Abstract
Spondweni virus (SPONV) is the most closely related known flavivirus to Zika virus (ZIKV). Its pathogenic potential and vector specificity have not been well defined. SPONV has been found predominantly in Africa, but was recently detected in a pool of Culex quinquefasciatus mosquitoes in Haiti. Here we show that SPONV can cause significant fetal harm, including demise, comparable to ZIKV, in a mouse model of vertical transmission. Following maternal inoculation, we detected infectious SPONV in placentas and fetuses, along with significant fetal and placental histopathology, together suggesting vertical transmission. To test vector competence, we exposed Aedes aegypti and Culex quinquefasciatus mosquitoes to SPONV-infected bloodmeals. Aedes aegypti could efficiently transmit SPONV, whereas Culex quinquefasciatus could not. Our results suggest that SPONV has the same features that made ZIKV a public health risk.
Keywords: Arbovirus, Flavivirus, Spondweni virus, Zika virus, Aedes aegypti, Vector competence, Congenital zika syndrome
1. Introduction
Zika virus (ZIKV) was originally isolated over seventy years ago, and was thought to cause a mild, self-limiting, febrile illness (Dick et al., 1952; Simpson, 1964). Not until the outbreak in the Americas in 2015 and 2016 was ZIKV identified as a cause of significant adverse pregnancy outcomes (Johansson et al., 2016; Melo et al., 2016). Before the definition of congenital Zika syndrome (CZS) in 2016, gestational arbovirus infection was not associated with birth defects. Spondweni virus (SPONV) is the closest known relative to ZIKV, but whether SPONV is an emerging threat to pregnant women and their babies is unknown. It was previously thought that SPONV was geographically confined to Africa and caused only mild disease in rare human infections, reminiscent of the consensus around ZIKV in the decades following its discovery, but recent data suggest that it may be spreading beyond Africa (White et al., 2018). SPONV may therefore be poised to harm pregnancies in new, immunologically naive populations. To do this, SPONV would need to fulfill two major criteria: it would need to be vertically transmitted and cause fetal harm, and be transmitted between humans by the urban mosquito vector Aedes aegypti, which is associated with large-scale outbreaks of related arboviruses.
The first identification of SPONV was thought to have occurred in 1955 in South Africa (Theiler and Downs, 1973; Wolfe et al., 1982). However, it was later recognized that SPONV was in fact isolated three years earlier in Nigeria, but was misidentified at the time as a strain of ZIKV because of serological cross-reactivity (Haddow et al., 1964; Simpson, 1964; Draper, 1965). Serological cross-reactivity with ZIKV and other flaviviruses likely still confounds accurate diagnostics today. As a result, only six well-documented clinical cases of SPONV infection have ever been described (Macnamara, 1954; Kokernot et al., 1957; Draper, 1965). It is likely that many infections have gone unrecognized—serosurveys have detected evidence of SPONV infection in 10 countries throughout Sub-Saharan Africa (Kokernot et al., 1965a, 1965b; Brottes et al., 1966; Ardoin et al., 1976; Wolfe et al., 1982). Still, these six cases indicate that infection with SPONV typically involves an acute, self-limiting mild to moderate febrile illness (Wolfe et al., 1982), but a subset (4/6) of cases are believed to progress to more serious disease, including vascular leakage and neurological involvement (Macnamara, 1954; Mcintosh et al., 1961; Draper, 1965; Wolfe et al., 1982; Haddow and Woodall, 2016); these symptoms resemble those of other infections common in the same regions (Haddow et al., 1964; Boorman and Draper, 1968; Wolfe et al., 1982). Nothing is known about the risks posed by SPONV infection during pregnancy in humans. Powassan virus and West Nile virus, ZIKV-related neurotropic flaviviruses, can infect human placental explants and cause fetal demise in mice (Platt et al., 2018). In addition, intrauterine infection with St. Louis encephalitis virus resulted in severe neurological outcomes in mice that was dependent on the gestational day of challenge (Andersen and Hanson, 1970). In pregnant mice whose type 1 interferon responses were blocked by antibody treatment, SPONV did not cause fetal demise, but the placenta and fetus were infected (Salazar et al., 2019).
Potential vectors for SPONV have not been identified, although SPONV has been isolated from several mosquito genera (Mcintosh et al., 1961; Worth et al., 1961; Mcintosh et al., 1972). SPONV is considered to be exclusively mosquito-borne, but recent mouse experiments suggest that it may have limited potential to be sexually transmitted (Mcdonald et al., 2017)—human cases of sexually transmitted SPONV have not been described. Whether SPONV could spill over into a human-mosquito cycle involving Aedes aegypti is unclear. One recent study suggested strains of Aedes albopictus, Aedes aegypti, and Culex quinquefasciatus were all unable to transmit SPONV (Haddow et al., 2016). Overall, with only limited studies evaluating SPONV's pathogenic potential and vector specificity, there is little data to guide our expectations for the potential of SPONV to cause fetal harm and adapt to an urban or peri-urban transmission cycle involving Aedes aegypti and/or other human-biting mosquito species.
We aimed to better characterize both the pathogenic potential of SPONV during pregnancy and to also identify potential vectors of the virus. Using an established vertical transmission model in mice (Jaeger et al., 2019), we assessed fetal outcomes after infection at embryonic day 7.5 with SPONV as compared to both ZIKV and dengue virus (DENV). We found that SPONV caused fetal harm, similar to what is observed from ZIKV infection in this model. Vector competence experiments showed that Ae. aegypti could transmit SPONV when exposed to bloodmeal titers that approximate physiological titers, while Cx. quinquefasciatus could not. Our study contributes to the characterization of SPONV pathogenesis and identifies a potential urban vector for the virus, collectively suggesting that this esoteric virus has features that could portend medically significant future outbreaks.
2. Results
2.1. Type I interferon deficient mice are susceptible to SPONV infection
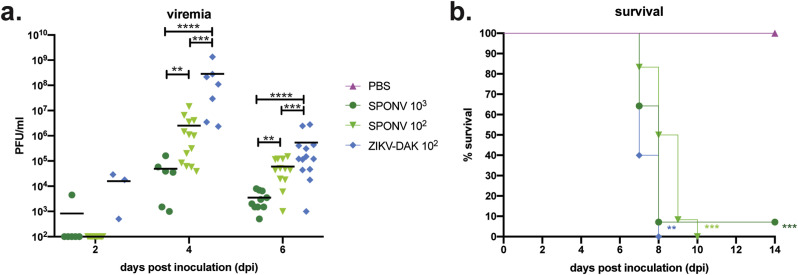
Recent studies have demonstrated that SPONV replicates in AG129 mice deficient in type I and II interferon (Mcdonald et al., 2017) and in mice treated with an Ifnar1-blocking monoclonal antibody (mAb) (Salazar et al., 2019). We sought to establish a model that was less immunocompromised than AG129 mice, and one in which transplacental ZIKV infection and fetal damage had been demonstrated (Miner et al., 2016; Yockey et al., 2018; Jaeger et al., 2019) to better understand SPONV pathologic outcomes during pregnancy. First, to confirm the route and doses that would result in productive infection, groups of non-pregnant, mixed sex six- to eleven-week-old mice lacking type I interferon signaling (Ifnar1 −/−) were inoculated subcutaneously (s.c.) in the footpad with 103 or 102 PFU of SPONV strain SA Ar94 (this is the only strain used in these studies, so it will be referred to hereafter as SPONV); or 102 PFU of the highly pathogenic African-lineage ZIKV strain DAK AR 41524 (ZIKV-DAK) (Jaeger et al., 2019). Since contemporary SPONV isolates from Haiti do not exist, we used the only available low-passage isolate, SPONV strain SA Ar94. This strain is 98.8% nucleotide identical with the SPONV genome recovered from mosquitoes in Haiti (Genbank:MG182017). Serum was collected at 2, 4, and 6 days post-inoculation (dpi) to confirm infection and determine the replication kinetics of SPONV in non-pregnant Ifnar1 −/− mice. We also collected and tested serum at 7, 14, and 21 days from mice surviving SPONV inoculation, because sustained vRNA loads were observed with the Ifnar1-blocking mAb model (Salazar et al., 2019). SPONV viral titer in the serum peaked at 4 dpi (Fig. 1 a), and in surviving animals there was no detectable viremia at 7, 14, or 21 dpi. Higher serum titers were observed in animals inoculated with the lowest dose of SPONV (102 PFU). We postulate that this could be the result of higher inoculating doses causing a rapid initial rise in viremia, which in turn induces a more robust immune response, leading to more rapid clearance of virus from the serum, but confirmation will require further studies. ZIKV-DAK viremia also peaked at 4 dpi and reached significantly higher titers at 4 dpi than either SPONV-inoculated group (one-way ANOVA with Tukey's multiple comparisons test; SPONV 103 vs. ZIKV-DAK: p < 0.0001, SPONV 102 vs. ZIKV-DAK: p = 0.0004).
Fig. 1.
Characterization of SPONV in non-pregnant Ifnar1−/−mice. (a) Serum was collected from mice during three independent replicates at 2, 4, and/or 6 days post inoculation and titered via plaque assay. Assay limit of detection was 100 PFU. Viremia peaked at 4 dpi for all virus groups, with ZIKV-DAK replicating to significantly higher titers at 4 dpi than SPONV (one-way ANOVA). ****p < 0.0001; ***p < 0.0005; **p < 0.006 (b) Survival curves of six-to eleven-week old Ifnar1−/− mice s.c. inoculated with 103 PFU of SPONV, 102 PFU of SPONV, 102 PFU ZIKV-DAK, or a PBS control. SPONV 103: n = 14; SPONV 102: n = 13, ZIKV-DAK: n = 5; PBS: n = 6). All virus infections caused significant mortality by 14 dpi as compared to PBS controls (Fisher's exact test). ***p < 0.0005; **p < 0.005.
All virus-inoculated groups showed significant mortality as compared to PBS-inoculated, age-matched controls (Log-rank test, p < 0.002) (Fig. 1b). Regardless of dose, SPONV-inoculated mice exhibited signs of illness by 5–6 dpi. We paid particular attention to the footpad used for inoculation because Salazar et al. (2019) recently noted ipsilateral footpad swelling in mice treated with an Ifnar1-blocking mAb and SPONV inoculation (Salazar et al., 2019). However, we did not observe any notable swelling of the ipsilateral footpad during the course of our studies. The typical signs of illness we observed were more consistent with ZIKV-associated clinical signs, including weight loss, lethargy, hunched posture, and unilateral hindlimb paralysis (Lazear et al., 2016).
