Abstract
Several uncertainties exist regarding how we will conduct our clinical, didactic, business, and social activities as the coronavirus disease 2019 (COVID-19) global pandemic abates and social distancing guidelines are relaxed. We anticipate changes in how we interact with our patients and other providers, how patient workflow is designed, the methods used to conduct our teaching sessions, and how we perform procedures in different clinical settings. The objective of the present report is to review some of the changes to consider in the clinical and academic oral and maxillofacial surgery workflow and, allow for a smoother transition, with less risk to our patients and healthcare personnel. New infection control policies should be strictly enforced and monitored in all clinical and nonclinical settings, with an overall goal to decrease the risk of exposure and transmission. Screening for COVID-19 symptoms, testing when indicated, and establishing the epidemiologic linkage will be crucial to containing and preventing new COVID-19 cases until a vaccine or an alternate solution is available. Additionally, the shortage of essential supplies such as drugs and personal protective equipment, the design and ventilation of workspaces and waiting areas, the increase in overhead costs, and the possible absence of staff, if quarantine is necessary, must be considered. This shift in our workflow and patient care paths will likely continue in the short-term at least through 2021 or the next 12 to 24 months. Thus, we must prioritize surgery, balancing patient preferences and healthcare personnel risks. We have an opportunity now to make changes and embrace telemedicine and other collaborative virtual platforms for teaching and clinical care. It is crucial that we maintain COVID-19 awareness, proper surveillance in our microenvironments, good clinical judgment, and ethical values to continue to deliver high-quality, economical, and accessible patient care.
In the past 2 decades, beta coronaviruses have caused 3 major infection outbreaks, severe acute respiratory syndrome (SARS)-associated coronavirus (CoV) in 2002 to 2003, Middle East respiratory syndrome (MERS)-CoV in 2012 and, more recently, SARS-CoV2 in 2019 and 2020. In 2005, Michael Osterholm from the Center for Infectious Research and Policy in Minneapolis, and other infectious disease experts, predicted the occurrence of an unavoidable influenza pandemic and recognized how unprepared our current healthcare systems were to manage such an event.1 Unlike the previous 2 outbreaks, which were regional epidemics, coronavirus disease 2019 (COVID-19), caused by SARS-CoV2, spread within a few weeks across the globe, disrupting the social, economic, and emotional well-being of our societies. This rapid spread to every continent resulted, in part, from the effects of traveling and globalization and, in part, from the greater infectivity and transmission of SARS-CoV2.2 , 3 Fauci et al4 reported that each infected person was infecting ∼2 people in March 2020 (R0 basic reproduction number, 2.2). SARS-CoV2 is a B lineage beta coronavirus, which is an enveloped, positive-sense, single-stranded RNA virus. Similar to SARS-CoV, the angiotensin-converting enzyme 2 (ACE2) receptor is the main entry path for SARS-CoV2. Protease activation on the S (spike) protein facilitates coronavirus entry into the cells. The higher affinity (10- to 20-fold) of the S protein of SARS-CoV2 for the human ACE2 receptor might explain the rapid human-to-human transmission compared with that of SAR-CoV.5
In the absence of a vaccine or effective antiviral therapies, the management of COVID-19 has centered on supportive care for those with severe symptoms, and the use of physical means such as quarantine and “social distancing” for mitigation of spread. Implementing the required physical distancing measures to prevent transmission of the novel coronavirus (SARS-CoV2) has been disruptive, to say the least, to our daily lives, patient care, business operations, and, to a lesser extent, to didactic activities. Concern exists that relaxing the social distancing arrangements too early, as the pandemic abates, could lead to a second wave of COVID-19 cases, extending the suffering and socioeconomic uncertainty in the long-term. The risk this could pose to our finite, already overburdened, healthcare personnel (HCP) and hospitals that have reached capacity should not be overlooked. Although these consequences are possible, some time-sensitive patient care activities are necessary to avoid worsening the prognosis of debilitating or life-threatening disorders.6, 7, 8
As we transition from our strict physical distancing guidelines to less stringent regulations and return to our clinical care activities, several uncertainties remain. Unfortunately SARS-CoV2 is a novel virus, and many deficiencies exist in the understanding of its behavior. These include 1) the exact case fatality rate is uncertain due to incomplete information on actual numbers of cases; 2) the timing of infectiousness relative to the onset of symptoms; 3) the large number of presymptomatic or asymptomatic infectious patients; 4) the duration of the infectious period; and 5) the accuracy of commercially available rapid diagnostic tests to determine whether a patient has COVID-19.9 Second, all surgical and nonsurgical procedures performed by oral-maxillofacial surgeons convey a high risk to HCP. Although some of these uncertainties and challenges will be resolved as the data regarding the virus and the disease improve, we all have some apprehension and reservations regarding delivering care in such an environment.
However, we have all experienced the positive effects that have resulted from this enforced change during the COVID-19 crisis. These have included collaborative online lectures, the use of telemedicine, and the emergence of numerous technological innovations (eg, voice user interface, chatbots) in healthcare delivery.10 The fact that we have all adopted these technologies rapidly is verification that “necessity is the mother of invention.” Such opportunities in a time of crisis can have tremendous effects in the short-term. The changes will be even more beneficial if we can refine and adopt these venerable technologies in the future into our mainstream workflow.
The aim of this report is to review some of the considerations in oral and maxillofacial surgery (OMS) practice as we move from the current situation of strict social and physical distancing rules to phased clinical care during this evolving global pandemic. We included some pragmatic suggestions to leverage telemedicine for emergency care and nonurgent consultations, to adopt and scale machine-learning tools and chatbots to improve surgical workflow efficiency in the operating room (OR), changes in infection control policies, surgical prioritization, screening and testing for COVID-19, and considerations for effective didactic curriculum changes, research opportunities, and business considerations. The suggestions for infection control policies, optimization of personal protective equipment (PPE), diagnostic testing, and surgical prioritization should be considered in conjunction with, and be guided by one's institution, the local, state, and/or federal (ie, Centers for Disease Control and Prevention [CDC], Centers for Medicare and Medicaid Services [CMS]) authorities, and other joint professional organizations.
Considerations for Oral and Maxillofacial Surgeons for Reentry in COVID-19 Era
Planning to return to our new normal requires engagement with our institutions and local and state administrative authorities and awareness of the COVID-19 situation and policies in our region (Table 1 ). It is essential to have a good understanding of 1) the current prevalence and incidence of new cases of COVID-19 in our region compared with the rest of the United States and the world; 2) the mode of transmission of SARS-CoV2; and 3) the risk of exposure to HCP. Creating an electronic standard operating procedures manual or blueprint for operations will help us to succeed during the next 12 to 24 months and, keep all personnel cognizant of the policies developed.
Table 1.
Considerations for Reentry in COVID-19 Era
| Surveillance of patients and HCP for COVID-19 (screening, testing, COVID-19 status reporting) |
| HCP training |
| Infection prevention and control policies |
| PPE courses for staff, including donning and doffing |
| Proper use of disinfectants and disinfection |
| Managing essential supplies: drugs and PPE inventory |
| Patient care |
| Telemedicine triage protocols for emergencies and/or clinic visits |
| Prioritizing surgical care; phased timetables for ambulatory and inpatient surgeries |
| Ambulatory anesthesia protocols |
| Protocols for AGPs |
| Changes to administrative and business operations |
| Modification of administrative personnel schedules and staffing models |
| Cost saving plans |
| Changes in training curriculum for students and residents |
Abbreviations: AGPs, aerosol-generating procedures; COVID-19, coronavirus disease 2019; HCP, healthcare personnel; PPE, personal protective equipment.
Evolution of the COVID-19 Pandemic (How Is the Pandemic Growing?)
