Abstract
The identification of thyroid cancers among children after the Chernobyl nuclear power plant accident propelled concerns regarding long-term radiation effects on thyroid cancer in children affected by the Fukushima Daiichi nuclear power plant accident in Fukushima, Japan. Herein we consider the potential association between absorbed dose in the thyroid and the risk of developing thyroid cancer as detected by ultrasonography on 300 473 children and adolescents aged 0–18 years in Fukushima. The absorbed dose mentioned in the present study indicates the sum of that from external exposure and that from internally deposited radionuclides. We grouped participants according to estimated absorbed doses in each of 59 municipalities in Fukushima Prefecture, based on The United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2013 report. The 59 municipalities were assigned to quartiles by dose. We limited our analyses to participants aged ≥6 years because only one case of thyroid cancer was observed in participants aged ≤5 years; 164 299 participants were included in the final analysis. Compared with the lowest dose quartile, the age- and sex-adjusted rate ratios (95% confidence intervals) for the low-middle, high-middle and highest quartiles were 2.00 (0.84–4.80), 1.34 (0.50–3.59) and 1.42 (0.55–3.67) for the 6–14-year-old groups and 1.99 (0.70–5.70), 0.54 (0.13–2.31) and 0.51 (0.12–2.15) for the >15-year-old group, respectively. No dose-dependent pattern emerged from the geographical distribution of absorbed doses by municipality, as estimated by UNSCEAR, and the detection of thyroid cancer among participants within 4–6 years after the accident. Ongoing surveillance might further clarify the effects of low-dose radiation exposure on thyroid cancer in Fukushima.
INTRODUCTION
The Fukushima Daiichi nuclear power plant (NPP) accident, a consequence of the 2011 Great East Japan Earthquake and subsequent tsunami, triggered an environmental release of radioisotopes. Significantly more radioisotopes were released by the Chernobyl NPP in 1986, after which many childhood thyroid cancer cases were reported [1]. Although internal exposures to 131I among children in Fukushima were much smaller than those in Chernobyl, the long-term effects of low-dose radiation exposure on thyroid cancer incidence among residents in Fukushima is of concern. Therefore, in Fukushima Prefecture, investigations began 6 months after the NPP accident to assess potential abnormalities in the thyroid glands of children aged ≤18 years, starting with ultrasound examinations. The aim was to identify long-term effects of total absorbed dose to thyroid on thyroid cancer incidence and to address concerns and anxiety among Fukushima residents in a constructive way.
Recently, we reported the absence of any association between regional and individual external radiation doses and thyroid cancer detection rates among children in Fukushima within 4–6 years of the NPP accident [2]. However, it is necessary to investigate possible associations with internal radiation doses, because internal radiation exposure was reported to be significantly related to the incidence of thyroid cancer in children following the Chernobyl accident [1]. This prospective study uses ultrasonography and follow-up investigations to consider any association between absorbed doses in the thyroid glands of Fukushima children and adolescents, including that from internally deposited radionuclides as well as that from external exposure, and the risk of subsequently developing thyroid cancer.
MATERIALS AND METHODS
Under the Fukushima Health Management Survey, thyroid gland examinations were offered to 367 685 people who were 0–18 years of age at the time of the NPP accident, as previously described [3]. Initial/baseline thyroid examinations proceeded from October 2011 to April 2015, with 300 473 subjects (82%) participating, including evacuees living in other prefectures [2, 4]. Japan’s civil registration system and the Fukushima Health Management Survey database allowed us to link thyroid cancer diagnoses to original municipalities of residence. Follow-up examinations were conducted in those who had no thyroid cancer detected at baseline and who subsequently consented to participate in a follow-up survey that finished in June 2017. Thereafter, 245 530 participants underwent follow-up investigations, of whom 2049 were recommended for secondary confirmatory examinations. Of these, 1,670 (81.5%) completed follow-up, including 214 (10.4%) who underwent fine-needle aspiration cytology (FNAC). Among these, 70 presented with nodules that were classified either as malignant or suspicious for malignancy, and 52 received surgical treatment. Study approvals (#1318 and #1294) came from the Ethics Committee of Fukushima Medical University, which is guided by local policy, national law and the World Medical Association Declaration of Helsinki. Informed consent was obtained from legal guardians of all children participating in the survey.