2.2. SPONV infection in Ifnar1−/− mice during pregnancy leads to fetal demise
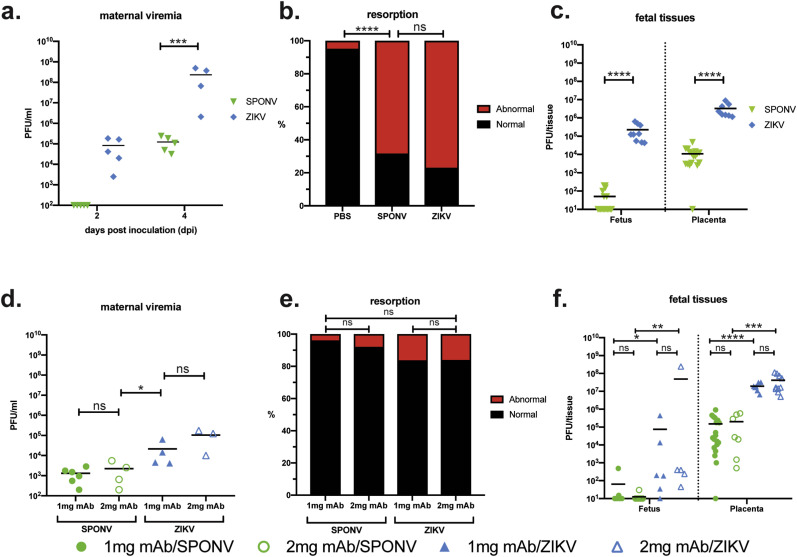
To characterize the range of pathogenic outcomes of congenital SPONV infection, we first used a previously established murine pregnancy model for ZIKV (Miner et al., 2016; Yockey et al., 2018; Jaeger et al., 2019), in which Ifnar1 −/− dams were crossed with wildtype sires to produce heterozygous offspring with one intact Ifnar1 allele to more closely mimic the immune status of a human fetus, i.e., the fetus and placenta have intact IFN-ɑ/β signaling. Timed-mated dams were s.c. inoculated in the footpad on embryonic day 7.5 (E7.5) with 102 PFU of SPONV or 102 PFU ZIKV-DAK. Based on our preliminary experiments with SPONV in non-pregnant animals, and the results from our past studies (Jaeger et al., 2019), we chose this dose to minimize the potential confounding impacts of maternal illness on fetal outcomes. We collected serum samples from dams at 2 and 4 dpi to confirm maternal infection. All dams were productively infected, with detectable viremia for all groups by 4 dpi (Fig. 2 a). ZIKV-DAK replicated to significantly higher titers at 4 dpi as compared to SPONV (Student's t-test p-value = 0.0008, t = 5.641, df = 7). Dams were monitored daily for clinical signs until the time of necropsy at E14.5, 7 days after inoculation. Mild clinical signs were evident in both SPONV- and ZIKV-infected dams at time of necropsy and included reduced activity, squinted eyes, ruffled fur, and hunched posture. Interestingly, clinical signs were milder in comparison to non-pregnant animals that received an equivalent inoculum dose, and none of the pregnant animals met euthanasia criteria.
Fig. 2.
Outcomes after maternal infection at E7.5. (a–c) Time-mated Ifnar1−/− dams were inoculated with 102 SPONV or ZIKV at E7.5. (a) Maternal infection with SPONV and ZIKV was confirmed by plaque assay on days 2 and 4 post inoculation (dpi). Assay limit of detection was 100 PFU. ZIKV infected dams had significantly higher viremia at 4 dpi than SPONV infected dams. ***p < 0.005 (Student's t-test).-(b) Rate of grossly normal (black) versus abnormal (red) fetuses at E14.5 after maternal infection at E7.5. An abnormal fetus was defined as in any stage of resorption. Data presented are for individual fetuses from 4 to 6 litters per treatment group. ****p < 0.0001; ns, not significant (Fisher's exact test). (c) Viral titer was measured by plaque assay for a subset of individual homogenized fetuses and placentas. Assay limit of detection was 10 PFU. Symbols represent individual fetuses and placentas from 5 to 6 individual experiments (litters) from each treatment group. Bars represent the mean viral burden from each treatment group. Viral titers were significantly higher in ZIKV placentas and fetuses as compared to SPONV. ****p < 0.0001 (one-way ANOVA). (d–f) Time-mated C57BL6 dams were treated with 1 or 2 mg of anti-IFNAR1 mAb at E6.5 and inoculated with 105 SPONV or ZIKV at E7.5. (d) Maternal infection with SPONV and ZIKV was confirmed by plaque assay 4 dpi. ZIKV infected dams had significantly higher viremia than SPONV infected dams. Maternal viremia did not differ significantly between 1 and 2 mg mAb treatments. *p < 0.05; ns, not significant (one-way ANOVA). (e) Rates of grossly normal (black) versus abnormal (red) fetuses at E14.5 after maternal infection at E7.5. Data presented are for individual fetuses from 3 to 6 litters per treatment group. Resorption rates did not differ between any treatment group. ns, not significant (Fisher's exact test). (f) Viral titer was measured by plaque assay for a subset of individual homogenized fetuses and placentas. Infectious virus was detectable in all treatment groups. Viral titers were not significantly different based on mAb dose. Viral titers were significantly higher in ZIKV infected fetuses and placentas as compared to SPONV. ****p < 0.0001; ***p < 0.0005; **p < 0.005; *p < 0.05; ns, not significant (one-way ANOVA).
Next, to assess fetal outcomes, dams were necropsied on E14.5. Similar to what we have reported previously with different strains of ZIKV (Jaeger et al., 2019), gross examination of fetuses and placentas at time of necropsy revealed overt differences among fetuses within pregnancies and with uninfected counterparts. In general, fetuses appeared either grossly normal or abnormal, defined as undergoing embryo resorption (Fig. 2b) (Flores et al., 2014). At the time of necropsy, we observed high rates of resorption from both ZIKV-DAK- and SPONV-infected pregnancies. Resorption rates from ZIKV-DAK- and SPONV-infected pregnancies were not significantly different (ZIKV-DAK: 76.92% vs. SPONV: 68.29%, Fisher's exact test, p = 0.457). Resorption rates for both SPONV and ZIKV-DAK were significantly higher than PBS-inoculated controls (p < 0.0001). Despite significantly higher maternal viremia observed at 4 dpi with ZIKV-DAK-infected dams, the fact that resorption rates did not significantly differ between the two groups indicates that both ZIKV-DAK and SPONV have a propensity to harm the developing fetus that is independent of the amount of replication in maternal blood. Surprisingly, and in contrast to the results described by Salazar et al. where no fetal resorption was observed (Salazar et al., 2019), maternal SPONV infection in our model resulted in high rates of fetal demise.
2.3. ZIKV or SPONV infection in anti-Ifnar1 monoclonal antibody treated dams does not lead to fetal demise
To further characterize the range of pathogenic outcomes of congenital SPONV infection and to assess differences between models, we repeated experiments by treating dams with anti-Ifnar1 mAb (MAR1-5A3; Leinco Technologies, Inc.) one day prior to subcutaneous footpad inoculation with ZIKV-DAK or SPONV (Sheehan et al., 2006). This model has been used previously for assessing both ZIKV and SPONV pathogenesis during pregnancy, but does not result in fetal resorption (Miner et al., 2016; Sapparapu et al., 2016; Jagger et al., 2019) unless mouse-adapted ZIKV is used (Mclennan and Wheal, 1976; Richner et al., 2017; Hassan et al., 2019). Here, we treated C57BL/6 dams with 1 or 2 mg of anti-Ifnar1 mAb at E6.5 and challenged with virus on E7.5. Serum was collected from dams at 4 dpi to confirm infection, and all dams were productively infected with SPONV or ZIKV-DAK following treatment with either dose of mAb ( Fig. 2 d). Maternal viremia did not significantly differ between treatment groups (SPONV/1 mg vs. SPONV/2 mg: p = 0.996; ZIKV/1 mg vs. ZIKV/2 mg: p = 0.35; one-way ANOVA with Tukey's correction for multiple comparisons). ZIKV-DAK titers, however, were significantly higher than SPONV titers (SPONV/1 mg vs. ZIKV/1 mg: p = 0.04; SPONV/2 mg vs. ZIKV/2 mg: p = 0.006). Next, adhering to our previously established experimental timeline, dams were necropsied on E14.5 to assess and compare fetal outcomes. At the time of necropsy, we observed no significant resorption from either ZIKV or SPONV infected pregnancies, after either dose of mAb ( Fig. 2 e), consistent with the results described by Salazar et al. observed after E6.5 virus challenge and E13.5 or E18.5 dam sacrifice (Salazar et al., 2019). Resorption rates from ZIKV-DAK- and SPONV-infected pregnancies were not significantly different (Fisher's exact test, p > 0.06 for all comparisons).
2.4. Fetal harm is specific to infection with ZIKV and SPONV
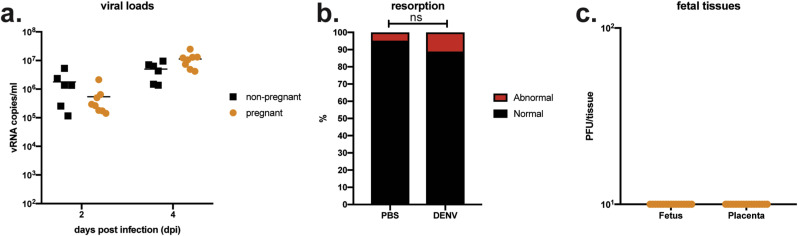
It is possible that the differences in outcomes in these two models may be due to the transient nature of mAb treatment versus knockout lines to abrogate interferon responses. Still, it was possible that the phenotype observed in our more immunocompromised model was instigated by IFN-ɑ/β signaling at the maternal-interface due to the single intact interferon allele from the wildtype sires (Casazza and Lazear, 2018; Yockey et al., 2018) and not a specific effect of infection with ZIKV or SPONV. To test this possibility, we inoculated Ifnar1 −/− pregnant mice with dengue virus serotype 2 (DENV-2) on E7.5. DENV is a flavivirus that is closely related to both ZIKV and SPONV and it is not known to cause adverse pregnancy outcomes in humans. To examine whether maternal DENV-2 infection is sufficient to induce fetal resorption, we s.c. inoculated pregnant dams on E7.5 with 7.5 × 104 PFU of DENV-2. Prior to studies in pregnant animals, we confirmed that this route and dose would result in productive infection in non-pregnant animals (Fig. 3 a). All dams were productively infected with DENV-2 with detectable vRNA loads at 2 and 4 dpi (Fig. 3a). Importantly, fetuses continued to develop as examined on E14.5, and rates of resorption were not significantly different when compared to PBS-inoculated controls (DENV-2: 11.1%, PBS: 4.8%, Fisher's exact test, p = 0.665) (Fig. 3b). These observations confirm that fetal harm was specifically associated with ZIKV-DAK and SPONV infection, but because DENV-2 infected mice do not show clinical signs, we cannot exclude the possibility that the more severe fetal outcomes observed with ZIKV-DAK and SPONV are the result of poor maternal health singly or in combination with other causes of birth defects like direct pathogenic effects of the virus infection.