The Johns Hopkins University has created a real-time dashboard for tracking COVID-19 (reported by Dong et al11 and available at: https://coronavirus.jhu.edu/map.html). Since the first case in the United States was reported on January 20, 2020, COVID-19 has spread rapidly, affecting people in all 50 states, with 18 states reporting more than 10,000 cases (available at: https://www.cdc.gov/covid-data-tracker/index.html). To date there are more than 6 million cases and 350,000 deaths in the world, with the United States accounting for 1.8 million cases and greater than 100,000 fatalities. The leadership in OMS has made tremendous efforts at the local, state, and national level to educate, disseminate, and share information about COVID-19 through national web conferences and the American Association of Oral and Maxillofacial Surgeons website. Oral and maxillofacial surgeons in various hospitals and academic medical centers rapidly made schedule changes to manage dental emergencies, odontogenic infections, maxillofacial injuries, and oncologic patients and take the necessary precautions. These precautions include airborne infection isolation rooms, negative pressure rooms, telemedicine triage, and the use of appropriate PPE for aerosol-generating procedures (including eye protection and filtered face respirators [N95], face shields, and powered air-purifying respirators [PAPRs]).
COVID-19 Surveillance (Screening, Testing, Tracing)
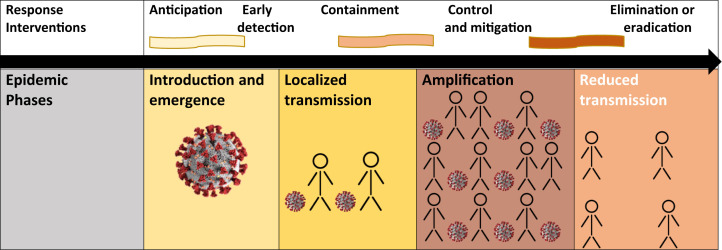
Ongoing surveillance is crucial to containing any epidemic (Fig 1 ). Surveillance includes screening, testing, tracing, and monitoring the incidence of new cases. The CDC has recommended that all frontline HCP should be trained and prepared to screen patients for COVID-19. The results of testing should be reported to the local or state authorities and the CDC to allow for proper surveillance. Symptom questionnaires must be completed before scheduling an outpatient clinic visit and updated on arrival of the patient to capture all presymptomatic cases. COVID-19 screening should include questions regarding recent travel to hotspots, recent exposure to an infected individual, previous testing for, or a diagnosis of COVID-19, any current investigation (self or family member), close contact (≥15 minutes within 6 ft) with anyone with a diagnosis of COVID-19, and, any symptoms experienced within the previous 2 to 14 days. These symptoms include fever, cough, shortness of breath or difficulty breathing, chills, repeated shaking with chills, muscle pain, headache, sore throat, and/or a new loss of taste or smell. The most common symptoms have been fever (83 to 99% of cases), cough (59 to 82%), fatigue (44 to 70%), and anorexia (40 to 84% of cases).12 , 13
Figure 1.
Epidemic phases and response interventions. Reprinted, with permission, from the World Health Organization: Managing epidemics: Key facts about major deadly diseases. Geneva: World Health Organization; 2018.
Studies from China and early reports from other regions showed that the median incubation period is 5 days (range, 4.5 to 5.8 days), similar to SARS. Most individuals who develop symptoms will manifest them within 11.5 days (range, 8.8 to 15.6 days) after exposure.14 Patients could have abnormalities found on chest imaging before the onset of symptoms. These abnormal findings include ground glass opacification with or without consolidation on chest imaging studies obtained as a part of a diagnostic workup for maxillofacial trauma or oral malignancy.
Testing
Identifying the asymptomatic or presymptomatic carriers will be impossible without testing. Many questions have ensured regarding testing, including the availability of validated rapid tests, who should be tested, and the process of obtaining specimens. The CDC has recommended that clinicians work with their local and state health departments to coordinate testing through public health laboratories. Also physicians should use clinical judgment to determine whether a patient who presents for care has symptoms of an influenza-like illness or symptoms compatible with COVID-19 to make a decision regarding testing (guidelines available at: https://www.cdc.gov/coronavirus/2019-ncov/downloads/priority-testing-patients.pdf). For patients scheduled for in-patient surgery the guidance for testing by each institution and/or state should be followed. As of March 24, the CDC guidance for testing priority showed that oral and maxillofacial surgeons (healthcare workers and first responders) should be considered priority 3.
In a recent white paper, Siddarth and Weyl15 proposed the removal of 3 major obstacles to scale up testing for COVID-19 in the United States: 1) the need to have PPE to collect specimens; 2) the need to transport the samples as biohazardous waste; and 3) the need for reagents to purify RNA if possible. These could be removed by 1) having patients collect saliva samples at home and validating this process with nasal swabs and/or throat swabs; 2) using a viral inactivation buffer in the test tube used to collect the sample.15 They reported that the Food and Drug Administration granted the Rutgers University Cell and DNA Repository Infinite Biologics an emergency use authorization on April 13, 2020, for a saliva kit test that can be self-administered.15 Wang et al,16 from the Chinese National Institute for Viral Disease Control and Prevention, documented that specimens with the greatest yield were bronchoalveolar lavage fluid (14 of 15; 93%), followed by sputum (72 of 104; 72%), nasal swabs (5 of 8; 63%), fiberoptic bronchoscope brush biopsy (6 of 13; 46%), pharyngeal swabs (126 of 398; 32%), feces (44 of 153; 29%), and blood (3 of 307; 1%).
Mode of Human-to-Human Transmission
SARS-CoV2 is transmitted from 1 individual to another predominantly via respiratory droplets through direct or indirect routes.3 Indirect transmission can occur through contact with inanimate objects (fomite) contaminated by the droplets.17 Transmission through oral–fecal contamination has also been reported.18, 19, 20 Direct transmission occurs when respiratory droplets (>5 μm in size) carrying the virus particles or pathogen spread from the infected host to the susceptible mucosal surface of the recipient. Studies have revealed that respiratory viruses enter via the nasal mucosa and conjunctiva and, less often, through the mouth. Droplets generated while talking, coughing, or sneezing are usually believed to travel up to a distance of 1 meter (3 ft) but the distance can be farther than 1 meter. Experience during the previous SARS outbreaks revealed that the droplets could travel up to 6 ft. Hence, the recommendation for the use of a face mask when within 6 to 10 feet of another person. Factors to consider include the droplet size, source, density of secretions, temperature, humidity, velocity, and mechanism by which the droplets were propelled. Procedures such as an injection of a local anesthetic or examination of the oropharynx can cause patients to cough or sneeze, propelling droplets 6 ft or more. In contrast, droplet nuclei (dried droplets containing the pathogen), which are 5 μm or less in size have been associated with airborne transmission that can remain in the air for long periods and be transmitted over greater distances.21 , 22 Airborne transmission is more likely to occur during aerosol-generating procedures (AGPs) with and without high-speed drills and during endoscopic airway manipulation, endotracheal intubation, and suctioning. The newer models of respiratory droplet transmission have been based on the concept of a high momentum turbulent puff or cloud that carries droplets for longer distances, as far as 7 to 8 meters or 23 to 27 ft. The current masks have not been tested for their ability to withstand such high-momentum, multiphase gas cloud ejections caused by a cough or sneeze.23
Indirect transmission through a contaminated intermediate object (fomite) has been associated with nosocomial spread or super-spreading events.20 van Doremalen et al24 assessed the 50% tissue-culture infectious dose (TCID50) of viable virus/1 mL of collection medium for SARS-CoV1 and SARS-CoV2. They found that the half-lives of both viruses, measured by exponential decay at a temperature of 21° to 23°C and 40% humidity, were lowest for copper (4 to 8 hours), stainless steel (5.6 hours), plastic (6.8 hours), and highest for cardboard (>8 hours). They found that viable virus can be detected on these inanimate objects for up to 72 hours, with implications for how we use and dispose packing materials such as plastic and cardboard and which disinfectants should be used to cleanse our operatory.24
HCP Exposure Risk
Oral and maxillofacial surgeons, like other specialists in otolaryngology, ophthalmology, and plastic surgery working in the head neck region and the ancillary staff working in these settings, have a high risk of exposure to the novel SARS-CoV2.25, 26, 27 During the SARS epidemic in 2003, HCP accounted for one fifth of all infected cases globally.