The United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) estimated absorbed doses in the thyroid gland for individuals throughout Japan by age group (i.e., adults, 10-year-old children and 1-year-old infants) during the first year following the Fukushima Daiichi NPP accident [5]. Here, the absorbed dose estimated by UNSCEAR was the sum of that from external exposure and that from internally deposited radionuclides. Many residents living in areas surrounding the Fukushima Daiichi NPP were forced to evacuate because of elevated radiation levels from the nuclear accident. Absorbed doses for these evacuees were estimated according to their evacuation scenarios. Owing to the fact that there were several evacuation scenarios in each municipal area, we considered the highest and lowest values as the maximum and minimum representative values, respectively, for each municipality [5], and we applied the values by age group to render individual absorbed doses. We enumerated participants by estimated absorbed doses in their thyroid glands in each of Fukushima’s 59 municipalities. Subsequently, we classified these 59 municipalities into quartiles by dose (Figs 1 and 2).
Fig. 1.
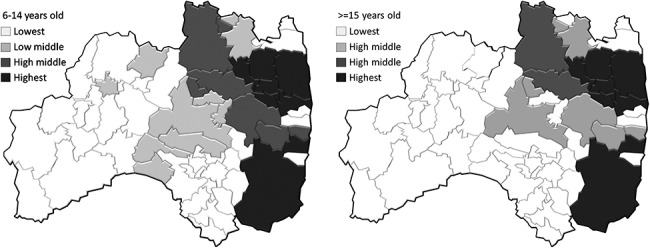
Geographical distribution of the highest, high-middle, low-middle and lowest dose areas based on maximum estimates by UNSCEAR, stratified by age groups.
Fig. 2.
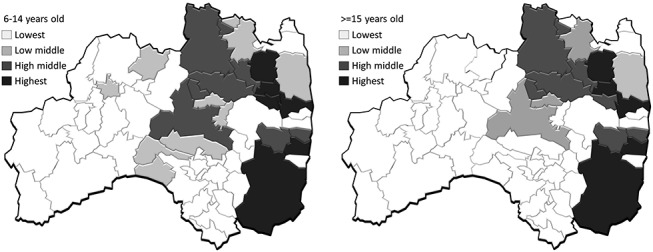
Geographical distribution of the highest, high-middle, low-middle and lowest dose areas based on minimum estimates by UNSCEAR, stratified by age groups.
To calculate person-years, observation times from baseline primary examination to follow-up primary examination were summed. Because all cases of thyroid cancer were found by ultrasonograpy and were asymptomatic, we used detection rate rather than incident rate. We calculated the rate ratios (RRs) and 95% confidence intervals (CIs) for thyroid cancer development in all areas. The quartile with the lowest radiation dose was used as a control reference; using Poisson regression models, we then adjusted for age, sex and examination year. Furthermore, we limited our analyses to participants aged ≥6 years because (unlike Chernobyl) only one case of thyroid cancer was observed in participants aged ≤5 years. As a result, 164 299 participants were included in the final analysis. In addition, we analysed the data stratified by age groups. UNSCEAR considered the following three age groups for dose estimation: adults, children and infants (>15, 6–15 and 0–5 years, respectively). Different doses were assigned to each age group in accordance with age-dependent dose factors [5]. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
RESULTS AND DISCUSSION
Table 1 presents the adjusted RRs (95% CIs) for thyroid cancer according to maximum estimated absorbed doses by quartile. In comparison to the lowest quartile, age- and sex-adjusted RRs for the 6- to 14-year-old group (95% CIs) with respect to the low-middle, high-middle and highest quartiles were 2.00 (0.84–4.80), 1.34 (0.50–3.59) and 1.42 (0.55–3.67), respectively (P for trend = 0.76). Corresponding values for the ≥15-year-old group were 1.99 (0.70–5.70), 0.54 (0.13–2.31) and 0.51 (0.12–2.15), respectively (P for trend = 0.89). After further adjusting for examination year, the RR for the low-middle and high-middle quartiles decreased to 1.42 (0.52–3.86) and 0.90 (0.29–2.77) for the 6–14-year-old group and 1.37 (0.43–4.34) and 0.38 (0.08–1.72) for the ≥15-year-old group, respectively.
Table 1.