Fig. 3.
DENV outcomes in non-pregnant and pregnant Ifnar1−/−mice. (a) Serum viral loads from non-pregnant (black symbols) and pregnant (orange) Ifnar1−/− mice at 2 and 4 days post infection with 7.5 × 104 PFU of DENV. Viral loads were measured by QRT-PCR. All mice had detectable serum viremia at both 2 and 4 dpi. (b) Rates of grossly abnormal (red) versus normal (black) fetuses at E14.5 after maternal infection at E7.5. Data presented are for individual fetuses from 6 litters from DENV infected dams. Resorption rates did not significantly differ between DENV and PBS controls. ns, not significant (Fisher's exact test). (c) Viral titer was measured by plaque assay for a subset of individual homogenized fetuses and placentas. There was no detectable infectious DENV virus present in any tested tissue.
2.5. SPONV causes placental and fetal histopathology
To begin to understand the potential for SPONV to be vertically transmitted, a subset of placentas and fetuses were collected for plaque assay at time of necropsy from all virus treatment groups. From the Ifnar1 +/- tissues, infectious virus was detected in 100% of ZIKV-DAK placentas and fetuses screened (Fig. 2c). Virus was detected in all but one SPONV placenta and 35% of fetuses (Fig. 2c). Viral titers were significantly higher in SPONV placentas than their corresponding fetuses (one-way ANOVA with Tukey's multiple comparisons; p < 0.0001), as were ZIKV-DAK placenta viral titers as compared to ZIKV-DAK fetuses (p = 0.006). In addition, ZIKV placenta and fetal viral titers were significantly higher than SPONV titers (p < 0.0001).
Vertical transmission was also supported by the presence of infectious virus in fetal and placental tissues from dams treated with anti-Ifnar1 mAb ( Fig. 2 f). Antibody dose did not affect the viral titer present in fetuses or placentas after either SPONV- or ZIKV-DAK-inoculation (p > 0.9 for all comparisons; one-way ANOVA with Tukey's multiple comparisons). In general, fetal and placenta tissue titers were significantly higher in ZIKV-DAK challenge groups as compared to SPONV challenge groups, with a more significant difference in placenta tissue titers than fetal tissue titers ( Fig. 2 f). Of note, infectious SPONV was detected in fetuses from both mAb treatment groups, which is in contrast to the previous report from Salazar et al., where no infectious SPONV was detected in fetal tissues (Salazar et al., 2019). Placental viral titers were so high that we cannot fully exclude the possibility that virus was introduced into fetal tissues during dissection. Therefore, we do not have robust evidence of vertical transmission in either experimental model. Fetal and placental tissues from DENV-2 infected pregnancies were also screened for infectious virus via plaque assay. Infectious virus was not detected in any of the screened fetal and placental tissues, further suggesting the specificity of fetal harm to ZIKV and SPONV ( Fig. 3 c).
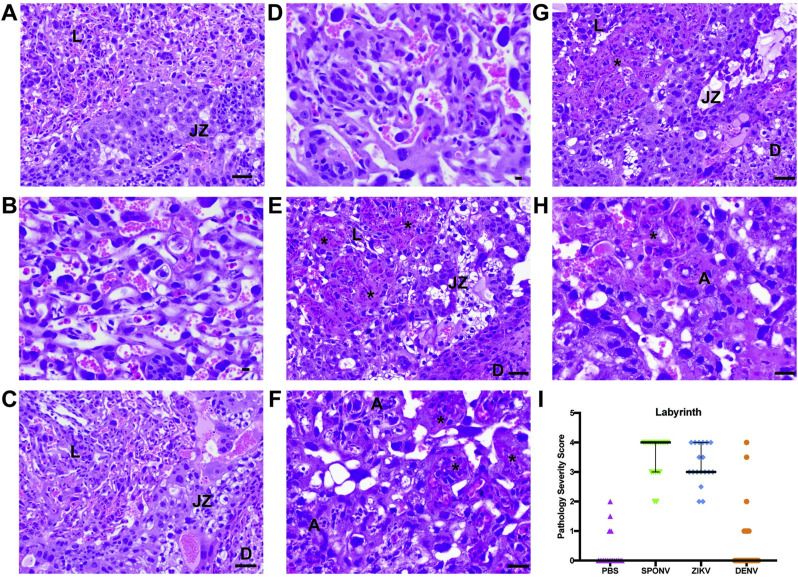
To better understand the impact of in utero SPONV exposure, tissues from the developing Ifnar1 +/- placenta and fetus were evaluated microscopically. In PBS- and DENV-inoculated dams, we observed normal decidua, junctional zone, and labyrinth with normal maternal and fetal blood spaces (Fig. 4 ). In contrast, ZIKV-DAK- and SPONV-inoculated dams displayed varying degrees of placental pathology with severe effects predominantly observed in the labyrinth zone, including vascular injury involving maternal and/or fetal vascular spaces, infarction (obstructed blood flow), necrosis, apoptosis, and hemorrhage (Fig. 4). Overall, the severity of the vascular injury in the labyrinth zone was similar between ZIKV-DAK and SPONV placentas.
Fig. 4.
Placenta histopathology analysis: Hematoxylin and eosin (H&E) staining of placenta labyrinth zone. (a–d) Normal histologic features of the labyrinth (L), junctional zone (JZ), and decidua (D) of the placenta at E14.5 from dams inoculated with PBS (a and b) or DENV-2 (c and d). (e) The interface between the L, the JZ, and the D from a SPONV placenta with numerous intravascular thrombi (*) with apoptotic debris (A) and loss of both maternal and fetal vascular spaces. (f) Higher magnification view of L from a SPONV placenta that shows numerous vascular thrombi (*) with apoptotic debris (A) and loss of both maternal and fetal vascular spaces. (g) The interface of the L, JZ, and D from ZIKV-DAK placenta with numerous intravascular thrombi (*) with apoptotic debris and loss of both maternal and fetal vascular spaces. (h) Higher magnification view of L from a ZIKV-DAK placenta with numerous intravascular thrombi (*) with apoptotic debris and loss of both maternal and fetal vascular spaces. (i) The degree of placental pathology was rated on a scale of 0–4 based on the overall percent of vascular injury and/or loss in both the fetal and maternal vascular spaces of the labyrinth zone: 0 represents 0–5% vasculature injured and/or lost and 4 represents >50% injury/loss. Only labyrinth scores are shown because this was where pathology was primarily observed. Error bars represent 95% confidence interval from the median. Data are representative of 3–6 independent experiments for each treatment group.
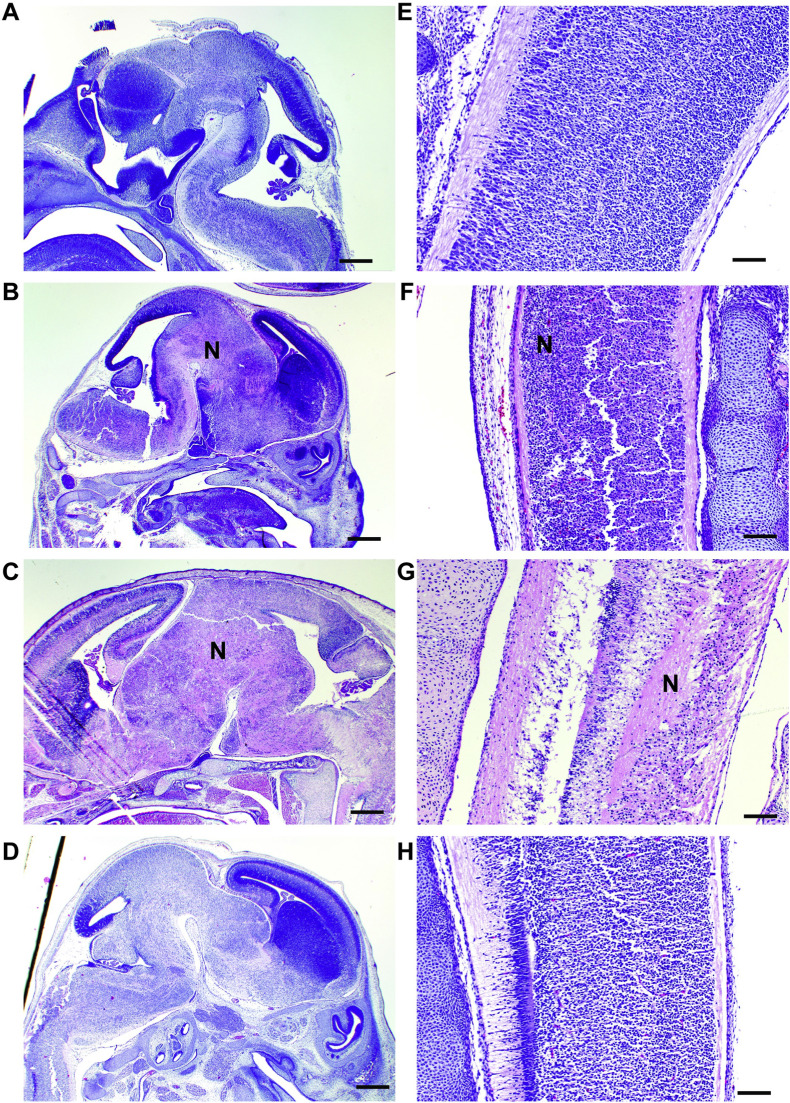
In the fetuses, there was no significant microscopic pathology from PBS- and DENV-inoculated dams. In contrast, fetuses from ZIKV-DAK- and SPONV-inoculated dams demonstrated varying degrees of pathology. In fetuses from the SPONV-inoculated dams, fetal injury was evident as mild pulmonary inflammation and mild to moderate segmental necrosis of the brain and spinal cord (Fig. 5 ). These data provide indirect evidence that vertical transmission did occur. Pathologic findings were more widespread and severe in fetuses from ZIKV-DAK-inoculated dams and included severe necrosis and inflammation of the lung, liver, kidney, brain, and spinal cord.