Although SARS and COVID-19 are both transmitted by droplets, it is now clear that the infectivity and extent of spread of COVID-19 will far exceed that of SARS.28 The onset and duration of viral shedding and the infectious period relative to onset of symptoms are still not well understood for SARS-CoV2. In a recent study by Wölfel et al,29 the greatest amounts of viral shedding from the upper respiratory tract were during the first 5 days as symptoms were developing. Also, live virus was isolated from 83% of the sputum samples but rarely (17%) from throat swabs in the first 8 days.29 Similarly, Chen and Li30 showed that a higher viral load was present during the early phase of disease and in older individuals. Joynt and Ku31 pointed out that the presence of viral RNA in the specimens does not correlate with viral transmissibility and that previous studies in an animal model of H1N1 infection showed that a negative viral culture coincided with a decrease in the infectious period rather than the absence of viral RNA.
The proportion of case fatalities among HCP during the SARS-CoV and MERS-CoV epidemics was 12% (12 of 98) and 7% (9 of 128), respectively.32 In the United States, from February 12, to April 9, 2020, of the 315,531 COVID-19 cases reported to the CDC, 9282 (3%) were identified as occurring in HCP. Most HCP with COVID-19 (6760; 90%) were not hospitalized. Severe outcomes, including 27 deaths, occurred across all age groups, although death frequently occurred in HCP aged 65 years or older. These preliminary findings have highlighted that, regardless of whether HCP acquire the infection at work or in the community, it is necessary to protect the health and safety of this essential national workforce (Table 2 ). If HCP have been exposed to a patient with COVID-19 or a patient suspected to have COVID-19, the recommended data collection forms available should be used to assess the healthcare worker's risk of exposure and, any breaches in infection prevention and control policies. The World Health Organization emphasizes the importance of direct face-to-face communication with HCP in a blame free environment. A daily self-check method should be established for all HCP. The CDC self-checker is an example of such a survey that can be used by the staff (available at: https://apps.who.int/iris/bitstream/handle/10665/331496/WHO-2019-nCov-HCW_risk_assessment-2020.2-eng.pdf; https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html; and https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/health-workers). Generally, 5 strategies represented by a hierarchical model are included to protect personnel from exposure to occupational hazards: 1) elimination, 2) substitution, 3) engineering controls (ie, ventilation, pressure differential of rooms, ultraviolet [UV] germicidal irradiation [UVGI]), 4) administrative controls (ie, staggering work schedules of staff to limit exposure and crowding of offices, facilitating working from home when feasible), and 5) PPE. Elimination offers the best protection, and the use of PPE offers the least protection. These strategies can be implemented concurrently and/or sequentially.
Table 2.
How to Protect Healthcare Personnel
| Implement source control-facemasks for everyone entering a healthcare facility (eg, HCP, patients, visitors), regardless of symptoms |
| Actively screen everyone for fever and symptoms of COVID-19 |
| Install barriers to limit contact with patients at triage |
| Limit the numbers of staff providing patient care |
| Emphasize hand hygiene |
| Follow standard and transmission-based precautions |
| Use appropriate PPE, including (PAPR or surgical respirator masks, face shield, eye protection, fluid-resistant gowns, booties) for AGPs |
| Understand sequence of donning and doffing of PPE and mask fitting |
Abbreviations: AGPs, aerosol-generating procedures; COVID-19, coronavirus disease 2019; HCP, healthcare personnel; PAPR, powered air-purifying respirator; PPE, personal protective equipment.
Infection Prevention and Control
The infection prevention and control (IPC) guidelines should be clear, concise, and applicable to the type of clinical facility. They must also follow the institutional, state, and national guidelines. All HCP, including administrative staff working in the facility or office, should be trained in the IPC policies to create behavioral changes and a mindset of COVID-19 awareness. Until a vaccine is available, our options for infection control include basic handwashing and decontamination, the proper use of PPE, administrative controls to alter staffing schedules to maintain the minimum effective number of people working at any time in a space, and the use of engineering controls to eliminate aerosol and droplets in the air.33 It is equally important to monitor these policies and to define indicators that will help to track the performance of HCP to maintain an effective IPC plan (available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html).
Personal Protective Equipment
Clinical considerations for PPE selection while of great importance, probably require less change from our established practice than might be first thought. One of the consequences of HIV/AIDS epidemic of the 1980's and 1990s was an understanding of “Universal Precautions”, the presumption that all patients were an infection risk, and acting accordingly. The primary modification in the current environment is the more thorough use of PPE, including a well fitting respirator. The most common types of respirators in healthcare are N95 filtering face piece respirators (FFRs), surgical N95 FFRs, and PAPRs (available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/using-ppe.html). Of these 3 options, PAPRs have a higher assigned protection factor than that of the reusable elastomeric nonpowered air-purifying half face piece (half mask) or disposable N95 FFRs (Table 3 ; additional information available at: https://multimedia.3m.com/mws/media/1794572O/surgical-n95-vs-standard-n95-which-to-consider.pdf). PAPRs reduce the aerosol concentration inhaled by the wearer to at least 1/25th of that in the air compared with the 1/10th reduction for FFRs and elastomeric half face piece air-purifying respirators. PAPRS can be used during procedures in which HCP are exposed to a greater risk of aerosolized pathogens33, 34, 35 [available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/burn-calculator.html; personal protective equipment (PPE) burn rate calculator Excel icon (3 sheets)].
Table 3.
Comparison of PPE for AGPs
| Advantages of surgical N95 respirator |
| Filters ≥95% of particles <5 μm in diameter |
| Blocks both aerosol (<5 μm) and droplet-size (5-50 μm) particles |
| Allows for use of head lights, face shield, stethoscopes |
| Does not generate sound or noise |
| Does not require a power source |
| More readily available and manufactured than PAPR |
| Disadvantages of surgical N95 respirator |
| Requires an initial and periodic fit testing |
| Not oil resistant |
| Possibility of leak owing to inadequate fit (eg, presence of facial hair) |
| Potential for contamination of exposed face and neck without face shield |
| Not well tolerated by users because of breathing resistance |
| Heat and moisture build up |
| High cost of maintaining an inventory of different types and sizes |
| Advantages of PAPR |
| Can filter ≥99.97% of particles 0.3 μm in diameter |
| Allows airborne precautions |
| Cartridges and filters are oil proof and color coded (eg, P100 is purple) |
| Provides head and neck protection |
| Does not require fit testing |
| Approved for use with facial hair |
| Good for long OR procedures or continuous bedside care of a patient |
| Disadvantages of PAPR |
| Requires power; battery-powered blower can fail |
| Filter or cartridge must be replaced |
| Difficulty communicating when wearing |
| Sound of air blowing causes difficultly hearing |
| Can result in difficulties with multiple operators due to bulky head piece |
| Cannot use headlight or stethoscope |
| Potential33 risk to individuals34 reprocessing reusable respirators |
Engineering Controls
Good ventilation, air filtration, UVGI, and pressurization (pressure differentials) are methods that can be used to reduce spread of aerosol and droplets and, mitigate the risk of transmission of airborne viral and bacterial pathogens.
Ventilation
All waiting areas should be well ventilated with distance and space between patients and appointments to minimize waiting times.
Filtration
Portable fan devices with high-efficiency particulate air (HEPA) filtration can increase the effective air changes per hour of clean air into the patient's room, reducing the risk to individuals entering the room without respiratory protection. The National Institute for Occupational Safety and Health (NIOSH) developed guidance for using portable HEPA filtration systems to create expedient patient isolation rooms in hospitals. It might be possible to use the expedient patient isolation room approach developed by NIOSH in outpatient clinic settings, if properly engineered. It involves establishing a high-ventilation-rate, negative-pressure, inner isolation zone that sits within a “clean,” larger ventilated zone. Placement of the HEPA filter in the correct position will allow the aerosolized inner zone air to be drawn toward the vent.
