute lung injury (ALI) was associated with improved outcomes in a retrospective study [97].
It has to be acknowledged that a positive fluid balance could be a marker of disease rather than a pure iatrogenic or preventable problem and it would be erroneous to assume the default position of under-resuscitation. Indeed, inadequate resuscitation due to insufficient fluid administration may result in poorer tissue perfusion and hence organ dysfunction and failure, particularly in the early phase of treatment. A balance needs to be achieved, such that each patient receives sufficient, but not excessive, fluid for her/his needs. Crucially, different patients will have different needs and baseline fluid status depending on multiple factors including age, co-morbid disease and current diagnosis. In addition, it is mandatory to consider indices of fluid tolerance, such as CVP, lung water, oxygenation and hemoglobin levels. Fluid requirements vary during the course of illness. As such, fluids must be prescribed on an individual patient basis; the prescription should be regularly reviewed and tailored to the evolving clinical stage. The answer to the question of whether fluid overload is a problem in the ICU will thus depend on when it is asked. In the acute resuscitation/salvage phase, fluid administration is generous. While fluid overload should always be a concern, a positive fluid balance is a specific target of this phase.
Is deresuscitation/de-escalation the solution?
The term deresuscitation/de-escalation was first suggested in 2012 [98] and finally coined in 2014 [16]. It specifically refers to ‘Late Goal-Directed Fluid Removal’, which involves “aggressive and active fluid removal through diuretics and renal replacement therapy with net ultrafiltration”. Deresuscitation/de-escalation is sometimes also used to more loosely refer to the phase of critical illness and/or the care of a critically ill patient, after initial resuscitation, stabilization, and optimization. It is characterized by the discontinuation of invasive therapies and a reduction of a spurious fluid balance. Late conservative fluid management is defined as 2 consecutive days of negative fluid balance within the first week of ICU stay, and is an independent predictor of survival in ICU patients [16].
Fluid overload and a positive cumulative fluid balance are associated with increased morbidity and worse outcomes, as previously discussed. The natural course of events after a first insult (such as infection, trauma, etc.) is a systemic inflammatory response with increased capillary permeability and organ dysfunction [98]. The presence of fluid overload and interstitial edema may thus trigger a vicious cycle. This is what has been referred to as the Ebb phase of shock [16]. In the majority of patients, shock reversal occurs (with correct antibiotics and proper source control) and excess fluids can be mobilized: this is called the Flow phase [16]. However, some patients will not transfer spontaneously from the Ebb to Flow phase and will remain in a state of unresolved shock with positive cumulative fluid balance, and this is where active deresuscitation/de-escalation might have an important role.
It is unclear which is the best therapeutic option for deresuscitation/de-escalation. The administration of albumin in combination with diuretics (20% albumin to achieve a serum albumin levels of 30 g/L and furosemide bolus of 60 mg followed by continuous infusion of 10 mg/h) and the association of this strategy with the sequential application of PEEP set to counteract intra-abdominal pressure (IAP) have been proposed [97]. In addition, renal replacement therapy and aggressive ultrafiltration can be used to achieve a negative fluid balance in selected patients [99]. When it comes to deresuscitation/de-escalation, it is important to decide on when, how and for how long. For this purpose, we need to use the right targets to reach our goals. “Over-deresuscitation” has its drawbacks and may cause neurologic dysfunction in the long run [100].
In conclusion, it is crucial to ensure that the indication for fluid resuscitation no longer exists (e.g., absence of vasopressor, no lactate, adequate venous oxygen saturation of hemoglobin) before starting with deresuscitation. Furthermore, the 5 steps of Deresuscitation/De-Escalation need to be kept in mind: (1) define a clinical endpoint (e.g., improvement in oxygenation); (2) set a fluid balance goal (e.g., 1 L negative balance in 24 h); (3) set perfusion and renal safety precautions (e.g., vasopressor need, 25% serum creatinine increase); (4) re-evaluate after 24 h unless safety limits reached; (5) adjust the plan accordingly.
The 4 phases of fluid therapy and the R.O.S.E. or S.O.S.D. concept
Two articles were published recently, almost simultaneously, referring to the dynamics of fluid therapy [16, 101]. These conceptual models identified four dynamic phases. The Acute Dialysis Quality Initiative (ADQI) group proposed S.O.S.D. (Salvage, Optimization, Stabilization, De-escalation) as acronym [101]. However, during the International Fluid Academy Day (IFAD) meetings there was a clear preference for the R.O.S.E. acronym (Resuscitation, Optimization, Stabilization, Evacuation) as summarized below, in Fig. 1 and Table 3. We tried to suggest endpoints and targets for the different phases; however, it was decided not to include them because there cannot be a specific target of cardiac index and PPV must be considered only if cardiac output is low. A high PPV is often a physiological state and defining a “normal” state when a low PPV value is reached might lead to unnecessary fluid infusion [102]. Also, defining a given preload level as a target of resuscitation is senseless as it may shift from patient to patient and from time to time.
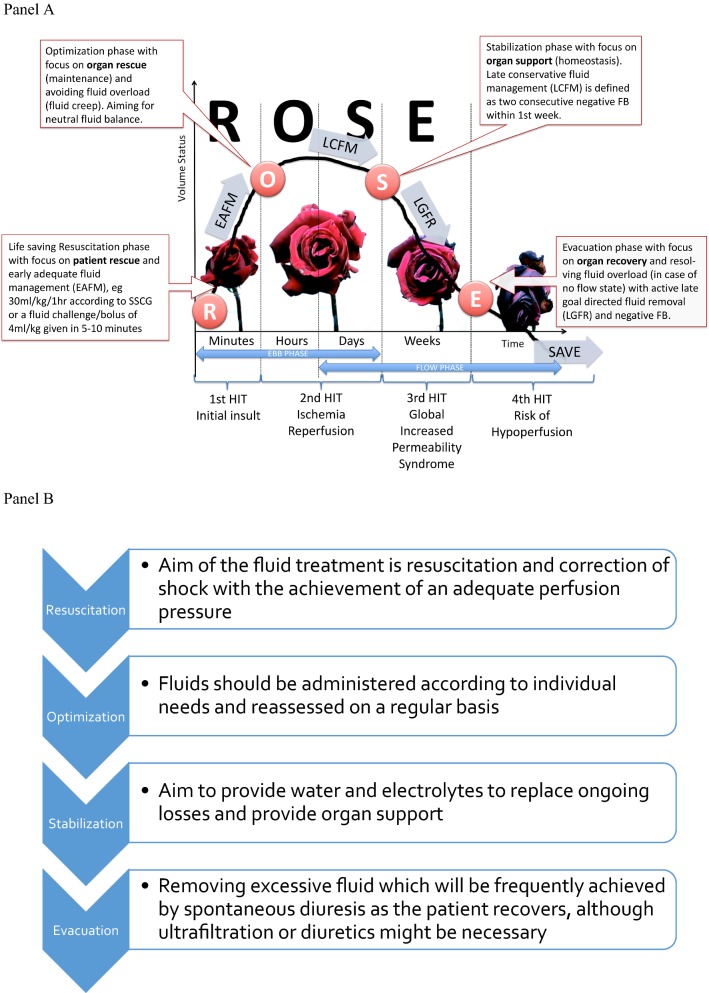
Fig. 1.
The R.O.S.E. concept and the 4 phases of Fluid Therapy. Adapted according to the Open Access CC BY Licence 4.0 with permission from Malbrain et al. [9]. a Graph showing the four-hit model of shock with evolution of patients’ cumulative fluid volume status over time during the five distinct phases of resuscitation: Resuscitation (R), Optimization (O), Stabilization (S), and Evacuation (E) (ROSE), followed by a possible risk of Hypoperfusion in case of too aggressive deresuscitation. On admission patients are hypovolemic, followed by normovolemia after fluid resuscitation (EAFM, early adequate fluid management), and possible fluid overload, again followed by a phase going to normovolemia with late conservative fluid management (LCFM) and late goal-directed fluid removal (LGFR) or deresuscitation. In case of hypovolemia, O2 cannot get into the tissues because of convective problems, in case of hypervolemia O2 cannot get into the tissue because of diffuse problems related to interstitial and pulmonary edema, gut edema (ileus and abdominal hypertension). b The role of fluids within the R.O.S.E. concept
Table 3.
The 4 dynamic phases of fluid therapy according to the ROSE concept.