Median (interquartile range), characteristics of participants and adjusted RRs (95% CIs) for thyroid cancer according to maximum absorbed doses in thyroid glands, as estimated by UNSCEAR
| Lowest quartile | Low middle | High middle | Highest quartile | Total | |
|---|---|---|---|---|---|
| Age 6–14 years | |||||
| Absorbed dose (mGy), range | 14.00–18.81 | 18.82–22.82 | 23.96–28.73 | 31.16–58.0 | |
| No. at risk | 35 175 | 44 555 | 33 387 | 32 390 | 145 507 |
| Sex (female), % | 49.2 | 49.5 | 49.1 | 49.7 | 49.4 |
| Age at NPP accident (years), median (25–75%) | 10 (8–12) | 10 (8–12) | 10 (8–12) | 10 (8–12) | 10 (8–12) |
| Age at baseline thyroid examination (years), median (25–75%) | 14 (12–16) | 14 (12–16) | 13 (11–16) | 14 (12–16) | 14 (12–16) |
| Participants needing confirmatory testing, n (%) | 341 (0.97) | 448 (1.01) | 328 (0.98) | 320 (0.99) | 1437 (0.99) |
| Participants completing confirmatory testing, n (%) | 271 (0.77) | 357 (0.80) | 273 (0.82) | 269 (0.83) | 1170 (0.80) |
| Participants getting FNAC, n (%)a | 20 (7.4) | 45 (12.6) | 44 (16.1) | 31 (11.5) | 140 (12.0) |
| Examination year | |||||
| 2014 (%) | 7645 (21.7) | 38 370 (86.1) | 33 366 (99.9) | 8083 (25.0) | 87 464 (60.1) |
| 2015–17 (%) | 27 530 (78.3) | 6185 (13.9) | 21 (0.1) | 24 307 (75.0) | 58 043 (39.9) |
| Interval from baseline to follow-up examination (years), median (25–75%) | 2.0 (1.9–2.1) | 2.1 (2.0–2.2) | 2.1 (2.1–2.2) | 2.3 (2.1–2.6) | 2.1 (2.0–2.3) |
| No. of cases | 7 | 18 | 9 | 11 | 45 |
| Detection rate per 100 000 person-years | 10.1 | 19.2 | 12.5 | 14.7 | 14.5 |
| Age- and sex-adjusted RR (95% CI) | Ref. | 2.00 (0.84–4.80) | 1.34 (0.50–3.59) | 1.42 (0.55–3.67) | (Trend P = 0.76) |
| Age-, sex- and examination year-adjusted RR (95% CI) | Ref. | 1.42 (0.52–3.86) | 0.90 (0.29–2.77) | 1.41 (0.55–3.64) | (Trend P = 0.89) |
| Age ≥15 years | |||||
| Absorbed dose (mGy), range | 7.88–11.45 | 12.48–15.00 | 15.42–16.30 | 17.35–35.00 | |
| No. at risk | 4599 | 4827 | 4430 | 4936 | 18 792 |
| Sex (female), % | 57.9 | 55.5 | 56.7 | 56.2 | 56.6 |
| Age at NPP accident (years), median (25–75%) | 16 (15–17) | 16 (15–17) | 16 (15–17) | 16 (15–17) | 16 (15–17) |
| Age at baseline thyroid examination (years), median (25–75%) | 21 (20–22) | 20 (19–21) | 20 (19–21) | 21 (20–22) | 20 (19–21) |
| Participants needing confirmatory testing, n (%) | 125 (2.72) | 134 (2.78) | 109 (2.46) | 146 (2.96) | 514 (2.74) |
| Participants completing confirmatory testing, n (%) | 105 (2.28) | 113 (2.34) | 88 (1.99) | 114 (2.31) | 420 (2.23) |
| Participants getting FNAC, n (%)a | 7 (6.7) | 18 (15.9) | 14 (15.9) | 7 (6.1) | 46 (11.0) |
| Examination year | |||||
| 2014 (%) | 1778 (38.7) | 4825 (99.96) | 4426 (99.9) | 1349 (27.3) | 12 378 (65.9) |
| 2015–17 (%) | 2821 (61.3) | 2 (0.04) | 4 (0.1) | 3587 (72.7) | 6414 (34.1) |
| Interval from baseline to follow-up examination (years), median (25–75%) | 2.1 (2.0–2.3) | 2.1 (2.0–2.6) | 2.1 (2.0–2.2) | 2.2 (2.0–2.6) | 2.1 (2.0–2.5) |
| No. of cases | 5 | 12 | 3 | 3 | 23 |
| Detection rate per 100 000 person-years | 51.0 | 108.2 | 30.9 | 26.6 | 54.9 |
| Age- and sex-adjusted RR (95% CI) | Ref. | 1.99 (0.70–5.70) | 0.54 (0.13–2.31) | 0.51 (0.12–2.15) | (Trend P = 0.10) |
| Age-, sex- and examination year-adjusted RR (95% CI) | Ref. | 1.37 (0.43–4.34) | 0.38 (0.08–1.72) | 0.55 (0.13–2.33) | (Trend P = 0.08) |
aParticipants who underwent FNAC/participants who completed confirmatory testing.