UV Germicidal Irradiation
Several infectious disease specialists have suggested considering existing options such as UVGI in high-risk areas such as emergency rooms, intensive care units (ICUs), and procedure rooms. Such interventions could be cost-effective and have high yield given the scarcity of PPE.36 Two different types of UVGI are used in healthcare settings: 1) upper room air irradiation; and 2) duct irradiation. UVGI is based on the principle of air cycling by convection between irradiated air in the upper zone (clean) and the patient care area or lower zone (dirty). Upper room air UVGI devices are placed on the ceiling or mounted on the wall. These devices have been shown to effectively reduce the transmission of airborne bacterial infections in hospitals.37, 38, 39
Pressurization
Pressurization of side-by-side rooms with a pressure differential—positive pressure (ideally, > +2.5 to 8 Pa) in 1 room and negative pressure (> −2.5 Pa) in the next room (Table 4 ) can be used to treat patients with airborne infections. Typically, these rooms will require more than 12 cycles of air changes/hour and efficient filtrations systems that will allow air to flow away from the clean area or positive pressure room toward the negative pressure or depressurized area or less clean room (eg, room with the patient with an airborne disease). The times required for airborne contaminant removal from a room with 12 cycles of air changes/hour with 99% and 99.9% efficiency are 23 and 35 minutes, respectively.40 We should consider accommodating for additional time if using negative pressure rooms for AGPs in the outpatient setting.
Table 4.
Differences Between Positive and Negative Pressure Areas
| Engineering Characteristic | Positive Pressure Areas (eg, PE) | Negative Pressure Areas (eg, AII) |
|---|---|---|
| Pressure differential | > +2.5 Pa (0.01-in. water gauge) | > −2.5 Pa (0.01-in. water gauge) |
| Air changes per hour | >12 | ≥12 (for renovation or new construction) |
| Filtration efficiency | ||
| Supply: 99.97% at 0.3 μm DOP | Supply: 90% (dust spot test) | |
| Return: none required (if patient requires both PE and AII, return air should be HEPA-filtered or otherwise exhausted to outside) | Return: 99.97% at 0.3 μm DOP (HEPA filtration of exhaust air from AII rooms should not be required, provided exhaust has been properly located to prevent re-entry into building) | |
| Room airflow direction | Out to adjacent area | In to room |
| Clean-to-dirty airflow in room | Away from patient (high-risk patient, immunosuppressed patient) | Toward patient (airborne disease patient) |
| Ideal pressure differential40 | > +8 Pa | > −2.5 Pa |
Note: Adapted from Streifel.40
Abbreviations: AII, airborne infection isolation; DOP, dioctylphthalate particles (0.3 μm in diameter); HEPA, high-efficiency particulate air; PE, protective environment.
In a study of air sample analysis for SARS-CoV2 RNA concentrations, higher levels (18 to 42 copies/m3) were found in the rooms used for removal of protective clothing, with much lower levels (6 copies/m3) found in patient treatment areas such as ICUs and wards designated for patients with COVID-19. This likely resulted from the high air exchange rates in these negative pressure areas.41 In the hospital setting, ORs and ICUs have usually been designed to accommodate these pressure differentials; thus, the option will be more feasible in the hospital setting. For outpatient clinics, the use of HEPA filtration and upper room UVGI methods, along with appropriate PPE, might be good options to decrease infection from AGPs.
Surface Decontamination
When choosing a disinfection product, the active ingredient, surface type, contact time, and type of clinical setting should be considered (Table 5 ). Coronaviruses are a subgroup of enveloped, single-stranded RNA viruses. Studies have shown that SARS-CoV is sensitive to ultraviolet rays and heat at 56°C for 30 minutes, in addition to ether, 75% ethanol, chlorine-containing disinfectant, peracetic acid, chloroform, and other fatty solvents, but not chlorhexidine.42
Table 5.
Selection of Claimed Surface Decontamination Products for Use Against SARS-CoV2
| EPA Registration No.; Name | Active Ingredient | Contact Time (min) | Surface Type | Virus | Use Site |
|---|---|---|---|---|---|
| 10492-4; Palermo Healthcare LLC | Quaternary ammonium isopropanol | 0.5 | Hard nonporous | Human coronavirus | Healthcare, institutional, residential |
| 10492-5; Palermo Healthcare LLC | Quaternary ammonium isopropanol | 0.5 | Hard nonporous | Human coronavirus | Healthcare, institutional, residential |
| 777-136; Reckitt Benckiser | Ethanol | 0.5 | Hard nonporous | Human coronavirus | Healthcare, institutional, residential |
| 8383-14; Contec Inc | Hydrogen peroxide, peroxyacetic acid | 0.5 | Hard nonporous | Human coronavirus | Healthcare, institutional, residential |
Abbreviations: EPA, Environmental Protection Agency; SARS-CoV2, severe acute respiratory syndrome-associated coronavirus 2.
Antiseptic Agents
The antimicrobial action of povidone iodine (PVP-I) has been well established in surgery for surgical skin site preparation (7 to 10% PVP-I). The free iodine in PVP-I is able to inactivate proteins, oxidize nucleic acids, and destroy microbes. The experience during the SARS-CoV and MERS-CoV epidemics has shown that PVP-I has virucidal effects in vitro, and it can potentially be used as an antiseptic rinse.43 Kariwa et al,42 in 2006, evaluated the efficacy of several PVP-I products and a number of other chemical agents and various physical conditions to inactivate the SARS coronavirus in vitro. They reported that treatment of SARS-CoV with 0.23 to 1% PVP-I products for 2 minutes reduced the viral infectivity from 1.17 × 106 TCID50/mL to an undetectable level.42 They reported that the efficacy of 70% ethanol was equivalent to that of the PVP-I products.42 In a recent in vivo study in the United Kingdom, Eggers et al44 tested the use of a 0.5% PVP-I solution (0.55 mg/mL available iodine) applied to the oral, oropharyngeal, and nasopharyngeal mucosa of patients with presumed or confirmed COVID-19 and as a rinse for HCP in close contact with this patient cohort. They aimed to decrease the risk to HCP and destroy the virus that had entered the upper aerodigestive tract before it had an opportunity to infect the host.44 Preparation of the oral and/or nasal mucosal with 0.23 to 1% PVP-I for 2 minutes in patients with COVID-19 and unknown COVID-19 status can potentially decrease viral infectivity.
OMS Patient Care
As we plan our patient care activities, different workflows for each setting—emergency room, outpatient clinic with and without ambulatory anesthesia, OR, and inpatient wards/ICU— must be considered. Surgical procedures themselves offer limited scope for change without impacting clinical outcomes. The primary modifications in the current environment are: 1) more thorough use of PPE and 2) use of telemedicine and digital workflows.
Alternate Workflow for Managing Oral and Maxillofacial Emergencies
The COVID-19 crisis has forced us to develop alternate paths to provide urgent and emergent care to our patients with limited in-person interaction, optimize the use of PPE, and minimize the risk of exposure and transmission of SARS-CoV2. This has allowed oral-maxillofacial surgeons to support our institutions in the treatment of patients with COVID-19 while we treated patients with maxillofacial injuries and head and neck infections, among many other conditions, using a hybrid model of telemedicine and in-person evaluations. The emergency room can be a potentially high-risk area for COVID-19 transmission and other infections requiring the use of significant PPE for airborne flu-droplet precautions. Thus, teleconsultation services should be considered as an alternative to our current workflow to increase efficiency and decrease the risk of exposure to infection, especially given the inconvenience of performing procedures in areas of the hospital not dedicated to oral and maxillofacial surgical care.
The potential incorporation of telemedicine OMS consultation services in the emergency room must include input from multiple entities, including emergency physicians, trauma surgeons, other sister specialties (ie, otolaryngology, plastic surgery), billing and compliance officers, and healthcare information technology teams. The designation of pathways of care for synchronous or asynchronous telemedicine consultations versus in-person consultations must be discussed and determined, with all contingents in agreement. Oral-maxillofacial surgeons might also require additional availability in their outpatient practices to provide timely consultations and procedural care for emergency room patients discharged after a telemedicine consultation. The financial effects of such remote digital healthcare services when treating patients without medical or dental insurance must also be considered. The alterations in this emergency room consultation delivery care model will initially require additional attending level expertise in training programs and increased communication with the office management staff involved in scheduling.