Adapted from Malbrain et al. [4] with permission
| Resuscitation (R) | Optimization (O) | Stabilization (S) | Evacuation (E) | ||
|---|---|---|---|---|---|
| HIT | First | Second | Second | Third | Fourth |
| Cause | Inflammatory insult, e.g., sepsis, severe acute pancreatitis (SAP), burns, trauma, etc. | Ischemia and reperfusion | Ischemia and reperfusion | GIPS (global increased permeability syndrome) | Hypoperfusion |
| Phase | Ebb | Flow | Flow/no flow | No flow | No flow |
| Type | Severe shock | Unstable | Stable | Recovering | Unstable |
| Example | Septic shock, major trauma, hemorrhagic shock, ruptured abdominal aortic aneurysm, severe acute pancreatitis, severe burns (> 25% TBSA) | Intra- and perioperative goal-directed therapy, less severe burns (< 25% TBSA), diabetic keto-acidosis, severe gastro-intestinal losses (vomiting, gastroenteritis) | Postoperative patient (nil per mouth or combination of total enteral plus parenteral nutrition), abdominal vacuum-assisted closure, replacement of losses in less-severe pancreatitis | Patient on full enteral feed in recovery phase of critical illness, polyuric phase after recovering from acute tubular necrosis | Patient with cirrhosis and anasarca edema (GIPS) and no Flow state, hepatosplanchnic hypoperfusion |
| Question | When to start fluids? | When to stop fluids? | When to stop fluids? | When to start unloading? | When to stop unloading? |
| Subquestion | Benefits of fluids? | Risks of fluids? | Risks of fluids? | Benefits of unloading? | Risks of unloading? |
| O2 transport | Convective problems | Euvolemia, normal diffusion | Diffusion problems | Euvolemia, normal diffusion | Convective problems |
| Fluids | Mandatory | Biomarker of critical illness | Biomarker of critical illness | Toxic | |
| Fluid therapy | Rapid bolus (4 ml/kg/10–15 min) | Titrate maintenance fluids, conservative use of fluid bolus | Minimal maintenance if oral intake inadequate, provide replacement fluids |
Oral intake if possible Avoid unnecessary IV fluids |
Avoid hypoperfusion |
| Fluid balance | Positive | Neutral | Neutral/negative | Negative | Neutral |
| Result | Life saving (rescue, salvage) | Organ rescue (maintenance) | Organ support (homeostasis) | Organ recovery (removal) | Organ support |
| Targets | Macrohemodynamics (MAP, CO); lactate; volumetric preload (LVEDAI); functional hemodynamics; fluid responsiveness (PLR, EEO) | Organ macroperfusion (MAP, APP, CO, ScvO2); volumetric preload (GEDVI, RVEDVI); GEF correction; R/L shunt; think of polycompartment syndrome, CARS | Organ function (EVLWI, PVPI, IAP, APP); biomarkers (NGAL, cystatin-C, citrullin); capillary leak markers (colloid oncotic pressure, osmolality, CLI, RLI); daily and cumulative FB, body weight |
Organ function evolution (P/F ratio, EVLWI, IAP, APP, PVPI) Body composition (ECW, ICW, TBW, VE) |
Organ microperfusion (pHi, ScvO2, lactate, ICG-PDR); Biomarkers; Negative cumulative fluid balance |
| Monitoring tools | Arterial-line, central venous-line, PPV or SVV (manual or via monitor), uncalibrated CO, TTE, TEE | Calibrated CO (TPTD, PAC) | Calibrated CO (TPTD); Balance; BIA (ECW, ICW, TBW, VE) | Calibrated CO (TPTD); balance; BIA; DE-escalation | LiMON, Gastric tonometry, micro-dialysis |
| Goals | Correct shock (EAFM—early adequate fluid management) | Maintain tissue perfusion | Aim for zero or negative fluid balance (LCFM—late conservative fluid management) | Mobilize fluid accumulation (LGFR—late goal-directed fluid removal = emptying) or DE-resuscitation | Maintain tissue perfusion |
| Timeframe | Minutes | Hours | Days | Days to weeks | Weeks |
APP abdominal perfusion pressure, = MAP − IAP, BIA bio-electrical impedance analysis, CARS cardio-abdominal renal syndrome, CLI capillary leak index, = serum CRP divided by serum albumin, CO cardiac output, ECW extracellular water, EEO end-expiratory occlusion test, EVLWI extravascular lung water index, GEDVI global end-diastolic volume index, GEF global ejection fraction, GIPS global increased permeability syndrome, IAP intra-abdominal pressure, ICG-PDR indocyaninegreen plasma disappearance rate, ICW intracellular water, IV intravenous, LVEDAI left ventricular end-diastolic area index, MAP mean arterial pressure, P/F pO2 over FiO2 ratio, PLRT passive leg raising, PPV pulse pressure variation, PVPI pulmonary vascular permeability index, RLI renal leak index, = urine albumin divide by urine creatinine, R/L right to left shunt, RVEDVI right ventricular enddiastolic volume index, SAP severe acute pancreatitis, ScvO2 central venous oxygen saturation, SVV stroke volume variation, TBSA total burned surface area, TBW total body water, TEE transesophageal echocardiography, TPTD transpulmonary thermodilution, TTE transthoracic echocardiograph, VE volume excess
Resuscitation phase (R) or salvage phase (S)
In the first, salvage/resuscitation phase, when a patient presents with hemodynamic shock, the aim of the treatment is resuscitation and correction of shock with the achievement of an adequate perfusion pressure. A rapid fluid bolus should be given (although the exact amount can vary, usually 3–4 mL/kg given over 10 to 15 min and repeated when necessary), normally in association with vasopressor administration. In parallel, emergency procedures to resolve any obvious underlying cause should be performed, with hemodynamic monitoring initiated. In this phase, the goal is early adequate goal-directed fluid management: fluid balance must be positive. We do not support blind adherence to the surviving sepsis campaign guidelines adagio to administer 30 ml/kg of fluids within the first hour for all patients, as explained previously [9]. This may lead to either over- or under resuscitation in some patients. Every patient needs an individual and personalized approach.
Optimization phase (O)
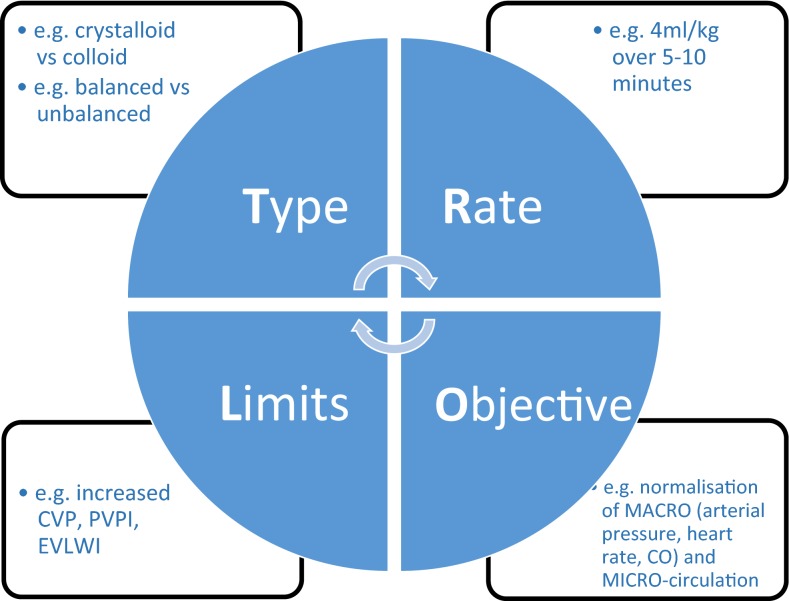
The optimization phase starts when the patient is no longer in overt absolute/relative hypovolemia, but remains hemodynamically unstable. Some form of monitoring will by now be in place. Fluids should be administered according to individual needs, reassessed on a regular basis, e.g., using fluid challenge techniques [103, 104]. Fluid challenges must be conducted carefully, bearing in mind the four essential components (TROL): Type of fluid (e.g., a balanced crystalloid-like PlasmaLyte); Rate (e100–200 mL over 10 min); Objective (e.g., normal arterial pressure or heart rate); and Limits (e.g., high central venous pressure level) (Fig. 2) [105]. The aim of this phase is to optimize and maintain adequate tissue perfusion and oxygenation in order to prevent and limit organ damage. The patient must be carefully monitored during the optimization phase: often several types of monitoring (e.g., arterial catheter, echocardiography, central venous pressure, arteriovenous blood gas) are required to obtain the most complete picture of a patient’s hemodynamic status.