The adjusted RRs (95% CIs) for thyroid cancer according to the minimum estimated absorbed doses exhibited the same trends as those for the maximum absorbed doses (Table 2). The age- and sex-adjusted RRs (95% CIs) for the low-middle, high-middle and highest quartiles compared with the lowest ones were 1.62 (0.61–4.32), 1.68 (0.74–3.83) and 1.35 (0.52–3.50) for the 6–14-year-old group (P for trend = 0.54) and 2.03 (0.72–5.74), 0.66 (0.17–2.51) and 0.23 (0.03–1.97) for the ≥15-year-old group (P for trend = 0.09), respectively.
Table 2.
Median (interquartile range), characteristics of participants and adjusted RRs (95% CIs) for thyroid cancer according to minimum absorbed doses in thyroid glands, as estimated by UNSCEAR
| Lowest quartile | Low middle | High middle | Highest quartile | Total | |
|---|---|---|---|---|---|
| Age 6–14 years | |||||
| Absorbed dose (mGy), range | 12.00–18.81 | 18.82–22.61 | 22.82–28.73 | 29.00–58.00 | |
| No. at risk | 38 262 | 23 037 | 57 547 | 26 661 | 145 507 |
| Sex (female), % | 49.3 | 49.7 | 49.1 | 49.7 | 49.4 |
| Age at NPP accident (years), median (25–75%) | 10 (8–12) | 10 (8–12) | 10 (8–12) | 10 (8–12) | 10 (8–12) |
| Age at baseline thyroid examination (years), median (25–75%) | 14 (12–16) | 13 (11–16) | 14 (11–16) | 14 (12–16) | 14 (12–16) |
| Participants needing confirmatory testing, n (%) | 380 (0.99) | 251 (1.09) | 551 (0.96) | 255 (0.96) | 1,437 (0.99) |
| Participants completing confirmatory testing, n (%) | 302 (0.79) | 213 (0.92) | 444 (0.77) | 211 (0.79) | 1,170 (0.80) |
| Participants getting FNAC, n (%)a | 25 (8.3) | 23 (10.8) | 69 (15.5) | 23 (10.9) | 140 (12.0) |
| Examination year | |||||
| 2014 (%) | 10 728 (28.0) | 16 876 (73.3) | 57 501 (99.9) | 2359 (8.8) | 87 464 (60.1) |
| 2015–17 (%) | 27 534 (72.0) | 6161 (26.7) | 46 (0.1) | 24 302 (91.2) | 58 043 (39.9) |
| Interval from baseline to follow-up examination (years), median (25–75%) | 2.0 (1.9–2.1) | 2.1 (2.0–2.4) | 2.1 (2.0–2.2) | 2.2 (2.1–2.5) | 2.1 (2.0–2.3) |
| No. of cases | 8 | 8 | 20 | 9 | 45 |
| Detection rate per 100 000 person-years | 10.4 | 15.9 | 16.4 | 14.8 | 14.5 |
| Age- and sex-adjusted RR (95% CI) | Ref. | 1.62 (0.61–4.32) | 1.68 (0.74–3.83) | 1.35 (0.52–3.50) | (Trend P = 0.54) |
| Age-, sex- and examination year-adjusted RR (95% CI) | 1.22 (0.43–3.49) | 1.12 (0.43–2.95) | 1.60 (0.59–4.33) | (Trend P = 0.43) | |
| Age ≥15 years | |||||
| Absorbed dose (mGy), range | 7.20–11.12 | 11.28–12.62 | 13.31–16.30 | 17.35–34.00 | |
| No. at risk | 4604 | 5160 | 4989 | 4039 | 18 792 |
| Sex (female), % | 58.1 | 54.8 | 57.2 | 56.3 | 56.6 |
| Age at NPP accident (years), median (25–75%) | 16 (15–17) | 16 (15–17) | 16 (15–17) | 16 (15–17) | 16 (15–17) |
| Age at baseline thyroid examination (years), median (25–75%) | 21 (20–22) | 20 (19–21) | 20 (19–21) | 21 (20–22) | 20 (19–21) |
| Participants needing confirmatory testing, n (%) | 124 (2.69) | 144 (2.79) | 128 (2.57) | 118 (2.92) | 514 (2.74) |
| Participants completing confirmatory testing, n (%) | 103 (2.24) | 120 (2.33) | 104 (2.08) | 93 (2.30) | 420 (2.23) |
| Participants getting FNAC, n (%)a | 7 (6.8) | 23 (19.2) | 15 (14.4) | 1 (1.1) | 46 (11.0) |
| Examination year | |||||
| 2014 (%) | 1784 (38.7) | 5156 (99.9) | 4985 (99.9) | 453 (11.2) | 12 378 (65.9) |
| 2015–17 (%) | 2820 (61.3) | 4 (0.1) | 4 (0.1) | 3586 (88.8) | 6414 (34.1) |
| Interval from baseline to follow-up examination (years), median (25–75%) | 2.1 (2.0–2.4) | 2.1 (2.0–2.6) | 2.1 (2.0–2.3) | 2.1 (2.0–2.5) | 2.1 (2.0–2.5) |
| No. of cases | 5 | 13 | 4 | 1 | 23 |
| Detection rate per 100 000 person-years | 50.1 | 107.6 | 36.0 | 11.5 | 54.9 |