Patient Evaluation in Outpatient Settings
We will be able to continue to perform some consultations for orthognathic surgery, obstructive sleep apnea (OSA), and dentoalveolar surgery via telephone- or video-assisted visits and choose to have in-person interactions during the pre- and postoperative period to decrease the risk of exposure and improve the workflow efficiency. When preparing patients for orthognathic, trauma, or temporomandibular joint surgery, alterations to traditional practice using a digital workflow model should be considered. During a preoperative planning visit, all attempts should be made to avoid the use of molded impressions and any intraoral imaging procedures that can lead to coughing and/or gagging and the generation of aerosol or droplets that will pose a risk to the staff. Instead, digital impressions, when the capability exists, should be considered or having the data transmitted from the referring orthodontist. Additionally, cone-beam computed tomography and virtual surgical planning will become an essential part of the workflow, if these have not already been incorporated as a standard part of the present workflow.
Decisions to Prioritize Surgery
Prioritizing surgical care is a major dilemma, as we gradually transition to patient care, especially for those patients who will require hospital admission and usage of more resources. During the COVID-19 pandemic, the CMS and other professional medical societies and organizations decided to halt all “elective,” “nonessential,” and “nonemergent” surgeries and preventive services. The CMS suggested a 3-tiered approach according to the acuity of the treatment or service, and conveyed that each organization or local institution should consider shared decision-making on a case-by-case basis. However, medical ethicists, Caplan and Thomas, argued that the terms “nonessential” and “elective” are fraught with confusion and emotion owing to the vagueness of these terms, making it difficult to decide who should qualify for surgery. Instead, they suggested a framework that would answer 2 crucial decision-making questions: 1) whether the procedure is life-saving requiring immediate or urgent action; and 2) whether the procedure is not life sustaining and does not require significant resources but will improve the patient's quality of life.45 Stahel46 suggested that we implement algorithms that are clinically relevant and driven by patient safety to make surgical treatment decisions (as shown in his Fig 1). He suggested stratification according to risk and resource usage. Thus, elective procedures can be divided into “essential” if an adverse outcome could occur owing to a delay in performing the procedure for an undetermined period and “nonessential” or “discretionary” if the procedure is not time sensitive.
Prachand et al47 developed a more comprehensive scoring system for medically necessary and time sensitive procedures (MeNTS) using the principles of maximizing benefit and minimizing harm and risk and considering patient, procedure, and disease factors. The MeNTS scoring system has 21 questions with a 5-point scale (score range, 21 to 105), which considers each patient's medical comorbidities, COVID-19 status and/or exposure risk, alternative nonoperative options, including no treatment and the effects of delay in treatment on the degree of difficulty of the procedure, and the outcome. The lower the score, the less risk to the patient, surgical team, and healthcare system as a whole (available at: https://www.facs.org/media/press-releases/2020/covid-scoring-system0414/worksheet). The developers are currently validating the MeNTS scoring system.47 It might serve as a good tool that can be implemented for OMS prioritization after further iterations and validation. The American College of Surgeons has recommended using this method or a similar method applicable to each institution. Ultimately, surgeons must be fair to their patients and make the best clinical and most ethical decision for their patients. For oral-maxillofacial surgeons, among the several medically necessary conditions we treat, apart from emergencies, the most time-sensitive procedures include treatment of malignancy (eg, oral cancer, salivary gland malignancy, osteosarcoma), neurologic injuries, aggressive benign jaw tumors, congenital craniofacial anomalies (eg, cleft palate repair before speech development), and chronic pain conditions recalcitrant to medical therapy.
Using these algorithms or scoring methods can help us monitor outcomes closely during the evolving COVID-19 situation. The threshold to allow surgery should decrease gradually as the threat of overusage of resources and the threat of COVID-19 becomes lower. Given that all our procedures will be high risk, we must weigh the patient and disease factors carefully. We must evaluate our facility needs and the risks to our patients and the alternative options of nonoperative treatment versus the implications of delaying treatment. For example, consider the case of a patient with severe OSA who is intolerant to continuous positive airway pressure (CPAP) and would like to consider surgical options. Maxillo-mandibular advancement is a high-risk AGP with a greater requirement for resources owing to the possible necessity for postoperative ICU care or prolonged intubation in the case of a complication and the increased risk to the healthcare team and the patient. Having a frank discussion with these patients to consider compliance with a nonoperative alternative therapy, such as a CPAP with a different type of mask, at least temporarily or a hybrid method of CPAP with an oral device might pose less risk to all. However, delaying treatment for a patient who is noncompliant and unable to tolerate CPAP can increase the risk of cardiovascular and cerebrovascular events, which would result in an overall bad outcome and the usage of more healthcare system resources.
When we consider the procedure, patient, and disease factors in the context of minor OMS procedures performed in the ambulatory setting, the risk is more favorable. For example, a 65-year-old man has presented for extraction of multiple (5 to 6) carious, nonrestorable teeth with periodontal infection and moderately well-controlled hypertension and diabetes, controlled with oral medications. The procedure is time sensitive with no alternative options. Also, a delay of 2 to 6 weeks could make the outcome moderately worse but the difficulty of the operation would not change. Using the MeNTS scoring system, if the length of a procedure is short and can be performed in an ambulatory setting, the score would sum to 31, denoting a favorable risk to personnel and resource usage. Similarly, procedures such as the placement of implants and dentoalveolar surgery, which are ambulatory procedures, pose a greater risk to HCP but have lower resource usage, possibly implying an overall favorable risk. Whether we modify and use the MeNTS scoring method or an alternative, such scores must be validated for OMS procedures. In contrast, consider the options for a relatively healthy, young female patient who requires a Botox injection for intractable migraine headaches. The procedure has low to moderate risk, low resource usage, can directly improve the patient's quality of life, and, indirectly, decrease the use of other resources by possibly avoiding an emergency room visit.
Head and neck oncology patients pose the greatest challenge given the time-sensitive nature of their condition, their age, medical comorbidities, and frailty along with high resource usage and risk to HCP. Shuman et al7 proposed an ethical framework for head and neck cancer care affected by COVID-19. They highlighted the conflicting interests of clinical ethics versus public health ethics when considering population versus individual interests.7 They suggested considering the metrics of tumor progression, the risk of delay, and the use of alternative therapies (eg, radiotherapy) when applicable and feasible.7 In general, they suggested having a well-thought-out plan through a collective decision-making process with members of the tumor board and with consideration of the patient's preferences.7 For such situations, one could study treatment models developed for limited resource settings with a high volume of cancer care, such as the model described by Pramesh and Badwe8 in the Tata Memorial Cancer Center, where more than 70,000 new patients with cancer, including a vast number with oral cancer, are treated annually. This disruption in surgical services resulting from COVID-19 for our patients with cancer is also likely to stimulate further interest in considering immunotherapies that are more novel. This is also the time to evaluate other existing technologies such as mobile telemedicine to diagnose cancer at early stages by decreasing the time required to refer patients to a specialist.
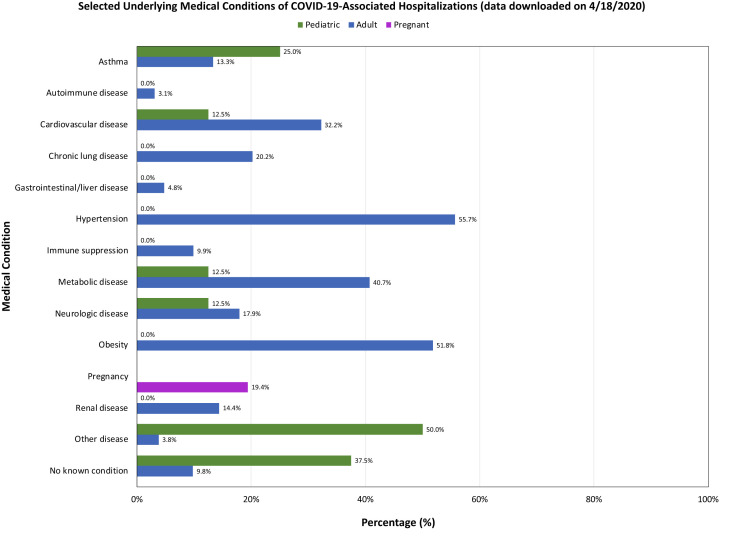
Being aware of the COVID-19 hotspots and understanding the population demographics and the risk factors of the individuals susceptible to COVID-19 will be critical to successful planning. Data from the CDC have shown that the overall cumulative COVID-19–associated hospitalization rate has been 29.2 per 100,000 persons, with the highest rates for persons aged 65 years and older (95.5 per 100,000 persons) and 50 to 64 years (47.2 per 100,000 persons). Hypertension, obesity, and diabetes were the most common underlying comorbid conditions (Fig 2 ). Oral-maxillofacial surgeons working in inner city hospitals and outpatient clinics at academic institutions often see high-risk populations who have limited access to oral healthcare and who live in group homes, correctional facilities, nursing homes, or temporary shelters. These patients also often have chronic diseases such as poorly controlled diabetes, hypertension, and renal failure, as well as other chronic conditions and immunosuppressive disorders. In addition, to the limited access to healthcare, these people have other socioeconomic disadvantages, live in densely populated neighborhoods, have limited disposable income, and must frequently visit stores for food and essential supplies, increasing their risk of exposure and transmission of infection. Cases of COVID-19 have been increasing among incarcerated individuals and workers in state prisons in Texas, Georgia, California, Massachusetts, Michigan, Connecticut, Pennsylvania, and Washington.48
Figure 2.