Fig. 2.
The TROL mnemonic of fluid challenge: considerations for administration of a fluid bolus in critically ill patients. CO cardiac output; CVP central venous pressure; EVLWI extra vascular lung water index; PVPI pulmonary vascular permeability index
(Adapted from Vincent and Weil [97])
Although a resuscitation based on microcirculatory endpoints is expected to result in analogous amelioration in the microcirculation, a lack of coherence may exist between macro- and microcirculation. Thus, markers of hypoperfusion should include also lactate, prolonged capillary refill time and mottling score [106].
Stabilization phase (S)
Once the patient is stable, the stabilization phase begins and evolves over days. In this phase, the aim of fluid management is to ensure water and electrolytes to replace ongoing losses and provide organ support. The target should be a zero or slightly negative fluid balance. It might be of interest, in this context, to underline the fact that in the major trials suggesting a harmful effect of starches [2, 107], these colloids were given abundantly also in the stabilization phase, i.e., in a phase that possibly did not require these drugs.
Evacuation phase (E) or de-escalation phase (D)
The final phase is evacuation or de-escalation, with the purpose of removing excessive fluid. This will be frequently achieved by spontaneous diuresis as the patient recovers, although ultrafiltration or diuretics might be necessary. Of note, it was recently shown that diuretics might favor the recruitment of microcirculation, thus decreasing diffusion distances and improving oxygen extraction [108].
Fluid management in trauma and burns
Fluid management in acute hemorrhagic shock following trauma
Although traumatic brain injury remains the commonest cause of death following severe blunt injury, concomitant major hemorrhage will result in cerebral hypoperfusion, which undoubtedly contributes to secondary brain injury and death. As such, hemorrhage remains the most preventable cause of trauma mortality.
An adequate intravascular volume, hemoglobin concentration and oxygen saturation are essential to maintain aerobic metabolism. Humans do not tolerate anaerobic metabolism and 90% of oxygen consumption is used in the formation of adenosine triphosphate (ATP), the major energy source for cell function. Rapid reversal of anaerobic metabolism is imperative to restore ATP and prevent irreversible cellular apoptosis and death [109].
Recognizing that hypovolemia is the consequence of hemorrhagic shock, past strategies utilized crystalloids to restore intravascular volume, followed by blood transfusion. Crystalloids, however, do not carry oxygen, and oxygen delivery may only be enhanced by an adequate hemoglobin concentration. Furthermore, major hemorrhage is accompanied by a unique coagulation disorder, the Acute Coagulopathy of Trauma and Shock (ACoTS) [110], leading to poor clot formation, as a result of increased binding of thrombin to thrombomodulin and enhanced fibrinolysis. Dilution of coagulation factors, acidosis, and hypothermia play a secondary role in this scenario. The approach to resuscitation must therefore be proactive and not reactive with the combined administration of packed red blood cells, plasma, platelets, and cryoprecipitate. The use of clear resuscitation fluids should be minimized. Based on military experience, the recommended ratio of packed red blood cells to plasma and platelets should be 1:1:1. The endpoints for hemoglobin concentration of 10 g/dL, a platelet count of > 50,000, an INR < 1.5 and a fibrinogen concentration of > 1 g/L cannot be generally recommended. In addition, the ionized calcium level should be > 1.0 mmol/L.
While the above is a general recommendation, not all patients will require such an aggressive approach [111]. Indeed, over-zealous transfusion is associated with unwanted complications.
The standard approach has been to use conventional laboratory coagulation testing to determine the need for component therapy. These, however, are performed at room temperature and do not reflect individual steps in coagulation. Thromboelastometry has now been recognized as an essential tool to monitor coagulopathy in trauma [112]. This device reflects the entire process of coagulation and can graphically determine the need for specific coagulation factors. Unlike laboratory coagulation studies, modern thromboelastometry machines may be set to the patient’s core temperature and accurately reflect the in vivo coagulation status. These instruments should be the standard of care in centers handling major trauma.
Following the CRASH-2 trial indicating the benefit of tranexamic acid given within 3 h from injury, such treatment has been included in many protocols for major hemorrhage [112]. In the presence of a sophisticated trauma system, the benefits are doubtful and further data are warranted [113].
Fluid management in burns
The understanding of burn shock pathophysiology and subsequent development of fluid resuscitation strategies resulted in dramatic outcome improvements in burn care during the last decades [114]. However, while under-resuscitation has become rare in clinical practice, there is growing concern that over-resuscitation, leading to increased morbidity and mortality, has become more of an issue in burn care. In the late sixties of the previous century, Baxter and Shires developed their landmark formula at the Parkland Memorial Hospital, which has lasted decades as the gold standard for fluid resuscitation in acute burn care across the world [115]. The formula advocates 4 ml crystalloids per kg per % of total body surface area for 24 h, of which half is given during the first 8 h. Diuresis (target 1 ml/kg/h) is used to guide the amount of intravenous fluids. During the second 24 h of resuscitation, colloids are allowed, and resuscitation volume is adapted according to diuresis (with a gradual decrease if diuresis is adequate).
However, over the last 15 years, multiple centers have reported excess fluid administration [116, 117]. This fluid excess often leads to “resuscitation morbidity”, a group of complications linked to fluid overload, such as delayed wound healing, delayed recovery of gastro-intestinal function (with ileus), pulmonary edema (due to capillary leak and increased extravascular lung water), limb compartment syndrome, orbital compartment syndrome, intra-abdominal hypertension and abdominal compartment syndrome leading to multiple organ failure [118–120].
This discrepancy between the predicted and the administered fluid is known as “fluid creep”, a term brought to life by Basil Pruitt [119].
Recommendations for fluid resuscitation in burns are listed in Table 4. The most well-known adverse effect of NaCl 0.9% is hyperchloremic metabolic acidosis. Given the large infusion volumes administered to burn patients, balanced solutions are preferred. Indeed, since the beginning of burn resuscitation, most formulae advocate the use of balanced crystalloid solutions. Of note, an observational study reported lower Sequential Organ Failure Assessment (SOFA) scores in severely burned patients resuscitated with acetated Ringer’s [121].
Table 4.
Recommendations regarding fluid resuscitation in severe burns’ patients.
Adapted from Peeters et al. [106] with permission
| Type of fluid | Recommendation |
|---|---|
| 1. Normal saline | Given the fact that fluid resuscitation in burn management requires large volumes, the use of saline cannot be recommended in a burn resuscitation protocol |
| 2. Balanced crystalloid | Based on the available evidence, balanced crystalloid solutions are a pragmatic initial resuscitation fluid in the majority of acutely ill (and burn) patients |
| 3. Semi-synthetic colloids | Given the recent data concerning the use of semi-synthetic colloids (and starches in particular), their use in critically ill patients, including burn patients, cannot be recommended |
| 4. Albumin | Based on the available evidence the use of albumin 20% can be recommended in severe burns, especially in the deresuscitation phase guided by indices of capillary leak, body weight, (cumulative) fluid balance, fluid overload, extravascular lung water and IAP |
| 5. Hypertonic solutions | To this day, there is insufficient evidence to reach consensus regarding the safety of hypertonic saline in burn resuscitation. Whenever using hypertonic saline in clinical practice, however, close monitoring of sodium levels is highly advised |
The use of colloids in the first 24 h has been controversial since it was thought that the existing capillary leak would allow large molecules to leak out into the extravascular space and exert an osmotic pull increasing the formation of edema [122]. In the last 15 years, renewed interest in colloids has arisen during burn resuscitation, instigated by the awareness of morbidity related to resuscitation and fluid creep. Until recently, the low molecular weight HES solutions were widely used as a resuscitation fluid in critically ill ICU, surgery and burn patients. However, after large fluid trials, including the CHEST and 6S trials, showing increased mortality and a higher rate of renal replacement therapy have raised alarming conclusions regarding the safety of HES solutions, starches can no longer be used in burn injuries as recommended by the Pharmacovigilance Risk Assessment Committee (PRAC) [2, 107, 123].
Albumin is a natural plasma protein that contributes most to intravascular oncotic pressure in humans (see above). The most common solutions are 4%, 5% or 20% albumin. It is a relatively expensive solution and its availability may be limited in some countries. Although albumin resuscitation has been used with some reservations, especially in the acute phase of burn resuscitation, trials provide promising data regarding the use of albumin as an adjunctive therapy in burn resuscitation [124, 125]. Similarly, hypertonic saline has been used for decades in burn resuscitation; theoretically, it expands the circulating volume by an intravascular water shift. Proponents claim that this process will decrease tissue edema and will lower the rate of complications. This hypothesis, however, needs to be confirmed by further studies.