Fig. 5.
Fetus histopathology analysis: Hematoxylin and eosin (H&E) staining of fetal brain and spinal cord. (a–d) Fetal brains at E14.5 from PBS- (a), SPONV- (b), ZIKV- (c), and DENV- (d) inoculated dams. Brains from PBS- and DENV-inoculated animals showed no signs of pathology. Significant histopathologic injury was observed in fetal brains from SPONV- and ZIKV-inoculated dams, as indicated by large necrotic regions (N). Scale bar (a–d), 500 μm. (e–h) Spinal cord at E14.5 from PBS (e), SPONV (f), ZIKV (g), and DENV (h) inoculated dams. Fetal spinal cords from ZIKV and SPONV infections exhibited histopathologic injury as indicated by necrosis (N). Scale bar (e–h), 100 μm.
2.6. Aedes aegypti transmits SPONV whereas Culex quinquefasciatus does not
To understand the potential risk for SPONV spillover into an urban epidemic cycle and because SPONV RNA was detected in a pool of Cx. quinquefasciatus in Haiti, we compared the relative abilities of Ae. aegypti and Cx. quinquefasciatus from Florida to transmit SPONV in the laboratory. SPONV titers in naturally infected hosts—to which feeding mosquitoes might be exposed in nature—are undefined. Therefore, we conducted our experiments with blood meal titers ranging from ~106-108 PFU/ml. We considered these doses to be physiologically relevant based on studies with DENV (50% mosquito infectious doses = 105.68-107.21 viral cDNA copies/ml) (Duong et al., 2015) and ZIKV (50% mosquito infectious doses = 106.1-107.5 PFU/ml) (Ciota et al., 2017). To assess vector competence, mosquitoes were exposed to viremic bloodmeals via water-jacketed membrane feeder maintained at 36.5 °C. Infection, dissemination, and transmission rates were assessed at 7 and 14 days post feeding (dpf) using an in vitro transmission assay (Aliota et al., 2016a, 2016b; Dudley et al., 2017). Ae. aegypti were susceptible to infection (10–39%) and had moderate transmission rates with all three bloodmeal concentrations (8–36%) at both 7 and 14 dpf ( Table 1 ). Infection efficiency indicates the proportion of mosquitoes with virus-positive bodies among the tested ones. Dissemination efficiency indicates the proportion of mosquitoes with virus-positive legs, and transmission efficiency indicates the proportion of mosquitoes with infectious saliva among the tested ones. In contrast, Cx. quinquefasciatus were susceptible to infection (0–6%) and some of these infected mosquitoes had disseminated infections (2–3%). However, none of the infected Cx. quinquefasciatus were capable of transmitting virus by 14 dpf (Table 1). Ae. aegypti that had been exposed to ZIKV-DAK at the same time demonstrated that the assay was capable of detecting virus-positive mosquitoes at these bloodmeal concentrations.
Table 1.
SPONV vector competence of Aedes aegypti and Culex quinquefasciatus mosquitoes.
|
Mosquito species |
Virus | Dose, log10 PFU/ml | 7 days post exposure |
14 days post exposure |
||||
|---|---|---|---|---|---|---|---|---|
| I | D | T | I | D | T | |||
| Ae. aegypti | ZIKV-DAK | 8.4–8.5* | 44/78 (56%) | 23/40 (58%) | 4/40 (10%) | 33/40 (84%) | 33/40 (84%) | 31/40 (78%) |
| Ae. aegypti | SPONV | 8.2 | 26/75 (35%) | 23/75 (31%) | 9/75 (12%) | 27/70 (39%) | 25/70 (36%) | 25/70 (36%) |
| Ae. aegypti | SPONV | 7.3 | 13/40 (33%) | 10/40 (25%) | 1/40 (3%) | 13/40 (33%) | 13/40 (33%) | 7/40 (18%) |
| Ae. aegypti | SPONV | 5.9 | 6/40 (15%) | 3/40 (8%) | 1/40 (3%) | 4/40 (10%) | 4/40 (10%) | 3/40 (8%) |
| Cx. quinquefasciatus | SPONV | 7.8 | 0/40 (0%) | – | – | 2/40 (5%) | 1/40 (3%) | 0/40 (0%) |
| Cx. quinquefasciatus | SPONV | 7.2 | 2/28 (7%) | 1/28 (3%) | 0/28 (0%) | 1/16 (6%) | 1/16 (6%) | 0/16 (0%) |
| Cx. quinquefasciatus | SPONV | 7.3 | 2/40 (5%) | 1/40 (3%) | 0/40 (0%) | 0/40 (0%) | – | – |
I, % infected; D, % disseminated; T, % transmitting.
*, Range indicates two independent experiments.
3. Discussion
SPONV is the flavivirus most closely related to ZIKV. It may be spreading beyond Africa (White et al., 2018), but little is known about its biology. The potential of SPONV to emerge and cause congenital infections in humans as ZIKV did depends on its capacity for vertical transmission in mammals and its ability to be transmitted by human-preferring mosquitoes like Ae. aegypti. To date, only limited attempts have been made to evaluate its pathogenic potential and identify potential vector mosquitoes, e.g., (Haddow et al., 2016; Mcdonald et al., 2017; Salazar et al., 2019). Here, we characterized congenital SPONV infection in a mouse model and evaluated vector competence for SPONV in two mosquito species—Ae. aegypti and Cx. quinquefasciatus—that feed on humans and are ubiquitous throughout the tropics. Cx. quinquefasciatus was evaluated primarily because SPONV RNA was detected in a pool of these mosquitoes during routine arbovirus surveillance activities in Haiti in 2016 (White et al., 2018). Our results demonstrated that maternal infection of Ifnar1 −/− dams with SPONV caused significant fetal harm and is consistent with ZIKV infections in this same model. Importantly, we confirmed that outcomes were specific to SPONV and ZIKV infection, because we observed no fetal harm in dams infected with DENV-2. Although there are limitations regarding the translational relevance of this model, this same mouse platform—coincident with epidemiological data (Rasmussen et al., 2016) and other lines of evidence—is widely regarded as helping to establish a direct causal link between ZIKV and fetal abnormalities in 2016 (Miner et al., 2016). In the less severely immunocompromised anti-Ifnar1 mAb model of pregnancy, we show that although ZIKV and SPONV do not cause fetal demise, maternal infection still results in vertical transmission of virus. These results are consistent with prior studies using the anti-Ifnar1 mAb model to study ZIKV pathogenesis during pregnancy (Miner et al., 2016; Sapparapu et al., 2016; Jagger et al., 2019). Together our data show that infection with SPONV during pregnancy can lead to fetal harm, with varying levels of damage to maternal, placental, and fetal tissues. We therefore speculate that SPONV infection during pregnancy in humans poses a risk to the developing neonate, and suggest that clinicians in areas endemic for Ae. aegypti caring for non-ZIKV adverse outcomes consider the possibility of congenital SPONV infection. We also speculate that SPONV could follow in the path of other Ae. aegypti-transmitted arboviruses, because of our data demonstrating that Ae. aegypti—which also transmits DENV, ZIKV, yellow fever virus, and chikungunya virus—could transmit SPONV. Ae. aegypti is a highly domesticated urban mosquito that lives in close proximity to humans, feeds on humans, and lays eggs in containers made by humans (Gubler, 2011). As a result, Ae. aegypti thrives in conditions created by high population density in tropical cities and thus facilitates epidemic arbovirus transmission.
In this study we found that SPONV infection of nonpregnant Ifnar1 −/− mice was nearly uniformly lethal. This finding mirrors observations from other studies using AG129 mice, which lack both type I and II interferon receptors (Mcdonald et al., 2017), and mice treated with an anti-Ifnar1 mAb to transiently abrogate type I interferon signaling (Salazar et al., 2019). Pregnant animals that received this anti-Ifnar1 mAb treatment prior to SPONV inoculation showed infection of the placenta and fetus, suggesting vertical transmission, but not increased rates of fetal resorption, recapitulating and expanding on the results reported from Salazar et al. by including a 2 mg mAb dose and ZIKV infection groups (Salazar et al., 2019). In contrast, SPONV-inoculated Ifnar1 −/− dams had significant rates of fetal resorption and severe pathology at both the maternal-fetal interface and in the developing fetus in our study, consistent with ZIKV-inoculated controls and with what we and others have reported previously for ZIKV in this model (Miner et al., 2016; Yockey et al., 2018; Jaeger et al., 2019). We speculate that the difference in outcomes between these two models could be due to type I IFN signaling at anatomic barriers like the placenta (Lazear et al., 2019). For example, anti-Ifnar1 mAb treatment is systemic and therefore may not dampen type I IFN signaling in the placenta, whereas Ifnar1 −/− dams have a more profound immune deficiency that manifests different tissue-specific effects (Yockey et al., 2018). Future investigation into the role of both timing and dose of anti-Ifnar1 mAb treatment during fetal and placental development could help further illuminate these immune mechanisms during SPONV and ZIKV infection.
Differences observed in fetal outcomes and histopathology across ZIKV and SPONV murine pregnancy models may also be due to the use of a different virus strain, dose, or mouse platform. It should be noted, however, that our Ifnar1 −/− mice are on the same genetic background as our anti-Ifnar1 mAb-treated mice: C57BL/6, therefore differences in outcome are not likely to be due to variation in host loci outside the IFNAR1 gene. The SA Ar94 strain used here was also used in previous mouse studies (Mcdonald et al., 2017; Salazar et al., 2019), though differences in culture and passage history could result in small, but biologically important, phenotypic differences. Regardless of these potential disparities in experimental approaches, broadly consistent results have been observed using these murine pregnancy models for ZIKV, including fetal harm and vertical transmission.