| Age- and sex-adjusted RR (95% CI) | Ref. | 2.03 (0.72–5.74) | 0.66 (0.17–2.51) | 0.23 (0.03–1.97) | (Trend P = 0.09) |
| Age-, sex- and examination year-adjusted RR (95% CI) | 1.66 (0.47–5.86) | 0.54 (0.12–2.45) | 0.26 (0.03–2.42) | (Trend P = 0.08) | |
aParticipants who underwent FNAC/participants who completed confirmatory testing.
No dose-dependent pattern emerged according to geographical distribution of absorbed doses (by municipality, as estimated by UNSCEAR) for the detection rate of thyroid cancer among participants within 4–6 years after the Fukushima Daiichi NPP accident. We previously reported that regional and individual external radiation doses were not associated with the prevalence and/or detection rate of thyroid cancer after the accident [2, 4, 6, 7]. Therefore, our current results underscore that both external radiation exposure and the absorbed dose in the thyroid, including that from internally deposited radionuclides, shows no association with an increased risk of thyroid cancer among children in Fukushima.
In contrast, a recent ecological study suggested a positive correlation between the average June 2011 radiation dose-rates in Fukushima Prefecture’s 59 municipalities and the corresponding thyroid cancer detection rates from October 2011 to March 2016 [8]. However, this discrepancy can be explained by how radiation doses were estimated and by variations in the fraction of cases that proceeded to FNAC examination in confirmatory testing among Fukushima’s 59 municipalities. More specifically, the previous ecological study [8] based associations on average dose rates in the municipalities according to ground deposition densities of cesium measured in June 2011. On the other hand, our absorbed dose estimates take account of evacuation scenarios for those residents near the Fukushima Daiichi NPP who were evacuated shortly after the NPP accident. Therefore, we were able to analyse the associations between radiation doses and detection of thyroid cancer more accurately.
In addition, the FNAC rates among those receiving confirmatory testing varied by municipality [9]. Municipalities that had lower exposure rates also tended to have lower rates of FNAC. Therefore, rates of thyroid cancer detection in these lower-exposed municipalities were likely lower because the fraction of those receiving FNAC is strongly associated with the detection rate of thyroid cancer. In other words, such underestimation may have contributed to an erroneous conclusion. Despite the fact that age- and sex-adjusted RRs in the low-middle and high-middle quartiles, compared with the lowest quartile, were 2.00 and 1.34, respectively, for the 6–14-year-old group (Table 1), the adjusted RRs decreased to 1.42 and 0.90 after further adjustment for the examination year. In fact, this adjustment is strongly associated with the respective FNAC rates. Furthermore, the rates of FNAC for 6–14-year-old participants in the low-middle, high-middle and highest quartiles were higher (low-middle, 12.6%; high-middle, 16.1%; and highest, 11.5%) than those in the lowest quartile (7.4%; Table 1). On the other hand, our findings did not reveal any significant difference in the proportions of participants needing additional confirmatory testing among the groups: 0.97, 1.01, 0.98 and 0.99 for the lowest, low-middle, high-middle and the highest quartile, respectively (Table 1). These findings may support our hypothesis, although the underlying causes for the distinct differences in the FNAC rates among the quartile groups are not completely elucidated.