Take home messages and considerations prior to IV fluid prescription
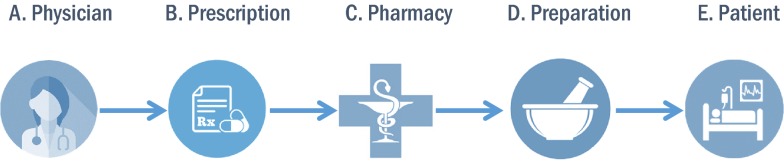
Consider the 5 Ps of fluid prescription as shown in Fig. 3 and tailor the IV fluids to the patient’s need via individualized and personalized care (Table 5) [126]. Prescription safety can be summarized by the ‘4 Ds’ principle as explained above [4]:
Drug—which fluid.
Dose—calculate how much to give.
Duration—duration of the IV fluid therapy.
De-escalation—stop it as soon as possible.
Fig. 3.
The 5 Ps of fluid administration. a Physician: All starts with the physician’s participation in making decisions related to fluid management. b Prescription: The physician should engage in writing a prescription that accounts for drug, dose, duration and whenever possible de-escalation. c Pharmacy: The prescription is sent to the pharmacy and is checked for inconsistencies by the pharmacist to get a more holistic view. d Preparation: The process by which the prescription is prepared and additions (e.g., electrolytes) made. e Patient: The filled prescription goes back to the patient and fluid stewards should observe administration, response, and debrief
Table 5.
The four stages to check for appropriateness of IV fluid therapy.
Adapted with permission from Malbrain ML et al. [126]
| Stage of evaluation | Audit standard |
|---|---|
| 1. Assessment |
The patient’s fluid balance (via fluid chart with input and output) is assessed on admission in the hospital and on a day-by-day basis The patient’s weight is assessed within the last 3 days of fluid prescription The patient’s fluid and electrolyte needs are assessed as part of every ward review The assessment of the patient’s fluid status (hypo/eu/hypervolemia) includes the use of clinical judgement, vital signs and fluid balance with urine output Recent lab results with urea and electrolytes (within 24 h of fluid prescription) If possible sodium balance should be reported |
| 2. Indication |
A. Resuscitation For patients in need of fluid resuscitation: The cause of the fluid deficit is identified An assessment of shock or hypoperfusion was made A fluid bolus of 4 mL/kg of balanced crystalloids is given Fluid responsiveness is assessed with functional hemodynamics, passive leg raising test or end-expiratory occlusion test, or a combination Mean arterial pressure and cardiac output are monitored continuously via pulse contour analysis allowing assessment of beat-to-beat variations Patients who have received initial fluid resuscitation are reassessed within 30 min Care is upgraded in patients who have already been given > 2000 mL of crystalloids and still need fluid resuscitation after reassessment Patients who have not had > 2000 mL of crystalloids and who still need fluid resuscitation after reassessment receive 2–4 mL/kg of crystalloids and have a further reassessment |
|
B. Maintenance If patients need IV fluids for routine maintenance alone, the initial prescription is restricted to 25–30 mL/kg/day (1 mL/kg/h) of water and Approximately 1 mmol/kg/day of potassium (K+) and Approximately 1–1.5 mmol/kg/day of sodium (Na+) and Approximately 1 mmol/kg/day of chloride and Approximately 50–100 g/day (1–1.5 g/kg/day) of glucose to limit starvation ketosis Definition of inappropriateness in case of electrolyte disturbances Solutions not containing adequate amount of sodium in case of hyponatremia (Na < 135 mmol/L) Solutions not containing adequate amount of potassium in case of hypokalemia (K < 3.5 mmol/L) Solutions containing too much sodium in case of hypernatremia (Na > 145 mmol/L) Solutions containing too much potassium in case of hypokalemia (K > 5 mmol/L) The amount of fluid intake via other sources should be subtracted from the basic maintenance need of 1 ml/kg/h: Enteral or parenteral nutrition Fluid creep (see further) | |
|
C. Replacement and redistribution If patients have ongoing abnormal losses or a complex redistribution problem, the fluid therapy is adjusted for all other sources of fluid and electrolyte losses (e.g., normal saline may be indicated in patients with metabolic alkalosis due to gastro-intestinal losses) | |
|
D. Fluid creep All sources of fluids administered need to be detailed: crystalloids, colloids, blood products, enteral and parenteral nutritional products, and oral intake (water, tea, soup, etc.) Precise data on the concentrated electrolytes added to these fluids or administered separately need to be collected Fluid creep is defined as the sum of the volumes of these electrolytes, the small volumes to keep venous lines open (saline or glucose 5%), and the total volume used as a vehicle for medication | |
| 3. Prescription |
The following information is included in the IV fluid prescription: The type of fluid The rate of fluid infusion The volume or dose of fluid The IV fluid prescription is adapted to current electrolyte disorders and other sources of fluid intake |
| 4. Management |
Patients have an IV fluid management plan, including a fluid and electrolyte prescription over the next 24 h The prescription for a maintenance IV fluid only changes after a clinical exam, a change in dietary intake or evaluation of laboratory results |
The bottom line is “Give the right fluid in the right dose to the right patient at the right time”
Conclusions
The prescription of fluid therapy is one of the most common medical acts in hospitalized patients but many of the aspects of this practice are surprisingly complex. It is time to introduce fluid stewardship in your ICU. To avoid fluid-induced harm, we recommend a careful evaluation of the chosen solution and a phase-wise approach to its administration, taking into account the clinical course of the disease or surgical procedure. Fluids should be prescribed with the same care as any other drug and every effort should be made to avoid their unnecessary administration.
Acknowledgements
MLNGM and NVR are co-founders of the International Fluid Academy (IFA). This open access article is endorsed by the IFA. The mission statement of the IFA is to foster education, promote research on fluid management and hemodynamic monitoring, and thereby improve survival of critically ill by bringing together physicians, nurses, and others from throughout the world and from a variety of clinical disciplines. The IFA is integrated within the not-for-profit charitable organization iMERiT, International Medical Education and Research Initiative, under Belgian law. The content of the IFA website (http://www.fluidacademy.org) is based on the philosophy of FOAM (Free Open Access Medical education—#FOAMed). The site recently received the HONcode quality label for medical education (https://www.healthonnet.org/HONcode/Conduct.html?HONConduct519739).
Abbreviations
- 4 Ds
Drug—Dose—Duration—De-escalation
- ANP
Atrial natriuretic peptide
- ATP
Adenosine triphosphate
- CI
Confidence interval
- ERAS
Enhanced recovery after surgery
- HES
Hydroxyethyl starch
- IAP
Intra-abdominal pressure
- ICU
Intensive care unit
- PEEP
Positive end-expiratory pressure
- ROSE
Resuscitation—Optimization—Stabilization–Evacuation
- SID
Strong ion difference
- SOFA
Sequential Organ Failure Assessment
Authors’ contributions
MLNGM wrote the concept. MLNGM wrote first draft. All other authors reviewed and edited the manuscript. All authors read and approved the final manuscript.
Authors’ information
Dr. Manu Malbrain is professor at the faculty of Medicine and Pharmacy at the Vrije Universiteit Brussels (VUB) and member of the Executive Committee of the Abdominal Compartment Society, formerly known as the World Society of Abdominal Compartment Syndrome (https://www.wsacs.org/). He is a co-founder, past-president and current treasurer of WSACS. He is a co-founder of the International Fluid Academy (IFA).