Despite similar fetal outcomes, SPONV and ZIKV may not necessarily utilize the same pathways or molecular mechanisms to harm the fetus. Histopathological analyses demonstrated significant fetal and placental pathology in SPONV- and ZIKV-inoculated groups as compared to PBS- or DENV-inoculated controls. But ZIKV-DAK-inoculated dams displayed the most severe histologic phenotype that corresponded with higher placenta and fetus titers in both pregnancy models (Fig. 2). SPONV histopathology was more heterogeneous and severity scores were reminiscent of what we have previously observed with congenital Asian-lineage ZIKV infection (Jaeger et al., 2019). The labyrinth zones of both ZIKV and SPONV placentas showed similar degrees of vascular injury/loss, such that, despite this heterogeneity, the final outcome of fetal demise did not differ between these two viruses. While SPONV fetal histopathology in the CNS and developing brain, as well as the detection of virus in fetal tissues, provide indirect evidence of vertical transmission, this does not preclude the possibility that SPONV-induced fetal harm was the result of placental insufficiency. Both vertical transmission and placental insufficiency can lead to fetal harm, and for ZIKV, placental insufficiency is now being recognized as a potential contributor to fetal harm (Hirsch et al., 2018; Walker et al., 2019). As a result, SPONV could still cause adverse pregnancy outcomes in the absence of direct infection of the fetus via pathophysiology at the maternal-fetal interface. Importantly, we only assessed outcomes following a single infection timepoint at E7.5 and infection outcomes may differ depending on the timing of infection (Jagger et al., 2017). More studies are needed to better understand antiviral signaling at the maternal-fetal interface and pathways these viruses exploit to traffic to the feto-placental unit to cause harm during pregnancies. Translational nonhuman primate models will be valuable for such studies.
SPONV's ability to spread and broadly infect new human populations depends on the mosquito vector that transmits it. One recent study suggested that strains of Ae. aegypti, Ae. albopictus, and Cx. quinquefasciatus were refractory to SPONV infection (Haddow et al., 2016). However, the blood on which mosquitoes fed in these experiments harbored moderate SPONV infectivity of ~105 PFU/ml. For related arboviruses like DENV, it is known that the level of viremia is one of the most important determinants of human infectiousness to mosquitoes (Nguyet et al., 2013; Carrington and Simmons, 2014). ZIKV 50% mosquito infectious doses are in the 106.1-107.5 PFU/ml range (Ciota et al., 2017). If SPONV is similar, then bloodmeal titers of ~105 PFU/ml are too low to achieve mosquito infection. In addition, some studies have shown that freeze-thawing flaviviruses impacts mosquito infection and transmission rates (Miller, 1987; Ciota et al., 2017). As a result, we chose a virus dose that we estimated to be what a mosquito might encounter in nature based on previous studies with DENV and ZIKV (Duong et al., 2015; Ciota et al., 2017), the flaviviruses most closely related to SPONV. In addition, we prepared our infectious bloodmeals using fresh virus supernatant to avoid the confounding effects of freeze-thawing virus stocks. These experiments demonstrated that Ae. aegypti could be productively infected and could transmit SPONV—as indicated by infectious virus in the saliva, suggesting the ability to transmit to a vertebrate host. In contrast, Cx. quinquefasciatus were susceptible to infection but none of these infected mosquitoes were capable of transmitting virus by day 14 post-feeding. These data therefore suggest that the wild-caught Haiti mosquitoes likely contained trace amounts of viremic blood in their midguts indicating that they fed upon a SPONV-infected human or animal. Further, the Haiti data also are consistent with field epidemiological reports for which it has been difficult to detect DENV-, CHIKV-, and/or ZIKV-infected mosquitoes because of low infection rates in field-collected mosquitoes, even during periods of active human outbreaks (Dzul-Manzanilla et al., 2016; Grubaugh et al., 2017; Ramesh et al., 2019). Finally, we used only a single combination of mosquito and virus genotype. Vector competence of many mosquito species for certain viruses likely is governed by vector genotype x virus genotype interactions in genetically diverse, natural mosquito populations (reviewed in (Lambrechts, 2011)). These data at the very least argue for more studies—both experimental and epidemiological—assessing different SPONV-mosquito combinations.
Together, our data show that SPONV possesses worrisome properties that should prompt further investigation into its epidemic potential and risk to pregnant women. The current coronavirus disease (COVID-19) pandemic underscores that RNA viruses can emerge unpredictably to cause human disease on a global scale. Characterizing viruses with potential emergence risk is a critical part of defending against future viral threats (Menachery et al., 2015; Goodin et al., 2016). The adaptation of SPONV to an urban or peri-urban cycle, involving Ae. aegypti and/or other mosquitoes in the Stegomyia subgenus (e.g., Ae. albopictus) should therefore be a public health concern (Musso et al., 2015). Given the continuing difficulties in differentiating between flaviviruses in diagnostic assays, understanding SPONV's prevalence in the expanding landscape of cross-reacting, co-endemic mosquito-borne viruses could also be considered a critical public health priority.
4. Materials and methods
Ethical approval
This study was approved by the University of Minnesota, Twin Cities Institutional Animal Care and Use Committee (Protocol Number, 1804–35828).
4.1. Cells and viruses
African Green Monkey kidney cells (Vero; ATCC #CCL-81) were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 2 mM L-glutamine, 1.5 g/L sodium bicarbonate, 100 U/ml penicillin, 100 μg/ml of streptomycin, and incubated at 37 °C in 5% CO2. Aedes albopictus mosquito cells (C6/36; ATCC #CRL-1660) were maintained in DMEM supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 2 mM L-glutamine, 1.5 g/L sodium bicarbonate, 100 U/ml penicillin, 100 μg/ml of streptomycin, and incubated at 28 °C in 5% CO2. The cell lines were obtained from the American Type Culture Collection, were not further authenticated, and were not specifically tested for mycoplasma.
ZIKV strain DAK AR 41524 (ZIKV-DAK; GenBank:KY348860) was originally isolated from Aedes africanus mosquitoes in Senegal in 1984, with a round of amplification on Aedes pseudocutellaris cells, followed by amplification on C6/36 cells, followed by two rounds of amplification on Vero cells, was obtained from BEI Resources (Manassas, VA). SPONV strain SA Ar94 (GenBank:KX227370) was originally isolated from a Mansonia uniformis mosquito in Lake Simbu, Natal, South Africa in 1955, with five rounds of amplification with unknown culture conditions followed by a single round of amplification on Vero cells. Virus stocks were prepared by inoculation onto a confluent monolayer of C6/36 mosquito cells. DENV-2 strain BID-V594 (GenBank:EU482725), originally isolated from a human in Puerto Rico in 2006 with a single round of amplification on C6/36 cells, was obtained from BEI Resources (Manassas, VA). BEI amplified the virus on C6/36 cells and virus stocks were prepared by inoculation onto a confluent monolayer of C6/36 mosquito cells. We deep sequenced our virus stocks to verify the expected origin (see next section for details). The SPONV, ZIKV-DAK, and DENV-2 stocks matched the GenBank sequences (MG182017, KY348860, EU482725, respectively) of the parental viruses; but a single nucleotide position in the 3’ UTR (site 10,603) of the DENV-2 stock contained a 79/21 ratio of T-to-A nucleotide substitutions and a variant at site 3710 in the ZIKV-DAK stock encodes a nonsynonymous change (A to V) in NS2A.
4.2. Deep sequencing
A vial of the viral stocks used for primary challenge (ZIKV-DAK, SPONV, DENV-2), were each deep sequenced by preparing libraries of fragmented double-stranded cDNA using methods similar to those previously described (Lauck et al., 2013). Briefly, the sample was centrifuged at 5000 rcf for 5 min. The supernatant was then filtered through a 0.45-μm filter. Viral RNA was isolated using the QIAamp MinElute Virus Spin Kit (Qiagen, Germantown, MD), omitting carrier RNA. Eluted vRNA was then treated with DNAse I. Double-stranded DNA was prepared with the Superscript Double-Stranded cDNA Synthesis kit (Invitrogen, Carlsbad, CA) and priming with random hexamers. Agencourt Ampure XP beads (Beckman Coulter, Indianapolis, IN) were used to purify double-stranded DNA. The purified DNA was fragmented with the Nextera XT kit (Illumina, Madison, WI), tagged with Illumina-compatible primers, and then purified with Agencourt Ampure XP beads. Purified libraries were then sequenced with 2 × 300 bp kits on an Illumina MiSeq.
4.3. Sequence analysis
Viral stock sequences were analyzed using a modified version of the viral-ngs workflow developed by the Broad Institute (http://viral-ngs.readthedocs.io/en/latest/description.html) implemented in DNANexus and using bbmap local alignment in Geneious Pro (Biomatters, Ltd., Auckland, New Zealand). Briefly, using the viral-ngs workflow, host-derived reads that map to a human sequence database and putative PCR duplicates were removed. The remaining reads were loaded into Geneious Pro and mapped to NCBI Genbank Zika (GenBank:KX601166), Spondweni (Genbank:MG182017), or dengue virus (GenBank:EU482725) reference sequences using bbmap local alignment. Mapped reads were aligned using Geneious global alignment and the consensus sequence was used for intra sample variant calling. Variants were called that fit the following conditions: have a minimum p-value of 10e-60, a minimum strand bias of 10e-5 when exceeding 65% bias, and were nonsynonymous.
4.4. Plaque assay
All ZIKV and SPONV screens from mouse tissue and titrations for virus quantification from virus stocks were completed by plaque assay on Vero cell cultures. Duplicate wells were infected with 0.1 ml aliquots from serial 10-fold dilutions in growth media and virus was adsorbed for 1 h. Following incubation, the inoculum was removed, and monolayers were overlaid with 3 ml containing a 1:1 mixture of 1.2% oxoid agar and 2X DMEM (Gibco, Carlsbad, CA) with 10% (vol/vol) FBS and 2% (vol/vol) penicillin/streptomycin. Cells were incubated at 37 °C in 5% CO2 for four days for plaque development for ZIKV and five days for SPONV. Cell monolayers then were stained with 3 ml of overlay containing a 1:1 mixture of 1.2% oxoid agar and 2X DMEM with 2% (vol/vol) FBS, 2% (vol/vol) penicillin/streptomycin, and 0.33% neutral red (Gibco). Cells were incubated overnight at 37 °C and plaques were counted.
4.5. Viral RNA isolation
DENV-2 viral RNA was extracted from sera using the Viral Total Nucleic Acid Kit (Promega, Madison, WI) on a Maxwell 48 RSC instrument (Promega, Madison, WI). RNA was then quantified using quantitative RT-PCR. Viral load data from serum are expressed as vRNA copies/mL. Viral load data from tissues are expressed as vRNA copies/tissue.