Comorbid risk factors in patients with coronavirus disease 2019 (COVID-19).
Source: Centers for Disease Control CDC.
Outpatient OMS clinics, especially in hospital settings, will require a new approach to maintain patient volume. The schedules must be modified to limit crowding and maintain safe distances in designated waiting areas. One method to accomplish this would be to continue video telemedicine triage services for consultations and to schedule surgical procedures more efficiently. The schedules should accommodate a patient's needs, and definitive treatment should be rendered whenever possible to avoid multiple visits. Streamlining procedures will allow for the performance of high-risk AGPs in designated areas with provisions for germicidal UV light disinfection, in addition to chemical disinfection, or in negative pressure rooms, using appropriate PPE (ie, surgical N95 respirator, eye protection, face shield, fluid-resistant disposable gown, booties). Performing OMS procedures during the pandemic and in the near future with additional PPE and new infection control guidelines can be time consuming, demanding, and tedious. Oral-maxillofacial surgeons should be careful to plan for additional time, delegate tasks, and request help from colleagues to reduce medical errors when working in stressful situations.49 Using a customized workflow for the specialist and the type of procedure with machine learning tools and chatbots could potentially make the tasks for the OR staff more straightforward and allow for seamless operation during shift changes and breaks.
Ambulatory Anesthesia and Drug Shortages
The American Society of Dental Anesthesiology has provided a guidance document for office-based anesthesia in the setting of COVID-19. It is a useful resource for the consent process in the COVID-19 era50 (available at: https://www.asdahq.org/sites/default/files/Guidance%20ASDA%204.14.20.pdf).
In addition to the severe shortages of PPE and other medical equipment, which have been discussed ad nauseam during this pandemic, several drug shortages have developed, which will be of paramount importance when we begin to return to our practices after the pandemic. From the current trends in usage globally, the following drugs are likely to be affected: oxygen, propofol, midazolam, fentanyl, antibiotics, muscle relaxants, and steroids. Treatment of patients with COVID-19 requires the delivery of oxygen as the main treatment, which has implications for the functioning of hospitals and ambulatory surgery centers. One should consider the possible delays that could occur from supply chain interruptions and prepare accordingly.
Global pandemics such as COVID-19 will not only expose several bottlenecks in the supply chain in the pharmaceutical industry, but will also collapse the delicate balance that exists between supply and demand at the end-user units. Typically, drug shortages can result from several causes. These include both business and market factors:
-
•
A lack of transparency or communication about actual or probable product shortages
-
•
A lack of business incentives to enter a specific product market
-
•
Unpredictable changes in product demand, such as in the current situation
-
•
A reallocation of production lines
-
•
Consolidation of companies
That 80% of raw materials in the pharmaceutical industry originate from outside the United States can be problematic. This will be especially true if single-source active product ingredients or raw material manufacturers face disruption to acquisition because of 1) political instability and/or government interference; 2) natural disasters or pandemics; and 3) contamination during drug production, storage, or transport. Furthermore, the distribution of drugs can be affected directly by barriers to the international transport of medications and inventory practices by healthcare facilities and supply chain entities. These factors will affect smaller OMS practices because such practices often have little to no inventory cushion to compensate for short-term shortages. In addition, their inventory procurement capabilities will be vastly different from those of large healthcare facilities, and they cannot maintain an excess inventory. Also, a large gray market exists that influences the availability of different medications on a regular basis. Thus, oral-maxillofacial surgeons should plan to monitor their current supplies and orders and to estimate the costs and need for anesthetic drugs, such as propofol, fentanyl, and oxygen.
Implications for OMS Education
The COVID-19 pandemic has paused almost all our traditional methods of education and training in our profession. This brings us to the question of whether the current training programs will meet the needs of the next generation of oral and maxillofacial surgeons and whether we will be able to relate the new knowledge base to undergraduate and postgraduate OMS education and training. Can we relate a national curriculum to the knowledge base for the board certification and recertification process? This might be the best time to create the national OMS curriculum for undergraduate and graduate training.
Perhaps some of the most disruptive changes that have resulted from this pandemic have been in the area of resident and student education. Although massive open online courses have been in existence for more than a decade, they have not been used as much nor have they been evaluated for didactic surgical training. Online education and collaborations through data sharing platforms have been an integral part of fields such as engineering and business (eg, Github, Kaggle). These have led to numerous innovations and connected and benefited students across the globe. Equivalent platforms and methods of learning and teaching have been less common in medicine by the sheer nature of how we must practice medicine. However, as we move into an era of cloud-sharing platforms, virtual simulation, augmented reality and intelligence, chatbots, and robotic surgery, it might be feasible to collaborate and share knowledge and interact with others in the field without barriers.51 National and international online collaborative teaching courses have been having a tremendous effect on our trainees and transforming how they learn. These methods of audio- and video-assisted teaching and learning will need proper evaluation, with milestones for further validation, before incorporating them into the mainstream. Several undergraduate faculty across the United States have developed problem-based learning tools and simulation models for clinical scenarios in oral surgery (eg, objective structured clinical evaluation) to determine the clinical competency of our dental students at all levels. During the next few months as patient care resumes, we might have to rely more frequently on small group clinical teaching sessions (groups of 4 to 5 students) and problem-based learning and simulation-based teaching models, rather than on direct patient care.
Research Opportunities
“Never let a good crisis go to waste”
Sir Winston Churchill
Challenges that were considered unsurmountable can now present as opportunities. At the top of our list are those relating to the best practices for OMS as we return to a new normal for caring for our patients and educating the next generation. The effects of the changes we make to provide safe quality care will be significant and should be supported by science. The research questions can be divided into the direct and indirect effects of COVID-19 (eg, affecting patient care, workflow, education). Teams of academic OMS programs in their respective geographic regions can address these questions, some of which we have included but are certainly not limited to those listed:
-
1.How do we prioritize patient care?
- Is a method such as the MeNTS score, proposed by Prachand et al,47 valid? or should a modified score be applied to the OMS setting?
- Assessment of the comparative effectiveness of nonsurgical treatment options and surgical treatment options for OSA (what is the role of modified therapy with CPAP and oral repositioning devices relative to maxillo-mandibular advancement).
- Assess the value of immunotherapy in oral cancer care- Does it have a role when there is delay in surgical treatment of malignancy?
-
2.What impact does life style alteration have on type and cause of maxillofacial trauma? What are treatment outcomes of such trauma managed during the COVID-19 pandemic (8-week period)?
- Combining the data from regional hospital databases to assess these outcomes can be valuable.
-
3.Should preoperative testing be performed for patients undergoing both inpatient and office-based surgery procedures?
- What are the implications of invalid information on how we should provide our care to the patients.
-
4.
Can we use digital tracing applications be used to monitor our office staff and HCP?
-
5.
What are best practices for PPE in OMS that will allow us to provide patient care safely, efficiently, and comfortably?
A study to assess the benefits and cost of various types of PPE in different OMS settings could be considered.
-
6.