Funding
Not applicable.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
MLNGM is a member of the medical advisory Board of Pulsion Medical Systems (now fully integrated in Getinge, Solna, Sweden) and Serenno Medical (Tel Aviv, Israel), consults for Baxter, Maltron, ConvaTec, Acelity, Spiegelberg and Holtech Medical. NVR has received speaker’s fees from Baxter Belgium and resided in a medical advisory board organized by Baxter Healthcare, US. PE is a member of the executive committee of IFA, founder of acidbase.org, and has received speaker’s fees from Baxter Belgium and an unrestricted education grant from BBraun. TL has received speaker’s fees from Bbraun. RGH holds a research grant from Grifols for the study of 20% albumin. MM is Director of the UCL Discovery Lab. His University Chair is sponsored by Smiths Medical. He is Co-Director Duke-UCL Consortium (The Morpheus Project); a paid Consultant for Deltex Medical and Edwards Lifesciences; a Director of the Bloomsbury Innovation Group (BiG); a Director and Chair of Evidence Based Perioperative Medicine (EBPOM) Community Interest Company; Share holder and Scientific Advisor Medical Defense Technologies LLC (Gastrostim and Entarik); Share holder and Director Clinical Hydration Solutions ltd (Patent holder “QUENCH”); GIFTASUP guidelines—Senior Author; NICE—Expert Advsior IV Fluids—Guideline 174. PC has received speaker’s fees from Bbraun, Baxter, and Octapharma and resided in the Critical Care Scientific Advisory Committee organized by Werfen group, and in a medical advisory board organized by Baxter. The other authors have no potential conflict of interest with regard to the content of this review paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manu L. N. G. Malbrain and Thomas Langer contributed equally to the manuscript
Pietro Caironi and Niels Van Regenmortel contributed equally to the manuscript
Contributor Information
Manu L. N. G. Malbrain, Email: manu.malbrain@uzbrussel.be
Thomas Langer, Email: thomas.langer@unimib.it.
Djillali Annane, Email: djillali.annane@aphp.fr.
Luciano Gattinoni, Email: gattinoniluciano@gmail.com.
Paul Elbers, Email: p.elbers@amsterdamumc.nl.
Robert G. Hahn, Email: r.hahn@telia.com
Inneke De laet, Email: inneke.delaet@zna.be.
Andrea Minini, Email: Andrea.Minini@uzbrussel.be.
Adrian Wong, Email: avkwong@mac.com.
Can Ince, Email: c.ince@amc.uva.nl.
David Muckart, Email: davidmuckart@gmail.com.
Monty Mythen, Email: m.mythen@ucl.ac.uk.
Pietro Caironi, Email: pietro.caironi@unito.it.
Niels Van Regenmortel, Email: niels.vanregenmortel@zna.be.
References
- 1.Van Regenmortel N, Jorens PG, Malbrain ML. Fluid management before, during and after elective surgery. Curr Opin Crit Care. 2014;20(4):390–395. doi: 10.1097/MCC.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 2.Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Aneman A, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367(2):124–134. doi: 10.1056/NEJMoa1204242. [DOI] [PubMed] [Google Scholar]
- 3.Langer T, Santini A, Scotti E, Van Regenmortel N, Malbrain ML, Caironi P. Intravenous balanced solutions: from physiology to clinical evidence. Anaesthesiol Intensive Ther. 2015;47(Spec No):s78–s88. doi: 10.5603/AIT.a2015.0079. [DOI] [PubMed] [Google Scholar]
- 4.Malbrain ML, Van Regenmortel N, Owczuk R. It is time to consider the four D’s of fluid management. Anaesthesiol Intensive Ther. 2015;47(Spec No):s1–s5. doi: 10.5603/AIT.a2015.0070. [DOI] [PubMed] [Google Scholar]
- 5.Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308(15):1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 6.Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. 2013;369(13):1243–1251. doi: 10.1056/NEJMra1208627. [DOI] [PubMed] [Google Scholar]
- 7.Padhi S, Bullock I, Li L, Stroud M, National Institute for H. Care Excellence Guideline Development G Intravenous fluid therapy for adults in hospital: summary of NICE guidance. BMJ. 2013;347:7073. doi: 10.1136/bmj.f7073. [DOI] [PubMed] [Google Scholar]
- 8.Langer T, Limuti R, Tommasino C, van Regenmortel N, Duval E, Caironi P, et al. Intravenous fluid therapy for hospitalized and critically ill children: rationale, available drugs and possible side effects. Anaesthesiol Intensive Ther. 2018;50(1):49–58. doi: 10.5603/AIT.a2017.0058. [DOI] [PubMed] [Google Scholar]
- 9.Malbrain M, Van Regenmortel N, Saugel B, De Tavernier B, Van Gaal PJ, Joannes-Boyau O, et al. Principles of fluid management and stewardship in septic shock: it is time to consider the four D’s and the four phases of fluid therapy. Ann Intensive Care. 2018;8(1):66. doi: 10.1186/s13613-018-0402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Regenmortel N, De Weerdt T, Van Craenenbroeck AH, Roelant E, Verbrugghe W, Dams K, et al. Effect of isotonic versus hypotonic maintenance fluid therapy on urine output, fluid balance, and electrolyte homeostasis: a crossover study in fasting adult volunteers. Br J Anaesth. 2017;118(6):892–900. doi: 10.1093/bja/aex118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moritz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med. 2015;373(14):1350–1360. doi: 10.1056/NEJMra1412877. [DOI] [PubMed] [Google Scholar]
- 12.Van Regenmortel N, Verbrugghe W, Roelant E, Van den Wyngaert T, Jorens PG. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: a retrospective study in a tertiary mixed ICU population. Intensive Care Med. 2018;44(4):409–417. doi: 10.1007/s00134-018-5147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choo WP, Groeneveld AB, Driessen RH, Swart EL. Normal saline to dilute parenteral drugs and to keep catheters open is a major and preventable source of hypernatremia acquired in the intensive care unit. J Crit Care. 2014;29(3):390–394. doi: 10.1016/j.jcrc.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364(26):2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 15.Byrne L, Obonyo NG, Diab SD, Dunster KR, Passmore MR, Boon AC, et al. Unintended consequences: fluid resuscitation worsens shock in an ovine model of endotoxemia. Am J Respir Crit Care Med. 2018;198(8):1043–1054. doi: 10.1164/rccm.201801-0064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46(5):361–380. doi: 10.5603/AIT.2014.0060. [DOI] [PubMed] [Google Scholar]
- 17.Stewart PA. Independent and dependent variables of acid–base control. Respir Physiol. 1978;33(1):9–26. doi: 10.1016/0034-5687(78)90079-8. [DOI] [PubMed] [Google Scholar]
- 18.Kellum JA, P.W.G. E. Stewartìs textbook of acid–base Lulucom. 2009.