4.6. Quantitative reverse transcription PCR (QRT-PCR)
For DENV-2, vRNA from serum was quantified by QRT-PCR using the following primers: Forward: CAGATCTCTGATGAAYAACCAACG Reverse: AGTYGACACGCGGTTTCTCT Probe: 6-Fam-CGCGTTTCAGCATATTGAA-BHQ1. IUPAC nucleotide codes are as follows: Y: C or T; B: C or G or T; R: A or G. The RT-PCR was performed using the SuperScript III Platinum One-Step Quantitative RT-PCR system (Invitrogen, Carlsbad, CA) on a LightCycler 480 instrument (Roche Diagnostics, Indianapolis, IN). The primers and probe were used at final concentrations of 600 nm and 100 nm respectively, along with 150 ng random primers (Promega, Madison, WI). Cycling conditions were as follows: 37 °C for 15 min, 50 °C for 30 min and 95 °C for 2 min, followed by 50 cycles of 95 °C for 15 s and 60 °C for 1 min. Viral RNA concentration was determined by interpolation onto an internal standard curve composed of seven 10-fold serial dilutions of a synthetic DENV-2 RNA fragment based the WHO type strain, New Guinea C that shares ~94% similarity at the nucleotide level to the Puerto Rican strain used in the infections described in this manuscript.
5. Mice
Female Ifnar1 −/− mice on the C57BL/6 background were bred in the specific pathogen-free animal facilities of the University of Minnesota College of Veterinary Medicine. Non-pregnant C57BL/6 mice were purchased from Jackson Laboratories. Timed matings between female Ifnar1 −/− mice and male C57BL/6 mice resulted in Ifnar1 -/+ progeny.
5.1. Subcutaneous inoculation
Non-pregnant Ifnar1 −/− mice were between six and eleven weeks of age. Non-pregnant mice were inoculated in the left hind foot pad with either 102 PFU of ZIKV, 102 or 103 PFU of SPONV, or 7.5 × 104 PFU of DENV in 20–25 μl of sterile PBS. Submandibular blood draws were performed 2, 4, and/or 6 days post inoculation and serum was collected to confirm viremia and compare replication kinetics.
All pregnant dams were between six and nine weeks of age. Littermates were randomly assigned to infected and control groups. Matings between female Ifnar1 −/− dams and wildtype sires were timed by checking for the presence of a vaginal plug, indicating a gestational age E0.5. At embryonic day 7.5 (E7.5), Ifnar1 −/− dams were inoculated in the left hind foot pad with 102 PFU of ZIKV or SPONV, in 20 μl of sterile PBS, with 7.5 × 104 PFU of DENV in 25 μl of sterile PBS, or with 25 μl of sterile PBS alone to serve as experimental controls. Sub-mandibular blood draws were performed 2 and 4 days post inoculation and serum was collected to verify viremia. Timed-mated wildtype dams were treated with a 1 or 2 mg dose of interferon-blocking monoclonal antibody (MAR1-5A3) intraperitoneally at E6.5. 24 h later at E7.5, dams were inoculated in the left hind foot pad with 105 PFU of ZIKV or SPONV in 25–32 μl of sterile PBS. Sub-mandibular blood draws were performed 4 days post inoculation and serum was collected to verify viremia.
All animals were closely monitored by laboratory staff for adverse reactions and signs of disease. Non-pregnant mice were humanely euthanized at the end of the experiment or if they met early end-point euthanasia criteria. Pregnant dams were humanely euthanized and necropsied at E14.5.
5.2. Mouse necropsy
Following inoculation with SPONV, ZIKV, or PBS, mice were sacrificed at E14.5. Tissues were carefully dissected using sterile instruments that were changed between each mouse to minimize possible cross contamination. For all mice, each organ/neonate was evaluated grossly in situ, removed with sterile instruments, placed in a sterile culture dish, and further processed to assess viral burden and tissue distribution or banked for future assays. Briefly, uterus was first removed, and then dissected to remove each individual conceptus (i.e, fetus and placenta when possible). Fetuses and placentas were either collected in PBS supplemented with 20% FBS and penicillin/streptomycin (for plaque assays) or fixed in 4% PFA or 10% Neutral Buffered Formalin for imaging. We characterized an embryo as in the resorption process if it met the following criteria: significant growth retardation compared to litter mates and controls accompanied by clearly evident developmental delay, i.e., morphology was ill defined; or visualization of a macroscopic plaque in the uterus (Flores et al., 2014).
6. Histology
Tissues were fixed in 4% paraformaldehyde for 24 h and transferred into cold, sterile DPBS until alcohol processed and embedded in paraffin. Paraffin sections (5 μm) were stained with hematoxylin and eosin (H&E). Pathologists were blinded to gross pathological findings when tissue sections were evaluated microscopically. The degree of pathology at the maternal-fetal interface was rated on a scale of 0–4: 0 – no lesions (normal); 1 – mild changes (1–2 focal lesions or 10–15% of zone involved); 2 – mild to moderate changes (3–4 focal lesions or 10–15% of zone involved); 3 – moderate to severe changes (4–6 focal lesions or 15–25% of zone involved); 4 – severe (>6 focal lesions or >25% of zone involved). The final score was dependent upon the greater of two parameters (# of lesions or % zone involved). This was an identical scoring system to what we reported previously (Jaeger et al., 2019). The final scores were determined as a consensus score of two independent pathologists. For each zone in the placenta (myometrium, decidua, junctional zone, labyrinth, and chorionic plate/membranes) a ‘General’ overall score was determined, a score for the amount of ‘Inflammation’, and a score for direct ‘Vascular Injury’. The ‘General’ score was based on an interpretation of the overall histopathologic findings in each placenta, which included features of necrosis, infarction, apoptosis, hemorrhage, thrombosis, mineralization, vascular injury, and inflammation. The ‘Inflammation’ score quantified the amount of inflammation in that layer. The ‘Vascular Injury’ score assessed vascular wall injury (fibrinoid necrosis, endothelial swelling), dilatation of the vessels or spaces, necrosis, loss of vascular lumen diameter, and intraluminal thrombi. The myometrial layer representing the uterine wall and the chorionic plate/membranes were often not present in histologic sections and therefore meaningful comparisons between strains could not be assessed. The decidual layer (maternal in origin), the junctional zone composed of fetal giant cells and spongiotrophoblast, and the labyrinth layer (the critical layer for gas and nutrient exchange between the fetal and maternal vascular systems) were scored. Since the percentage of injured/pathologic labyrinth zone is a predictor of poor fetal outcome, we also independently scored the labyrinth zone based only on the percentage of fetal and maternal vascular injury/loss using the following scoring system: 0-5%- 0 (background); 5-15%- 1 (mild); 15-30%- 2 (moderate); 30-50%- 3 (moderate to severe); and >50%- 4 (severe). Photomicrographs were obtained using a bright light microscope Olympus BX43 and Olympus BX46 (Olympus Inc., Center Valley, PA) with attached Olympus DP72 digital camera (Olympus Inc.) and Spot Flex 152 64 Mp camera (Spot Imaging), and captured using commercially available image-analysis software (cellSens DimensionR, Olympus Inc. and spot software 5.2).
6.1. Mosquito strain and colony maintenance
All mosquitoes used in this study were maintained at the University of Minnesota, Twin Cities as described (Christensen and Sutherland, 1984) in an environmental chamber at 26.5 ± 1 °C, 75% ± 5% relative humidity, and with a 12 h light and 12 h dark photoperiod with a 30 min crepuscular period at the beginning of each light cycle. The Aedes aegypti Gainesville strain used in this study was obtained from Mike Smanski (University of Minnesota, Twin Cities, St. Paul, MN), and was originally derived from the USDA “Gainesville” strain. Culex quinquefasciatus used in this study were obtained from Greg Ebel (Colorado State University, Ft. Collins, CO) and were originally collected in Sebring County, FL in 1988. Three-to six-day-old female mosquitoes were used for all experiments.
6.2. Vector competence studies
Mosquitoes were exposed to SPONV- or ZIKV-infected bloodmeals via water-jacketed membrane feeder maintained at 36.5 °C (Rutledge et al., 1964). Bloodmeals consisted of defibrinated sheep blood (HemoStat Laboratories, Inc.) and fresh virus supernatant, yielding infectious bloodmeal titers ranging from ~106-108 PFU/ml. Bloodmeal titer was determined after feeding. Infection, dissemination, and transmission rates were determined for individual mosquitoes and sample sizes were chosen using long established procedures (Aliota et al., 2016a, 2016c; Dudley et al., 2017). Briefly, mosquitoes were sucrose starved for 14–16 h prior to bloodmeal exposure. Mosquitoes that fed to repletion were randomized, separated into cartons in groups of 40–50, and maintained on 0.3 M sucrose in a Conviron A1000 environmental chamber at 26.5 ± 1 °C, 75% ± 5% relative humidity, with a 12 h photoperiod within the Veterinary Isolation Facility BSL3 Insectary at the University of Minnesota, Twin Cities. All samples were screened by plaque assay on Vero cells.
6.3. Statistical analysis
All analyses were performed using GraphPad Prism. For survival analysis, Kaplan-Meier survival curves were analyzed by the log-rank test. Unpaired Student's t-test was used to determine significant differences in maternal viremia and fetal and placental tissue viremia. Fisher's exact test was used to determine differences in rates of normal vs. abnormal concepti.
Data availability
Virus stock sequence data have been deposited in the Sequence Read Archive (SRA) with accession codes pending. The authors declare that all other data supporting the findings of this study are available within the article.
assays. R.M.V. and S.L.O. developed and performed the deep sequencing pipeline. D.M.S. and M.K.F. performed histological analysis.
CRediT authorship contribution statement
Anna S. Jaeger: Conceptualization, Validation, Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Visualization. Andrea M. Weiler: Investigation. Ryan V. Moriarty: Investigation. Sierra Rybarczyk: Investigation. Shelby L. O'Connor: Methodology, Resources, Data curation, Writing - review & editing, Supervision, Project administration. David H. O'Connor: Methodology, Resources, Data curation, Writing - review & editing, Supervision, Project administration. Davis M. Seelig: Investigation. Michael K. Fritsch: Formal analysis, Investigation. Thomas C. Friedrich: Methodology, Resources, Writing - review & editing, Supervision, Project administration. Matthew T. Aliota: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Writing - original draft, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the University of Minnesota, Twin Cities BSL3 Program for facilities and Neal Heuss for support. We thank Natalie Benett for her contribution in mosquito maintenance, and the University of Minnesota, Twin Cities Comparative Pathology Shared Resource for preparation of histological sections. Funding for this project came from DHHS/PHS/NIH R21AI131454 and R01AI132563 to M.T.A. The publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or NIH.