The strengths of this study are the inclusion of a relatively large number of residents in a prospective manner, including both evacuees and non-evacuees following the Fukushima accident. Ultrasonography was used to investigate the thyroid glands of participants in a baseline survey and subsequent follow-ups. Furthermore, to avoid an ecology-based fallacy, we analysed associations between absorbed radiation doses and detection rate of thyroid cancer after adjusting for confounding factors, such as age, sex and examination year, whereas previous ecological research could not adjust for such factors [8, 10]. Nevertheless, there are potential limitations of the study that need to be considered. First, although absorbed doses were estimated by UNSCEAR according to evacuation scenarios, individual absorbed doses could not be uniquely established. In fact, UNSCEAR has stated that dose estimates in their 2013 report may be higher than actual exposures as a result of the limited information available before UNSCEAR had completed its 2013 report [5]. Therefore, associations between the absorbed doses and the detection rate of thyroid cancer may be underestimated/overestimated. Second, although all participants who underwent secondary confirmatory examinations at any location and were diagnosed with thyroid cancer were counted, some who did not undergo primary examination or secondary confirmatory examination at follow-up could have undocumented thyroid cancer in the present study. This could modify the results. However, if the proportion of such participants did not differ among the absorbed-dose quartile groups, associations between doses to the thyroid and detection rate of thyroid cancer would not be affected by this issue. Furthermore, because of the short timeframe used to assess all thyroid cancers, further long-term follow-up surveys need to be conducted to clarify the effects of low-dose radiation exposure on thyroid cancer in Fukushima.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
FUNDING
This work was supported by the National Health Fund for Children and Adults Affected by the Nuclear Incident that facilitated the efficient design and conduction of our study.
ACKNOWLEDGMENTS
The authors thank the staff of the Fukushima Health Management Survey for their significant contributions. The authors also thank Professor Gen Suzuki (International University of Health and Welfare), Professor Tomotaka Sobue (Osaka University) and Dr. Kota Katanoda (National Cancer Center Japan) for their valuable advice on the manuscript. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the Fukushima Prefecture Government.
REFERENCES
- 1. Iglesias ML, Schmidt A, Ghuzlan AA et al. Radiation exposure and thyroid cancer: A review. Arch Endocrinol Metab 2017;61:180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ohira T, Ohtsuru A, Midorikawa S et al. External radiation dose, obesity, and risk of childhood thyroid cancer after the Fukushima Daiichi nuclear power plant accident: A prospective study of the Fukushima health management survey. Epidemiology 2019;30:853–60. [DOI] [PubMed] [Google Scholar]
- 3. Yasumura S, Hosoya M, Yamashita S et al. Study protocol for the Fukushima health management survey. J Epidemiol 2012;22:375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ohira T, Takahashi H, Yasumura S et al. Associations between childhood thyroid cancer and external radiation dose after the Fukushima Daiichi nuclear power plant accident. Epidemiology 2018;29:e32–4. [DOI] [PubMed] [Google Scholar]
- 5. UNSCEAR 2013 REPORT Vol. I Sources, effects and risks of ionizing radiation. https://www.unscear.org/unscear/en/publications/b2013_1.html (15 November 2019, date last accessed)
- 6. Ohira T, Takahashi H, Yasumura S et al. Comparison of childhood thyroid cancer prevalence among 3 areas based on external radiation dose after the Fukushima Daiichi nuclear power plant accident: The Fukushima health management survey. Medicine (Baltimore) 2016;95:e4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suzuki S, Suzuki S, Fukushima T et al. Comprehensive survey results of childhood thyroid ultrasound examinations in Fukushima in the first four years after the Fukushima Daiichi nuclear power plant accident. Thyroid 2016;26:843–51. [DOI] [PubMed] [Google Scholar]
- 8. Yamamoto H, Hayashi K, Scherb H. Association between the detection rate of thyroid cancer and the external radiation dose-rate after the nuclear power plant accidents in Fukushima, Japan. Medicine (Baltimore) 2019;98:e17165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. The 28th prefectural oversite committee meeting for Fukushima health management survey. A report of thyroid ultrasound examinations (the first full-scale thyroid screening program). http://fmu-global.jp/download/thyroid-ultrasound-examinations-first-full-scale-thyroid-screening-program/?wpdmdl=3608 (15 November 2019, date last accessed).
- 10. Tsuda T, Tokinobu A, Yamamoto E et al. Thyroid cancer detection by ultrasound among residents ages 18 years and younger in Fukushima, Japan: 2011 to 2014. Epidemiology 2016;27:316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