- 19.Langer T, Scotti E, Carlesso E, Protti A, Zani L, Chierichetti M, et al. Electrolyte shifts across the artificial lung in patients on extracorporeal membrane oxygenation: interdependence between partial pressure of carbon dioxide and strong ion difference. J Crit Care. 2015;30(1):2–6. doi: 10.1016/j.jcrc.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Morgan TJ, Venkatesh B, Hall J. Crystalloid strong ion difference determines metabolic acid–base change during in vitro hemodilution. Crit Care Med. 2002;30(1):157–160. doi: 10.1097/00003246-200201000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Langer T, Ferrari M, Zazzeron L, Gattinoni L, Caironi P. Effects of intravenous solutions on acid–base equilibrium: from crystalloids to colloids and blood components. Anaesthesiol Intensive Ther. 2014;46(5):350–360. doi: 10.5603/AIT.2014.0059. [DOI] [PubMed] [Google Scholar]
- 22.Langer T, Carlesso E, Protti A, Monti M, Comini B, Zani L, et al. In vivo conditioning of acid–base equilibrium by crystalloid solutions: an experimental study on pigs. Intensive Care Med. 2012;38(4):686–693. doi: 10.1007/s00134-011-2455-2. [DOI] [PubMed] [Google Scholar]
- 23.Carlesso E, Maiocchi G, Tallarini F, Polli F, Valenza F, Cadringher P, et al. The rule regulating pH changes during crystalloid infusion. Intensive Care Med. 2011;37(3):461–468. doi: 10.1007/s00134-010-2095-y. [DOI] [PubMed] [Google Scholar]
- 24.Scheingraber S, Rehm M, Sehmisch C, Finsterer U. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology. 1999;90(5):1265–1270. doi: 10.1097/00000542-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Drobin D, Hahn RG. Kinetics of isotonic and hypertonic plasma volume expanders. Anesthesiology. 2002;96(6):1371–1380. doi: 10.1097/00000542-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Hahn RG. Influences of red blood cell and platelet counts on the distribution and elimination of crystalloid fluid. Medicina. 2017;53(4):233–241. doi: 10.1016/j.medici.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Aksu U, Bezemer R, Yavuz B, Kandil A, Demirci C, Ince C. Balanced vs unbalanced crystalloid resuscitation in a near-fatal model of hemorrhagic shock and the effects on renal oxygenation, oxidative stress, and inflammation. Resuscitation. 2012;83(6):767–773. doi: 10.1016/j.resuscitation.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 28.Hansen PB, Jensen BL, Skott O. Chloride regulates afferent arteriolar contraction in response to depolarization. Hypertension (Dallas, Tex: 1979). 1998;32(6):1066–1070. doi: 10.1161/01.hyp.32.6.1066. [DOI] [PubMed] [Google Scholar]
- 29.Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Investig. 1983;71(3):726–735. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfortmueller CA, Fleischmann E. Acetate-buffered crystalloid fluids: current knowledge, a systematic review. J Crit Care. 2016;35:96–104. doi: 10.1016/j.jcrc.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Potura E, Lindner G, Biesenbach P, Funk GC, Reiterer C, Kabon B, et al. An acetate-buffered balanced crystalloid versus 0.9% saline in patients with end-stage renal disease undergoing cadaveric renal transplantation: a prospective randomized controlled trial. Anesth Analg. 2015;120(1):123–129. doi: 10.1213/ANE.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 32.Shaw AD, Bagshaw SM, Goldstein SL, Scherer LA, Duan M, Schermer CR, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg. 2012;255(5):821–829. doi: 10.1097/SLA.0b013e31825074f5. [DOI] [PubMed] [Google Scholar]
- 33.Lobo DN, Stanga Z, Aloysius MM, Wicks C, Nunes QM, Ingram KL, et al. Effect of volume loading with 1 liter intravenous infusions of 0.9% saline, 4% succinylated gelatine (Gelofusine) and 6% hydroxyethyl starch (Voluven) on blood volume and endocrine responses: a randomized, three-way crossover study in healthy volunteers. Crit Care Med. 2010;38(2):464–470. doi: 10.1097/CCM.0b013e3181bc80f1. [DOI] [PubMed] [Google Scholar]
- 34.Reid F, Lobo DN, Williams RN, Rowlands BJ, Allison SP. (Ab)normal saline and physiological Hartmann’s solution: a randomized double-blind crossover study. Clin Sci (London, England: 1979). 2003;104(1):17–24. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 35.Lobo DN, Awad S. Should chloride-rich crystalloids remain the mainstay of fluid resuscitation to prevent ‘pre-renal’ acute kidney injury?: con. Kidney Int. 2014;86(6):1096–1105. doi: 10.1038/ki.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marik PE. Iatrogenic salt water drowning and the hazards of a high central venous pressure. Ann Intensive Care. 2014;4:21. doi: 10.1186/s13613-014-0021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams EL, Hildebrand KL, McCormick SA, Bedel MJ. The effect of intravenous lactated Ringer’s solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesth Analg. 1999;88(5):999–1003. doi: 10.1097/00000539-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Wilkes NJ, Woolf R, Mutch M, Mallett SV, Peachey T, Stephens R, et al. The effects of balanced versus saline-based hetastarch and crystalloid solutions on acid–base and electrolyte status and gastric mucosal perfusion in elderly surgical patients. Anesth Analg. 2001;93(4):811–816. doi: 10.1097/00000539-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Young P, Bailey M, Beasley R, Henderson S, Mackle D, McArthur C, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: The SPLIT randomized clinical trial. JAMA. 2015;314(16):1701–1710. doi: 10.1001/jama.2015.12334. [DOI] [PubMed] [Google Scholar]
- 40.Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(9):829–839. doi: 10.1056/NEJMoa1711584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Self WH, Semler MW, Wanderer JP, Wang L, Byrne DW, Collins SP, et al. Balanced crystalloids versus saline in non-critically ill adults. N Engl J Med. 2018;378(9):819–828. doi: 10.1056/NEJMoa1711586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Semler MW, Kellum JA. Balanced crystalloid solutions. Am J Respir Crit Care Med. 2019;199(8):952–960. doi: 10.1164/rccm.201809-1677CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Aspects Med. 2012;33(3):209–290. doi: 10.1016/j.mam.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Caironi P, Gattinoni L. The clinical use of albumin: the point of view of a specialist in intensive care. Blood Transfus. 2009;7(4):259–267. doi: 10.2450/2009.0002-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caironi P, Langer T, Gattinoni L. Albumin in critically ill patients: the ideal colloid? Curr Opin Crit Care. 2015;21(4):302–308. doi: 10.1097/MCC.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 46.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454(3):345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng Y, Zhang XF, Fu BM, Tarbell JM. The role of endothelial surface glycocalyx in mechanosensing and transduction. Adv Exp Med Biol. 2018;1097:1–27. doi: 10.1007/978-3-319-96445-4_1. [DOI] [PubMed] [Google Scholar]
- 48.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 49.Guerci P, Ergin B, Uz Z, Ince Y, Westphal M, Heger M, et al. Glycocalyx degradation is independent of vascular barrier permeability increase in non-traumatic hemorrhagic shock in rats. Anesth Analg. 2019;129(2):598–607. doi: 10.1213/ANE.0000000000003918. [DOI] [PubMed] [Google Scholar]
- 50.Ince C, Mayeux PR, Nguyen T, Gomez H, Kellum JA, Ospina-Tascon GA, et al. The endothelium in sepsis. Shock. 2016;45(3):259–270. doi: 10.1097/SHK.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubio-Gayosso I, Platts SH, Duling BR. Reactive oxygen species mediate modification of glycocalyx during ischemia–reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;290(6):H2247–H2256. doi: 10.1152/ajpheart.00796.2005. [DOI] [PubMed] [Google Scholar]
- 52.Hasselgren E, Zdolsek M, Zdolsek JH, Bjorne H, Krizhanovskii C, Ntika S, et al. Long intravascular persistence of 20% albumin in postoperative patients. Anesth Analg. 2019;129(5):1232–1239. doi: 10.1213/ANE.0000000000004047. [DOI] [PubMed] [Google Scholar]
- 53.Nemme J, Hahn RG, Krizhanovskii C, Ntika S, Sabelnikovs O, Vanags I. Minimal shedding of the glycocalyx layer during abdominal hysterectomy. BMC Anesthesiol. 2017;17(1):107. doi: 10.1186/s12871-017-0391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hippensteel JA, Uchimido R, Tyler PD, Burke RC, Han X, Zhang F, et al. Intravenous fluid resuscitation is associated with septic endothelial glycocalyx degradation. Crit Care. 2019;23(1):259. doi: 10.1186/s13054-019-2534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caironi P, Tognoni G, Masson S, Fumagalli R, Pesenti A, Romero M, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370(15):1412–1421. doi: 10.1056/NEJMoa1305727. [DOI] [PubMed] [Google Scholar]