References
- Aliota M.T., Peinado S.A., Osorio J.E., Bartholomay L.C. Culex pipiens and Aedes triseriatus mosquito susceptibility to Zika virus. Emerg. Infect. Dis. 2016;22:1857–1859. doi: 10.3201/eid2210.161082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliota M.T., Peinado S.A., Velez I.D., Osorio J.E. The wmel strain of wolbachia reduces transmission of Zika virus by Aedes aegypti. Sci. Rep. 2016;6:28792. doi: 10.1038/srep28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliota M.T., Walker E.C., Uribe Yepes A., Velez I.D., Christensen B.M., Osorio J.E. The wmel strain of wolbachia reduces transmission of chikungunya virus in Aedes aegypti. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen A.A., Hanson R.P. Experimental transplacental transmission of st. Louis encephalitis virus in mice. Infect. Immun. 1970;2:320–325. doi: 10.1128/iai.2.3.320-325.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardoin P., Rodhain F., Hannoun C. Epidemiologic study of arboviruses in the Arba-Minch district of Ethiopia. Trop. Geogr. Med. 1976;28:309–315. [PubMed] [Google Scholar]
- Boorman J.P., Draper C.C. Isolations of arboviruses in the Lagos area of Nigeria, and a survey of antibodies to them in man and animals. Trans. R. Soc. Trop. Med. Hyg. 1968;62:269–277. doi: 10.1016/0035-9203(68)90168-5. [DOI] [PubMed] [Google Scholar]
- Brottes H., Rickenbach A., Brès P., Salaün J.J., Ferrara L. [Arboviruses in the Cameroon. Isolation from mosquitoes] Bull. World Health Organ. 1966;35:811–825. [PMC free article] [PubMed] [Google Scholar]
- Carrington L.B., Simmons C.P. Human to mosquito transmission of dengue viruses. Front. Immunol. 2014;5:290. doi: 10.3389/fimmu.2014.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza R.L., Lazear H.M. Antiviral immunity backfires: pathogenic effects of type I interferon signaling in fetal development. Sci. Immunol. 2018;3 doi: 10.1126/sciimmunol.aar3446. [DOI] [PubMed] [Google Scholar]
- Christensen B.M., Sutherland D.R. Exsheathment and midgut penetration in Aedes aegypti. Trans. Am. Microsc. Soc. 1984;103:423–433. [Google Scholar]
- Ciota A.T., Bialosuknia S.M., Zink S.D., Brecher M., Ehrbar D.J., Morrissette M.N., Kramer L.D. Effects of Zika virus strain and Aedes mosquito species on vector competence. Emerg. Infect. Dis. 2017;23:1110–1117. doi: 10.3201/eid2307.161633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick G.W., Kitchen S.F., Haddow A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- Draper C.C. Infection with the chuku strain of Spondweni virus. W. Afr. Med. J. 1965;14:16–19. [PubMed] [Google Scholar]
- Dudley D.M., Newman C.M., Lalli J., Stewart L.M., Koenig M.R., Weiler A.M., Semler M.R., Barry G.L., Zarbock K.R., Mohns M.S., Breitbach M.E., Schultz-Darken N., Peterson E., Newton W., Mohr E.L., Capuano S., Iii, Osorio J.E., O’connor S.L., O’connor D.H., Friedrich T.C., Aliota M.T. Infection via mosquito bite alters Zika virus tissue tropism and replication kinetics in rhesus macaques. Nat. Commun. 2017;8:2096. doi: 10.1038/s41467-017-02222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong V., Lambrechts L., Paul R.E., Ly S., Lay R.S., Long K.C., Huy R., Tarantola A., Scott T.W., Sakuntabhai A., Buchy P. Asymptomatic humans transmit dengue virus to mosquitoes. Proc. Natl. Acad. Sci. U. S. A. 2015;112:14688–14693. doi: 10.1073/pnas.1508114112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzul-Manzanilla F., Martínez N.E., Cruz-Nolasco M., Gutiérrez-Castro C., López-Damián L., Ibarra-López J., Martini-Jaimes A., Bibiano-Marín W., Tornez-Benitez C., Vazquez-Prokopec G.M., Manrique-Saide P. Evidence of vertical transmission and co-circulation of chikungunya and dengue viruses in field populations of Aedes aegypti (L.) from Guerrero, Mexico. Trans. R. Soc. Trop. Med. Hyg. 2016;110:141–144. doi: 10.1093/trstmh/trv106. [DOI] [PubMed] [Google Scholar]
- Flores L.E., Hildebrandt T.B., Kühl A.A., Drews B. Early detection and staging of spontaneous embryo resorption by ultrasound biomicroscopy in murine pregnancy. Reprod. Biol. Endocrinol. 2014;12:38. doi: 10.1186/1477-7827-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodin M.M., Hatfull G.F., Malik H.S. A diversified portfolio. Annu. Rev. Virol. 2016;3 doi: 10.1146/annurev-vi-3-100316-100011. vi-viii. [DOI] [PubMed] [Google Scholar]
- Grubaugh N.D., Ladner J.T., Kraemer M.U.G., Dudas G., Tan A.L., Gangavarapu K., Wiley M.R., White S., Thézé J., Magnani D.M., Prieto K., Reyes D., Bingham A.M., Paul L.M., Robles-Sikisaka R., Oliveira G., Pronty D., Barcellona C.M., Metsky H.C., Baniecki M.L., Barnes K.G., Chak B., Freije C.A., Gladden-Young A., Gnirke A., Luo C., Macinnis B., Matranga C.B., Park D.J., Qu J., Schaffner S.F., Tomkins-Tinch C., West K.L., Winnicki S.M., Wohl S., Yozwiak N.L., Quick J., Fauver J.R., Khan K., Brent S.E., Reiner R.C., Lichtenberger P.N., Ricciardi M.J., Bailey V.K., Watkins D.I., Cone M.R., Kopp E.W., Hogan K.N., Cannons A.C., Jean R., Monaghan A.J., Garry R.F., Loman N.J., Faria N.R., Porcelli M.C., Vasquez C., Nagle E.R., Cummings D.A.T., Stanek D., Rambaut A., Sanchez-Lockhart M., Sabeti P.C., Gillis L.D., Michael S.F., Bedford T., Pybus O.G., Isern S., Palacios G., Andersen K.G. Genomic epidemiology reveals multiple introductions of Zika virus into the United States. Nature. 2017;546:401–405. doi: 10.1038/nature22400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler D.J. Dengue, urbanization and globalization: the unholy trinity of the 21(st) century. Trop. Med. Health. 2011;39:3–11. doi: 10.2149/tmh.2011-S05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow A.D., Nasar F., Guzman H., Ponlawat A., Jarman R.G., Tesh R.B., Weaver S.C. Genetic characterization of Spondweni and Zika viruses and susceptibility of geographically distinct strains of Aedes aegypti, Aedes albopictus and Culex quinquefasciatus (Diptera: Culicidae) to Spondweni virus. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0005083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow A.D., Woodall J.P. Distinguishing between Zika and Spondweni viruses. Bull. World Health Organ. 2016;94 doi: 10.2471/BLT.16.181503. 711-711a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow A.J., Williams M.C., Woodall J.P., Simpson D.I., Goma L.K. Twelve isolations of Zika virus from Aedes (Stegomyia) africanus (theobald) taken in and above A Uganda forest. Bull. World Health Organ. 1964;31:57–69. [PMC free article] [PubMed] [Google Scholar]
- Hassan A.O., Dmitriev I.P., Kashentseva E.A., Zhao H., Brough D.E., Fremont D.H., Curiel D.T., Diamond M.S. A Gorilla adenovirus-based vaccine against Zika virus induces durable immunity and confers protection in pregnancy. Cell Rep. 2019;28:2634–2646. doi: 10.1016/j.celrep.2019.08.005. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A.J., Roberts V.H.J., Grigsby P.L., Haese N., Schabel M.C., Wang X., Lo J.O., Liu Z., Kroenke C.D., Smith J.L., Kelleher M., Broeckel R., Kreklywich C.N., Parkins C.J., Denton M., Smith P., Defilippis V., Messer W., Nelson J.A., Hennebold J.D., Grafe M., Colgin L., Lewis A., Ducore R., Swanson T., Legasse A.W., Axthelm M.K., Macallister R., Moses A.V., Morgan T.K., Frias A.E., Streblow D.N. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nat. Commun. 2018;9:263. doi: 10.1038/s41467-017-02499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger A.S., Murrieta R.A., Goren L.R., Crooks C.M., Moriarty R.V., Weiler A.M., Rybarczyk S., Semler M.R., Huffman C., Mejia A., Simmons H.A., Fritsch M., Osorio J.E., Eickhoff J.C., O’connor S.L., Ebel G.D., Friedrich T.C., Aliota M.T. Zika viruses of African and Asian lineages cause fetal harm in a mouse model of vertical transmission. PLoS Neglected Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger B.W., Dowd K.A., Chen R.E., Desai P., Foreman B., Burgomaster K.E., Himansu S., Kong W.P., Graham B.S., Pierson T.C., Diamond M.S. Protective efficacy of nucleic acid vaccines against transmission of Zika virus during pregnancy in mice. J. Infect. Dis. 2019;220:1577–1588. doi: 10.1093/infdis/jiz338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger B.W., Miner J.J., Cao B., Arora N., Smith A.M., Kovacs A., Mysorekar I.U., Coyne C.B., Diamond M.S. Gestational stage and ifn-λ signaling regulate zikv infection in utero. Cell Host Microbe. 2017;22:366–376. doi: 10.1016/j.chom.2017.08.012. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M.A., Mier-Y-Teran-Romero L., Reefhuis J., Gilboa S.M., Hills S.L. Zika and the risk of microcephaly. N. Engl. J. Med. 2016;375:1–4. doi: 10.1056/NEJMp1605367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokernot R.H., Casaca V.M., Weinbren M.P., Mcintosh B.M. Survey for antibodies against arthropod-borne viruses in the sera of indigenous residents of Angola. Trans. R. Soc. Trop. Med. Hyg. 1965;59:563–570. doi: 10.1016/0035-9203(65)90159-8. [DOI] [PubMed] [Google Scholar]
- Kokernot R.H., Smithburn K.C., Muspratt J., Hodgson B. Studies on arthropod-borne viruses of Tongaland. Viii. Spondweni virus, an agent previously unknown, isolated from Taeniorhynchus (Mansonioides) uniformis. S. Afr. J. Med. Sci. 1957;22:103–112. [PubMed] [Google Scholar]