The effectiveness of pressure differential rooms and room cleaning methods (eg, UVGI) and the use of a preoperative oral antiseptic rinse with PVP-I should be evaluated. Any methods chosen must have good science and validation behind the recommendations. Some of the guidelines developed for hospital ORs and even general dental offices should not be transferred to our OMS practices without evidence.
-
7.
Can machine learning technology and chatbots such as Alexa or Siri play a role in training OR staff and HCP to perform repetitive tasks and improve the workflow efficiency?
-
8.
The benefits of virtual collaborative platforms for interdisciplinary care? (eg, virtual tumor board for cancer care, OSA, orthognathic surgery, cleft and craniofacial care, prosthetic rehabilitation, implant dentistry) should be evaluated and validated.
-
9.
What is the value of telemedicine in OMS emergency triage workflow? The experience of providers and patients during real-time video versus audio, “store-and-forward” versus hybrid models for routine clinic visits should be assessed.
-
10.
What are the applications of augmented and artificial intelligence, virtual reality, and simulation technologies for surgical education and patient care in oral and maxillofacial surgery?
Business Changes to Consider for OMS Practices
From the standpoint of practice business, no good comparison is available for the situation of a global pandemic. We can extrapolate some aspects from our experiences during natural disasters such as Hurricane Katrina or similar events. During these uncertain times, the observed financial risk should not tempt us to restart our clinical operations earlier than the recommendations from our local and state authorities. It is imperative to follow their guidance, to avoid the risk of exposure of our HCP and patients, and to preserve the necessary resources for our professional colleagues during this period of scarcity.
However, this is a great opportunity to think about how we practice and to test new digital platforms to collaborate with other specialists. One might consider requesting one's accountant to calculate the cash flow and expenses and provide a financial analysis and projection for the year ahead. Early conversations with one's banker regarding a line of credit for the business, the consolidation of loans with more favorable terms, and changes to current loan covenants can be helpful. Many banks will be open to loan forgiveness or interest-only payments. Also, contacting major vendors of practice equipment and negotiating loans and payment schedules can reduce some of the financial burden in the immediate future. It will be important to keep communication open with billing team members to decrease the accounts receivable and maximize the revenue from previous services. Before planning any HCP contract changes or furloughing staff, such items should be discussed with one's practice lawyer and the state labor laws should be reviewed to understand one's staff's rights, and one's own responsibilities. Insurance agents can review the business interruption insurance and coverage for a pandemic should be considered, if not already included, for the future. If still conducting business partially via televisits, the documentation and billing codes for these remote visits should be reviewed. It is a good time to reach out to professional colleagues to find out what they are doing, or as a way of more efficiently shepherding resources.
Support the Professional Community
It is also important to keep our staff and referring dentists engaged and to keep them informed about any plans for reopening one's practice. We should reach out to our referring dentists and physicians to provide clinical support for emergency care. We should also keep them informed of our plans on how we intend to prioritize treatment for patients in accordance with our facility and state guidelines. The staff should be educated and trained to use the COVID-19 tools from state and federal websites regarding the new infection control policies, screening and testing for COVID-19. We should incorporate digital services and telemedicine to deliver care and create an inventory tool to estimate and calculate essential practice items (ie, PPE and drugs). Being a part of the broader solution is important, because any reentry must be in-line with others in the supply chain, including patients, community, ancillary staff, industry, and government. It would be prudent for oral-maxillofacial surgeons to take a fresh look at worst-case scenarios and develop contingency plans. Having a “living revival document” that can easily be updated or withdrawn as necessary is vital. Preparation for (or at least discussion about) the next crisis (or the next phase of the present crisis) among stakeholders in each practice at this time is highly recommended. Just as debriefings after emergency practice drills will be the most informative, we learn the most while the crisis is unfolding. The challenges, responses, and scientific evidence should be well-documented.
Revenue Implications for OMS Training Programs
All educational institutions and academic medical centers have lost revenue as the result of suspension of their programs and the increased costs of supporting the employees of the institutions. In addition, the economic downturn will continue the losses. This could lead to layoffs, which will be disruptive to many OMS programs, which do not have extensive employee numbers. We must find ways to reduce costs to protect the core and character of our programs. Hiring freezes, travel restrictions, and elimination of cost of living increases could all be cost-saving methods. Capital budget requests will probably be put on hold, most likely for 2 years. University and program endowments are often restricted to specific purposes set by the donors and cannot be used as a reserve fund. The decline in the investment market has resulted in a decline in the market value of the endowments. The monies spun off the principal of these endowments, which are used to support the institution's operations will also require cost reductions by individual operating units.
It is important to undertake a very deliberate and transparent review of every aspect of an OMS program's budget, with the intent of eliminating unnecessary expenditures for the next 2 years. This process will not be easy. However, if undertaken immediately and by the OMS program leadership, cost reduction decisions will have fewer effects on the program. Leaders of OMS programs must give special attention to the budget processes to ensure that the professorships that are supported by institutional funds stay intact, providing solid justification for mission critical positions to be filled, and providing meaningful feedback and support to the faculty in lieu of salary increases for the next 2 years. They should prioritize and only support faculty travel determined to be essential to the mission of the program, looking to philanthropy to support capital budget projects that are aligned with the institution's master capital plan.
In conclusion, these challenges we face will continue for at least the foreseeable future, including the next 12 to 24 months. The COVID-19 pandemic has opened our minds and forced us to mobilize and transition to a new and possibly more efficient healthcare delivery model through telemedicine and increased virtual collaboration. Digital technologies could have a high impact on healthcare delivery if we adopt, validate, and scale them for incorporation into our daily workflow. Prioritizing surgery will require objective methods and good clinical and ethical judgment on the part of the surgeon. The COVID-19 global pandemic has exposed the vulnerabilities of our healthcare systems. OMS professionals and leaders have an unprecedented opportunity to work with community, institutional, and professional leadership to implement care standards that address some of the flaws that have been recognized in our current healthcare system. High-quality care that is appropriate, accessible, and economically feasible, delivered with pride and in a transparent fashion, might be much closer to reality than it ever has been.
Footnotes
Conflict of Interest Disclosures: None of the authors have any relevant financial relationship(s) with a commercial interest.
References
- 1.Osterholm M.T. Preparing for the next pandemic. N Engl J Med. 2005;352:1839. doi: 10.1056/NEJMp058068. [DOI] [PubMed] [Google Scholar]
- 2.Kucharski A.J., Russell T.W., Diamond C. Early dynamics of transmission and control of COVID-19: A mathematical modelling study. Lancet Infect Dis. 2020;20:553. doi: 10.1016/S1473-3099(20)30144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fauci A.S., Lane H.C., Redfield R.R. Covid-19—navigating the uncharted. N Engl J Med. 2020;382:1268. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ou X., Liu Y., Lei X. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arduino P.G., Conrotto D., Broccoletti R. The outbreak of novel coronavirus disease (COVID-19) caused a worrying delay in the diagnosis of oral cancer in north-west Italy: the Turin metropolitan area experience. https://doi.org/10.1111/odi.13362 [e-pub ahead of print]. Oral Dis. accessed April 29, 2020. [DOI] [PMC free article] [PubMed]
- 7.Shuman A.G., Campbell B.H., AHNS Ethics & Professionalism Service Ethical framework for head and neck cancer care impacted by COVID-19. https://doi.org/10.1002/hed.26193 [e-pub ahead of print]. Head Neck. accessed April 29, 2020. [DOI] [PMC free article] [PubMed]
- 8.Pramesh C.S., Badwe R.A. Cancer management in India during COVID-19. N Engl J Med. 2020;382:e61. doi: 10.1056/NEJMc2011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson R.M., Heesterbeek H., Klinkenberg D., Hollingsworth T.D. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395:931. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keesara S., Jonas A., Schulman K. COVID-19 and health care's digital revolution. https://doi.org/10.1056/NEJMp2005835 [e-pub ahead of print]. N Engl J Med. accessed April 3, 2020. [DOI] [PubMed]
- 11.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan W.J., Ni Z., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lauer S.A., Grantz K.H., Bi Q. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: Estimation and application. Ann Intern Med. 2020;172:577. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siddarth D., Weyl G.E. COVID-19 Rapid Response Impact Initiative: Why We Must Test Millions a Day. Boston, MA: Edmond J. Safra Center for Ethics, Harvard University; 2020. https://ethics.harvard.edu/covid-19-response Available at: Accessed April 22, 2020.