- 56.Zazzeron L, Gattinoni L, Caironi P. Role of albumin, starches and gelatins versus crystalloids in volume resuscitation of critically ill patients. Curr Opin Crit Care. 2016;22(5):428–436. doi: 10.1097/MCC.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 57.Investigators SS, Finfer S, McEvoy S, Bellomo R, McArthur C, Myburgh J, et al. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Med. 2011;37(1):86–96. doi: 10.1007/s00134-010-2039-6. [DOI] [PubMed] [Google Scholar]
- 58.Lewis SR, Pritchard MW, Evans DJ, Butler AR, Alderson P, Smith AF, et al. Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database Syst Rev. 2018;8:CD000567. doi: 10.1002/14651858.CD000567.pub7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frenette AJ, Bouchard J, Bernier P, Charbonneau A, Nguyen LT, Rioux JP, et al. Albumin administration is associated with acute kidney injury in cardiac surgery: a propensity score analysis. Crit Care. 2014;18(6):602. doi: 10.1186/s13054-014-0602-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guidet B, Ghout I, Ropers J, Aegerter P. Economic model of albumin infusion in septic shock: The EMAISS study. Acta Anaesthesiol Scand. 2020. https://onlinelibrary.wiley.com/doi/epdf/10.1111/aas.13559 [DOI] [PubMed]
- 61.Brandstrup B, Tonnesen H, Beier-Holgersen R, Hjortso E, Ording H, Lindorff-Larsen K, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238(5):641–648. doi: 10.1097/01.sla.0000094387.50865.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hahn RG. Arterial pressure and the rate of elimination of crystalloid fluid. Anesth Analg. 2017;124(6):1824–1833. doi: 10.1213/ANE.0000000000002075. [DOI] [PubMed] [Google Scholar]
- 63.Li Y, Yi S, Zhu Y, Hahn RG. Volume kinetics of Ringer’s lactate solution in acute inflammatory disease. Br J Anaesth. 2018;121(3):574–580. doi: 10.1016/j.bja.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 64.Chowdhury AH, Lobo DN. Fluids and gastrointestinal function. Curr Opin Clin Nutr Metab Care. 2011;14(5):469–476. doi: 10.1097/MCO.0b013e328348c084. [DOI] [PubMed] [Google Scholar]
- 65.Coe AJ, Revanas B. Is crystalloid preloading useful in spinal anaesthesia in the elderly? Anaesthesia. 1990;45(3):241–243. doi: 10.1111/j.1365-2044.1990.tb14696.x. [DOI] [PubMed] [Google Scholar]
- 66.Jackson R, Reid JA, Thorburn J. Volume preloading is not essential to prevent spinal-induced hypotension at caesarean section. Br J Anaesth. 1995;75(3):262–265. doi: 10.1093/bja/75.3.262. [DOI] [PubMed] [Google Scholar]
- 67.Lilot M, Ehrenfeld JM, Lee C, Harrington B, Cannesson M, Rinehart J. Variability in practice and factors predictive of total crystalloid administration during abdominal surgery: retrospective two-centre analysis. Br J Anaesth. 2015;114(5):767–776. doi: 10.1093/bja/aeu452. [DOI] [PubMed] [Google Scholar]
- 68.Chappell D, Jacob M, Hofmann-Kiefer K, Conzen P, Rehm M. A rational approach to perioperative fluid management. Anesthesiology. 2008;109(4):723–740. doi: 10.1097/ALN.0b013e3181863117. [DOI] [PubMed] [Google Scholar]
- 69.Hopf HW, Morrissey C. Perioperative fluid management: turning art to science. Anesthesiology. 2019;130(5):677–679. doi: 10.1097/ALN.0000000000002663. [DOI] [PubMed] [Google Scholar]
- 70.Minto G, Mythen MG. Perioperative fluid management: science, art or random chaos? Br J Anaesth. 2015;114(5):717–721. doi: 10.1093/bja/aev067. [DOI] [PubMed] [Google Scholar]
- 71.Myles PS, McIlroy DR, Bellomo R, Wallace S. Importance of intraoperative oliguria during major abdominal surgery: findings of the Restrictive versus Liberal Fluid Therapy in Major Abdominal Surgery trial. Br J Anaesth. 2019;122(6):726–733. doi: 10.1016/j.bja.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 72.Thacker JK, Mountford WK, Ernst FR, Krukas MR, Mythen MM. Perioperative fluid utilization variability and association with outcomes: considerations for enhanced recovery efforts in sample US surgical populations. Ann Surg. 2016;263(3):502–510. doi: 10.1097/SLA.0000000000001402. [DOI] [PubMed] [Google Scholar]
- 73.Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, et al. Guidelines for perioperative care in elective colorectal surgery: enhanced recovery after surgery (ERAS((R))) society recommendations: 2018. World J Surg. 2019;43(3):659–695. doi: 10.1007/s00268-018-4844-y. [DOI] [PubMed] [Google Scholar]
- 74.Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland G, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014;311(21):2181–2190. doi: 10.1001/jama.2014.5305. [DOI] [PubMed] [Google Scholar]
- 75.Rochwerg B, Alhazzani W, Sindi A, Heels-Ansdell D, Thabane L, Fox-Robichaud A, et al. Fluid resuscitation in sepsis: a systematic review and network meta-analysis. Ann Intern Med. 2014;161(5):347–355. doi: 10.7326/M14-0178. [DOI] [PubMed] [Google Scholar]
- 76.Trof RJ, Sukul SP, Twisk JW, Girbes AR, Groeneveld AB. Greater cardiac response of colloid than saline fluid loading in septic and non-septic critically ill patients with clinical hypovolaemia. Intensive Care Med. 2010;36(4):697–701. doi: 10.1007/s00134-010-1776-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Annane D, Siami S, Jaber S, Martin C, Elatrous S, Declere AD, et al. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA. 2013;310(17):1809–1817. doi: 10.1001/jama.2013.280502. [DOI] [PubMed] [Google Scholar]
- 78.Raiman M, Mitchell CG, Biccard BM, Rodseth RN. Comparison of hydroxyethyl starch colloids with crystalloids for surgical patients: a systematic review and meta-analysis. Eur J Anaesthesiol. 2016;33(1):42–48. doi: 10.1097/EJA.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 79.Annane D, Fuchs-Buder T, Zoellner C, Kaukonen M, Scheeren TWL. EMA recommendation to suspend HES is hazardous. Lancet. 2018;391(10122):736–738. doi: 10.1016/S0140-6736(18)30254-X. [DOI] [PubMed] [Google Scholar]
- 80.Doshi P. Data too important to share: do those who control the data control the message? BMJ. 2016;352:i1027. doi: 10.1136/bmj.i1027. [DOI] [PubMed] [Google Scholar]
- 81.Priebe HJ, Malbrain ML, Elbers P. The great fluid debate: methodology, physiology and appendicitis. Anaesthesiol Intensive Ther. 2015;47(5):437–440. doi: 10.5603/AIT.a2015.0067. [DOI] [PubMed] [Google Scholar]
- 82.Futier E, Garot M, Godet T, Biais M, Verzilli D, Ouattara A, et al. Effect of hydroxyethyl starch vs saline for volume replacement therapy on death or postoperative complications among high-risk patients undergoing major abdominal surgery: The FLASH randomized clinical trial. JAMA. 2020;323(3):225–236. doi: 10.1001/jama.2019.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McNab S, Duke T, South M, Babl FE, Lee KJ, Arnup SJ, et al. 140 mmol/L of sodium versus 77 mmol/L of sodium in maintenance intravenous fluid therapy for children in hospital (PIMS): a randomised controlled double-blind trial. Lancet. 2015;385(9974):1190–1197. doi: 10.1016/S0140-6736(14)61459-8. [DOI] [PubMed] [Google Scholar]
- 84.Scales K. NICE CG 174: intravenous fluid therapy in adults in hospital. Br J Nurs. 2014;23(8):S6–S8. doi: 10.12968/bjon.2014.23.Sup8.S6. [DOI] [PubMed] [Google Scholar]
- 85.Van Regenmortel N, Hendrickx S, Roelant E, Baar I, Dams K, Van Vlimmeren K, et al. 154 compared to 54 mmol per liter of sodium in intravenous maintenance fluid therapy for adult patients undergoing major thoracic surgery (TOPMAST): a single-center randomized controlled double-blind trial. Intensive Care Med. 2019;45(10):1422–1432. doi: 10.1007/s00134-019-05772-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Magder S. Volume and its relationship to cardiac output and venous return. Crit Care. 2016;20:271. doi: 10.1186/s13054-016-1438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miller TE, Roche AM, Mythen M. Fluid management and goal-directed therapy as an adjunct to Enhanced Recovery After Surgery (ERAS) Can J Anaesth. 2015;62(2):158–168. doi: 10.1007/s12630-014-0266-y. [DOI] [PubMed] [Google Scholar]
- 88.Guyton AC, Lindsey AW. Effect of elevated left atrial pressure and decreased plasma protein concentration on the development of pulmonary edema. Circ Res. 1959;7(4):649–657. doi: 10.1161/01.res.7.4.649. [DOI] [PubMed] [Google Scholar]
- 89.Kolsen-Petersen JA. The endothelial glycocalyx: the great luminal barrier. Acta Anaesthesiol Scand. 2015;59(2):137–139. doi: 10.1111/aas.12440. [DOI] [PubMed] [Google Scholar]