- Kokernot R.H., Szlamp E.L., Levitt J., Mcintosh B.M. Survey for antibodies against arthropod-borne viruses in the sera of indigenous residents of the Caprivi Strip and Bechuanaland Protectorate. Trans. R. Soc. Trop. Med. Hyg. 1965;59:553–562. doi: 10.1016/0035-9203(65)90158-6. [DOI] [PubMed] [Google Scholar]
- Lambrechts L. Quantitative genetics of Aedes aegypti vector competence for dengue viruses: towards a new paradigm. Trends Parasitol. 2011;27:111–114. doi: 10.1016/j.pt.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Lauck M., Switzer W.M., Sibley S.D., Hyeroba D., Tumukunde A., Weny G., Taylor B., Shankar A., Ting N., Chapman C.A., Friedrich T.C., Goldberg T.L., O’connor D.H. Discovery and full genome characterization of two highly divergent simian immunodeficiency viruses infecting black-and-white colobus monkeys (Colobus guereza) in Kibale National Park, Uganda. Retrovirology. 2013;10:107. doi: 10.1186/1742-4690-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear H.M., Govero J., Smith A.M., Platt D.J., Fernandez E., Miner J.J., Diamond M.S. A mouse model of Zika virus pathogenesis. Cell Host Microbe. 2016;19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear H.M., Schoggins J.W., Diamond M.S. Shared and distinct functions of type I and type iii interferons. Immunity. 2019;50:907–923. doi: 10.1016/j.immuni.2019.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnamara F.N. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1954;48:139–145. doi: 10.1016/0035-9203(54)90006-1. [DOI] [PubMed] [Google Scholar]
- Mcdonald E.M., Duggal N.K., Brault A.C. Pathogenesis and sexual transmission of Spondweni and Zika viruses. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcintosh B.M., Jupp P.G., De Sousa J. Further isolations of the arboviruses from mosquitoes collected in Tongaland, South Africa, 1960-1968. J. Med. Entomol. 1972;9:155–159. doi: 10.1093/jmedent/9.2.155. [DOI] [PubMed] [Google Scholar]
- Mcintosh B.M., Kokernot R.H., Paterson H.E., De Meillon B. Isolation of Spondweni virus from four species of culicine mosquitoes and a report of two laboratory infections with the virus. S. Afr. Med. J. 1961;35:647–650. [PubMed] [Google Scholar]
- Mclennan H., Wheal H.V. The interaction of glutamic and aspartic acids with excitatory amino acid receptors in the mammalian central nervous system. Can. J. Physiol. Pharmacol. 1976;54:70–72. doi: 10.1139/y76-011. [DOI] [PubMed] [Google Scholar]
- Melo A.S., Aguiar R.S., Amorim M.M., Arruda M.B., Melo F.O., Ribeiro S.T., Batista A.G., Ferreira T., Dos Santos M.P., Sampaio V.V., Moura S.R., Rabello L.P., Gonzaga C.E., Malinger G., Ximenes R., De Oliveira-Szejnfeld P.S., Tovar-Moll F., Chimelli L., Silveira P.P., Delvechio R., Higa L., Campanati L., Nogueira R.M., Filippis A.M., Szejnfeld J., Voloch C.M., Ferreira O.C., Brindeiro R.M., Tanuri A. Congenital Zika virus infection: beyond neonatal microcephaly. Jama Neurol. 2016;73:1407–1416. doi: 10.1001/jamaneurol.2016.3720. [DOI] [PubMed] [Google Scholar]
- Menachery V.D., Yount B.L., Debbink K., Agnihothram S., Gralinski L.E., Plante J.A., Graham R.L., Scobey T., Ge X.Y., Donaldson E.F., Randell S.H., Lanzavecchia A., Marasco W.A., Shi Z.L., Baric R.S. A Sars-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat. Med. 2015;21:1508–1513. doi: 10.1038/nm.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B.R. Increased yellow fever virus infection and dissemination rates in Aedes aegypti mosquitoes orally exposed to freshly grown virus. Trans. R. Soc. Trop. Med. Hyg. 1987;81:1011–1012. doi: 10.1016/0035-9203(87)90381-6. [DOI] [PubMed] [Google Scholar]
- Miner J.J., Cao B., Govero J., Smith A.M., Fernandez E., Cabrera O.H., Garber C., Noll M., Klein R.S., Noguchi K.K., Mysorekar I.U., Diamond M.S. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell. 2016;165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso D., Cao-Lormeau V.M., Gubler D.J. Zika virus: following the path of dengue and chikungunya. Lancet. 2015;386:243–244. doi: 10.1016/S0140-6736(15)61273-9. [DOI] [PubMed] [Google Scholar]
- Nguyet M.N., Duong T.H., Trung V.T., Nguyen T.H., Tran C.N., Long V.T., Dui L.T., Nguyen H.L., Farrar J.J., Holmes E.C., Rabaa M.A., Bryant J.E., Nguyen T.T., Nguyen H.T., Nguyen L.T., Pham M.P., Nguyen H.T., Luong T.T., Wills B., Nguyen C.V., Wolbers M., Simmons C.P. Host and viral features of human dengue cases shape the population of infected and infectious Aedes aegypti mosquitoes. Proc. Natl. Acad. Sci. U. S. A. 2013;110:9072–9077. doi: 10.1073/pnas.1303395110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt D.J., Smith A.M., Arora N., Diamond M.S., Coyne C.B., Miner J.J. Zika virus-related neurotropic flaviviruses infect human placental explants and cause fetal demise in mice. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aao7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh A., Jeffries C.L., Castanha P., Oliveira P.A.S., Alexander N., Cameron M., Braga C., Walker T. No evidence of Zika, dengue, or chikungunya virus infection in field-caught mosquitoes from the Recife Metropolitan Region, Brazil, 2015. Wellcome Open Res. 2019;4:93. doi: 10.12688/wellcomeopenres.15295.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S.A., Jamieson D.J., Honein M.A., Petersen L.R. Zika virus and birth defects--reviewing the evidence for causality. N. Engl. J. Med. 2016;374:1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- Richner J.M., Jagger B.W., Shan C., Fontes C.R., Dowd K.A., Cao B., Himansu S., Caine E.A., Nunes B.T.D., Medeiros D.B.A., Muruato A.E., Foreman B.M., Luo H., Wang T., Barrett A.D., Weaver S.C., Vasconcelos P.F.C., Rossi S.L., Ciaramella G., Mysorekar I.U., Pierson T.C., Shi P.Y., Diamond M.S. Vaccine mediated protection against Zika virus-induced congenital disease. Cell. 2017;170:273–283. doi: 10.1016/j.cell.2017.06.040. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge L.C., Ward R.A., Gould D.J. Studies on the feeding response of mosquitoes to nutritive solutions in a new membrane feeder. Mosqutio News. 1964;24:407–419. [Google Scholar]
- Salazar V., Jagger B.W., Mongkolsapaya J., Burgomaster K.E., Dejnirattisai W., Winkler E.S., Fernandez E., Nelson C.A., Fremont D.H., Pierson T.C., Crowe J.E., Screaton G.R., Diamond M.S. Dengue and Zika virus cross-reactive human monoclonal antibodies protect against Spondweni virus infection and pathogenesis in mice. Cell Rep. 2019;26:1585–1597. doi: 10.1016/j.celrep.2019.01.052. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapparapu G., Fernandez E., Kose N., Bin C., Fox J.M., Bombardi R.G., Zhao H., Nelson C.A., Bryan A.L., Barnes T., Davidson E., Mysorekar I.U., Fremont D.H., Doranz B.J., Diamond M.S., Crowe J.E. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature. 2016;540:443–447. doi: 10.1038/nature20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan K.C., Lai K.S., Dunn G.P., Bruce A.T., Diamond M.S., Heutel J.D., Dungo-Arthur C., Carrero J.A., White J.M., Hertzog P.J., Schreiber R.D. Blocking monoclonal antibodies specific for mouse Ifn-alpha/beta receptor subunit 1 (Ifnar-1) from mice immunized by in vivo hydrodynamic transfection. J. Interferon Cytokine Res. 2006;26:804–819. doi: 10.1089/jir.2006.26.804. [DOI] [PubMed] [Google Scholar]
- Simpson D.I. Zika virus infection in man. Trans. R. Soc. Trop. Med. Hyg. 1964;58:335–338. [PubMed] [Google Scholar]
- Theiler M., Downs W.G. 1973. The Arthropod-Borne Viruses of Vertebrates. An Account of Therockefeller Foundation Virus Program 1951-1970; p. 174. [Google Scholar]
- Walker C.L., Little M.E., Roby J.A., Armistead B., Gale M., Rajagopal L., Nelson B.R., Ehinger N., Mason B., Nayeri U., Curry C.L., Adams Waldorf K.M. Zika virus and the nonmicrocephalic fetus: why we should still worry. Am. J. Obstet. Gynecol. 2019;220:45–56. doi: 10.1016/j.ajog.2018.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S.K., Lednicky J.A., Okech B.A., Morris J.G., Dunford J.C. Spondweni virus in field-caught Culex quinquefasciatus mosquitoes, Haiti, 2016. Emerg. Infect. Dis. 2018;24:1765–1767. doi: 10.3201/eid2409.171957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe M.S., Calisher C.H., Mcguire K. Spondweni virus infection in a foreign resident of Upper Volta. Lancet. 1982;2:1306–1308. doi: 10.1016/s0140-6736(82)91511-2. [DOI] [PubMed] [Google Scholar]
- Worth C.B., Paterson H.E., De Meillon B. The incidence of arthropod-borne viruses in a population of culicine mosquitoes in Tongaland, Union of South Africa (January, 1956, through April, 1960) Am. J. Trop. Med. Hyg. 1961;10:583–592. doi: 10.4269/ajtmh.1961.10.583. [DOI] [PubMed] [Google Scholar]
- Yockey L.J., Jurado K.A., Arora N., Millet A., Rakib T., Milano K.M., Hastings A.K., Fikrig E., Kong Y., Horvath T.L., Weatherbee S., Kliman H.J., Coyne C.B., Iwasaki A. Type I interferons instigate fetal demise after Zika virus infection. Sci. Immunol. 2018;3 doi: 10.1126/sciimmunol.aao1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Virus stock sequence data have been deposited in the Sequence Read Archive (SRA) with accession codes pending. The authors declare that all other data supporting the findings of this study are available within the article.
assays. R.M.V. and S.L.O. developed and performed the deep sequencing pipeline. D.M.S. and M.K.F. performed histological analysis.