- 16.Wang W., Xu Y., Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J., Liao X., Qian S. Community transmission of severe acute respiratory syndrome coronavirus 2, Shenzhen, China, 2020. Emerg Infect Dis. 2020;26:1320. doi: 10.3201/eid2606.200239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferretti L., Wymant C., Kendall M. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. 2020;368:eabb6936. doi: 10.1126/science.abb6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hindson J. COVID-19: Faecal-oral transmission? Nat Rev Gastroenterol Hepatol. 2020;17:259. doi: 10.1038/s41575-020-0295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong S.W.X., Tan Y.K., Chia P.Y. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323:1610. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tran K., Cimon K., Severn M. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: A systematic review. PLoS One. 2012;7:e35797. doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization Modes of transmission of virus causing COVID-19: Implications for IPC precaution recommendations: Scientific brief, 27 March 2020. https://apps.who.int/iris/handle/10665/331601 Available at: Accessed March 31, 2020.
- 23.Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. https://doi.org/10.1001/jama.2020.4756 [e-pub ahead of print]. JAMA. accessed April 5, 2020. [DOI] [PubMed]
- 24.van Doremalen N., Bushmaker T., Morris D.H. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vukkadala N., Qian Z.J., Holsinger F.C. COVID-19 and the otolaryngologist: Preliminary evidence-based review. https://doi.org/10.1002/lary.28672 [e-pub ahead of print]. Laryngoscope. accessed April 27, 2020. [DOI] [PubMed]
- 26.Xu K., Lai X.Q., Liu Z. [Suggestions for prevention of 2019 novel coronavirus infection in otolaryngology head and neck surgery medical staff] Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020;55:E001. doi: 10.3760/cma.j.issn.1673-0860.2020.0001. [DOI] [PubMed] [Google Scholar]
- 27.Lu D., Wang H., Yu R. Integrated infection control strategy to minimize nosocomial infection of coronavirus disease 2019 among ENT healthcare workers. J Hosp Infect. 2020;104:454. doi: 10.1016/j.jhin.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tay J.K., Khoo M.L., Loh W.S. Surgical considerations for tracheostomy during the COVID-19 pandemic: Lessons learned from the severe acute respiratory syndrome outbreak. https://doi.org/10.1001/jamaoto.2020.0764 [e-pub ahead of print]. JAMA Otolaryngol Head Neck Surg. accessed April 19, 2020. [DOI] [PubMed]
- 29.Wölfel R., Corman V.M., Guggemos W. Virological assessment of hospitalized patients with COVID-2019. https://doi.org/10.1038/s41586-020-2196-x [e-pub ahead of print]. Nature. accessed March 31, 2020. [DOI] [PubMed]
- 30.Chen Y., Li L. SARS-CoV-2: Virus dynamics and host response. Lancet Infect Dis. 2020;20:515. doi: 10.1016/S1473-3099(20)30235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joynt G.M., Wu W.K. Understanding COVID-19: What does viral RNA load really mean? https://doi.org/10.1016/S1473-3099(20)30237-1 [e-pub ahead of print]. Lancet Infect Dis. accessed April 2, 2020. [DOI] [PMC free article] [PubMed]
- 32.Liu S., Chan T.-C., Chu Y.-T. Comparative epidemiology of human infections with Middle East respiratory syndrome and severe acute respiratory syndrome coronaviruses among healthcare personnel. PLoS One. 2016;11:e0149988. doi: 10.1371/journal.pone.0149988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daugherty E.L. Health care worker protection in mass casualty respiratory failure: Infection control, decontamination, and personal protective equipment. Respir Care. 2008;53:201. [PubMed] [Google Scholar]
- 34.Roberts V. To PAPR or not to PAPR? Can J Respir Ther. 2014;50:87. [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Preventions Using PAPRS in Clinical and Healthcare Settings. 2018. https://workersafety.3m.com/using-paprs-clinical-healthcare-settings/external icon Available at: Accessed April 17, 2020.
- 36.Nathavitharan R., Lederer P., Davis S. Innovation and knowledge sharing can transform COVID-19 infection prevention response. J Hosp Med. 2020;15:299. doi: 10.12788/jhm.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riley R.L., Nardell E.A. Clearing the air: The theory and application of ultraviolet air disinfection. Am Rev Respir Dis. 1989;139:1286. doi: 10.1164/ajrccm/139.5.1286. [DOI] [PubMed] [Google Scholar]
- 38.Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care facilities. MMWR Morb Mortal Wkly Rep. 1994;54:1. [Google Scholar]
- 39.Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care facilities. MMWR Morb Mortal Wkly Rep. 1994;43:1. [PubMed] [Google Scholar]
- 40.Streifel A.J. Design and maintenance of hospital ventilation systems and prevention of airborne nosocomial infection. In: Mayhall C.G., editor. Hospital Epidemiology and Infection Control. ed 2. Lippincott Williams & Wilkins; Philadelphia, PA: 1999. [Google Scholar]
- 41.Liu Y., Ning Z., Chen Y. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. https://doi.org/10.1038/s41586-020-2271-3 [e-pub ahead of print]. Nature. accessed April 27, 2020. [DOI] [PubMed]
- 42.Kariwa H., Fujii N., Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology. 2006;212(suppl 1):119. doi: 10.1159/000089211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eggers M., Eickmann M., Zorn J. Rapid and effective virucidal activity of povidone-iodine products against Middle East respiratory syndrome coronavirus (MERS-CoV) and modified vaccinia virus Ankara (MVA) Infect Dis Ther. 2015;4:491. doi: 10.1007/s40121-015-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirk-Bayley J., Sunkaraneni S., Challacombe S. The use of povidone iodine nasal spray and mouthwash during the current COVID-19 pandemic may reduce cross infection and protect healthcare workers (May 4, 2020). SSRN. Available at: Accessed April 27, 2020. [DOI]
- 45.Caplan A.L., Thomas S.A. A better way to prioritize "essential" vs. "elective" care during COVID-19. Perspective: Medscape Business and Medicine; April 15, 2020. https://www.medscape.com/viewarticle/928537 Available at: Accessed April 16, 2020.
- 46.Stahel P.F. How to risk-stratify elective surgery during the COVID-19 pandemic? Patient Saf Surg. 2020;14:8. doi: 10.1186/s13037-020-00235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prachand V.N., Milner R., Angelos P. Medically necessary, time-sensitive procedures: A scoring system to ethically and efficiently manage resource scarcity and provider risk during the COVID-19 pandemic. https://doi.org/10.1016/j.jamcollsurg.2020.04.011 [e-pub ahead of print]. J Am Coll Surg. accessed April 17, 2020. [DOI] [PMC free article] [PubMed]
- 48.Rubin R. The challenge of preventing COVID-19 spread in correctional facilities. https://doi.org/10.1001/jama.2020.5427 [e-pub ahead of print]. JAMA. accessed April 26, 2020. [DOI] [PubMed]
- 49.Ellis R., Hay-David A.G.C., Brennan P.A. Operating during the COVID-19 pandemic: How to reduce medical error. https://doi.org/10.1016/j.bjoms.2020.04.002 [e-pub ahead of print]. Br J Oral Maxillofac Surg. accessed April 20, 2020. [DOI] [PMC free article] [PubMed]
- 50.Fonner A., Fukami C., Ganzberg S. Interim Guidance for Dentist Anesthesiologists Practicing in the Office-Based Setting During the COVID-19 Pandemic. https://www.asdahq.org/sites/default/files/Guidance%20ASDA%204.14.20.pdf American Society of Dentist Anesthesiologists. Available at: Accessed April 22, 2020.
- 51.Patel R.J., Kejner A., McMullen C. Early institutional head and neck oncologic and microvascular surgery practice patterns across the United States during the SARS-CoV-2 (COVID19) pandemic. https://doi.org/10.1002/hed.26189 [e-pub ahead of print]. Head Neck. accessed April 28, 2020. [DOI] [PMC free article] [PubMed]