- 90.Hahn R, Stalberg H, Carlstrom K, Hjelmqvist H, Ullman J, Rundgren M. Plasma atrial natriuretic peptide concentration and renin activity during overhydration with 1.5% glycine solution in conscious sheep. Prostate. 1994;24(2):55–61. doi: 10.1002/pros.2990240202. [DOI] [PubMed] [Google Scholar]
- 91.Kamp-Jensen M, Olesen KL, Bach V, Schutten HJ, Engquist A. Changes in serum electrolyte and atrial natriuretic peptide concentrations, acid–base and haemodynamic status after rapid infusion of isotonic saline and Ringer lactate solution in healthy volunteers. Br J Anaesth. 1990;64(5):606–610. doi: 10.1093/bja/64.5.606. [DOI] [PubMed] [Google Scholar]
- 92.Norberg A, Hahn RG, Li H, Olsson J, Prough DS, Borsheim E, et al. Population volume kinetics predicts retention of 0.9% saline infused in awake and isoflurane-anesthetized volunteers. Anesthesiology. 2007;107(1):24–32. doi: 10.1097/01.anes.0000268387.34758.6d. [DOI] [PubMed] [Google Scholar]
- 93.Acheampong A, Vincent JL. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit Care. 2015;19:251. doi: 10.1186/s13054-015-0970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Oliveira FS, Freitas FG, Ferreira EM, de Castro I, Bafi AT, de Azevedo LC, et al. Positive fluid balance as a prognostic factor for mortality and acute kidney injury in severe sepsis and septic shock. J Crit Care. 2015;30(1):97–101. doi: 10.1016/j.jcrc.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 95.Silva JM, Jr, de Oliveira AM, Nogueira FA, Vianna PM, Pereira Filho MC, Dias LF, et al. The effect of excess fluid balance on the mortality rate of surgical patients: a multicenter prospective study. Crit Care. 2013;17(6):R288. doi: 10.1186/cc13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marik PE, Linde-Zwirble WT, Bittner EA, Sahatjian J, Hansell D. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med. 2017;43(5):625–632. doi: 10.1007/s00134-016-4675-y. [DOI] [PubMed] [Google Scholar]
- 97.Cordemans C, De Laet I, Van Regenmortel N, Schoonheydt K, Dits H, Martin G, et al. Aiming for a negative fluid balance in patients with acute lung injury and increased intra-abdominal pressure: a pilot study looking at the effects of PAL-treatment. Ann Intensive Care. 2012;2(Suppl 1):S15. doi: 10.1186/2110-5820-2-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cordemans C, De Laet I, Van Regenmortel N, Schoonheydt K, Dits H, Huber W, et al. Fluid management in critically ill patients: the role of extravascular lung water, abdominal hypertension, capillary leak, and fluid balance. Ann Intensive Care. 2012;2(Suppl 1 Diagnosis and management of intra-abdominal hypertension):S1. doi: 10.1186/2110-5820-2-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dabrowski W, Kotlinska-Hasiec E, Schneditz D, Zaluska W, Rzecki Z, De Keulenaer B, et al. Continuous veno-venous hemofiltration to adjust fluid volume excess in septic shock patients reduces intra-abdominal pressure. Clin Nephrol. 2014;82(1):41–50. doi: 10.5414/CN108015. [DOI] [PubMed] [Google Scholar]
- 100.Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185(12):1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoste EA, Maitland K, Brudney CS, Mehta R, Vincent JL, Yates D, et al. Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth. 2014;113(5):740–747. doi: 10.1093/bja/aeu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Silversides JA, Perner A, Malbrain M. Liberal versus restrictive fluid therapy in critically ill patients. Intensive Care Med. 2019;45(10):1440–1442. doi: 10.1007/s00134-019-05713-y. [DOI] [PubMed] [Google Scholar]
- 103.Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, et al. Fluid challenges in intensive care: the FENICE study: a global inception cohort study. Intensive Care Med. 2015;41(9):1529–1537. doi: 10.1007/s00134-015-3850-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vincent JL, Weil MH. Fluid challenge revisited. Crit Care Med. 2006;34(5):1333–1337. doi: 10.1097/01.CCM.0000214677.76535.A5. [DOI] [PubMed] [Google Scholar]
- 105.Vincent JL. Let’s give some fluid and see what happens” versus the “mini-fluid challenge. Anesthesiology. 2011;115(3):455–456. doi: 10.1097/ALN.0b013e318229a521. [DOI] [PubMed] [Google Scholar]
- 106.Bennett VA, Vidouris A, Cecconi M. Effects of fluids on the macro- and microcirculations. Crit Care. 2018;22(1):74. doi: 10.1186/s13054-018-1993-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367(20):1901–1911. doi: 10.1056/NEJMoa1209759. [DOI] [PubMed] [Google Scholar]
- 108.Uz Z, Ince C, Guerci P, Ince Y, Araujo RP, Ergin B, et al. Recruitment of sublingual microcirculation using handheld incident dark field imaging as a routine measurement tool during the postoperative de-escalation phase-a pilot study in post ICU cardiac surgery patients. Perioperat Med. 2018;7:18. doi: 10.1186/s13741-018-0091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Paxian M, Bauer I, Rensing H, Jaeschke H, Mautes AE, Kolb SA, et al. Recovery of hepatocellular ATP and “pericentral apoptosis” after hemorrhage and resuscitation. FASEB J. 2003;17(9):993–1002. doi: 10.1096/fj.02-0624com. [DOI] [PubMed] [Google Scholar]
- 110.Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64(5):1211–1217. doi: 10.1097/TA.0b013e318169cd3c. [DOI] [PubMed] [Google Scholar]
- 111.Schochl H, Maegele M, Solomon C, Gorlinger K, Voelckel W. Early and individualized goal-directed therapy for trauma-induced coagulopathy. Scand J Trauma Resuscitat Emerg Med. 2012;20:15. doi: 10.1186/1757-7241-20-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 113.Valle EJ, Allen CJ, Van Haren RM, Jouria JM, Li H, Livingstone AS, et al. Do all trauma patients benefit from tranexamic acid? J Trauma Acute Care Surg. 2014;76(6):1373–1378. doi: 10.1097/TA.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 114.Peeters Y, Vandervelden S, Wise R, Malbrain ML. An overview on fluid resuscitation and resuscitation endpoints in burns: past, present and future. Part 1—historical background, resuscitation fluid and adjunctive treatment. Anaesthesiol Intensive Ther. 2015;47(Spec No):s6–s14. doi: 10.5603/AIT.a2015.0063. [DOI] [PubMed] [Google Scholar]
- 115.Baxter CR, Shires T. Physiological response to crystalloid resuscitation of severe burns. Ann N Y Acad Sci. 1968;150(3):874–894. doi: 10.1111/j.1749-6632.1968.tb14738.x. [DOI] [PubMed] [Google Scholar]
- 116.Cartotto R, Zhou A. Fluid creep: the pendulum hasn’t swung back yet! J Burn Care Res. 2010;31(4):551–558. doi: 10.1097/BCR.0b013e3181e4d732. [DOI] [PubMed] [Google Scholar]
- 117.Strang SG, Van Lieshout EM, Breederveld RS, Van Waes OJ. A systematic review on intra-abdominal pressure in severely burned patients. Burns. 2014;40(1):9–16. doi: 10.1016/j.burns.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 118.Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39(7):1190–1206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pruitt BA., Jr Protection from excessive resuscitation: “pushing the pendulum back”. J Trauma. 2000;49(3):567–568. doi: 10.1097/00005373-200009000-00030. [DOI] [PubMed] [Google Scholar]
- 120.Sullivan SR, Ahmadi AJ, Singh CN, Sires BS, Engrav LH, Gibran NS, et al. Elevated orbital pressure: another untoward effect of massive resuscitation after burn injury. J Trauma. 2006;60(1):72–76. doi: 10.1097/01.ta.0000197657.25382.b2. [DOI] [PubMed] [Google Scholar]
- 121.Gille J, Klezcewski B, Malcharek M, Raff T, Mogk M, Sablotzki A, et al. Safety of resuscitation with Ringer’s acetate solution in severe burn (VolTRAB)—an observational trial. Burns. 2014;40(5):871–880. doi: 10.1016/j.burns.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 122.Baxter CR. Fluid volume and electrolyte changes of the early postburn period. Clin Plast Surg. 1974;1(4):693–703. [PubMed] [Google Scholar]
- 123.Guidet B, Martinet O, Boulain T, Philippart F, Poussel JF, Maizel J, et al. Assessment of hemodynamic efficacy and safety of 6% hydroxyethyl starch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care. 2012;16(3):R94. doi: 10.1186/cc11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cochran A, Morris SE, Edelman LS, Saffle JR. Burn patient characteristics and outcomes following resuscitation with albumin. Burns. 2007;33(1):25–30. doi: 10.1016/j.burns.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 125.Lawrence A, Faraklas I, Watkins H, Allen A, Cochran A, Morris S, et al. Colloid administration normalizes resuscitation ratio and ameliorates “fluid creep”. J Burn Care Res. 2010;31(1):40–47. doi: 10.1097/BCR.0b013e3181cb8c72. [DOI] [PubMed] [Google Scholar]
- 126.Malbrain ML, Rice T, Mythen M, Wuyts S. It is time for improved fluid stewardship. ICU Manag Pract. 2018;18(3):158–162. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.